Introduction
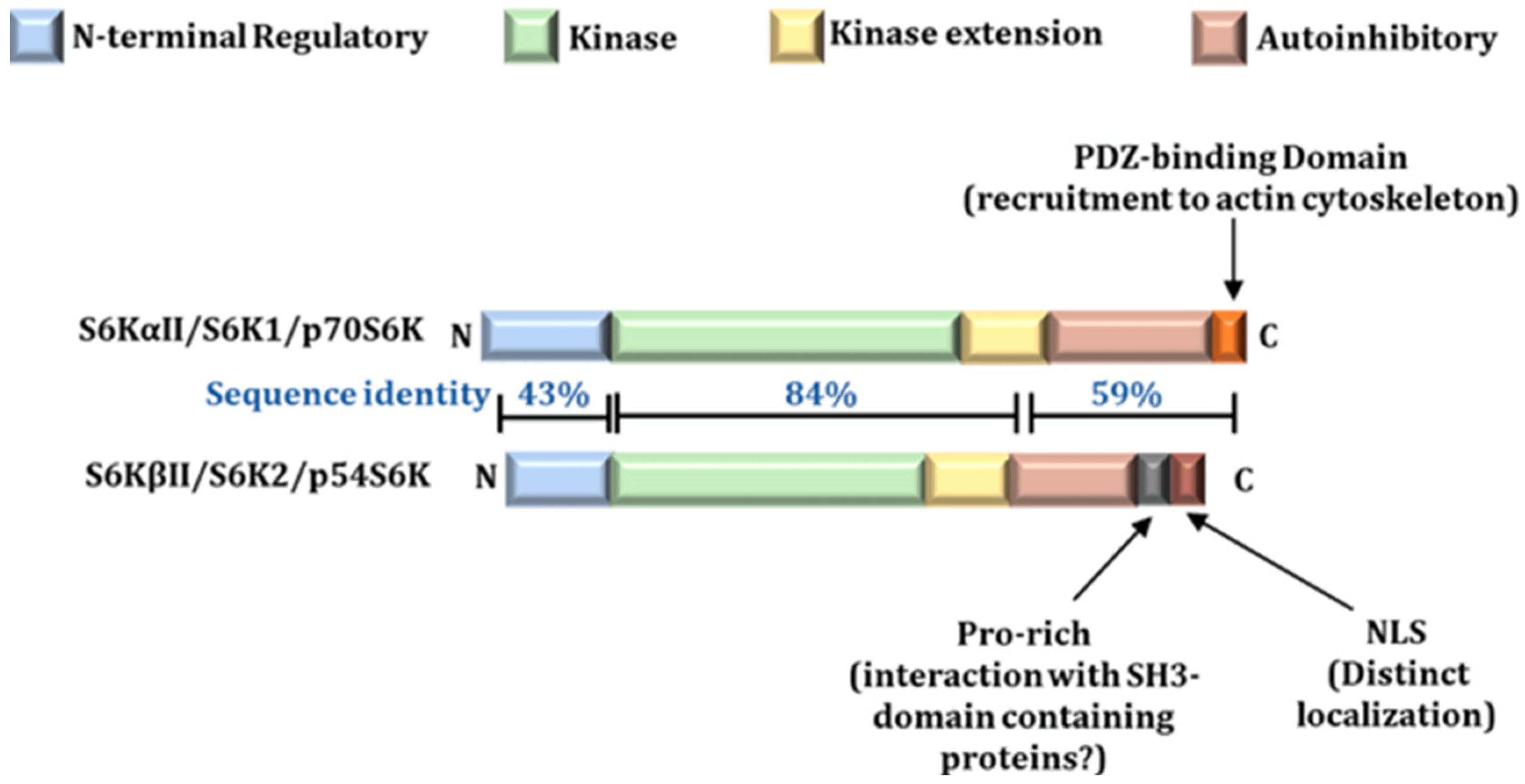
Protein kinases, acting as central nodes for cell signaling transduction, phosphorylate various downstream substrates both temporally and spatially to catalytically transmit signals originating from extracellular or intracellular changes. The AGC kinase subfamily is widely recognized as one of the most crucial hubs in response to growth factors. S6K1 and S6K2 share approximately 83% identity in their kinase domains (Sridharan and Basu, 2020). Nevertheless, lower similarity is observed in the N-terminal and C-terminal regions of the two proteins, with identities of 43% and 59%, respectively (Sridharan and Basu, 2020) (
Figure 1).
S6K1 (ribosomal protein S6 kinase beta-1) plays a crucial role in regulating various cellular processes, including protein synthesis, gene expression, lipid metabolism, and energy homeostasis, which are essential for cellular growth, proliferation, and migration (Saxton and Sabatini, 2017). In mammals, the S6K1 gene encodes several isoforms, including the two main variants, p85S6K1 and p70S6K1, produced through alternative translational start sites (Tavares et al., 2015). These isoforms differ in their subcellular localization and functional roles, with p85S6K1 predominantly localized in the nucleus and p70S6K1 primarily in the cytoplasm (Grove et al., 1991; Niwa et al., 2014). Another variant, p60-S6K1, exhibits a distinct mode of regulation, potentially independent of mTOR, the master regulator of cellular metabolism (Zaiets et al., 2018).
S6K1 has been implicated in various pathological conditions, including cancer, cardiovascular diseases, metabolic disorders, and neurodegenerative diseases, due to its involvement in key signaling pathways such as mTOR and PI3K/Akt. Dysregulation of S6K1 activity, whether through overexpression, aberrant phosphorylation, or altered isoform expression, can lead to uncontrolled cell growth, apoptosis resistance, and metabolic imbalances, contributing to disease progression (Catalán et al., 2015; Fenton and Gout, 2011). For instance, in cancer, S6K1 promotes oncogenic translation by phosphorylating components of the translation machinery, while in cardiovascular diseases, it influences vascular smooth muscle cell proliferation and inflammation (Mensah et al., 2019; Lee et al., 2020).
In the context of neurodegenerative diseases, recent studies have highlighted the role of S6K1 in modulating neuronal survival and stress responses. For example, Li et al. (2015) found that ULK1, a kinase involved in autophagy, inhibits S6K1 phosphorylation at T389, leading to reduced viability of dopaminergic neurons under MPP+ treatment, a model for Parkinson's disease. This suggests that S6K1 activity is tightly regulated in neurons, and its dysregulation may contribute to neurodegenerative processes. Similarly, Xu et al. (2014) demonstrated that inhibition of the mTOR/S6K1 pathway by Parkinson’s disease mimetics results in neuronal cell death, further implicating S6K1 in the pathology of neurodegenerative diseases.
Understanding the complex role of S6K1 in these diseases may open new avenues for therapeutic intervention, offering hope for better management and treatment of conditions such as Parkinson’s disease.
Structure of S6K1- A unique serine kinase
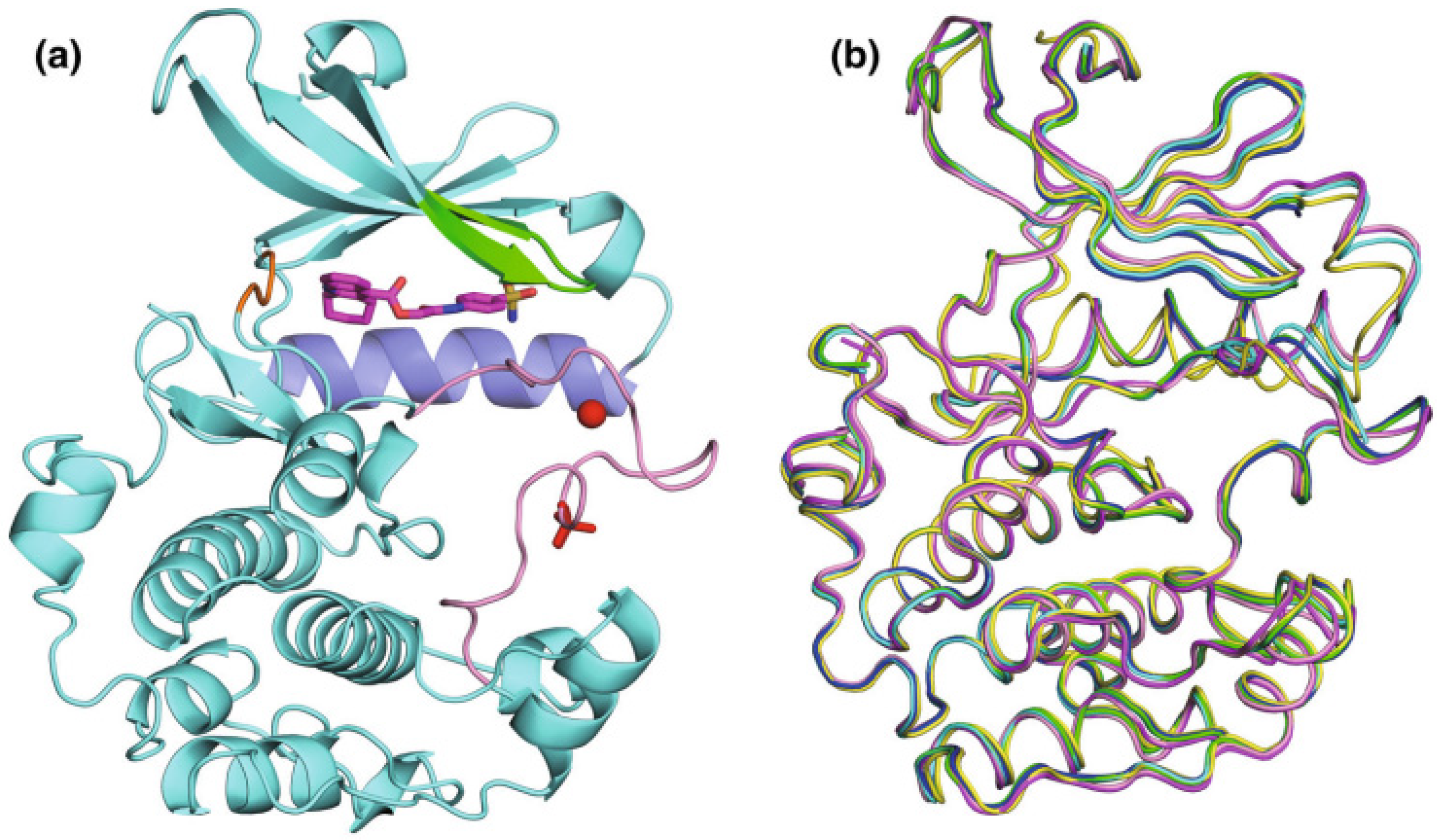
The first reported structure of S6K1 was of its kinase domain (S6K1KD) complexed with the non-specific inhibitor staurosporine. More recently, structures of S6K1 complexed with PF-4708671 have been published, including versions with either the S6K1KD protein alone or with the C-terminal extension containing the hydrophobic motif (S6K1HM) and its derivatives (Wang et al, 2013).
The crystals of S6K1KD co-crystallized with F108, F109, F179, and PF-4708671 exhibited octahedral shapes and belonged to the space group P41212. The unit cell parameters were similar to those of the S6K1KD-staurosporine crystal (PDB: 3A62). (Sunami et al, 2010). The crystal structures of S6K1KD with the inhibitors displayed the typical bilobal structures of protein kinases. The inhibitors occupied the ATP-binding pocket located between the small N-terminal lobe and the large C-terminal lobe (Niwa et al, 2014). Although the F176 and F177 complexes were crystallized in a different packing mode than the others, they exhibited a similar overall structure (
Figure 2).
SUBSTRATES AND MECHANISMS OF SUBSTRATE CATALYSIS FOR S6K1
A proteomic approach was taken to identify S6K1 targets based on the premise that these targets, like EPRS, will exhibit selective, high-affinity binding to S6K1 by Arif et al (2019). They reported that Cdk5-Mediated Phosphorylation of S6K1 at Ser424 and Ser429 in the C Terminus is required for differential target phosphorylation. Of the nine newly identified candidates in this study, three were shown to bind S6K1 in a Cdk5-dependent way, namely bifunctional coenzyme A (CoA) synthase (COASY), neutrophil gelatinase-associated lipocalin (LCN2), and Src substrate cortactin (CTTN).
Additionally, Juien et al. (2010) found that Rictor phosphorylation requires mTORC1 activity and, more specifically, the p70 ribosomal S6 kinase 1 (S6K1). Rictor at residue Thr1135 is directly phosphorylated by S6K1 in vitro and in vivo, in a rapamycin-sensitive manner. Phosphorylation of Rictor on Thr1135 did not affect mTORC2 assembly, kinase activity, or cellular localization. They report that cells expressing a Rictor T1135A mutant were found to have increased mTORC2-dependent phosphorylation of Akt. In addition, phosphorylation of the Akt substrates FoxO1/3a and glycogen synthase kinase 3α/β (GSK3α/β) was found to be increased in these cells, indicating that S6K1-mediated phosphorylation of Rictor inhibits mTORC2 and Akt signaling.
Also, Djouder et al. (2007) report that the prefoldin chaperone URI represents a mitochondrial substrate of S6K1. In growth factor-deprived or rapamycin-treated cells, URI forms stable complexes with protein phosphatase (PP)1γ at mitochondria, thereby inhibiting the activity of the bound enzyme. Growth factor stimulation induces disassembly of URI/PP1γ complexes through S6K1-mediated phosphorylation of URI at serine 371. This activates a PP1γ-dependent negative feedback program that decreases S6K1 activity and BAD phosphorylation, thereby altering the threshold for apoptosis. These findings establish URI and PP1γ as integral components of an S6K1-regulated mitochondrial pathway dedicated, in part, to oppose sustained S6K1 survival signaling and to ensure that the mitochondrial threshold for apoptosis is set in accord with nutrient and growth factor availability.
Moreover, Zhang et al. (2006) reported that S6K1 was the strongest candidate for the mTORC1-dependent kinase responsible for GSK3 phosphorylation in the absence of Akt signaling. S6K1 and S6K2 are the only members of the AGC kinase family, to which Akt belongs, known to be acutely sensitive to mTORC1 inhibition (Ghosh and Kapur, 2017). Akt and S6K1 have similar substrate specificities in vitro, and the Akt sites on GSK3α (S21) and -β (S9) closely resemble those of the S6K1 sites on eIF4B and ribosomal S6 (Zhang et al., 2006). They tested whether the constitutively active S6K1 from Tsc2−/− MEFs or HeLa cells with siRNA-mediated knockdown of TSC2 could directly phosphorylate GSK3. They found that endogenous S6K1 isolated from serum-starved TSC2-deficient cells, but not control cells, could phosphorylate recombinant GSK3β on S9 in vitro, and this activity is sensitive to pretreatment of the cells with rapamycin.
POST-TRANSCRIPTIONAL REGULATION OF S6K1
Alternative splicing of S6K1: Alternative splicing is a regulated process that allows a single gene to produce multiple different mRNA transcripts, and thus multiple different proteins. This process generates two isoforms (short and long isoforms) which vary in length through different methods. These methods include alternative 5' or 3' splice sites, exclusive exons, exon skipping, and intron retention. Alternative splicing has been implicated in so many diseases, including cancer. Studies have shown that alternative splicing of S6K1 modulates progression in different cancers. (Mei et al., 2016; Sridharan et al. 2020).
Ben-Hur et al (2013) demonstrated that human breast epithelial cells undergo transformation when short isoforms of S6K1 are overexpressed. The effects of the long S6K1 variant (Iso-1) were opposite. While its knockdown or deletion causes transformation, it prevents Ras-induced transformation and tumor formation, indicating that Iso-1 has tumor-suppressive properties. Additionally, human breast epithelial cells' transformation, anchorage-independent growth, cell motility, and growth-factor-independent 3D acinus formation were all improved by overexpressing mouse or human S6K1 short isoforms. Since it has been shown that S6K1 is a downstream effector of mTORC1, their research also revealed that the majority of the carcinogenic effects of S6K1 short isoforms are mediated by mTORC1 activation (Ben-Hur et al. 2013).
Regulation by microrna: microRNAs (miRNAs) are endogenous, 18–24 nucleotide noncoding RNAs that regulate gene expression by preventing mRNA translation and promoting mRNA instability (Bushati and Cohen, 2007). These miRNAs can bind to complementary regions in the 3' UTR of S6K1 mRNA, which can cause translational suppression or mRNA damage.
Zhong et al (2021) showed that miRNAs are crucial players in a variety of biological processes, because miRNAs negatively regulate the expression of target genes.They discovered that whereas miR-506-3p sponge raised S6K1 levels, overexpressing miR-506-3p mimic suppressed S6K1 expression. Furthermore, it was verified by dual-luciferase experiments that S6K1 has a conserved miR-506-3p binding site. In conjunction with previously released information, the findings show that miR-506-3p directly targets RPS6KB1 mRNA (Zhong et al. 2021).
TRANSCRIPTIONAL REGULATION OF S6K1
S6K1 is upregulated by estrogen via (ER)α: S6K1 is a kinase that plays a key role in cell growth and proliferation. Its upregulation in response to estrogen can contribute to the proliferation of ER-positive breast cancer cells (Zhou et al. 2014).
Maruani et al (2012) found that found that ERα mediates the estrogenic stimulation of S6K1 expression. The estrogen receptor (ER) typically binds DNA either directly through Estrogen Response Elements (EREs) or indirectly through other transcription factors. The interaction of ER with the ERE consensus sequence (CAGGTCAnnnTGACCTG) is thought to exhibit maximum ER transcriptional activity. While the promoter regions of human genes rarely include the ERE consensus sequence (Gruber et al., 2004), the promoter regions of genes that respond to estrogen typically contain many flawed palindromic sequences and extensive ERE half-sites, both of which have been demonstrated to function in concert. Because ERα activates the transcription of the S6K1 gene, the cell produces more S6K1 protein as a result of elevated S6K1 mRNA level (Maruani et al 2012).
S6K1 expression is elevated by 17q23 amplification: S6K1 expression is enhanced as a result of increased RPS6KB1 gene copy number caused by chromosomal 17q23 amplifications in breast cancer. Within the 17q23 amplicon, which also contains RPS6KB1, is the protooncogene that codes for SF2/ASF (SFRS1). Therefore, it is probable that the amplification of this area in breast cancer leads to both a rise in S6K1 levels and the development of this oncogenic S6K1 splice variant. Under low serum conditions, breast cancer cell lines with increased S6K1 expression as a result of RPS6KB1 gene amplification exhibit a significant proliferative advantage over those lines with normal S6K1 levels, while mice heterozygous for S6K1 are marginally smaller than their wild type littermates (Fenton and Gout 2011).
Upstream gene transcription regulators regulate S6K1 expression: Anaplastic oligoastrocytoma patients with poor survival status are considerably more likely to have overexpressed S6K1, which suggests that upstream gene transcription regulators such as mTOR (Mechanistic Target of Rapamycin) bind to the S6K1 gene promoter and increase S6K1 expression. A potential link between S6K1 overexpression and hypoxia in brain tumors has been suggested by the findings that S6K1 is co-overexpressed with recognized genes activated by hypoxia (Ismail, 2012).
Insulin upregulates S6K1 transcription: Many metabolic disorders, including insulin resistance and inflammation, that are linked to obesity can alter the expression of several genes, including S6K1. Under these circumstances, the S6K1 gene transcription can be elevated via transcription factors and signaling pathways that react to inflammatory and metabolic signals (Um et al 2006).
In the study conducted by Catalán et al (2015), it was shown that visceral adipose tissue (VAT) from obese patients has substantially higher levels of RPS6KB1 gene expression (which produces S6K1) than VAT from lean volunteers does. Additionally, there is a positive correlation between this upregulation and IL-6 and MCP-1, two indicators of systemic and VAT inflammation. Furthermore, the research shows that insulin therapy raises RPS6KB1 mRNA levels in human omental adipocytes in a concentration-dependent way (Catalán et al 2015).
POST-TRANSLATIONAL REGULATION OF S6K1
Regulation of S6K1 via phosphorylation at T389: There are several phosphorylation sites on S6K1, and mitogens, epidermal growth factor, and other growth factors which are kinases can phosphorylate these sites. Among these sites, a docking site for PDK1 is created when mTORC1 directly phosphorylates the hydrophobic motif at Thr389 in the linker region. Following PDK1's phosphorylation of the T-loop on Thr229, S6K1 activation occurs. The autoinhibitory pseudosubstrate domain located at the C-terminus has several proline-directed phosphorylation sites. For S6K1 to fully activate, this domain must be phosphorylated at Ser411, Ser418, Thr421, and Ser424 by ERK1/2, p38-MAPK, cdc2, and mTORC1 respectively. These kinases have all been linked to the phosphorylation of these residues (Magnuson et al 2012).
Regulation of S6K1 subcellular localization: Multiple phosphorylation/dephosphorylation events that are induced by external mitogenic stimuli regulate the S6K activity. An S6K1-binding protein, regulatory в-sub unit of Casein kinase 2 (CK2в), was studied using the yeast two-hybrid system. The formation of the complex between CK2 and S6K1 was confirmed in vitro. Also, it was shown that CK2 can phosphorylate Ser17 on S6K1. CK2 phosphorylation of S6K1 Ser17 is involved in the regulation of S6K1 export from the nucleus (Panasyuk et al 2006).
Regulation of S6K1protein stability: Acetylation and ubiquitination can interplay in complex ways to regulate S6K1 stability. They often target the same lysine residues (Lys240 and Lys389), leading to competition between these modifications. The balance between acetylation and ubiquitination on S6K1 can thus determine its stability and functional state (Yin et al 2021). Acetylation can inhibit or promote ubiquitination at specific sites, thereby influencing whether S6K1 is stabilized or targeted for degradation (Zhao et al 2015).
The discovery that the Roc1 ubiquitin ligase specifically interacts with and ubiquitinates S6K1 suggests that S6K1 and S6K2 are susceptible to degradation via different ubiquitin ligases, offering another mechanism for the differential regulation of these kinases, even though little is known about the pathways leading to S6K destabilization. Also, the discovery that S6Ks are acetylated as well and that administering deacetylase inhibitors to cells stabilizes S6K2 implies that, similar to other proteins like p53, lysine acetylation may be in opposition to the ubiquitination and degradation of S6Ks (Fenton and Gout, 2011).
PHYSIOLOGICAL ROLE OF S6K1
The functioning of living organisms relies heavily on S6K1 to regulate a variety of cellular processes. The physiological role of S6K1 refers to its essential and healthy activities within the body, these activities are important for maintaining cellular homeostasis and responding to environmental signals (Hac et al., 2021). S6K1 influences a wide array of physiological processes, ranging from proper regulation of protein synthesis to active participation in immune response (Garifulin et al., 2023).
Protein synthesis and cell growth
S6K1 phosphorylates ribosomal protein S6 and enhances the translation of a subset of mRNA, especially those with the 5’ terminal oligopyrimidine (5’TOP) motif (Kimball, 2002). Protein synthesis is a regulated cellular process where genetic information encoded in mRNA is translated into functional proteins. S6K1 supports the biosynthetic and metabolic activities necessary for cell development and growth (Zhao et al., 2024; Garifulin et al., 2023). Recent studies have explained the role of S6K1 in protein synthesis and its broader implications on cell metabolism and growth, this can be seen by the fact that the impact of S6K1 on cell development can be replicated in vitro through genome editing of cultured cells, indicating that S6K1 can self-sufficiently regulate cell growth (Kim and Choi, 2019; Fumagali and Pende, 2022; Bonucci et al., 2020).
Furthermore, a study by Arif et al., (2019) describes the phosphorylation of S6K1 and its impact on substrate selection, and the involvement of the Kinase in protein synthesis and cell growth (Arif et al., 2019). Another study by Jao et al., (2024) found out that Eukaryotic Initiation Factor 4A1 (Eif4A1) is a substrate of the CRL3-IBTK complex (a complex that mediates ubiquitination of target proteins) and that the signaling pathway involving mTOR/S6K1-IBTK-Eif4A1 regulates cap-dependent translation, which in turn supports the expression of oncoproteins and stimulates the growth of surrounding cells (Jiao et al., 2024).
Transcriptional Regulation and Cell Cycle Progression
As a key player in the mTOR signaling pathway, S6K1 influences various cellular processes including transcriptional regulation and cell cycle progression, this influence exerted by S6K1 affects gene expression by modulating the activities of transcription factors and other regulatory proteins (Marques-Ramos and Cervantes, 2023). S6K1 can phosphorylate cAMP Response Element-Binding Protein (CREB), a transcription factor that controls gene expression involved in cell metabolism, proliferation and survival (Jeon et al., 2022). During gene transcriptional regulation, S6K1 modulates chromatin structure and histone modification, which is crucial in controlling gene expression, this kinase also interacts with chromatin remodelers to alter nucleosome positioning, further regulating access for DNA transcription machinery (Jeon et al., 2022; Yi Sa et al., 2022).
Additionally, S6K1 phosphorylates Fork-head Box O Proteins (FOXO), they play crucial roles in apoptosis and cell cycle control (Gong et al., 2020). The series of event that takes place in a cell leading to its division and duplication is known as cell cycle, S6K1 phosphorylates various substrates involved in cells cycle progression, including cyclin-dependent Kinases (CDKs) and their inhibitors he can be helped (Amar-Schwatz et al., 2022).
Autophagy and Stress Response
Beyond its role in protein synthesis, S6K1 is also involved in the regulation of autophagy, a cellular process that breaks down and recycles cell components (Xu et al., 2017; Cao et al., 2020). Under nutrient-rich conditions, S6K1 activity inhibits autophagy by phosphorylating and inactivating key autophagy regulators (Cao et al., 2020). This inhibition ensures that cellular resources are used for growth rather than recycling (Xu et al., 2017). Autophagy breaks down misfolded proteins, like the mutated superoxide dismutase 1 (SOD1), which aggregates in motor neurons and is linked to amyotrophic lateral sclerosis (ALS) (Sinha, 2022). S6K1 inhibition through pathways involving mTOR allows cells to degrade and recycle damaged cells and misfolded proteins. A study by Xu et al. (2017) demonstrated that the inhibition of S6K1 using A77 1726, an active metabolite of leflunomide (an anti-inflammatory drug), resulted in unexpected outcomes in NSC34 cell (a hybrid mouse motor neuron cell line). The findings in this study suggested that S6K1 plays a more complex role in autophagy regulation than previously thought. Instead of inhibiting autophagy, blocking S6K1 might trigger other pathways that actually boost autophagic activities (Xu et al., 2017).
S6K1 also participates in cellular stress response, providing cells with the ability to manage a range of stressors such as DNA damage and oxidative stress (Cao et al., 2020; Zhong et al., 2021). Usually, oxidative stress leads to the activation of S6K1, which in turn affects various downstream targets that help curb damages caused by reactive oxygen species (ROS) (Chen, 2020). Cells must rewire their energy metabolism during times of stress to adjust to unfavorable conditions, such as nutrient deprivation or hypoxia. S6K1 helps cells effectively manage their energy reserve by controlling metabolic processes such as lipid metabolism and glycolysis (Chen, 2020; Wang et al., 2021). S6K1 interaction with signaling molecules such as p53 and Bcl-2 family proteins influences pathways that promote cell adaptability and survival (Gao et al., 2019; Ni et al., 2019).
Immune Response and Muscle Hypertrophy
The body’s defense against harmful substances, pathogens, and abnormal cells is known as the immune response; this process entails a complex network of cells, tissues, and organs cooperating to recognize and eradicate threats to or in the body (MedlinePlus, 2024). The immune response is divided into two major types: innate immunity and adaptive immunity. The former is the first line of defense that responds quickly to invaders, while the latter is more specific and develops over time as the body becomes exposed to different pathogens (Brady et al., 2020). S6K1 also has a crucial function in controlling the operation necessary for the activation and differentiation of immune cells, specifically T cells, which are vital for adaptive immunity (Li et al., 2020).
T cells require quick growth and stimulation when confronting pathogens, and these activities rely significantly on the production of proteins, a task in which S6K1 assists (Rich and Chaplin, 2019). S6K1 modulates the translation of specific mRNAs required for the growth and function of immune cells, ensuring an adequate immune response by promoting the proliferation of T cells in response to antigens and maintaining proper immune function (Rich and Chaplin, 2019).
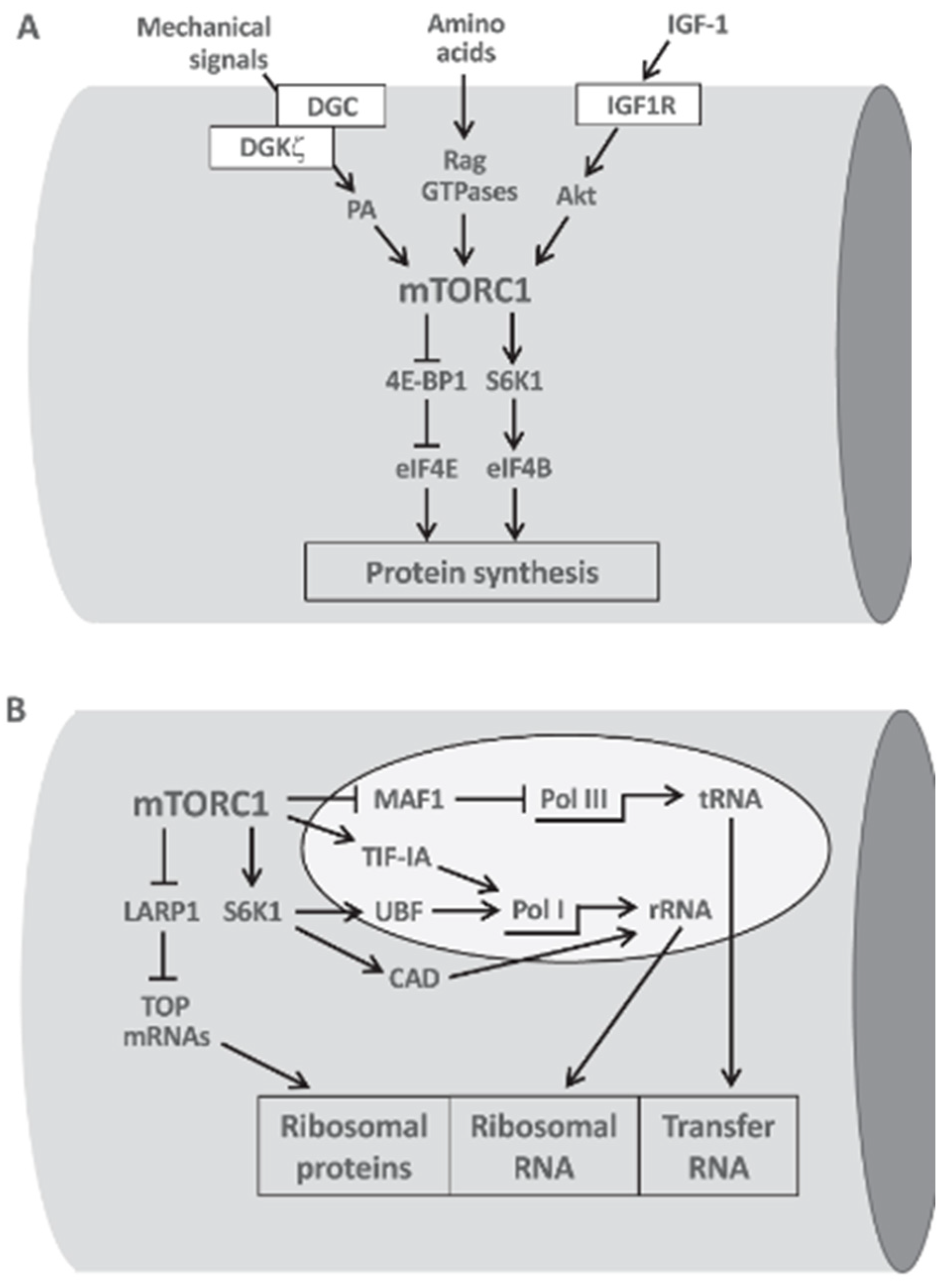
Similarly, S6K1 also plays a critical role in muscle hypertrophy. Increase in the size of muscle cells is known as muscle hypertrophy, which is primarily driven by the synthesis of new proteins (Schiaffino et al., 2021) (
Figure 3). This process is heavily influenced by S6K1, which acts as a downstream effector for the mTORC1 pathway responsible for regulating protein synthesis (Kim and Nader, 2019; Schiaffino et al., 2021) (
Figure 3). When activated by mTORC1, S6K1 phosphorylates the ribosomal protein S6 and other elements of the translation machinery, thereby enhancing the translation of mRNAs that contain the instructions for proteins necessary for muscle growth (Goodman, 2019).
The pathway is particularly active during resistance exercise and other forms of mechanical loading that stimulate muscle hypertrophy (Kim and Nader, 2019). A review article by Schiaffino et al. (2021) suggests that S6K1 not only contributes to the initial growth of muscle fiber but also plays a role in their maintenance and repair, making it a key regulator for muscle mass. The significance of S6K1’s interconnected functions highlight its importance in regulating the immune system and promoting muscle growth, emphasizing its wide-ranging impact on cellular processes.
PATHOLOGICAL ROLE OF S6K1
The ribosomal protein S6 kinase 1 (S6K1) is a central player in numerous cellular processes, as it plays indispensable roles in regulating protein synthesis, cell growth, transcriptional regulation, autophagy, stress response, immune function, and muscle hypertrophy (Sobhon et al., 2023). By mediating the mTOR signaling pathway, S6K1 ensures that cells adapt their metabolic activities according to nutrient availability and environmental stimuli, thereby maintaining cellular homeostasis and supporting organismal growth and development (Jiao et al., 2023; Goul et al., 2023). Physiologically, S6K1's involvement in translating specific mRNAs, particularly those related to cell growth and metabolism, as well as its roles in autophagy and stress response, illustrates its versatility and usefulness (Kyriakopoulos and McCullough, 2021; Fernandes and Demetriades, 2021). However, the dysregulation of S6K1 contributes to several pathological conditions such as cancer, metabolic disorders, and cardiovascular diseases. Its interference with insulin signaling pathways and excessive fat accumulation underscores the importance of regulating S6K1 (Jiao et al., 2023). The dual nature of S6K1’s role—being vital for normal physiological processes yet contributing to disease when dysregulated—highlights its importance as a therapeutic target (Fernandes and Demetriades, 2021). S6K1 stands at the crossroads of health and disease. Future research aimed at unraveling the complexities of S6K1 signaling will be crucial in developing innovative strategies to harness its physiological benefits and curb its pathological effects (Fernandes and Demetriades, 2021).
Cancer
The series of steps through which cancer develops from localized benign growth to a more aggressive malignant form that can invade surrounding tissues and spread to other parts of the body is known as cancer progression; this process is also called metastasis (Babalola et al., 2021a; Klein, 2020). The progression is often driven by cellular misbehavior, genetic mutations, and alterations in the cellular microenvironment (Lai et al., 2020).
Overexpression of S6K1 has been observed in various cancers, including breast, lung, and prostate cancers, where it correlates with increased tumor size and poor patient prognosis (Klein, 2020; Shen et al., 2020). One of the most significant roles of S6K1 in cancer is its ability to promote oncogenic translation. This is done by phosphorylating components of the translation machinery, including ribosomal protein S6 and the eukaryotic translation initiation factor 4B (eIF4B) (Wei et al., 2019). This phosphorylation enhances the translation of mRNAs that encode proteins essential for cell growth and proliferation, such as cyclin D1, c-Myc, and vascular endothelial growth factor (VEGF) (Lee et al., 2020). The enhanced translation of oncogenic proteins driven by S6K1 leads to accelerated cancer development and progression.
During metastasis, cells undergo different changes, including resistance to evasion of the immune system, apoptosis (programmed cell death), and progressive angiogenesis (the formation of new blood vessels to supply a tumor) (Lee et al., 2020). Although a major challenge in targeting S6K1 is its involvement in multiple cellular processes, which raises concern about potential side effects, understanding the mechanisms of cancer progression is crucial for developing targeted therapies and improving patient well-being (Tropée et al., 2021).
Additionally, over-expression of S6K1 has been shown to contribute to tumor development, progression, and poor prognosis in different types of cancers, such as breast, lung, and colorectal cancer (Lu et al., 2015; Artemenko, et al., 2022). Several studies indicated that S6K1 mediated cisplatin resistance in ovarian cancer (Nam et al., 2019) and selumetinib resistance in colorectal cancer (Grasso et al., 2014). In a previous study by Shen et al., (2020), it was shown that constitutive activation of S6K1 contributes to resistance against EGFR-TKIs in Non-Small Cell Lung Cancer (NSCLC) by facilitating MDM2 phosphorylation and stability (Shen et al., 2020). This suggests that targeting S6K1 may improve the efficacy of EGFR-TKIs in resistant NSCLC. Therefore, further understanding of the role of S6K1 in acquired EGFR-TKI resistance and the regulatory mechanisms that account for its increased activity in NSCLC. Knockdown of S6K1 in PDX-R cells significantly reduced cell viability and increased apoptosis compared to the control cells in the presence of gefitinib. Additionally, silencing S6K1 through siRNA transfection in PC9G cells also resulted in a decrease in the IC50 of gefitinib and a significantly greater reduction in cell viability compared to the control cells upon exposure to gefitinib. These results indicated that increased S6K1 activity contributes to the acquired resistance to gefitinib in PDX tumors and NSCLC cell lines.
Cardiovascular Diseases
Cardiovascular diseases (CVDs) are a group of disorders affecting the heart and blood vessels. They include conditions such as hypertension (high blood pressure), heart failure, peripheral artery diseases, and coronary artery diseases (Mensah et al., 2019). In recent years, the role of S6K1 in CVDs has gained significant attention due to its influence on vascular smooth muscle cells (VSMCs), inflammation, and endothelial function (Sanches-Silva et al., 2020). CVDs such as heart failure, atherosclerosis, and hypertension remain the leading cause of mortality worldwide. Understanding S6K1 is involved in the regulation of VSMC proliferation through its role in the mTOR signaling pathway (Mensah et al., 2019).
Inflammation takes part in the development and progression of CVDs, particularly atherosclerosis. S6K1 is involved in the regulation of inflammatory response through its effect on immune cells and cytokine production (Mensah et al., 2019; Sanches-Silva et al., 2020). S6K1 activation has been shown to enhance the production of pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-α), which adds to the inflammatory milieu in atherosclerosis (Du et al., 2020; Kaldirim et al., 2022).
Moreover, S6K1 is implicated in cardiac hypertrophy, a condition characterized by the enlargement of the heart muscle in response to increased workload, mostly due to hypertension (Gao et al., 2020). The kinase promotes the synthesis of proteins necessary for cardiac muscle growth, and its activation has been linked to pathological cardiac hypertrophy, which can progress to heart failure if left unchecked (Gao et al., 2020).
S6K1 plays a multifaceted role in the development and progression of cardiovascular diseases. Targeting the kinase could offer a novel therapeutic strategy for treating atherosclerosis, hypertension, and other cardiovascular conditions (Kaldirim et al., 2022). A study by Pinto et al. (2022) investigated the effects of mTORC1/S6K1 inhibition via rapamycin on liver and serum inflammation in overtrained mice. It found that rapamycin effectively reduced inflammation by decreasing IL-6 protein levels and increasing autophagy markers, suggesting that inhibiting mTORC1 can mitigate liver inflammation and enhance autophagy.
Neurodegenerative Diseases
Li et al. (2015) observed an upregulation of ULK1 mRNA and protein levels in MN9D cells treated with MPP+. The knockdown of ULK1 resulted in increased neuronal cell viability and specifically enhanced phosphorylation of S6K1 at the T389 site. They reported that T389A mutation improved the viability of MPP+-treated MN9D cells, whereas the T389D mutation reduced cell viability. These findings suggest that ULK1 inhibits S6K1 phosphorylation at T389, thereby reducing the viability of MN9D cells under MPP+ treatment. This study introduces a novel mechanism by which ULK1-mediated inhibition of S6K1 phosphorylation at T389 contributes to neurodegeneration in MPP+-treated MN9D cells and proposes a potential therapeutic approach for Parkinson's disease (PD).
In a related study, Xu et al. (2014) demonstrated that PD mimetics such as 6-hydroxydopamine, N-methyl-4-phenylpyridine, and rotenone inhibited the phosphorylation of mTOR, S6K1, and 4E-BP1, leading to reduced cell viability and activation of caspase-3 and PARP in PC12 cells and primary neurons. Overexpression of wild-type mTOR, constitutively active S6K1, or downregulation of 4E-BP1 partially prevented cell death in response to these toxins. This indicates that the inhibition of the mTOR-mediated S6K1 and 4E-BP1 pathways by PD toxins contributes to neuronal cell death.
CONCLUSION AND RECOMMENDATION
S6K1 is a versatile kinase that plays a fundamental role in various cellular processes, including protein synthesis, gene expression, and metabolic regulation. The structural complexity of S6K1, characterized by its multiple isoforms and post-translational modifications, allows it to participate in a wide range of physiological functions across different tissues. However, the dysregulation of S6K1, whether through aberrant expression, altered subcellular localization, or disrupted phosphorylation, is strongly associated with the pathogenesis of numerous diseases, including cancer, cardiovascular disorders, metabolic syndromes, and neurodegenerative conditions.
Given the critical role of S6K1 in various pathological conditions, particularly in neurodegenerative diseases, cancer, metabolic diseases and obesity, developing targeted inhibitors or modulators of S6K1 activity offers a promising therapeutic avenue (Babalola et al., 2021b; Adetobi et al., 2022; Babalola et al., 2024a; Otunba et al., 2021; Babalola et al., 2024b; Otunba et al., 2022; Babalola and Adegboyega, 2024). These therapies should focus on restoring normal S6K1 function or selectively inhibiting its dysregulated forms to prevent or mitigate disease progression. The specificity of these treatments could help minimize side effects while maximizing therapeutic efficacy.
There is a strong need for further research into the distinct roles of S6K1 isoforms, especially regarding their functions in different tissues and disease contexts. Each isoform may contribute uniquely to cellular processes and pathological states, and understanding these differences could inform the development of isoform-specific therapies. Such targeted approaches could reduce potential off-target effects and enhance the effectiveness of treatments for diseases linked to S6K1 dysregulation.
The regulation of S6K1 through post-translational modifications like phosphorylation and acetylation is a crucial area for further investigation. These modifications play significant roles in controlling S6K1 activity and stability. By unraveling the precise mechanisms behind these regulatory processes, new strategies could be developed to modulate S6K1 function in various disease contexts, potentially leading to novel therapeutic interventions.
To translate the growing body of knowledge on S6K1 into clinical practice, it is essential to identify reliable biomarkers that can monitor S6K1 activity, disease progression, and therapeutic response. These biomarkers would be invaluable in the development of precision medicine approaches, allowing treatments to be tailored to individual patient profiles and improving the accuracy and effectiveness of interventions for diseases involving S6K1 dysregulation.
Considering S6K1's involvement in multiple signaling pathways, exploring combinatorial therapies that target S6K1 alongside other key regulators, such as mTOR and Akt, may enhance therapeutic outcomes in complex diseases like cancer and neurodegeneration. By simultaneously addressing multiple pathways involved in disease progression, such combination therapies could offer a more comprehensive and effective treatment strategy, potentially improving patient outcomes and reducing the likelihood of resistance.
References
- Adetobi, E.T., Akinsuyi, S.O., Ahmed, O.A., Folajimi, E.O. and Babalola, B.A., 2022. In silico Evaluation of the Inhibitory Potential of Cymbopogonol from Cymbopogon citratus Towards Falcipain-2 (FP2) Cysteine Protease of Plasmodium falciparum. Tropical Journal of Natural Product Research, 6(10). [CrossRef]
- Ahmed, A. R., Owens, R. J., Stubbs, C. D., Parker, A. W., Hitchman, R., Yadav, R. B., ... & Sonenberg, N. (2019). Direct imaging of the recruitment and phosphorylation of S6K1 in the mTORC1 pathway in living cells. Scientific Reports, 9(1), 3408. [CrossRef]
- Amar-Schwartz, A., Ben Hur, V., Jbara, A., Cohen, Y., Barnabas, G. D., Arbib, E., ... & Tyler, J. K. (2022). S6K1 phosphorylates Cdk1 and MSH6 to regulate DNA repair. In N. Sonenberg & J. K. Tyler (Eds.), eLife, 11, e79128. [CrossRef]
- Arif, A., Jia, J., Willard, B., Li, X., & Fox, P. L. (2019). Multisite phosphorylation of S6K1 directs a kinase phospho-code that determines substrate selection. Molecular Cell, 73(3), 446-457.e6. [CrossRef]
- Artemenko, M.; Zhong, S.S.; To, S.K.; Wong, A.S. p70 S6 kinase as a therapeutic target in cancers: More than just an mTOR effector. Cancer Lett. 2022, 535, 215593. [CrossRef]
- Autophagic lipid metabolism sustains mTORC1 activity in TSC-deficient neural stem cells. (2019). Nature Metabolism. [CrossRef]
- Babalola, B.A. and Adegboyega, A.E. (2024). Computational Discovery of Novel Imidazole Derivatives as Inhibitors of SARS-CoV-2 Main Protease: An Integrated Approach Combining Molecular Dynamics and Binding Affinity Analysis. COVID, 4(6), pp.672-695. [CrossRef]
- Babalola, B.A., Adebami, G.E. and Akinsuyi, S.E. (2021a). Mechanistic basis for Cancer Immune Evasion and role of immune checkpoint blockades in Immuno-Oncology. Global Journal of Cancer Therapy, 7(1), pp.035-042. [CrossRef]
- Babalola, B.A., Adetobi, T.E., Akinsuyi, O.S., Adebisi, O.A. and Folajimi, E.O. (2021b). Computational study of the therapeutic potential of novel heterocyclic derivatives against SARS-CoV-2. Covid, 1(4), pp.757-774. [CrossRef]
- Babalola, B.A., Akinwande, A.I., Otunba, A.A., Adebami, G.E., Babalola, O. and Nwufo, C. (2024a). Therapeutic benefits of Carica papaya: A review on its pharmacological activities and characterization of papain. Arabian Journal of Chemistry, 17(1), p.105369. [CrossRef]
- Babalola, B.A., Malik, M., Sharma, L., Olowokere, O. and Folajimi, O. (2024b). Exploring the therapeutic potential of phenothiazine derivatives in medicinal chemistry. Results in Chemistry, p.101565. [CrossRef]
- Ben-Hur, V., Denichenko, P., Siegfried, Z., Maimon, A., Krainer, A., Davidson, B. and Karni, R., 2013. S6K1 alternative splicing modulates its oncogenic activity and regulates mTORC1. Cell reports, 3(1), pp.103-115. [CrossRef]
- Bhatia, V., & Sharma, S. (2021). Role of mitochondrial dysfunction, oxidative stress, and autophagy in the progression of Alzheimer’s disease. Journal of Neurological Sciences, 421, 117253. [CrossRef]
- Bonucci, M., Kuperwasser, N., Barbe, S., Koka, V., de Villeneuve, D., Zhang, C., ... & Liu, J. (2020). mTOR and S6K1 drive polycystic kidney by the control of Afadin-dependent oriented cell division. Nature Communications, 11(1), 3200. [CrossRef]
- Brady, J., Horie, S., & Laffey, J. G. (2020). Role of the adaptive immune response in sepsis. Intensive Care Medicine Experimental, 8(1), 1–19. [CrossRef]
- Bushati N, Cohen SM (2007) microRNA functions. Annu Rev Cell Dev Biol 23:175–205. [CrossRef]
- Cao, S., Shen, W. B., Reece, E. A., & Yang, P. (2020). Deficiency of the oxidative stress–responsive kinase p70S6K1 restores autophagy and ameliorates neural tube defects in diabetic embryopathy. American Journal of Obstetrics and Gynecology, 223(5), 753.e1-753.e14. [CrossRef]
- Catalán, V., Gómez-Ambrosi, J., Rodríguez, A., Ramírez, B., Andrada, P., Rotellar, F., Valentí, V., Moncada, R., Martí, P., Silva, C. and Salvador, J., 2015. Expression of S6K1 in human visceral adipose tissue is upregulated in obesity and related to insulin resistance and inflammation. Acta diabetologica, 52, pp.257-266. [CrossRef]
- Chen, H. (2020). Nutrient mTORC1 signaling contributes to hepatic lipid metabolism in the pathogenesis of non-alcoholic fatty liver disease. Liver Research, 4(1), 15–22. [CrossRef]
- Cho, S. Y. (2020). Post-Transcriptional Regulation of RNA Metabolism by mTOR Signaling (Doctoral dissertation, Weill Medical College of Cornell University).
- Clinical development of targeted and immune based anti-cancer therapies | Journal of Experimental & Clinical Cancer Research [Internet]. [cited 2024 Aug 2]. Available from: https://link.springer.com/article/10.1186/s13046-019-1094-2. [CrossRef]
- Das, F., Maity, S., Ghosh-Choudhury, N., Kasinath, B. S., & Choudhury, G. G. (2019). Deacetylation of S6 kinase promotes high glucose–induced glomerular mesangial cell hypertrophy and matrix protein accumulation. Journal of Biological Chemistry, 294(24), 9440–9460. [CrossRef]
- Di Pietrantonio, N., Palmerini, C., Pipino, C., Baldassarre, M. P. A., Bologna, G., Mohn, A., ... & Ceglia, V. (2021). Plasma from obese children increases monocyte-endothelial adhesion and affects intracellular insulin signaling in cultured endothelial cells: Potential role of mTORC1-S6K1. Biochimica et Biophysica Acta - Molecular Basis of Disease, 1867(4), 166076. [CrossRef]
- Djouder, N., Metzler, S. C., Schmidt, A., Wirbelauer, C., Gstaiger, M., Aebersold, R., Hess, D., & Krek, W. (2007). S6K1-mediated disassembly of mitochondrial URI/PP1γ complexes activates a negative feedback program that counters S6K1 survival signaling. Molecular Cell, 28(1):28-40. [CrossRef]
- Du, H., Wang, Y., Zeng, Y., Huang, X., Liu, D., Ye, L., Li, Y., Chen, X., Liu, T., Li, H. and Wu, J., 2020. Tanshinone IIA suppresses proliferation and inflammatory cytokine production of synovial fibroblasts from rheumatoid arthritis patients induced by TNF-α and attenuates the inflammatory response in AIA mice. Frontiers in pharmacology, 11, p.568. [CrossRef]
- Fenton, T.R. and Gout, I.T., 2011. Functions and regulation of the 70 kDa ribosomal S6 kinases. The international journal of biochemistry & cell biology, 43(1), pp.47-59. [CrossRef]
- Fernandes, S. A., & Demetriades, C. (2021). The multifaceted role of nutrient sensing and mTORC1 signaling in physiology and aging. Frontiers in Aging, 2. [CrossRef]
- Fig. 2: S6K1-mediated H2BS36 phosphorylation and H3K27 trimethylation suppress adiponectin expression. | Experimental & Molecular Medicine. [cited 2024 Aug 1]; Available from: https://www.nature.com/articles/s12276-022-00747-7/figures/2. [CrossRef]
- Fumagalli, S. and Pende, M., 2022. S6 kinase 1 at the central node of cell size and ageing. Frontiers in Cell and Developmental Biology, 10, p.949196. [CrossRef]
- Funkhouser, W. K. (2009). Pathology: The clinical description of human disease. Molecular Pathology, 197–207. [CrossRef]
- Gao, Q., Hou, B., Yang, H., & Jiang, X. (2019). Distinct role of 4E-BP1 and S6K1 in regulating autophagy and hepatitis B virus (HBV) replication. Life Sciences, 220, 1–7. [CrossRef]
- Gao, W., Guo, N., Zhao, S., Chen, Z., Zhang, W., Yan, F., ... & Ma, W. (2020). HTR2A promotes the development of cardiac hypertrophy by activating PI3K-PDK1-AKT-mTOR signaling. Cell Stress & Chaperones, 25(6), 899–908. [CrossRef]
- Garifulin, O. M., Zaiets, I. V., Kosach, V. R., Horak, I. R., Khoruzhenko, A. I., Gotsulyak, N. Y., Savinska, L. O., Kroupskaya, I. V., Martsynyuk, M. Y., Drobot, L. B., & Filonenko, V. V. (2023). Alterations in S6K1 isoforms expression induce epithelial to mesenchymal transition and estrogen receptor 1 silencing in human breast adenocarcinoma MCF-7 cells. Biopolymers and Cell, 39(3):189-200. [CrossRef]
- Ghosh, J., & Kapur, R. (2017). Role of mTORC1–S6K1 signaling pathway in regulation of hematopoietic stem cell and acute myeloid leukemia. *Experimental Hematology, 50*, 13-21. [CrossRef]
- Gong Y, Chen J, Jin Y, Wang C, Zheng M, He L. GW9508 ameliorates cognitive impairment via the cAMP-CREB and JNK pathways in APPswe/PS1dE9 mouse model of Alzheimer’s disease. Neuropharmacology. 2020 Mar 1;164:107899. [CrossRef]
- Goodman, C. A. (2019). Role of mTORC1 in mechanically induced increases in translation and skeletal muscle mass. Journal of Applied Physiology, 127(2), 581–590. [CrossRef]
- Goul, C., Peruzzo, R., & Zoncu, R. (2023). The molecular basis of nutrient sensing and signalling by mTORC1 in metabolism regulation and disease. Nature Reviews Molecular Cell Biology, 24(12), 857–875. [CrossRef]
- Grove, J. R., Banerjee, P., Balasubramanyam, A., Coffer, P. J., Price, D. J., Avruch, J., & Woodgett, J. R. (1991). Cloning and expression of two human p70 S6 kinase polypeptides differing only at their amino termini. *Molecular and Cellular Biology, 11*(11), 5541-5550. [CrossRef]
- Gruber, C.J., Gruber, D.M., Gruber, I.M., Wieser, F. and Huber, J.C., 2004. Anatomy of the estrogen response element. Trends in endocrinology & metabolism, 15(2), pp.73-78. [CrossRef]
- Hać, A., Pierzynowska, K., & Herman-Antosiewicz, A. (2021). S6K1 is indispensable for stress-induced microtubule acetylation and autophagic flux. Cells, 10(4), 929. [CrossRef]
- Holz, M. K. (2012). The role of S6K1 in ER-positive breast cancer. *Cell Cycle, 11*(17), 3159-3165. [CrossRef]
- IJMS | Free Full-Text | Insulin signal transduction perturbations in insulin resistance. (2021). International Journal of Molecular Sciences, 22(16), 8590. [CrossRef]
- Immune response: MedlinePlus Medical Encyclopedia [Internet]. [cited 2024 Aug 1]. Available from: https://medlineplus.gov/ency/article/000821.htm.
- Ismail, H. M. (2012). Overexpression of S6 Kinase 1 in brain tumours is associated with induction of hypoxia-responsive genes and predicts patients' survival. *Journal of Oncology, 2012*, 416927. [CrossRef]
- Jeon YJ, Yi SA, Lee J, Han JW. Nuclear S6K1 regulates cAMP-responsive element-dependent gene transcription through activation of mTOR signal pathway. Biochem Biophys Res Commun. 2022 Feb 26;594:101–8. [CrossRef]
- Jiao, D., Sun, H., Zhao, X., Chen, Y., Lv, Z., Shi, Q., ... & Li, X. (2024). mTORC1/S6K1 signaling promotes sustained oncogenic translation through modulating CRL3IBTK-mediated ubiquitination of eIF4A1 in cancer cells. eLife, 13. [CrossRef]
- Jiao, L., Liu, Y., Yu, X.Y., Pan, X., Zhang, Y., Tu, J., Song, Y.H. and Li, Y., 2023. Ribosome biogenesis in disease: new players and therapeutic targets. Signal Transduction and Targeted Therapy, 8(1), p.15. [CrossRef]
- Julien, L. A., Carriere, A., Moreau, J., & Roux, P. P. (2010). mTORC1-activated S6K1 phosphorylates Rictor on threonine 1135 and regulates mTORC2 signaling. Molecular and Cellular Biology, 30(4):908-921. [CrossRef]
- Kaldirim, M., Lang, A., Pfeiler, S., Fiegenbaum, P., Kelm, M., Bönner, F., ... & T. Müller, T. (2022). Modulation of mTOR signaling in cardiovascular disease to target acute and chronic inflammation. Frontiers in Cardiovascular Medicine, 9. [CrossRef]
- Karni, R., de Stanchina, E., Lowe, S. W., & Krainer, A. R. (2007). The gene encoding the splicing factor SF2/ASF is a proto-oncogene. *Nature Structural & Molecular Biology, 14*(3), 185–193. [CrossRef]
- Kim, A. R., & Choi, K. W. (2019). TRiC/CCT chaperonins are essential for organ growth by interacting with insulin/TOR signaling in Drosophila. Oncogene, 38(24), 4739–4754. [CrossRef]
- Kim, D., Akcakanat, A., Singh, G., Sharma, C., & Meric-Bernstam, F. (2009). Regulation and localization of ribosomal protein S6 kinase 1 isoforms. *Growth Factors, 27*(1), 12-21. [CrossRef]
- Kim, H. G., Guo, B., Nader, G. A. (2019) Regulation of Ribosome Biogenesis During Skeletal Muscle Hypertrophy. Exerc Sport Sci Rev. 47(2):91. [CrossRef]
- Kimball, S. R. (2002). Regulation of global and specific mRNA translation by amino acids. Journal of Nutrition, 132(5), 883–886. [CrossRef]
- Kispotta, S., Das, D. and Prusty, S.K., 2024. A recent update on drugs and alternative approaches for parkinsonism. Neuropeptides, p.102415. [CrossRef]
- Klein, C.A. (2020). Cancer progression and the invisible phase of metastatic colonization. Nat Rev Cancer 20, 681–694. [CrossRef]
- Kyriakopoulos, A. M., & McCullough, P. A. (2021). Synthetic mRNAs; their analogue caps and contribution to disease. Diseases, 9(3), 57. [CrossRef]
- Lai, K. P., Cheung, A., Ho, C. H., Tam, N. Y. K., Li, J. W., Lin, X., ... & Li, X. (2020). Transcriptomic analysis.
- Lee S, Roh HS, Song SS, Shin J, Lee J, Bhang DH, et al. Loss of S6K1 But Not S6K2 in the Tumor Microenvironment Suppresses Tumor Growth by Attenuating Tumor Angiogenesis. Transl Oncol. 2020 Apr 1;13(4):100767. [CrossRef]
- Lee-Fruman, K. K., Kuo, C. J., Lippincott, J., Terada, N., & Blenis, J. (1999). Characterization of S6K2, a novel kinase homologous to S6K1. *Oncogene, 18*(36), 5108-5114. [CrossRef]
- Li, K., Shen, X., Qiu, H., Zhao, T., Ai, K., Li, C., et al. (2020) S6K1/S6 axis-regulated lymphocyte activation is important for adaptive immune response of Nile tilapia. Fish Shellfish Immunol. 106:1120–30. [CrossRef]
- Li, Y., Zhang, J. and Yang, C., 2015. UNC-51-like kinase 1 blocks S6k1 phosphorylation contributes to neurodegeneration in Parkinson's disease model in vitro. Biochemical and biophysical research communications, 459(2), pp.196-200. [CrossRef]
- Lim, W. A., & Pawson, T. (2010). Phosphotyrosine signaling: evolving a new cellular communication system. *Cell, 142*(5), 661–667. [CrossRef]
- Lu, Q.; Wang, J.; Yu, G.; Guo, T.; Hu, C.; Ren, P. Expression and clinical significance of mammalian target of rapamycin/P70 ribosomal protein S6 kinase signaling pathway in human colorectal carcinoma tissue. Oncol. Lett. 2015, 10, 277–282. [CrossRef]
- Lyzogubov, V. V., Lytvyn, D. I., Dudchenko, T. M., Lubchenko, N. V., Pogrybniy, P. V., Nespryadko, S. V., Vinnitska, A. B., Usenko, V. S., Gout, I. T., & Filonenko, V. V. (2004). Immunohistochemical analysis of S6K1 and S6K2 expression in endometrial adenocarcinomas. *Experimental Oncology, 26*(4), 287-293.
- Magnuson, B., Ekim, B. and Fingar, D.C., 2012. Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks. Biochemical Journal, 441(1), pp.1-21. [CrossRef]
- Manning, G., Whyte, D. B., Martinez, R., Hunter, T., & Sudarsanam, S. (2002). The protein kinase complement of the human genome. *Science, 298*(5600), 1912-1934. [CrossRef]
- Marques-Ramos A, Cervantes R. Expression of mTOR in normal and pathological conditions. Mol Cancer. 2023 Jul 15;22(1):112. [CrossRef]
- Mei, H., Wang, Y., Fan, J. and Lin, Z., 2016. Alternative splicing of S6K1 promotes non-small cell lung cancer survival. Tumor Biology, 37, pp.13369-13376. [CrossRef]
- Mensah GA, Roth GA, Fuster V. The Global Burden of Cardiovascular Diseases and Risk Factors. J Am Coll Cardiol. 2019 Nov 19;74(20):2529–32. [CrossRef]
- Montagne, J., & Thomas, G. (2004). S6K integrates nutrient and mitogenic signals to control cell growth. Cold Spring Laboratory Press, Cold Spring Harbor, NY.
- Nam, K.H.; Yi, S.A.; Nam, G.; Noh, J.S.; Park, J.W.; Lee, M.G.; Park, J.H.; Oh, H.; Lee, J.; Lee, K.R.; et al. Identification of a novel S6K1 inhibitor, rosmarinic acid methyl ester, for treating cisplatin-resistant cervical cancer. BMC Cancer 2019, 19, 773. [CrossRef]
- Ni F, Tang H, Wang C, Zhang H, Zheng C, Zhang N, et al. Baohuoside I Inhibits the Proliferation of Pancreatic Cancer Cells via mTOR/S6K1-Caspases/Bcl2/Bax Apoptotic Signaling. Cancer Manag Res. 2019 Dec 19;11:10609–21. [CrossRef]
- Niwa, H., Mikuni, J., Tomabechi, Y., Honda, K., Ikeda, M., Ohsawa, N., Wakiyama, M., Shirouzu, M., Honma, T., & Tanaka, A. (2014). Crystal structures of the S6K1 kinase domain in complexes with inhibitors. *Journal of Structural and Functional Genomics, 15*(4), 153-164. [CrossRef]
- Otunba, A.A., Osuntoki, A.A., Okunowo, W., Olukoya, D.K. and Babalola, B.A. (2022). Characterization of novel bacteriocin PB2 and comprehensive detection of the pediocin gene ped-A1 from Pediococcus pentosaceus PB2 strain isolated from a sorghum-based fermented beverage in Nigeria. Biotechnology Reports, 36, p.e00772. [CrossRef]
- Otunba, A.A., Osuntoki, A.A., Olukoya, D.K. and Babalola, B.A. (2021). Genomic, biochemical and microbial evaluation of probiotic potentials of bacterial isolates from fermented sorghum products. Heliyon, 7(12). [CrossRef]
- Panasyuk, G.G., Nemazanyy, I.O., Zhyvoloup, A.M., Filonenko, V.V. and Gout, I.T., 2006. Regulation of S6K1 subcellular localization by Casein kinase 2. Biopolymers & Cell, 22(1), p.82. [CrossRef]
- Pardo, O. E., & Seckl, M. J. (2013). S6K2: The neglected S6 kinase family member. Frontiers in Oncology, 3:191. [CrossRef]
- Patra, T., Bose, S. K., Kwon, Y. C., Meyer, K., & Ray, R. (2021). Inhibition of p70 isoforms of S6K1 induces anoikis to prevent transformed human hepatocyte growth. Life Sciences, 265:118764. [CrossRef]
- Pearce, L. R., Komander, D., & Alessi, D. R. (2010). The nuts and bolts of AGC protein kinases. Nature Reviews Molecular Cell Biology, 11(1):9-22. [CrossRef]
- Pinto AP, da Rocha AL, Marafon BB, Nogueira JE, Branco LGS, Pauli JR, et al. Chronic rapamycin treatment decreases hepatic IL-6 protein, but increases autophagy markers as a protective effect against the overtraining-induced tissue damage. Clin Exp Pharmacol Physiol. 2022;49(8):893–902. [CrossRef]
- Rapaka, D., Bitra, V. R., Challa, S. R., Adiukwu, P. C. (2022) mTOR signaling as a molecular target for the alleviation of Alzheimer’s disease pathogenesis. Neurochem Int. 155:105311. [CrossRef]
- Razaviyan, J., Hadavi, R., Tavakoli, R., Kamani, F., Paknejad, M. and Mohammadi-Yeganeh, S., 2018. Expression of miRNAs targeting mTOR and S6K1 genes of mTOR signaling pathway including miR-96, miR-557, and miR-3182 in triple-negative breast cancer. Applied biochemistry and biotechnology, 186, pp.1074-1089. [CrossRef]
- Reinhard, C., Fernandez, A., Lamb, N. J. C., & Thomas, G. (1994). Nuclear localization of p85s6k: Functional requirement for entry into S phase. EMBO Journal, 13:1557-1565. [CrossRef]
- Rich, R. R. and Chaplin, D. D. (2019) 1 - The Human Immune Response, Editor(s): Robert R. Rich, Thomas A. Fleisher, William T. Shearer, Harry W. Schroeder, Anthony J. Frew, Cornelia M. Weyand, Clinical Immunology (Fifth Edition), Elsevier, Pages 3-17.e1.
- Rosner, M., & Hengstschläger, M. (2011). Nucleocytoplasmic localization of p70 S6K1, but not of its isoforms p85 and p31, is regulated by TSC2/mTOR. Oncogene, 30(44):4509-4522. [CrossRef]
- S6K regulates inflammageing, immunosenescence and lifespan through the endolysosomal system - Kölner UniversitätsPublikationsServer [Internet]. [cited 2024 Aug 1]. Available from: https://kups.ub.uni-koeln.de/70490/.
- Sanches-Silva A, Testai L, Nabavi SF, Battino M, Pandima Devi K, Tejada S, et al. Therapeutic potential of polyphenols in cardiovascular diseases: Regulation of mTOR signaling pathway. Pharmacol Res. 2020 Feb 1;152:104626. [CrossRef]
- Saxton, R. A., & Sabatini, D. M. (2017). mTOR signaling in growth, metabolism, and disease. Cell, 169(2):361-371. [CrossRef]
- Schiaffino S, Reggiani C, Akimoto T, Blaauw B. Molecular Mechanisms of Skeletal Muscle Hypertrophy. J Neuromuscul Dis. 2021 Jan 1;8(2):169–83. [CrossRef]
- Shen, H., Wang, G.C., Li, X., Ge, X., Wang, M., Shi, Z.M., Bhardwaj, V., Wang, Z.X., Zinner, R.G., Peiper, S.C. and Aplin, A.E. (2020). S6K1 blockade overcomes acquired resistance to EGFR-TKIs in non-small cell lung cancer. Oncogene, 39(49), pp.7181-7195. [CrossRef]
- Shum, M., Bellmann, K., St-Pierre, P., & Marette, A. (2016). Pharmacological inhibition of S6K1 increases glucose metabolism and Akt signalling in vitro and in diet-induced obese mice. Diabetologia, 59(3):592-603. [CrossRef]
- Shum, M., Houde, V. P., Bellemare, V., Junges Moreira, R., Bellmann, K., St-Pierre, P., et al. (2019). Inhibition of mitochondrial complex 1 by the S6K1 inhibitor PF-4708671 partly contributes to its glucose metabolic effects in muscle and liver cells. J Biol Chem. 294(32):12250–60. [CrossRef]
- Sinha D. Activation of mTOR signalling in the RPE cells induces alterations in melanogenesis by suppressing autophagy. Acta Ophthalmol (Copenh) [Internet]. 2022 [cited 2024 Jul 31];100(S275). Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1755-3768.2022.15353. [CrossRef]
- Sobhon P, Savedvanich G, Weerakiet S. Oxidative stress, inflammation, dysfunctional redox homeostasis and autophagy cause age-associated diseases. Explor Med. 2023 Feb 28;4(1):45–70. [CrossRef]
- Sridharan, S. and Basu, A., 2020. Distinct roles of mTOR targets S6K1 and S6K2 in breast cancer. International journal of molecular sciences, 21(4), p.1199. [CrossRef]
- Sunami, T., Byrne, N., Diehl, R. E., Funabashi, K., Hall, D. L., Ikuta, M., Patel, S. B., Shipman, J. M., Smith, R. F., Takahashi, I., Zugay-Murphy, J., Iwasawa, Y., Lumb, K. J., Munshi, S. K., & Sharma, S. (2010). Structural basis of human p70 ribosomal S6 kinase-1 regulation by activation loop phosphorylation. *Journal of Biological Chemistry, 285(8):4587-4594. [CrossRef]
- Tavares, M. R., Pavan, I. C., Amaral, C. L., Meneguello, L., Luchessi, A. D., & Simabuco, F. M. (2015). The S6K protein family in health and disease. Life Sciences, 131:1-10. [CrossRef]
- TBK1-mTOR Signaling Attenuates Obesity-Linked Hyperglycemia and Insulin Resistance | Diabetes | American Diabetes Association [Internet]. [cited 2024 Aug 2]. Available from: https://diabetesjournals.org/diabetes/article/71/11/2297/147456/TBK1-mTOR-Signaling-Attenuates-Obesity-Linked. [CrossRef]
- Tee, A. R., & Blenis, J. (2005). mTOR, translational control and human disease. Seminars in Cell & Developmental Biology, 16(1):29-37. [CrossRef]
- Tropée, R., de la Peña Avalos, B., Gough, M., Snell, C., Duijf, P.H. and Dray, E. (2021). The SWI/SNF subunit SMARCD3 regulates cell cycle progression and predicts survival outcome in ER+ breast cancer. Breast cancer research and treatment, 185, pp.601-614. [CrossRef]
- Um, S.H., D'Alessio, D. and Thomas, G., 2006. Nutrient overload, insulin resistance, and ribosomal protein S6 kinase 1, S6K1. Cell metabolism, 3(6), pp.393-402. [CrossRef]
- Wang X, Wei Z, Jiang Y, Meng Z, Lu M. mTOR Signaling: The Interface Linking Cellular Metabolism and Hepatitis B Virus Replication. Virol Sin. 2021 Dec 1;36(6):1303–14. [CrossRef]
- Wang, J., Zhong, C., Wang, F., Qu, F., & Ding, J. (2013). Crystal structures of S6K1 provide insights into the regulation mechanism of S6K1 by the hydrophobic motif. Biochemical Journal, 454(1):39-47. [CrossRef]
- Wei X, Luo L, Chen J. Roles of mTOR Signaling in Tissue Regeneration. Cells. 2019 Sep;8(9):1075. [CrossRef]
- Xu X, Sun §Jing, Song R, Doscas ME, Williamson AJ, Zhou J, et al. Inhibition of p70 S6 kinase (S6K1) activity by A77 1726, the active metabolite of leflunomide, induces autophagy through TAK1-mediated AMPK and JNK activation. Oncotarget. 2017 Mar 31;8(18):30438–54. [CrossRef]
- Xu, Y., Liu, C., Chen, S., Ye, Y., Guo, M., Ren, Q., Liu, L., Zhang, H., Xu, C., Zhou, Q. and Huang, S., 2014. Activation of AMPK and inactivation of Akt result in suppression of mTOR-mediated S6K1 and 4E-BP1 pathways leading to neuronal cell death in in vitro models of Parkinson's disease. Cellular signalling, 26(8), pp.1680-1689. [CrossRef]
- Yi SA, Jeon YJ, Lee MG, Nam KH, Ann S, Lee J, et al. S6K1 controls adiponectin expression by inducing a transcriptional switch: BMAL1-to-EZH2. Exp Mol Med. 2022 Mar;54(3):324–33. [CrossRef]
- Yin, S., Liu, L. and Gan, W., 2021. The roles of post-translational modifications on mTOR signaling. International Journal of Molecular Sciences, 22(4), p.1784. [CrossRef]
- Zaiets, I. V., Sivchenko, A. S., Khoruzhenko, A. I., & Filonenko, V. V. (2018). The Р60-S6K1 isoform of ribosomal protein S6 kinase 1 is a product of alternative mRNA translation. *Ukrainian Biochemical Journal, 90 (4):25-35. [CrossRef]
- Zhang, H. H., Lipovsky, A. I., Dibble, C. C., Sahin, M., & Manning, B. D. (2006). S6K1 regulates GSK3 under conditions of mTOR-dependent feedback inhibition of Akt. Molecular Cell, 24(2):185-197. [CrossRef]
- Zhang, P., Catterson, J. H., Grönke, S., & Partridge, L. (2024). Inhibition of S6K lowers age-related inflammation and increases lifespan through the endolysosomal system. Nature Aging, 4(4):491-509. [CrossRef]
- Zhao T, Fan J, Abu-Zaid A, Burley SK, Zheng XFS. Nuclear mTOR Signaling Orchestrates Transcriptional Programs Underlying Cellular Growth and Metabolism. Cells. 2024 Jan;13(9):781. [CrossRef]
- Zhao, J., Zhai, B., Gygi, S.P. and Goldberg, A.L., 2015. mTOR inhibition activates overall protein degradation by the ubiquitin proteasome system as well as by autophagy. Proceedings of the National Academy of Sciences, 112(52), pp.15790-15797. [CrossRef]
- Zhong FY, Li J, Wang YM, Chen Y, Song J, Yang Z, et al. MicroRNA-506 modulates insulin resistance in human adipocytes by targeting S6K1 and altering the IRS1/PI3K/AKT insulin signaling pathway. J Bioenerg Biomembr. 2021 Dec 1;53(6):679–92. [CrossRef]
- Zhong H, Yuan P, Li Y, Batonon-Alavo D, Deschamps C, Feng B, et al. Methionine Protects Mammary Cells against Oxidative Stress through Producing S-Adenosylmethionine to Maintain mTORC1 Signaling Activity. [cited 2024 Aug 1]; Available from: https://onlinelibrary.wiley.com/doi/10.1155/2021/5550196. [CrossRef]
- Zhong, F.Y., Li, J., Wang, Y.M., Chen, Y., Song, J., Yang, Z., Zhang, L., Tian, T., Hu, Y.F. and Qin, Z.Y., 2021. MicroRNA-506 modulates insulin resistance in human adipocytes by targeting S6K1 and altering the IRS1/PI3K/AKT insulin signaling pathway. Journal of Bioenergetics and Biomembranes, 53, pp.679-692. [CrossRef]
- Zhou, Z., Qiao, J. X., Shetty, A., Wu, G., Huang, Y., Davidson, N. E., & Wan, Y. (2014). Regulation of estrogen receptor signaling in breast carcinogenesis and breast cancer therapy. Cellular and Molecular Life Sciences, 71(8):1549. [CrossRef]
- Zhu Z, Yang C, Iyaswamy A, Krishnamoorthi S, Sreenivasmurthy SG, Liu J, et al. Balancing mTOR Signaling and Autophagy in the Treatment of Parkinson’s Disease. Int J Mol Sci. 2019 Jan;20(3):728. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).