1. Introduction
Brain metastasis is the most frequently occurring intracranial malignancy in adults [
1,
2]. The incidence of brain metastasis seems to have increased over the past decade and may be the paradoxical result of the effectiveness of drugs on primary cancer. In part, this is due to the fact that chemotherapeutic agents that show efficacy against systemic disease have poor penetration of the blood-tumor-barrier (BTB), which means that brain metastases are significantly undertreated [
3,
4,
5,
6]. Many brain metastasis patients exhibit multiple tumors at the time of diagnosis. Even in the event of a solitary metastasis, it is believed that the brain may be seeded with many radiographically invisible metastases [
7,
8]. Whole brain radiation therapy (WBRT) alone or in combination with surgical resection or stereotactic radiosurgery is thus the standard of care for brain metastasis patients. However, WBRT is often associated with neurological complications that preclude sufficient radiation doses to effectively treat the lesions. The prognosis for brain metastasis is extremely poor, with a median survival of 8-16 months even with combination treatment [
9,
10]. Thus, there is an urgent need to develop new diagnostic and therapeutic agents that are specific and effective for brain metastases.
Multiple strategies have been exploited to improve brain metastases access to systemic diagnostic and therapeutic agents [
11,
12]. However, limited success has been reported with the physical or chemical approaches to open BTB, primarily owing to inhomogeneous disruption of BTB and more importantly, unwanted damage of blood-brain-barrier (BBB) in normal brain, resulting in neurological toxicity from drugs or blood components entering the normal brain [
6]. Taking advantage of intravascular receptors that mediate transcytosis across the BBB, several ligands or antibodies that specifically bind to such receptors have been conjugated directly or indirectly with therapeutic compounds [
13]. Despite leading to increased drug concentrations to brain tumors, this strategy also causes toxicity to normal brain because the receptors such as transferrin, insulin and low-density lipoprotein receptor are widespread in normal brain [
14]. Clearly, discovery of a biomarker that is specific to brain metastasis will be critical for the development of brain metastasis-targeted diagnosis and therapy. Ideally, this biomarker needs to be accessible to systemically administered diagnostic and therapeutic agents. Thus, a vascular luminal surface-exposed biomarker might be particularly useful for developing brain metastasis-targeting agents, which otherwise have difficulty passing through the BTB to brain metastases.
Phosphatidylserine (PS), the most abundant anionic phospholipid of the cell membrane, is normally constrained to the inner leaflet of the plasma membrane of healthy cells. PS becomes externalized on the outer leaflet of the plasma membrane of cells during programmed cell death, apoptosis. However, PS exposure can occur in endothelial cells of tumor blood vessels and cancer cells [
15,
16]. These cells are viable and PS exposure is inducible and reversible, which is distinct from the irreversible process of PS externalization during apoptosis [
17,
18,
19]. We and others have previously reported various levels of PS-positive tumor blood vessels, ranging from 5% to 40% among multiple tumor models [
20,
21].
In the present study, we applied a monoclonal PS-targeting antibody, 1N11 to assess PS exposure on vasculature of brain metastases in mouse models. After systemic administration of 1N11, PS exposure on tumor vascular ECs was quantified by detecting 1N11+ CD31+ ECs with fluorescence microscopy imaging. Notably, 1N11+ fluorescence signal was only seen in brain metastases, coinciding well with CD31+ tumor vascular ECs. Examination of series of whole mount brain sections revealed that 1N11+ fluorescence signals clearly demarcated individual brain metastases. Since previous works suggest that PS exposure in non-apoptotic cells is possibly caused by oxidative stresses in the tumor microenvironment (TME), we investigated plausible contribution of oxidative stress-related factors including tumor hypoxia and inflammatory cytokines, tumor necrotic factor (TNF-α) and interleukin (IL)-1. To investigate the potential of targeting exposed PS for sensitive and specific imaging of brain metastases, we labeled 1N11 with a fluorescence dye, IRDye 800CW, or a radiotracer, I-125, to perform optical imaging and autoradiographic imaging of brain metastases in mice.
2. Materials and Methods
2.1. Reagents and Cell Lines
The human monoclonal antibody, 1N11, binds to PS, while Aurexis, a human monoclonal antibody, binds to an irrelevant antigen (S. aureus clumping factor A), serving as a negative control antibody. Both antibodies were generously provided by Drs. Philip Thorpe and Rolf Brekken (UT Southwestern Medical Center at Dallas). Murine breast cancer 4T1 cells and human umbilical vein endothelial cells (HUVEC) were purchased from American Type Culture Collection (ATCC, Manassas, VA). The brain-tropic human breast cancer MDA-MB-231/BR-GFP (231-Br) cell line was a kind gift from Dr. Patricia Steeg (NIH/NCI).
2.2. Breast Cancer Brain Metastasis Models
All animal procedures were approved by the Institutional Animal Care and Use Committee of Wake Forest University School of Medicine and University of Texas Southwestern Medical Center. Murine breast cancer 4T1 cells and brain-tropic human breast cancer 231-Br cells were incubated in Dulbecco’s modified Eagle’s medium (DMEM) with 10% FBS, 1% L-Glutamine and 1% penicillin-streptomycin at 37oC with 5% CO2 . Once 80% confluence was reached, the cells were harvested, and suspended in serum-free medium. Immunocompetent female BALB/c mice (n = 6; 6-8 weeks old; NCI, Frederick, MD) were used for the 4T1 model, while female nude mice (n = 16; BALB/c nu/nu, 6-8 weeks old; NCI or The Jackson Laboratory) were used for the human breast cancer 231-Br model. The mice were anesthetized with inhalation of 2% isoflurane. 2 × 105 4T1 cells or 231-BR cells (in 100 μl of serum free medium) were injected directly into the left ventricle of a mouse heart under the imaging guidance of a small animal ultrasound (Vevo 770, VisualSonics; Toronto, Canada).
2.3. Longitudinal MRI Monitoring of Development of Brain Metastases
MRI was initiated two weeks after tumor implantation and repeated once a week for up to three weeks. Animals were sedated with 3% isoflurane and maintained under general anesthesia (1.5% isoflurane). Animal body temperature and respiration were monitored and maintained constant throughout the experiment. MR measurements were performed using a 9.4T horizontal bore magnet with a Varian INOVA Unity system (Palo Alto, CA) or a 7T Bruker BioSpec 70/30 USR scanner (Bruker Biospin, Rheinstetten, Germany). A tail vein of mouse was catheterized using a 27G butterfly for Gd-DTPA (Magnevist®; Bayer HealthCare, Wayne, NJ) contrast agent administration. High resolution multi-slice (14 slices with 1mm-thick) T2-weighted coronal images, covering from the frontal lobe to the posterior fossa, were acquired with fast spin echo multiple slice sequence (TR/TE = 2500 ms/48 ms, 8 echo trains, matrix: 256 x 256, FOV 20 x 20 mm, resolution: 78 x 78 µm2 in plane). T1-weighted contrast enhanced images were acquired with spin echo multi-slice sequence (TR/TE = 400 ms/20 ms, matrix: 256 x 256, FOV 20 x 20 mm). We determined tumor volume on T2-weighted images by manually outlining the enhancing portion of the mass on each image by using standard ‘‘browser’’ software provided with the Varian Inova imaging system or the Bruker system.
2.4. Detection and Quantification of Exposed PS In Vivo
Immediately after the last MRI follow-up of the 231-Br mice (n=6), 150 μg of 1N11 or the control body Aurexis were injected i.v. and allowed to circulate for 4 h. The mice were anesthetized, exsanguinated, and perfused with heparinized saline. The mouse brains were dissected and frozen for preparation of cryosections. We analyzed the entire brain of each animal by immunohistochemistry. Vascular endothelium was stained using a rat anti-mouse CD31 antibody (1:20; Serotec Inc., Raleigh, NC, USA) followed by Alexa Fluor 350-labeled goat anti-rat IgG (1: 200; BD Biosciences, San Jose, CA). 1N11 or Aurexis was detected using goat anti-human IgG conjugated to Cy3 (1:500; Jackson Immunoresearch Laboratory, West Grove, PA). The immunostained sections of the entire brain were evaluated under Zeiss LSM510 (Carl Zeiss MicroImaging, Inc., Thornwood, NY), and the individual 10× fluorescence images were automatically stitched by using SoftWoRx (Applied Precision, Issaquah, WA). Doubly labeled endothelial cells (i.e. CD31 positive/1N11 positive) were identified on merged images. The percentage of doubly positive vessels was calculated as follows: (mean number of yellow vessels per field/mean number of total vessels) × 100. Ten random fields containing metastases were evaluated for each section.
2.5. Immunohistochemical Detection of Hypoxia and TNF-α in Brain Metastasis
To detect hypoxia, pimonidazole (60 mg/kg, Hypoxyprobe, Burlington, MA) was injected intravenously 60 mins before sacrifice. Pimonidazole was recognized by primary Mab1 (1:100; Hypoxyprobe) and followed by rabbit anti-mouse secondary conjugated to AMCA (1:300; Jackson Immunoresearch Laboratory). For TNF-α staining, anti-TNF-α (1:200; R&D Systems) was followed by horseradish peroxidase (HRP)-conjugated goat anti-rat secondary antibody (1:1000; Santa Cruz Biotechnology). After a PBST wash, sections were immersed in DAB (3,3´-diaminodbenzidine; Thermo Fisher Scientific) and then observed under microscope.
2.6. Near Infrared Fluorescence Imaging
1N11 and Aurexis F(ab’)2 fragments were generated by reacting antibodies with pepsin at a molar ratio of 1:130 (antibody:pepsin) for 1 h at 37oC. F(ab’)2 fragments (MW = 110 kDa) were purified by FPLC using an S-200 column (Pharmacia, Piscataway, NJ) and PBS running buffer. F(ab’)2 was then reacted with an N-hydroxysuccinimide ester derivative of IRDye 800CW (Li-COR, Lincoln, NE) at molar ratio of 1:10 (F(ab’)2:dye) for 2 h at room temperature. Unreacted dye was separated from the conjugate using a PD-10 desalting column (GE Healthcare, Uppsala, Sweden). Analyses of the final product, based on the absorbance of the dye at 778 nm and the absorbance of the F(ab’)2 at 280 nm, showed that it consisted of approximately 2 molecules of dye bound to each F(ab’)2 fragment. The products are referred to as 800CW-1N11 or 800CW-Aurexis throughout this manuscript. Immediately after the last MRI follow-up at 5 weeks post intracardiac injection, 800CW-1N11 or 800CW-Aurexis (2 nmol/mouse) was injected into a tail vein of the brain metastasis mice. Twenty-four hours later, the mice were sacrificed and perfused, and brains were dissected. Ex vivo fluorescence imaging (excitation, 671-705 nm; emission, 730-950 nm) was performed using a Maestro imaging system (CRI Inc. Woburn, MA). Brain tissues were then embedded in optimal cutting temperature compound (O.C.T.) and then transferred to –80oC freezer. On the second day, a series of coronal sections (10 µm) of the entire brain was cut with a cryostat. The sections containing tumor tissues were identified by H&E staining. The adjacent frozen sections were then used for ex vivo fluorescence imaging. The ex vivo images of the thin frozen sections were acquired by the same Maestro system. Typically, an exposure time of 2.0 s was applied for image acquisition. Fluorescence images were processed with the Maestro software 2.8. Lastly, the frozen sections were then stained with anti-CD31 and correlated with the 800CW-1N11 signals using an Envos microscope (AMG, Bothell, WA) equipped with near-infrared (NIR) filters.
2.7. Autoradiographic Imaging of I-125 Labeled 1N11
Iodine-125 (125I) was purchased from Perkin Elmer (Waltham, MA). Pre-coated iodination tubes and Protein A agarose were from Pierce Biotechnology (Rockford, IL). Instant thin-layer chromatography plates (ITLC-SG) were from Pall Life Sciences (East Hills, NY). F(ab’)2 fragments were generated by reacting antibodies with pepsin at a molar ratio of 1:130 (antibody:pepsin) for 1 h at 37oC. F(ab’)2 fragments were purified on a FPLC S-200 column (Pharmacia, Piscataway, NJ). The F(ab’)2 fragments were then radioiodinated using the indirect IODO-GEN method (Pierce Biotechnology). Briefly, 1-3 mCi of iodine were activated in 100 µl iodination buffer (125 mM Tris-HCL, pH 6.8, 150 mM NaCl) in a pre-coated iodination tube and then reacted with 0.2-0.6 mg F(ab’)2 in 100 µl iodination buffer in a separate uncoated tube. Free iodine was removed with Bio-Spin 6 gel filtration columns (Bio-Rad Laboratories, Hercules, CA) that were pre-blocked with iodination buffer containing 10% FBS. Radio-TLC analysis was used to determine iodination efficiency on a Rita Star Radioisotope TLC Analyzer (Straubenhardt, Germany) using ITLC-SG plates. Immediately after anatomic MRI, each mouse was given i.v. 60 µCi 125I-1N11 F(ab’)2. Forty-eight hours later, the mice were perfused, and mouse brains were dissected. Using a mouse brain matrix, 4 mm-thick brain bearing brain metastases, correlating with the MRI, were cut from each mouse brain and laid on an autoradiographic film. After 12 h incubation, the film was read using Cyclone Plus Storage Phosphor (PerkinElmer, Waltham, MA). Spatial localizations of ‘hot spots’ on radiographic images were correlated with MRI stacked images. Signal intensity of the tumor versus normal brain was also calculated.
2.8. Statistical Analysis
Statistical analysis was performed using Microsoft Excel. Data were presented as mean ± SD. Statistical significance was determined by Student’s t test. All t tests were one-tailed, unpaired and considered statistically significant if p < 0.05.
4. Discussion
A significant finding from this study is that abundant PS is externalized on the luminal surface of blood vessels of brain metastases, which can be recognized by the systemically administered PS-targeting antibody. While previous studies reported various levels of PS positive blood vessels among cancer types [
20], our data detected PS expression in ~90% of vascular ECs of brain metastases (
Figure 2 and
Figure 3). Importantly, PS exposure was found to be specific to brain metastases since there was no PS detected in normal brain, and the control antibody, Aurexis, showed no positive staining in blood vessels of brain metastases (
Figure 3). Unlike other tumor models, in which significant angiogenesis commonly leads to enhanced vascular permeability, the intracardiac 231-Br model contains many tumor lesions with an intact BTB, which was found impermeable to the small molecule MRI contrast agent, Gd-DTPA (
Figure 1 and
Figure 2). Along with micrometastases detected by histology, these small lesions may be comparable to those occult brain lesions in human. Notably, positive PS staining co-localized completely with CD31+ tumor vascular ECs. Neither PS antibody nor the control antibody, Aurexis, was seen at extravascular tumor tissues even in those brain metastases that were obviously enhanced by Gd-DTPA (
Figure 2). These data clearly indicated that biological macromolecules such as proteins were largely inaccessible to tumor parenchyma of the 231-Br brain metastases. These results are in good agreement with previous studies of correlating molecular weight with the BTB permeability in the 231-Br as well as other intracardiac models [
6]. Nonetheless, because PS externalization occurred in almost every tumor blood vessel but not normal vessel, positive immunostaining with the PS-targeting antibody successfully delineated individual brain metastases, even micrometastases, from normal brain tissues (
Figure 2 and
Figure 3).
We further demonstrated the development of PS-targeted imaging probes for sensitive and specific imaging of brain metastases. In addition to the 231-Br model, we also injected murine mammary cancer 4T1 cells intracardiacally into the immunocompetent mice. In contrast to multifocal lesions in the 231-Br mouse brain, fewer lesions, often a solitary lesion, were seen in the 4T1 mouse brain (
Figure 5). The 4T1 brain metastases were larger and enhanced on T1-w post contrast MRI (
Figure 5b). Despite most of the 800CW-1N11 probes that were detected on tumor vascular ECs, extravascular signals of 800CW-1N11 were observed (
Figure 5f), indicating that the BTB disruption is more severe in the 4T1 than the 231-Br brain metastases. Unlike many other cancer biomarkers, which are commonly confirmed with immunohistochemical or biological analyses of tissue specimen from biopsy or resection of a tumor, there is a concern that PS may become externalized during tissue resection and preparation process, and the tissues are no longer suitable for PS detection. Pre-injection of PS antibody to the patient will be necessary for accurate assessment. Alternatively, the development of an optical imaging probe targeting PS, as shown in this study, may be useful for pathological diagnosis of occult metastases. Labeling with a NIR fluorescence dye enables the light to penetrate deeper because it has lower tissue absorption and scattering of light, and is distinct from autofluorescence. Without the use of a secondary antibody for staining, the resected tissue chucks may be screened for the likelihood of brain metastases based on the observation of the optical signals (
Figure 5a). Subsequent tissue sectioning will likely provide definitive pathological diagnosis in conjunction with routine histology and immunohistochemistry (
Figure 5b). Moreover, NIR optical imaging has been increasingly applied for intraoperative detection of residual tumors and cancer metastases. The PS-targeting optical probe developed in this study may also be useful for neurosurgeons to identify occult metastases and tumor margins during surgical resection of brain metastasis.
In the present study, we have also labeled 1N11 F(ab’)
2 with I-125 and successfully applied it for autoradiography imaging of brain metastases, which provided a proof-of-concept for its potential for in vivo nuclear imaging of brain metastases. Indeed, we have previously radiolabeled a PS antibody with
74As, which is a long-lived positron emitter, and used it for PET imaging of prostate tumor in rats [
31]. Several other radioisotopes such as
64Cu for PET imaging and
111In for SPECT have also been used to image PS expression in various cancer models [
32]. Unlike metabolic radiotracers such as
18FDG for PET imaging, which often gives high signals in normal brain due to a high metabolic rate of neurons, the PS-radioisotope probe is more likely specific to brain metastases because PS exposes only in tumor blood vessels. Other imaging contrast agents may also be considered for attachment to the PS antibody. We have previously attached a MRI contrast agent, iron oxide to the PS antibody and demonstrated its feasibility of imaging tumor vasculature in a breast cancer model [
33]. Thus, it will be interesting to explore the utility of the PS-targeted imaging probes for PET/SPECT imaging or MRI of occult brain metastases.
Anti-cancer therapeutic effects of PS-targeting antibody have previously been studied. The antibodies bind to PS-exposed tumor vascular ECs and mediate antibody-dependent cell-mediated cytotoxicity (ADCC) by monocytes and macrophages, resulting in tumor blood vessel collapse and consequently tumor cell starvation and death [
34]. The level of PS exposure was found to increase after chemotherapy or radiotherapy, thus a combination with anti-PS antibodies enhanced treatment efficacy [
34,
35]. Given WBRT being the standard of care treatment for brain metastases in clinic, it will be interesting to investigate the combination of WBRT with anti-PS antibodies in brain metastases. PS is also known for its contribution to anti-inflammatory and immunosuppressed responses through the PS-PS receptor signaling [
36,
37]. By blocking the interaction, anti-PS antibodies were shown to successfully reprogram the immune cells in the TME and elicit anti-cancer innate and adaptive immunity [
36,
38,
39].
Other strategies may also be considered to exploit PS-targeting antibodies for brain metastases treatment. Alternative to the diagnostic radioisotopes, radiotherapeutic isotopes, such as β
—emitters, Yttrium-90 (
90Y) or Lutetium-177 (
177Lu) can be conjugated with PS-targeting antibodies. Advantages of β
- emitters include their relatively high energy and shorter emission range. After binding to PS on blood vessels of brain metastases, the radiotherapeutics emit high energy to kill tumor vascular ECs and surrounding tumor cells. Importantly, the shorter traveling distance of β
- emitters less likely causes unwanted damage to normal brain. Thus, the development of PS-targeting radiotherapeutics may be particularly useful to target clinically occult brain metastases. Another antibody-based therapeutic strategy is to conjugate the antibody with a chemo-drug. Antibody-drug conjugates (ADCs) have recently shown clinical success in treating several types of cancer. A recent preclinical study reported the efficacy of anti-HER2 antibody-tubulysin ADC on preventing brain metastases of HER2+ breast cancer in mouse models [
39]. We consider anti-PS antibody a good candidate for ADC. Moreover, nanoparticle (NPs) delivery systems may have advantages including high drug payload and increased binding affinity due to multiple targeting molecules assembled on the surface of NPs [
40,
41,
42]. We have recently developed various PS-targeted nano-delivery systems (PS-NPs) by functionalizing the nanoparticles with PS-targeting antibody. While examining different types of PS-NPs, we observed an interesting finding that after binding to the PS exposed cells, the lipid-based nanoparticles (LNPs), but not other types of NP, e.g., iron oxide, became internalized into the targeted cells [
33,
43,
44,
45]. It is believed that binding to the cell surface PS leads to close apposition and subsequent fusion between the lipid layers of LNPs and cell membrane. Thus, we expect that PS-LNP serves as an effective carrier of anti-cancer drugs to brain metastases through the receptor-mediated transcytosis.
Because PS is the same molecule and has the same distribution and regulation in all mammalian species, it is plausible that the mouse data will extrapolate to humans. While exact mechanisms underpinning the high-level expression of PS on blood vessels of brain metastases are unclear, the factors related to oxidative stress and inflammatory cytokines, such as TNF-α overexpression observed in this study (
Figure 4), are likely playing an important role in flipping PS to the cell surface.
Author Contributions
Conceptualization, L.W. and D.Z.; methodology, L.W., A.H.Z., C.A.A.; software, L.W., A.H.Z., C.A.A.; formal analysis, L.W., A.H.Z., C.A.A.; investigation, L.W., A.H.Z., C.A.A., D.Z.; resources, R.A.B., D.Z., M.D.C., F.X., A.A.H.; data curation, L.W., A.H.Z., C.A.A.; writing—original draft preparation, L.W., A.H.Z., D.Z.; writing—review and editing, all authors. All authors have read and agreed to the published version of the manuscript. Supervision. F.X., M.D.C., R.A.B., A.A.H., D.Z.
Figure 1.
Longitudinal MRI monitoring of intracranial distribution of brain metastases and permeability of the BTB in the 231-Br model. a. Three consecutive coronal sections of high-resolution MRI were obtained from a representative mouse brain bearing 231-Br metastases. At week 3, T2-w images revealed multiple hyperintense metastases across the brain (yellow arrowheads), only two of which were enhanced on T1-w post contrast images (green arrows), indicating a leaky BTB. At week 4, several new lesions appeared on T2-w images (yellow arrowheads), while those lesions seen on the prior scan became larger. An increased number of the contrast enhanced lesions were seen at this time (green arrows). b. A total of 464 231-Br brain metastases were identified by MRI including non-permeable (n = 304) and permeable (n = 160) metastases based on T1-w post contrast images. A plot of permeability versus size indicated that larger metastases tend to be leaky. However, there was no significant difference in tumor size between the permeable and non-permeable metastases (p = 0.1).
Figure 1.
Longitudinal MRI monitoring of intracranial distribution of brain metastases and permeability of the BTB in the 231-Br model. a. Three consecutive coronal sections of high-resolution MRI were obtained from a representative mouse brain bearing 231-Br metastases. At week 3, T2-w images revealed multiple hyperintense metastases across the brain (yellow arrowheads), only two of which were enhanced on T1-w post contrast images (green arrows), indicating a leaky BTB. At week 4, several new lesions appeared on T2-w images (yellow arrowheads), while those lesions seen on the prior scan became larger. An increased number of the contrast enhanced lesions were seen at this time (green arrows). b. A total of 464 231-Br brain metastases were identified by MRI including non-permeable (n = 304) and permeable (n = 160) metastases based on T1-w post contrast images. A plot of permeability versus size indicated that larger metastases tend to be leaky. However, there was no significant difference in tumor size between the permeable and non-permeable metastases (p = 0.1).
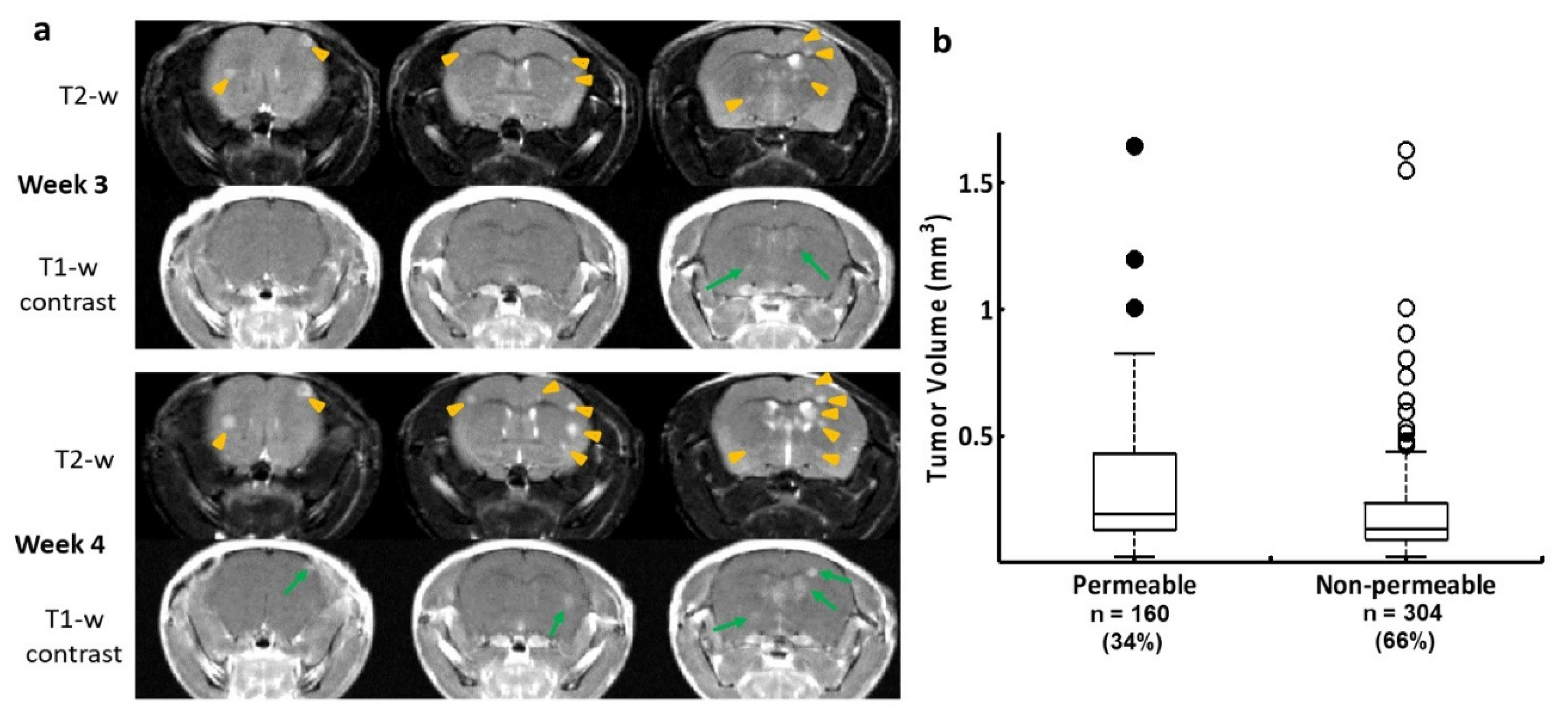
Figure 2.
PS exposed exclusively in vascular endothelial cells of brain metastases. a. Multiple brain lesions were detected on three consecutive coronal sections of T2-w images (arrow) in a 231-Br mouse, while a few lesions were enhanced on T1-w post contrast images (arrow). Immediately after MRI, the mouse was injected i.v. with 1N11 (150 µg). Four hour later, the mouse was perfused and the brain was dissected. b. Corresponding H&E sections depicted more microscopic lesions that were invisible by MRI. c-f. Diffuse brain metastases were labeled on one of the H&E sections (arrow; c). Immunofluorescence staining on a consecutive section showed that 1N11 (red; d) localized to every tumor lesion, even microscopic lesions identified by H&E staining (arrow; bar=1 mm; c). Binding of 1N11 to tumor vessels (CD31, blue; e) was confirmed by the magenta color in merged images (f). g-j. A region containing positive 1N11 (outlined in d) was selected and magnified (bar = 100 µm). The merged image showed that 1N11 (red, h) co-localized with almost every CD31-positive tumor vessel (blue, g) to give a magenta color (i). Vessels in nearby normal brain (star, i) were not stained by 1N11. The tumor regions were distinguished from normal brain by the presence of GFP in the tumor (j). .
Figure 2.
PS exposed exclusively in vascular endothelial cells of brain metastases. a. Multiple brain lesions were detected on three consecutive coronal sections of T2-w images (arrow) in a 231-Br mouse, while a few lesions were enhanced on T1-w post contrast images (arrow). Immediately after MRI, the mouse was injected i.v. with 1N11 (150 µg). Four hour later, the mouse was perfused and the brain was dissected. b. Corresponding H&E sections depicted more microscopic lesions that were invisible by MRI. c-f. Diffuse brain metastases were labeled on one of the H&E sections (arrow; c). Immunofluorescence staining on a consecutive section showed that 1N11 (red; d) localized to every tumor lesion, even microscopic lesions identified by H&E staining (arrow; bar=1 mm; c). Binding of 1N11 to tumor vessels (CD31, blue; e) was confirmed by the magenta color in merged images (f). g-j. A region containing positive 1N11 (outlined in d) was selected and magnified (bar = 100 µm). The merged image showed that 1N11 (red, h) co-localized with almost every CD31-positive tumor vessel (blue, g) to give a magenta color (i). Vessels in nearby normal brain (star, i) were not stained by 1N11. The tumor regions were distinguished from normal brain by the presence of GFP in the tumor (j). .
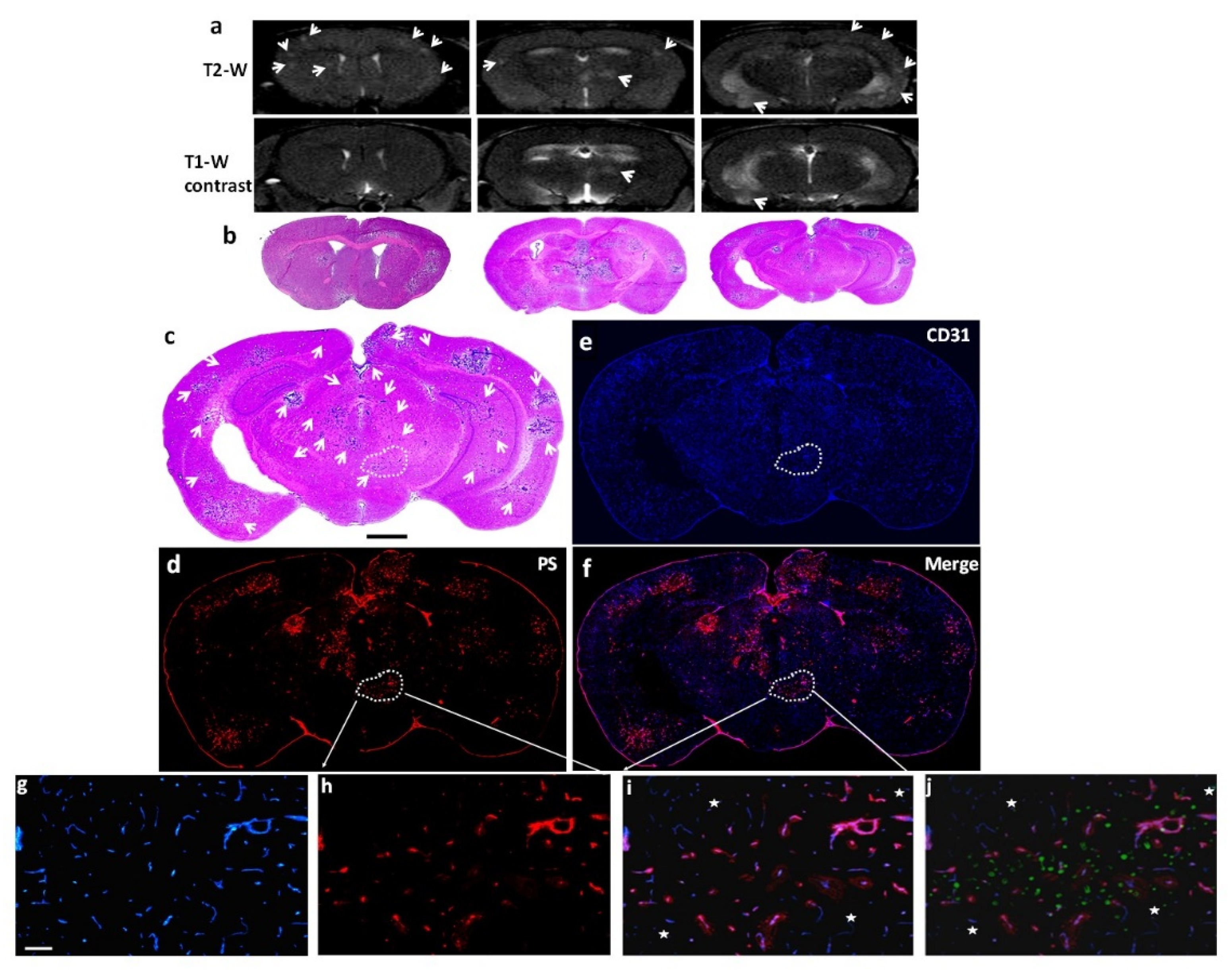
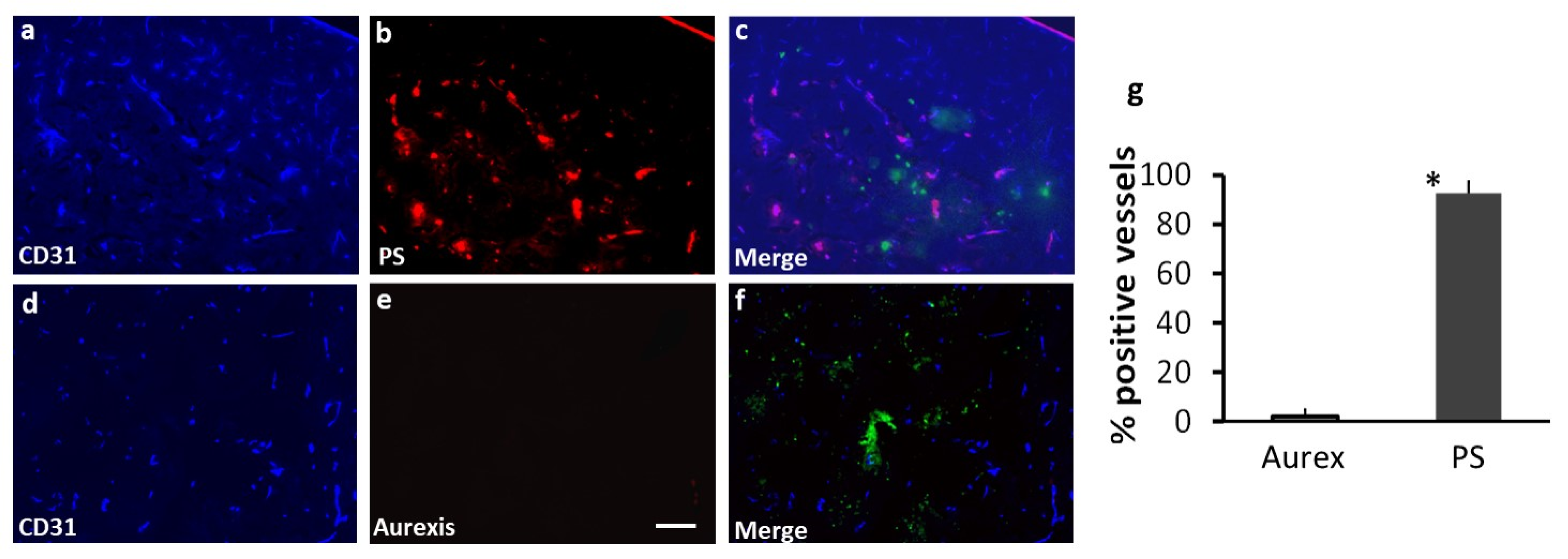
Figure 3.
Staining with 1N11 was antigen specific. 213-Br mice were injected i.v. with either 1N11 or the control antibody, Aurexis (150 µg). Four hour later, the mice were perfused and the brains were dissected. a-c. A brain region containing a tumor lesion was stained with anti-CD31 for blood vessels (a), and co-stained for 1N11 (b). The merged image showed that 1N11 co-localized with CD31-positive tumor vessels. Vessels in nearby normal brain (containing no GFP) were not stained by 1N11 (c). d-f. In contrast, the control antibody, Aurexis, showed no staining of blood vessels of brain metastases (bar = 100 µm). g. For the group of 231-Br mice, the percentage of PS-positive vessels in brain metastases was 93 ± 5%, while only 2 ± 2% for the control antibody Aurexis. * p < 0.01.
Figure 3.
Staining with 1N11 was antigen specific. 213-Br mice were injected i.v. with either 1N11 or the control antibody, Aurexis (150 µg). Four hour later, the mice were perfused and the brains were dissected. a-c. A brain region containing a tumor lesion was stained with anti-CD31 for blood vessels (a), and co-stained for 1N11 (b). The merged image showed that 1N11 co-localized with CD31-positive tumor vessels. Vessels in nearby normal brain (containing no GFP) were not stained by 1N11 (c). d-f. In contrast, the control antibody, Aurexis, showed no staining of blood vessels of brain metastases (bar = 100 µm). g. For the group of 231-Br mice, the percentage of PS-positive vessels in brain metastases was 93 ± 5%, while only 2 ± 2% for the control antibody Aurexis. * p < 0.01.
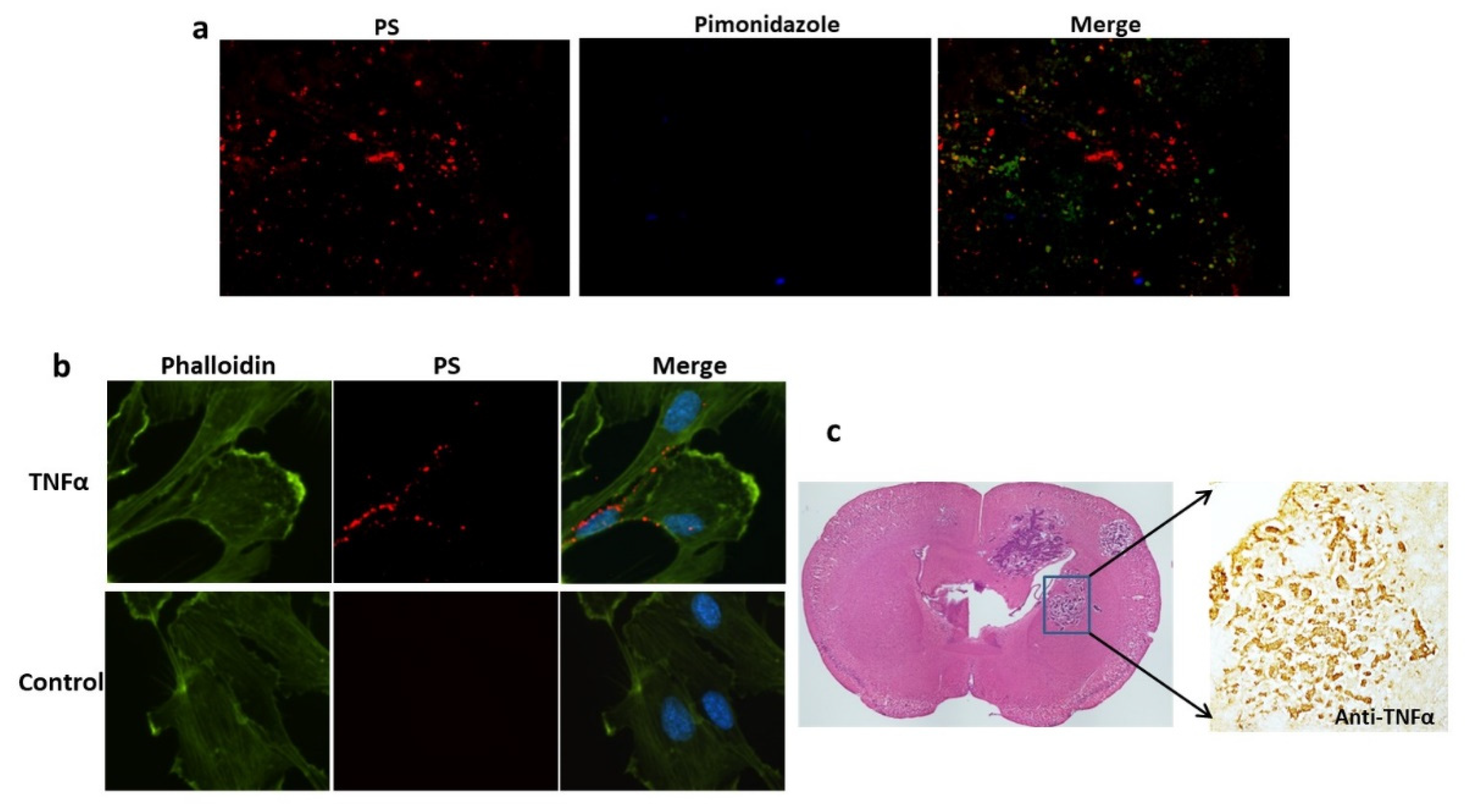
Figure 4.
TNF-α induced PS externalization in vascular endothelial cells and overexpression of TNF-α was detected in brain metastases. a. A group of 213-Br mice receiving i.v. 1N11 and followed by i.v. pimonidazole were used to study tumor hypoxia. A representative region containing positive 1N11 staining (red) was co-stained for pimonidazole (blue). There was essentially no positive pimonidazole staining in the brain metastasis (GFP). b. HUVEC cells were treated with/without TNFα (20 ng/ml) for 24 h before fixation and immunocytostained with 1N11 (red), cytoskeleton (phalloidin, green) and nuclear (DAPI, blue). The merged image showed numerous cell surface-exposed PS in the TNFα-treated cells, while no PS exposure in the control cells. c. Anti-TNF-α staining revealed marked expression of TNF-α in the 231-Br brain metastasis but not in normal brains.
Figure 4.
TNF-α induced PS externalization in vascular endothelial cells and overexpression of TNF-α was detected in brain metastases. a. A group of 213-Br mice receiving i.v. 1N11 and followed by i.v. pimonidazole were used to study tumor hypoxia. A representative region containing positive 1N11 staining (red) was co-stained for pimonidazole (blue). There was essentially no positive pimonidazole staining in the brain metastasis (GFP). b. HUVEC cells were treated with/without TNFα (20 ng/ml) for 24 h before fixation and immunocytostained with 1N11 (red), cytoskeleton (phalloidin, green) and nuclear (DAPI, blue). The merged image showed numerous cell surface-exposed PS in the TNFα-treated cells, while no PS exposure in the control cells. c. Anti-TNF-α staining revealed marked expression of TNF-α in the 231-Br brain metastasis but not in normal brains.
Figure 5.
PS-targeted NIR imaging of brain metastases. NIR dye, IRDye-800CW labeled 1N11 F(ab’)2, was injected into the mice bearing 231-Br or 4T1 brain metastases. a. Twenty-four hours later, ex vivo NIR imaging of the whole brain detected distinct 800CW signals from multiple regions of a 231-Br brain, while a clear contrast of a single brain lesion was seen in a 4T1 brain. b. A series of unstained coronal brain cryosections (10 µm, bottom row) was obtained after the brain in a was imaged. The NIR imaging revealed a clear tumor contrast on the series of brain sections, which correlated well with H&E staining (middle row) of tumors and T1-contrast enhanced MR images (top row). c. Quantification of the light intensity in the tumor versus the contralateral normal brain obtained a ratio of 5.7 ± 0.8 with 800CW-1N11, which was significantly higher than that of the control antibody, 800CW- Aurexis (1.5 ± 0.4; * p <0.05). d. Tumor vascular endothelial cells were immunostained with anti-CD31 (green) in the cryosections adjacent to those used in b. 800CW-1N11 signals (red) from the same field were detected with an NIR filter set (e). f. The merged image revealed extensive 800CW-1N11 bound to vascular endothelial cells, while some detected in the extravascular space (DAPI, blue).
Figure 5.
PS-targeted NIR imaging of brain metastases. NIR dye, IRDye-800CW labeled 1N11 F(ab’)2, was injected into the mice bearing 231-Br or 4T1 brain metastases. a. Twenty-four hours later, ex vivo NIR imaging of the whole brain detected distinct 800CW signals from multiple regions of a 231-Br brain, while a clear contrast of a single brain lesion was seen in a 4T1 brain. b. A series of unstained coronal brain cryosections (10 µm, bottom row) was obtained after the brain in a was imaged. The NIR imaging revealed a clear tumor contrast on the series of brain sections, which correlated well with H&E staining (middle row) of tumors and T1-contrast enhanced MR images (top row). c. Quantification of the light intensity in the tumor versus the contralateral normal brain obtained a ratio of 5.7 ± 0.8 with 800CW-1N11, which was significantly higher than that of the control antibody, 800CW- Aurexis (1.5 ± 0.4; * p <0.05). d. Tumor vascular endothelial cells were immunostained with anti-CD31 (green) in the cryosections adjacent to those used in b. 800CW-1N11 signals (red) from the same field were detected with an NIR filter set (e). f. The merged image revealed extensive 800CW-1N11 bound to vascular endothelial cells, while some detected in the extravascular space (DAPI, blue).
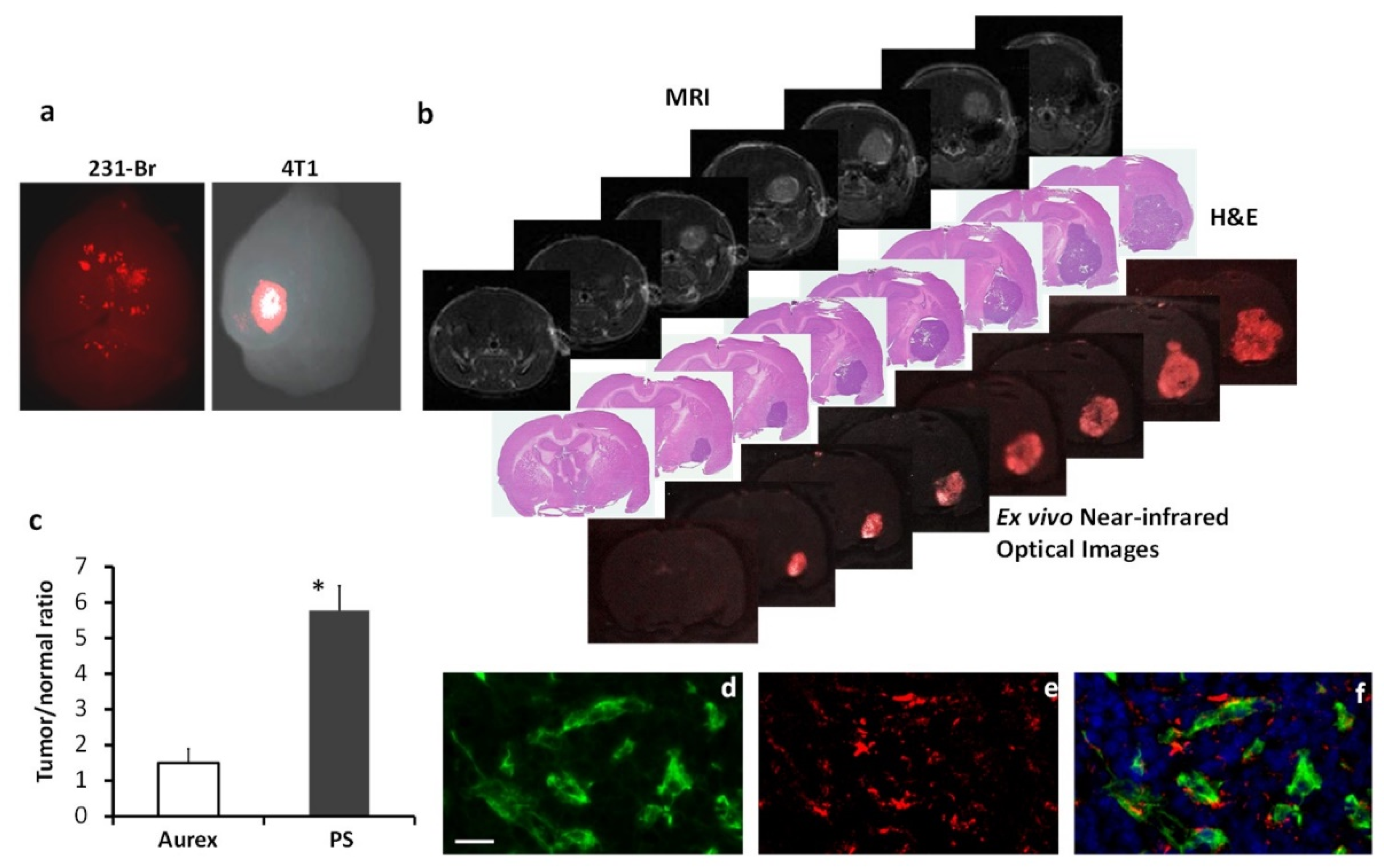
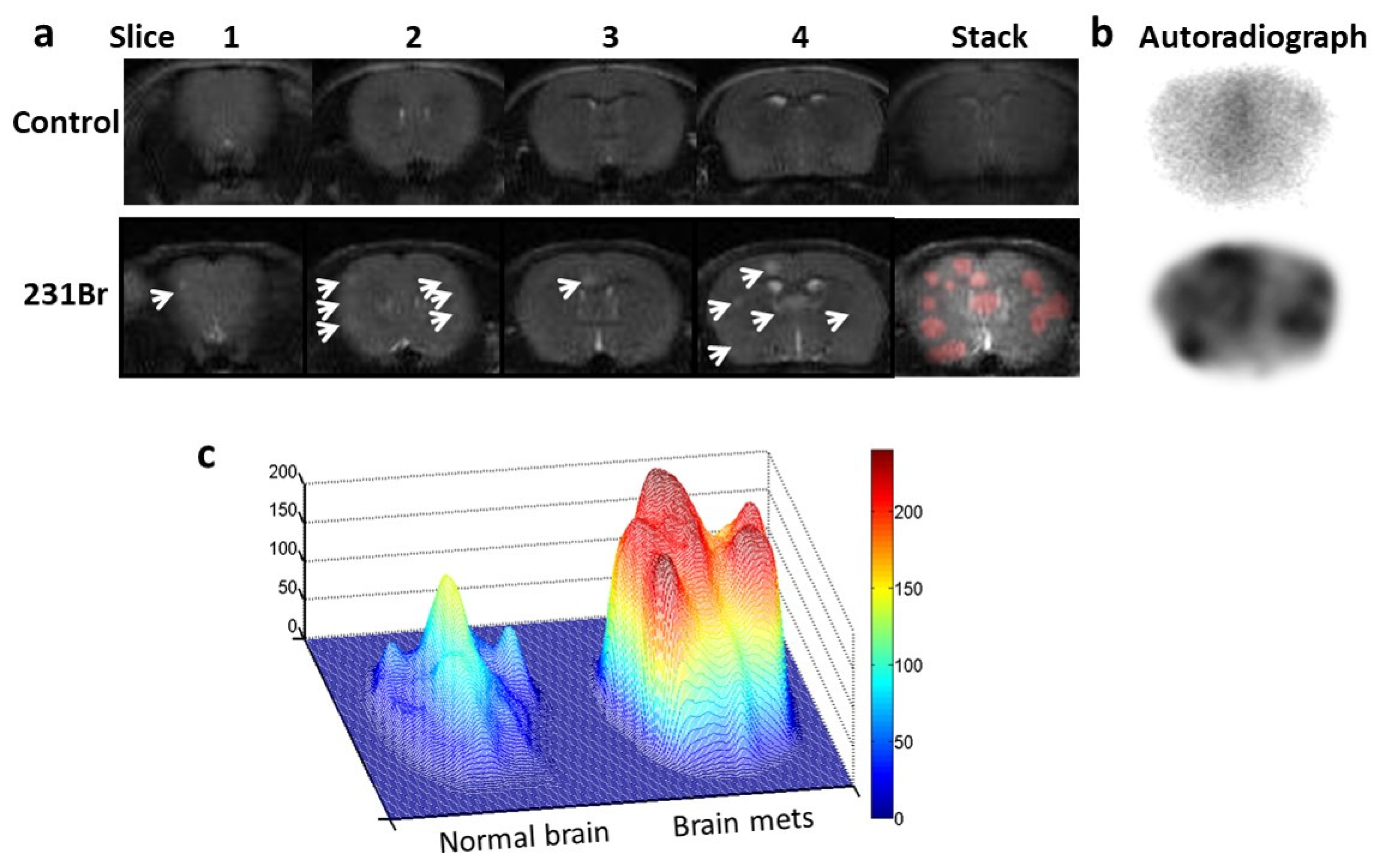
Figure 6.
Autoradiography imaging of I-125 labeled 1N11 in targeting brain metastases. a. T2-w MR images of 4 consecutive 1 mm thick coronal slices, covering 4mm brain tissues post bregma, were acquired from a normal control mouse brain (top) and a 231-Br brain (bottom), respectively. Multiple tumors in the 231-Br brain were identified (arrow). An image stack across the 4 slices was created for each animal. For the 231-Br brain, the tumor lesions (red) from each slice were projected on the stacked image to correlate with autoradiography study. b. After MRI, each mouse was given i.v. 60 µCi 125I-1N11. Forty-eight hours later, the mice were perfused and mouse brains were dissected. Using a mouse brain matrix, 4mm thick brain tissues post bregma, correlating with the MRI, were cut from each mouse brain and laid the cutting face on the autoradiograph film. After 12 h incubation, autoradiograph images showed multiple hot spots on the tumor brain, while clean background signal was observed on the normal brain. There was a general spatial correlation between tumor lesions on MRI and hot spots on the autoradiograph. c. Significantly higher uptake of 125I-1N11 was observed in individual brain metastases, as compared to normal brain tissues (a ratio of 3.6±0.8; p < 0.05).
Figure 6.
Autoradiography imaging of I-125 labeled 1N11 in targeting brain metastases. a. T2-w MR images of 4 consecutive 1 mm thick coronal slices, covering 4mm brain tissues post bregma, were acquired from a normal control mouse brain (top) and a 231-Br brain (bottom), respectively. Multiple tumors in the 231-Br brain were identified (arrow). An image stack across the 4 slices was created for each animal. For the 231-Br brain, the tumor lesions (red) from each slice were projected on the stacked image to correlate with autoradiography study. b. After MRI, each mouse was given i.v. 60 µCi 125I-1N11. Forty-eight hours later, the mice were perfused and mouse brains were dissected. Using a mouse brain matrix, 4mm thick brain tissues post bregma, correlating with the MRI, were cut from each mouse brain and laid the cutting face on the autoradiograph film. After 12 h incubation, autoradiograph images showed multiple hot spots on the tumor brain, while clean background signal was observed on the normal brain. There was a general spatial correlation between tumor lesions on MRI and hot spots on the autoradiograph. c. Significantly higher uptake of 125I-1N11 was observed in individual brain metastases, as compared to normal brain tissues (a ratio of 3.6±0.8; p < 0.05).