Submitted:
14 August 2024
Posted:
15 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
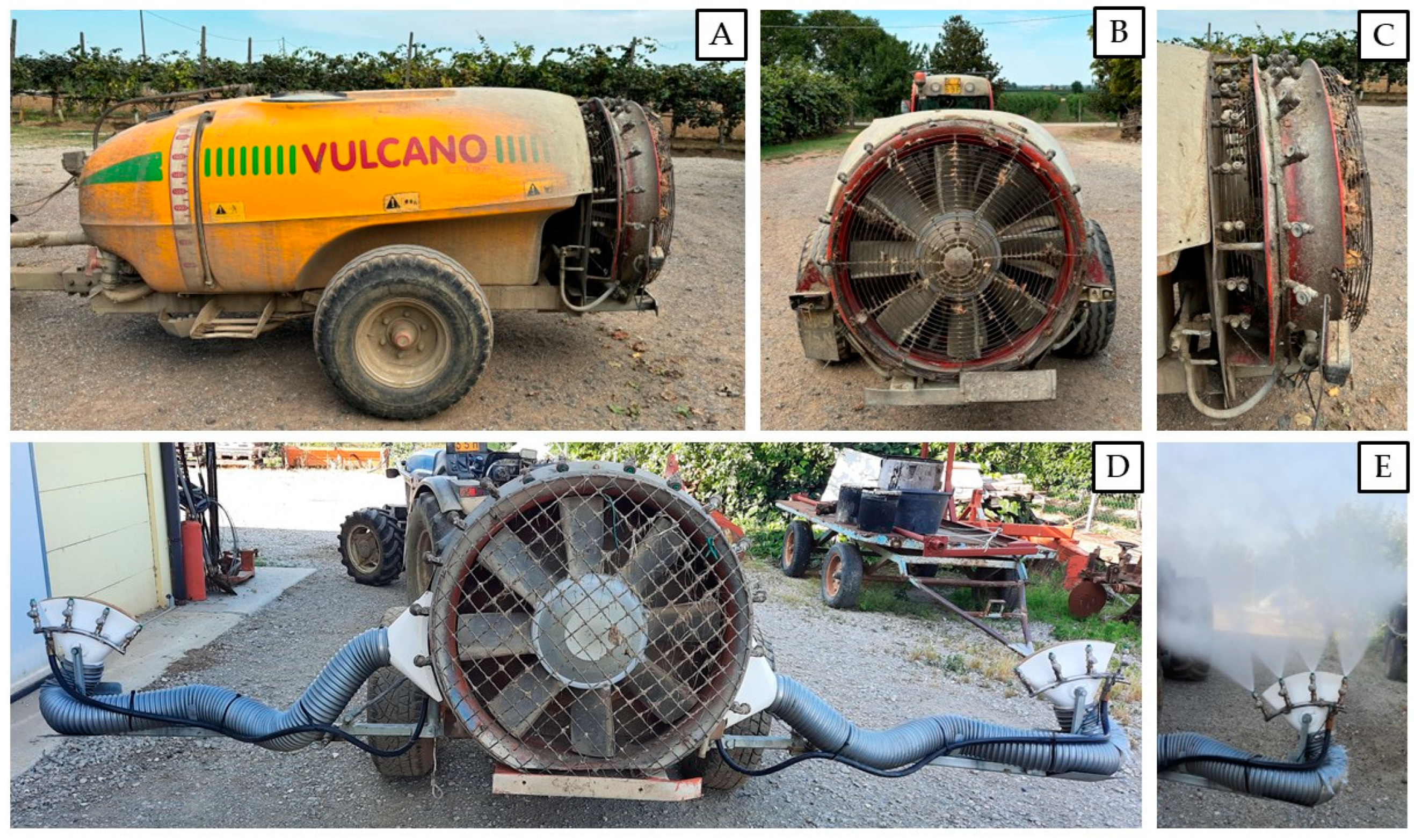
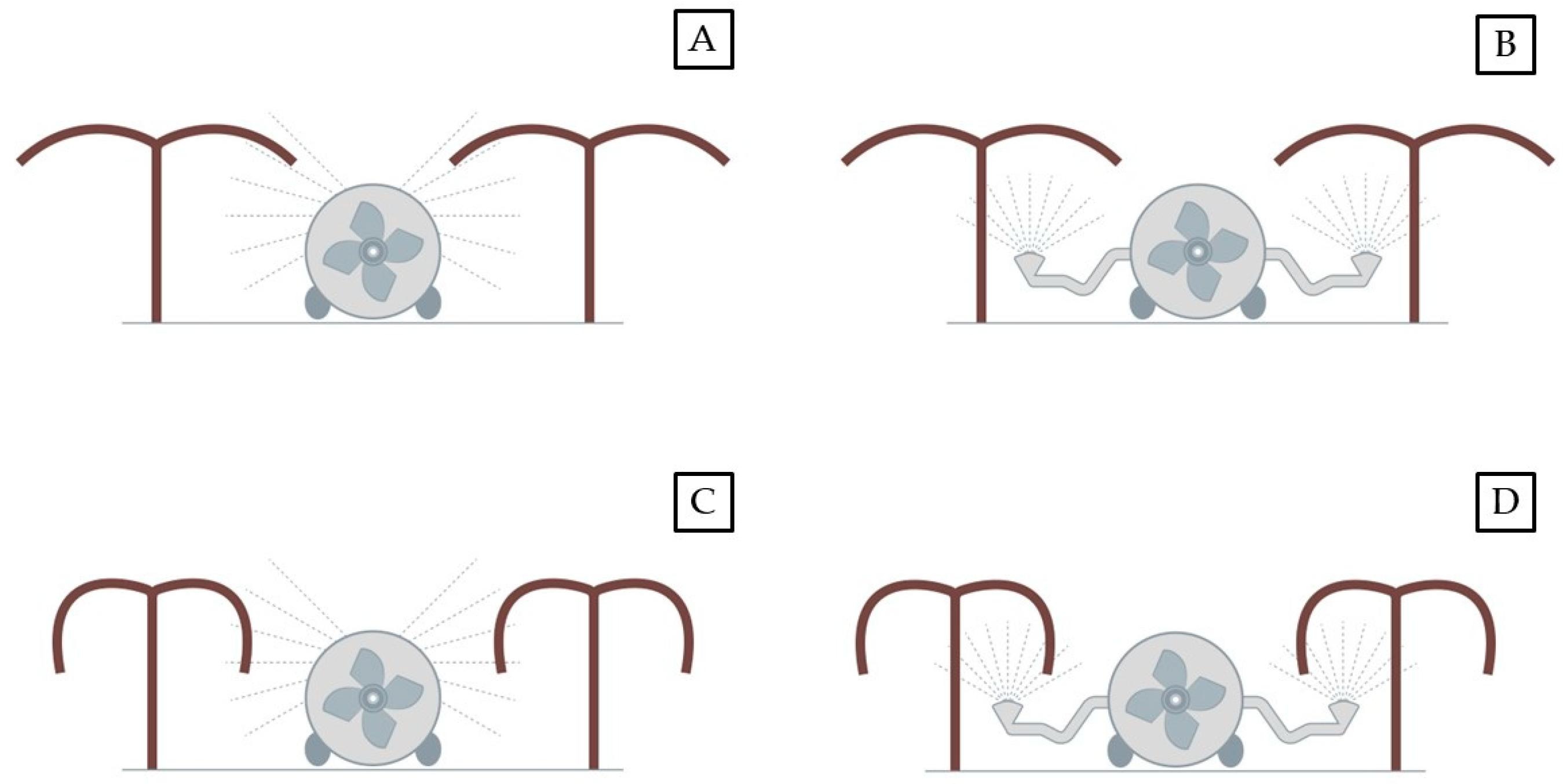
2.1. Spraying Machineries
2.2. Study Sites and Biometric Plant Parameters
2.3. Halyomorpha halys Mortality Experiments
2.4. Injury Assessment on Kiwifruit
2.5. Statistical Analyses
3. Results
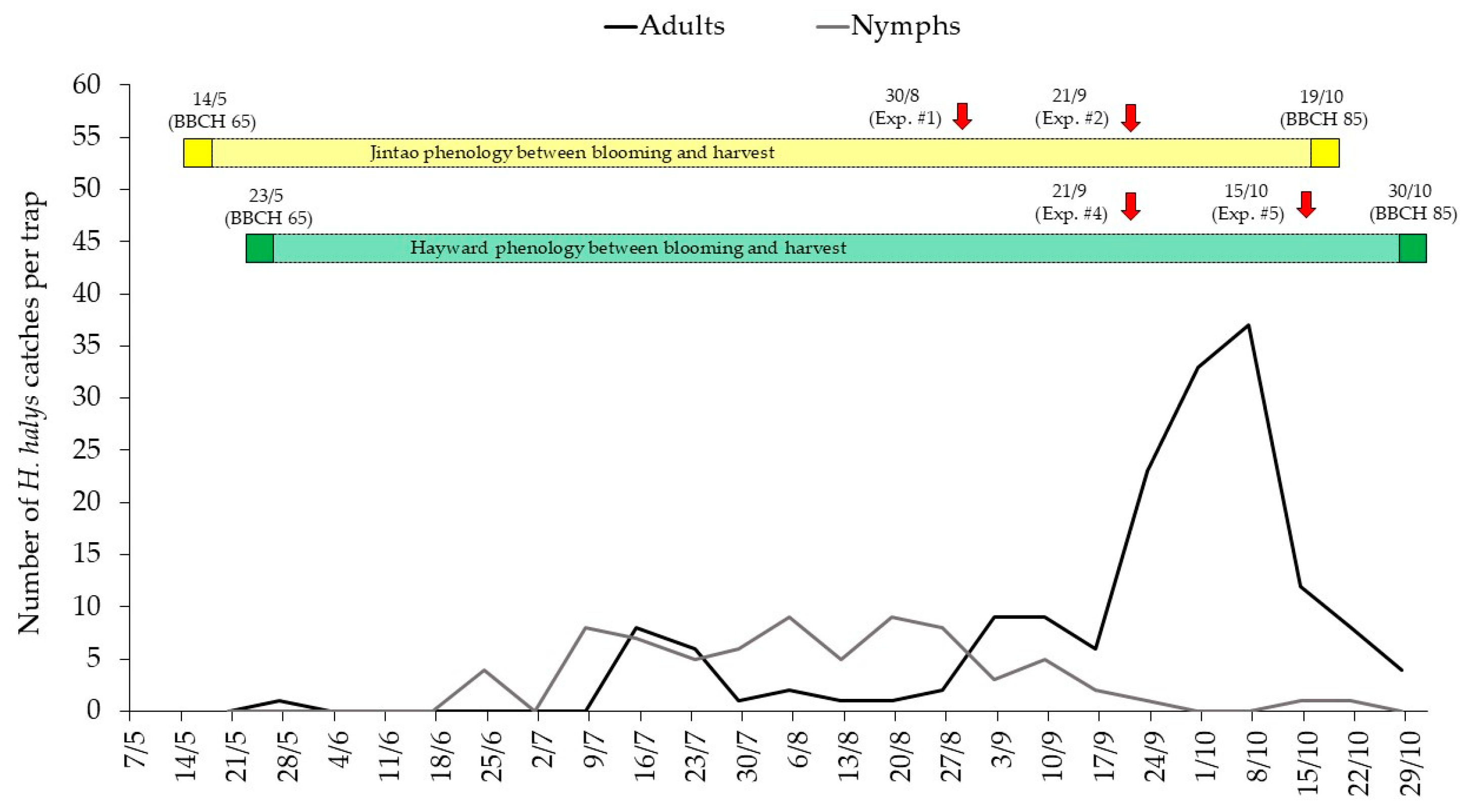
3.1. Effect of Insecticide Spary Technique on Halyomorpha halys Mortality in Conditions of Artificial Infestation
| Mean percent mortality (95% CI) | |||||
|---|---|---|---|---|---|
| Experiment | Treatment | 1 day | 3 days | 7 days | 10 days |
| #1 Deltamethrin, 30 Aug 2021 |
Control | 26.7 (17.8 - 35.6) | - | - | - |
| Conventional atomizer | 60.0 (48.5 - 71.5) | - | - | - | |
| Trumpet atomizer | 57.8 (44.7 - 70.8) | - | - | - | |
| #2 * Pyrethrins, 21 Sep 2021 |
Control | 0.0 (-) c | 4.4 (0.1 - 8.7) c | 28.9 (19.2 - 38.5) b | 28.9 (19.2 - 38.5) b |
| Conventional atomizer | 27.8 (19.1 - 36.5) a | 38.9 (29.6 - 48.2) a | 60.0 (53.1 - 66.9) a | 74.4 (65.4 - 83.5) a | |
| Trumpet atomizer | 11.1 (4.3 - 17.9) b | 23.3 (15.9 - 30.7) b | 54.4 (44.9 - 64.0) a | 74.4 (66.7 - 82.2) a | |
| #3 * Deltamethrin, 26 Sep 2022 |
Control | 2.2 (0.0 - 5.4) c | 6.7 (1.6 - 11.7) c | 24.4 (14.1 - 34.7) b | 43.3 (29.8 - 56.9) b |
| Conventional atomizer | 33.3 (21.5 - 45.1) b | 50.0 (38.8 - 61.2) b | 83.3 (76.2 - 90.5) a | 90.0 (84.2 - 95.8) a | |
| Trumpet atomizer | 67.8 (55.8 - 79.8) a | 67.8 (55.8 - 79.8) a | 82.2 (74.4 - 90.0) a | 90.0 (84.2 - 95.8) a | |
| Mean percent mortality (95% CI) | |||||
|---|---|---|---|---|---|
| Experiment | Treatment | 1 day | 3 days | 7 days | 10 days |
| #4 † Pyrethrins, 21 Sep 2021 |
Control (b) | 2.2 (0.0 - 5.4) | 11.1 (5.1 - 17.1) | 21.1 (12.8 - 29.4) | 37.8 (26.1 - 49.4) |
| Conventional atomizer (a) | 11.1 (3.6 - 18.7) | 23.3 (13.4 - 33.2) | 45.6 (35.5 - 55.6) | 57.8 (48.0 - 67.5) | |
| Trumpet atomizer (a) | 15.6 (7.7 - 23.4) | 30.0 (21.8 - 38.2) | 46.7 (38.9 - 54.4) | 72.2 (63.5 - 80.9) | |
| #5 * Deltamethrin, 15 Oct 2021 |
Control | 8.9 (1.6 - 16.1) c | 18.9 (7.2 - 30.5) c | 25.6 (12.6 - 38.5) c | 36.7 (22.3 - 51.0) c |
| Conventional atomizer | 43.3 (32.3 - 54.2) b | 47.8 (36.6 - 59.0) b | 47.8 (36.6 - 59.0) b | 58.9 (48.2 - 69.6) b | |
| Trumpet atomizer | 81.1 (73.3 - 88.9) a | 85.6 (78.5 - 92.6) a | 93.3 (88.3 - 98.4) a | 94.4 (89.7 - 99.2) a | |
| #6 † Deltamethrin, 26 Sep 2022 |
Control (c) | 4.4 (0.1 - 8.7) | 12.2 (6.1 - 18.3) | 22.2 (12.2 - 32.2) | 45.6 (34.5 - 56.6) |
| Conventional atomizer (b) | 46.7 (33.0 - 60.4) | 56.7 (43.9 - 69.4) | 71.1 (59.9 - 82.3) | 77.8 (68.3 - 87.2) | |
| Trumpet atomizer (a) | 83.3 (74.2 - 92.4) | 94.4 (89.7 - 99.2) | 100.0 (-) | 100.0 (-) | |
| Experiment | Factor | F | df1 | df2 | p value |
|---|---|---|---|---|---|
| #2 Pyrethrins, 21 Sep 2021 |
Treatment | 3.61 | 2 | 150.58 | 0.03 |
| Days of exposure | 48.97 | 3 | 276.83 | < 0.001 | |
| Treatment x Days of exposure | 3.43 | 6 | 273.97 | 0.002 | |
| #3 Deltamethrin, 26 Sep 2022 |
Treatment | 44.17 | 2 | 94.20 | < 0.001 |
| Days of exposure | 44.19 | 3 | 264.86 | < 0.001 | |
| Treatment x Days of exposure | 3.12 | 6 | 267.40 | 0.01 | |
| #4 Pyrethrins, 21 Sep 2021 |
Treatment | 12.80 | 2 | 95.63 | < 0.001 |
| Days of exposure | 49.39 | 3 | 254.10 | < 0.001 | |
| Treatment x Days of exposure | 0.98 | 6 | 259.59 | 0.44 | |
| #5 Deltamethrin, 15 Oct 2021 |
Treatment | 40.42 | 2 | 95.79 | < 0.001 |
| Days of exposure | 22.04 | 3 | 279.82 | < 0.001 | |
| Treatment x Days of exposure | 4.13 | 6 | 277.14 | < 0.001 | |
| #6 Deltamethrin, 26 Sep 2022 |
Treatment | 34.99 | 2 | 112.15 | < 0.001 |
| Days of exposure | 8.77 | 3 | 272.35 | < 0.001 | |
| Treatment x Days of exposure | 1.92 | 6 | 280.90 | 0.08 |
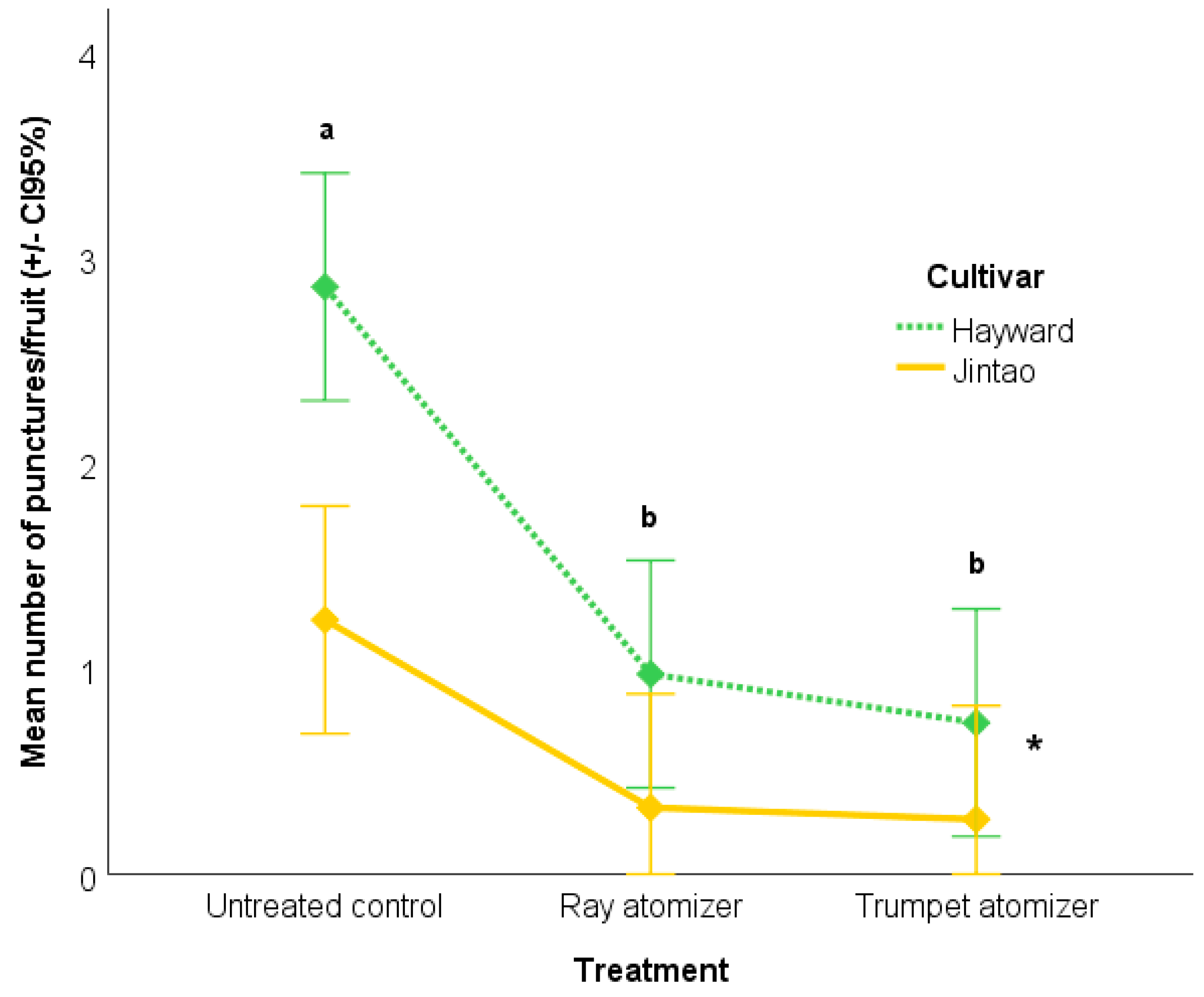
3.2. Effect of Insecticide Spary Technique On Fruit Damage Caused by Halyomorpha halys Natural Infestation
3.3. Actinidia chinensis Biometric Plant Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferguson, A. R., Huang, H., & Costa, G. History of Kiwifruit: Evolution of a Global Crop. In Kiwifruit: Botany, Production and Uses. Richardson A., Jeremy Burdon J., Ferguson A.R. (Eds). 2023, 1-15. CABI Publishing. [CrossRef]
- Ferguson, A. R. Kiwifruit: a botanical review. Janick, J. (Ed); Horticultural Reviews, 1984, 6.
- Ferguson, A. R. Kiwifruit: the wild and the cultivated plants. Advances in food and nutrition research, 2013, 68, 15-32. [CrossRef]
- Richardson, A. C., & Burdon, J. N. Kiwifruit: Future Potential, Risks and New Directions. In Kiwifruit: Botany, Production and Uses. Richardson A., Jeremy Burdon J., Ferguson A.R. (Eds). 2023, 385-390. CABI Publishing. [CrossRef]
- Bassi, I., Gori, E., Iseppi, L., & Troiano, S. Rasch model in agriculture: the attitude towards kiwifruit. In Proceedings of WRFASE International Conference, 2023, 37-43. Institute for Technology and Research (ITRESEARCH). ISBN 978-93-90150-32-8.
- Donati, I., Cellini, A., Sangiorgio, D., Vanneste, J. L., Scortichini, M., Balestra, G. M., & Spinelli, F. Pseudomonas syringae pv. actinidiae: Ecology, infection dynamics and disease epidemiology. Microbial Ecology, 2020, 80, 81-102. [CrossRef]
- Donati, I., Cellini, A., Sangiorgio, D., Caldera, E., Sorrenti, G., & Spinelli, F. Pathogens associated to kiwifruit vine decline in Italy. Agriculture, 2020, 10(4), 119. [CrossRef]
- Francati, S., Masetti, A., Martinelli, R., Mirandola, D., Anteghini, G., Busi, R., Dalmonte, F., Spinelli., F., Burgio, G., & Dindo, M. L. Halyomorpha halys (Hemiptera: Pentatomidae) on kiwifruit in northern Italy: Phenology, infestation, and natural enemies assessment. Journal of Economic Entomology, 2021, 114(4), 1733-1742. [CrossRef]
- FAOSTAT, 2024. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 9 August 2024).
- Testolin, R., & Ferguson, A. R. Kiwifruit (Actinidia spp.) production and marketing in Italy. New Zealand Journal of Crop and Horticultural Science, 2009, 37(1), 1-32. [CrossRef]
- Donati, I., Onofrietti, C., Raule, N., Cellini, A., Manzoni, L., Spinelli, F. and Xylogiannis, E. Effect of trunk girdling on fruit production, quality and storability in A. chinensis var. chinensis ‘Zesy002’ in Italy. Acta Horticulturae, 2023, 1366, 461-468. [CrossRef]
- Chamberlain J.E. Global industry and Markets. In Kiwifruit: Botany, Production and Uses. Richardson A., Jeremy Burdon J., Ferguson A.R. (Eds). 2023, 16-28. CABI Publishing. [CrossRef]
- Lee, D. H., Short, B. D., Joseph, S. V., Bergh, J. C., & Leskey, T. C. Review of the biology, ecology, and management of Halyomorpha halys (Hemiptera: Pentatomidae) in China, Japan, and the Republic of Korea. Environmental Entomology, 2013, 42(4), 627-641. [CrossRef]
- Haye, T., Gariepy, T., Hoelmer, K., Rossi, J. P., Streito, J. C., Tassus, X., & Desneux, N. Range expansion of the invasive brown marmorated stinkbug, Halyomorpha halys: an increasing threat to field, fruit and vegetable crops worldwide. Journal of Pest Science, 2015, 88, 665-673. [CrossRef]
- Lee, D. H. Current status of research progress on the biology and management of Halyomorpha halys (Hemiptera: Pentatomidae) as an invasive species. Applied Entomology and Zoology, 2015, 50, 277-290. [CrossRef]
- Leskey, T. C., & Nielsen, A. L. Impact of the invasive brown marmorated stink bug in North America and Europe: history, biology, ecology, and management. Annual Review of Entomology, 2018, 63(1), 599-618. [CrossRef]
- Andreadis, S. S., Navrozidis, E. I., Farmakis, A., & Pisalidis, A. First evidence of Halyomorpha halys (Hemiptera: Pentatomidae) infesting Kiwi fruit (Actinidia chinensis) in Greece. Journal of Entomological Science, 2018, 53(3), 402-405. [CrossRef]
- Moore, L. C. Population dynamics of the brown marmorated stink bug, Halyomorpha halys (Stal), in the Veneto region of Italy and its damage potential to kiwifruit and cherry. Doctoral dissertation, University of Georgia, 2018. https://getd.libs.uga.edu/pdfs/moore_logan_c_201808_ms.pdf.
- Tamburini, G., Laterza, I., Nardi, D., Mele, A., Mori, N., Pasini, M., Scaccini, D., Pozzebon, A., & Marini, L. Effect of landscape composition on the invasive pest Halyomorpha halys in fruit orchards. Agriculture, Ecosystems & Environment, 2023, 353, 108530. [CrossRef]
- Cianferoni, F., Graziani, F., Dioli, P., & Ceccolini, F. Review of the occurrence of Halyomorpha halys (Hemiptera: Heteroptera: Pentatomidae) in Italy, with an update of its European and World distribution. Biologia, 2018, 73, 599-607. [CrossRef]
- Bariselli, M., Bugiani, R., & Maistrello, L. Distribution and damage caused by Halyomorpha halys in Italy. Eppo Bulletin, 2016, 46(2), 332-334. [CrossRef]
- Maistrello, L., Vaccari, G., Caruso, S., Costi, E., Bortolini, S., Macavei, L., Foca, G., Ulrici, A., Bortolotti, P., P., Nannini, R., Casoli, L., Fornaciari, M., Mazzoli, G. L., & Dioli, P. Monitoring of the invasive Halyomorpha halys, a new key pest of fruit orchards in northern Italy. Journal of Pest Science, 2017, 90, 1231-1244. [CrossRef]
- Lara, J. R., Kamiyama, M., Hernandez, G., Lewis, M., & Hoddle, M. S. Laboratory assessment of feeding injury and preference of brown marmorated stink bug, Halyomorpha halys Stål (Hemiptera: Pentatomidae), for Actinidia chinensis var. deliciosa ‘Hayward’(Zespri® Green) and Actinidia chinensis var. chinensis ‘Zesy002’(Zespri® SunGold). New Zealand Entomologist, 2018, 41(1), 12-24. [CrossRef]
- Chen, J. H., Avila, G. A., Zhang, F., Guo, L. F., Sandanayaka, M., Mi, Q. Q., Shi, S. S., & Zhang, J. P. Field cage assessment of feeding damage by Halyomorpha halys on kiwifruit orchards in China. Journal of Pest Science, 2020, 93, 953-963. [CrossRef]
- Preti, M., Moretti, C., Landi, M., Bombardini, E., Tommasini, M. G., Foca, G., Ulrici, A., Maistrello, L. Stink bug study reveals Gold3 preference. NZ Kiwifruit Journal, 2021, 39-41. https://iris.unimore.it/handle/11380/1239788.
- Conti, E., Avila, G., Barratt, B., Cingolani, F., Colazza, S., Guarino, S., Hoelmer, K., Laumann, R. A., Maistrello, L., Martel, G., Peri, E., Rodriguez-Saona, C., Rondoni, G., Rostás, M., Roversi, P. F., Sforza, R. F.H., Tavella, L., & Wajnberg, E. Biological control of invasive stink bugs: review of global state and future prospects. Entomologia Experimentalis et Applicata, 2021, 169(1), 28-51. [CrossRef]
- Iacovone, A., Masetti, A., Mosti, M., Conti, E., & Burgio, G. Augmentative biological control of Halyomorpha halys using the native European parasitoid Anastatus bifasciatus: Efficacy and ecological impact. Biological Control, 2022, 172, 104973. [CrossRef]
- Zapponi, L., Tortorici, F., Anfora, G., Bardella, S., Bariselli, M., Benvenuto, L., Bernardinelli, I., Butturini, A., Caruso, S., Colla, R., Costi, E., Culatti, P., Di Bella, E., Falagiarda, M., Giovannini, L., Haye, T., Maistrello, L., Malossini, G., Marazzi, C., Marianelli, L., Mele, A., Michelon, L., Moraglio, S. T., Pozzebon, A., Preti, M., Salvetti, M., Scaccini, D., Schmidt, S., Szalatnay, D., Roversi, P. F., Tavella, L., Tommasini, M. G., Vaccari, G., Zandigiacomo, P., & Sabbatini-Peverieri, G. (2021). Assessing the distribution of exotic egg parasitoids of Halyomorpha halys in Europe with a large-scale monitoring program. Insects, 2021, 12(4), 316. [CrossRef]
- Avila, G. A., Chen, J., Li, W., Alavi, M., Mi, Q., Sandanayaka, M., Zhang, F., & Zhang, J. Seasonal abundance and diversity of egg parasitoids of Halyomorpha halys in kiwifruit orchards in China. Insects, 2021, 12(5), 428. [CrossRef]
- Candian, V., Pansa, M. G., Briano, R., Peano, C., Tedeschi, R., & Tavella, L. Exclusion nets: A promising tool to prevent Halyomorpha halys from damaging nectarines and apples in NW Italy. Bulletin of Insectology, 2018, 71(1), 21-30. ISSN 1721-8861.
- Candian, V.; Pansa, M.G.; Santoro, K.; Spadaro, D.; Briano, R.; Peano, C.; Tavella, L.; Tedeschi, R. First multi-target application of exclusion net in nectarine orchards: Effectiveness against pests and impact on beneficial arthropods, postharvest rots and fruit quality. Insects, 2021, 12, 210. [CrossRef]
- Fornasiero, D., Scaccini, D., Lombardo, V., Galli, G., & Pozzebon, A. Effect of exclusion net timing of deployment and color on Halyomorpha halys (Hemiptera: Pentatomidae) infestation in pear and apple orchards. Crop Protection, 2023, 172, 106331. [CrossRef]
- Preti, M., Bombardini, E., Landi, M., Rufolo, E., Fagioli, L., Manucci, F., Fabbri, G., & Pozzebon, A. Actinidia, reti multifunzionali contro la cimice asiatica. L’Informatore Agrario, 2024, 23, 39-44.
- Weber, D. C., Morrison, W. R., Khrimian, A., Rice, K. B., Leskey, T. C., Rodriguez-Saona, C., Nielsen, A. L., & Blaauw, B. R. Chemical ecology of Halyomorpha halys: discoveries and applications. Journal of Pest Science, 2017, 90, 989-1008. [CrossRef]
- Forresi, C., Gallinucci, E., Golfarelli, M., Maistrello, L., Preti, M., & Vaccari, G. A data platform for real-time monitoring and analysis of the brown marmorated stink bug in Northern Italy. Ecological Informatics, 2024, 102713. [CrossRef]
- Leskey, T. C., Lee, D. H., Short, B. D., & Wright, S. E. Impact of insecticides on the invasive Halyomorpha halys (Hemiptera: Pentatomidae): analysis of insecticide lethality. Journal of Economic Entomology, 2012, 105(5), 1726-1735. [CrossRef]
- Kuhar, T. P., & Kamminga, K. Review of the chemical control research on Halyomorpha halys in the USA. Journal of Pest Science, 2017, 90, 1021-1031. [CrossRef]
- Morrison III, W. R., Poling, B., & Leskey, T. C. The consequences of sublethal exposure to insecticide on the survivorship and mobility of Halyomorpha halys (Hemiptera: Pentatomidae). Pest Management Science, 2017, 73(2), 389-396. [CrossRef]
- Masetti, A., Depalo, L., & Pasqualini, E. Impact of triflumuron on Halyomorpha halys (Hemiptera: Pentatomidae): laboratory and field studies. Journal of Economic Entomology, 2021, 114(4), 1709-1715. [CrossRef]
- Masetti, A., Rathé, A., Robertson, N., Anderson, D., Walker, J., Pasqualini, E., & Depalo, L. Effects of three chitin synthesis inhibitors on egg masses, nymphs and adults of Halyomorpha halys (Hemiptera: Pentatomidae). Pest Management Science, 2023, 79(8), 2882-2890.
- Masetti, A., Morelli, A., Fagioli, L., Pradolesi, G., Nicoli, R., Scagliarini, O., Tommasini, M. G., & Preti, M. Evaluation of an Attract-and-Kill strategy using Long-Lasting Insecticide Nets for the management of the Brown Marmorated Stink Bug in Northern Italy. Insects, 2024, 15(8), 577. [CrossRef]
- Lee, D. H., Short, B. D., Nielsen, A. L., & Leskey, T. C. Impact of organic insecticides on the survivorship and mobility of Halyomorpha halys (Stål) (Hemiptera: Pentatomidae) in the laboratory. Florida Entomologist, 2014, 97(2), 414-421. [CrossRef]
- Preti, M., Landi, M., Bombardini, E., Moretti, C., Masetti, A., Pasqualini, E. Validation of an experimental protocol to evaluate the effect of insecticides with an artificial infestation of Halyomorpha halys in semi-field experiments. Atti Giornate Fitopatologiche, 2020, 1, 203-210.
- Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. European Union, 2009. Available online: https://eur-lex.europa.eu/eli/reg/2009/1107/oj (accessed on 9 August 2024).
- Marchand, P. A. EU chemical plant protection products in 2023: Current state and perspectives. Agrochemicals, 2023, 2(1), 106-117. [CrossRef]
- Patterson, K. J., & Thorp, G. Vine Management Systems. In Kiwifruit: Botany, Production and Uses. Richardson A., Jeremy Burdon J., Ferguson A.R. (Eds). 2023, 184-206. CABI Publishing. [CrossRef]
- Xiloyannis, C., Dichio, B., & Mininni, A. N. Vine Nutrition and Water Requirements. In Kiwifruit: Botany, Production and Uses. Richardson A., Jeremy Burdon J., Ferguson A.R. (Eds). 2023, 164-183. CABI Publishing. [CrossRef]
- ImageJ, 2024. Available online: https://imagej.net/ij/ (accessed on 9 August 2024).
- EPPO Standards PP1/313(2) - Halyomorpha halys on fruit tree crops. EPPO Bulletin, 2021. [CrossRef]
- Preti, M., Montanari, M., Mirossevich, L., Masetti, A., Pasqualini, E. Validation of an experimental protocol to evaluate the knockdown effect of insecticides on Halyomorpha halys in field experiments. Atti Giornate Fitopatologiche, 2018, 1, 383-394.
- Preti, M., Montanari, M., Landi, M., Cavazza, F., Franceschelli, F., Mirossevich, L., Nannini, R., Bortolotti, P. P. Screening in open field and laboratory condition of pyrethroids insecticide activity against Halyomorpha halys in Emilia-Romagna. Atti Giornate Fitopatologiche, 2018, 1, 403-414.
- Commission Regulation (EU) 2024/1342 of 21 May 2024 amending Annex II to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for deltamethrin, metalaxyl, thiabendazole and trifloxystrobin in or on certain products. European Union, 2024. Available online: https://eur-lex.europa.eu/legal-content/IT/TXT/?uri=OJ%3AL_202401342 (accessed on 9 August 2024).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





