Submitted:
15 August 2024
Posted:
20 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McLaughlin K, Jardine AG, Moss JG. Renal artery stenosis. BMJ. 2000;320(7242):1124-7.
- Gupta R, Syed M, Ashcherkin N, Chen K, Vaidya PP, Cooper CJ. Renal Artery Stenosis and Congestive Heart Failure: What Do We Really Know? Curr Cardiol Rep. 2019;21(8):74.
- Conlon PJ, Little MA, Pieper K, Mark DB. Severity of renal vascular disease predicts mortality in patients undergoing coronary angiography. Kidney Int. 2001;60(4):1490-7. [CrossRef]
- Conlon PJ, Athirakul K, Kovalik E, et al. Survival in renal vascular disease. Journal of the American Society of Nephrology. 1998;9(2):252-6. [CrossRef]
- Uzu T, Takeji M, Yamada N, et al. <B>Prevalence and Outcome of Renal Artery Stenosis in Atherosclerotic Patients with Renal Dysfunction</B>. Hypertension Research. 2002;25(4):537-42. [CrossRef]
- Mui K-W, Zeebregts CJ, van den Hout H, van Baal JG, Navis G, Jan-Woittiez A. Impact of incidental renal artery stenosis on long-term mortality in patients with peripheral arterial disease undergoing vascular procedure. J Vasc Surg. 2011;54(3):785-90. [CrossRef]
- Rifkin DE, Ix JH, Wassel CL, Criqui MH, Allison MA. Renal artery calcification and mortality among clinically asymptomatic adults. J Am Coll Cardiol. 2012;60(12):1079-85. [CrossRef]
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLOS Medicine. 2009;6(7):e1000097. [CrossRef]
- Wells GA, Wells G, Shea B, et al., editors. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses2014.
- Aqel RA, Zoghbi GJ, Baldwin SA, et al. Prevalence of renal artery stenosis in high-risk veterans referred to cardiac catheterization. J Hypertens. 2003;21(6):1157-62. [CrossRef]
- Bageacu S, Cerisier A, Isaaz K, Nourissat A, Barral X, Favre JP. Incidental Visceral and Renal Artery Stenosis in Patients Undergoing Coronary Angiography. European Journal of Vascular and Endovascular Surgery. 2011;41(3):385-90. [CrossRef]
- Buller CE, Nogareda JG, Ramanathan K, et al. The profile of cardiac patients with renal artery stenosis. J Am Coll Cardiol. 2004;43(9):1606-13. [CrossRef]
- Cohen MG, Pascua JA, Garcia-Ben M, et al. A simple prediction rule for significant renal artery stenosis in patients undergoing cardiac catheterization. Am Heart J. 2005;150(6):1204-11. [CrossRef]
- Dzielińska Z, Januszewicz A, Demkow M, et al. Cardiovascular risk factors in hypertensive patients with coronary artery disease and coexisting renal artery stenosis. J Hypertens. 2007;25(3):663-70. [CrossRef]
- El-Mawardy RH, Ghareeb MA, Mahdy MM, Sabet SS, Nammas WM. Prevalence and predictors of renal artery stenosis in hypertensive patients undergoing elective coronary procedures. 2008;10(11):844-9. [CrossRef]
- Ghaffari S, Sohrabi B, Siahdasht RB, Pourafkari L. Prevalence and predictors of renal artery stenosis in hypertensive patients undergoing coronary angiography. Hypertens Res. 2009;32(11):1009-14. [CrossRef]
- Imori Y, Akasaka T, Ochiai T, et al. Co-existence of carotid artery disease, renal artery stenosis, and lower extremity peripheral arterial disease in patients with coronary artery disease. Am J Cardiol. 2014;113(1):30-5. [CrossRef]
- Khatami MR, Edalati-Fard M, Sadeghian S, Salari-Far M, Bs MP. Renal artery stenosis in patients with established coronary artery disease: prevalence and predicting factors. Saudi J Kidney Dis Transpl. 2014;25(5):986-91. [CrossRef]
- Kobo O, Hammoud M, Makhoul N, Omary H, Rosenschein U. Screening, diagnosis, and treatment of renal artery stenosis by percutaneous transluminal renal angioplasty with stenting. Isr Med Assoc J. 2010;12(3):140-3.
- Liu B-C, Tang R-N, Feng Y, Wang Y-L, Yin L-F, Ma G-S. A single chinese center investigation of renal artery stenosis in 141 consecutive cases with coronary angiography. Am J Nephrol. 2004;24(6):630-4. [CrossRef]
- Marcantoni C, Rastelli S, Zanoli L, et al. Prevalence of renal artery stenosis in patients undergoing cardiac catheterization. Intern Emerg Med. 2013;8(5):401-8.
- Mirbolouk F, Salari A, Ashouri A, Mahdavi-Roshan M, Gholipour M. Frequency of renal artery stenosis and associated factors in patients undergoing coronary angiography. Journal of Nephropathology. 2019;8(2):14-. [CrossRef]
- Ollivier R, Boulmier D, Veillard D, et al. Frequency and predictors of renal artery stenosis in patients with coronary artery disease. Cardiovasc Revasc Med. 2009;10(1):23-9. [CrossRef]
- Omeish AF, Abbadi HH, Ghanma IM, Botoosh FA, Shwabkeh MK. Frequency of renal artery stenosis among cohort of Jordanians undergoing drive-by renal angiography at time of conventional cardiac catheterization. Saudi Med J. 2009;30(11):1459-64.
- Park S, Jung J-H, Seo H-S, et al. The prevalence and clinical predictors of atherosclerotic renal artery stenosis in patients undergoing coronary angiography. Heart Vessels. 2004;19(6):275-9. [CrossRef]
- Payami B, Jafarizade M, Beladi Mousavi SS, Sattari S-A, Nokhostin F. Prevalence and predictors of atherosclerotic renal artery stenosis in hypertensive patients undergoing simultaneous coronary and renal artery angiography; a cross-sectional study. J Renal Inj Prev. 2016;5(1):34-8. [CrossRef]
- Przewłocki T, Kabłak-Ziembicka A, Tracz W, et al. Renal artery stenosis in patients with coronary artery disease. Kardiol Pol. 2008;66(8):856-62; discussion 63-4. [CrossRef]
- Rihal CS, Textor SC, Breen JF, et al. Incidental renal artery stenosis among a prospective cohort of hypertensive patients undergoing coronary angiography. Mayo Clin Proc. 2002;77(4):309-16. [CrossRef]
- Rimoldi SF, de Marchi SF, Windecker S, Meier B, Allemann Y. Screening renal artery angiography in hypertensive patients undergoing coronary angiography and 6-month follow-up after ad hoc percutaneous revascularization. J Hypertens. 2010;28(4):842-7. [CrossRef]
- Rokni N, Salarifar M, Hakki Kazazi E, Goodarzynejad H. Frequency and predictors of renal artery stenosis in patients undergoing simultaneous coronary and renal catheterization. J Tehran Heart Cent. 2012;7(2):58-64.
- Salehi N, Firouzi A, Gholoobi A, et al. relationship between distribution of coronary artery lesions and renal artery stenosis in patients undergoing simultaneous coronary and renal angiography. Clin Med Insights Cardiol. 2011;5:35-40. [CrossRef]
- Sani SH, Hasanzadeh M Fau - Gholoobi A, Gholoobi A Fau - Alimi H, Alimi H Fau - allah Esmaily H, allah Esmaily H Fau - Gifani M, Gifani M. Relationship between coronary and renal artery disease and associated risk factors in hypertensive and diabetic patients undergoing coronary angiography. (1774-024X (Print)).
- Shukla AN, Madan TH, Jayaram AA, et al. Prevalence and predictors of renal artery stenosis in patients undergoing peripheral and coronary angiography. Int Urol Nephrol. 2013;45(6):1629-35. [CrossRef]
- Tumelero RT, Duda NT, Tognon AP, Thiesen M. Prevalence of renal artery stenosis in 1,656 patients who have undergone cardiac catheterization. Arq Bras Cardiol. 2006;87(3):248-53. [CrossRef]
- Vahedparast H, Pourbehi MR, Amini A, et al. Renal artery stenosis and its predictors in hypertensive patients undergoing coronary artery angiography. Iran J Radiol. 2011;8(4):235-40. [CrossRef]
- Wang Y, Ho DSW, Chen WH, et al. Prevalence and predictors of renal artery stenosis in Chinese patients with coronary artery disease. Intern Med J. 2003;33(7):280-5. [CrossRef]
- Weber-Mzell D. Coronary anatomy predicts presence or absence of renal artery stenosis. A prospective study in patients undergoing cardiac catheterization for suspected coronary artery disease. European Heart Journal. 2002;23(21):1684-91.
- Yamashita T, Ito F, Iwakiri N, Mitsuyama H, Fujii S, Kitabatake A. Prevalence and predictors of renal artery stenosis in patients undergoing cardiac catheterization. Hypertens Res. 2002;25(4):553-7. [CrossRef]
- Yorgun H, Kabakci G, Canpolat U, et al. Frequency and predictors of renal artery stenosis in hypertensive patients undergoing coronary angiography. Angiology. 2013;64(5):385-90. [CrossRef]
- Zandparsa A, Habashizadeh M, Moradi Farsani E, Jabbari M, Rezaei R. Relationship between Renal Artery Stenosis and Severity of Coronary Artery Disease in Patients with Coronary Atherosclerotic Disease. Int Cardiovasc Res J. 2012;6(3):84-7.
- Sani SHD, Hasanzadeh M, Gholoobi A, Alimi H, allah Esmaily H, Gifani M. Relationship between coronary and renal artery disease and associated risk factors in hypertensive and diabetic patients undergoing coronary angiography. EuroIntervention : journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. 2008;4(3):373-7. [CrossRef]
- Andersen UB, Borglykke A, Jørgensen T. Prevalence of renal artery stenosis in subjects with moderate hypertension. A population-based study. Blood Press. 2011;20(3):140-4. [CrossRef]
- Ozkan U, Oguzkurt L, Tercan F, Nursal TZ. The prevalence and clinical predictors of incidental atherosclerotic renal artery stenosis. Eur J Radiol. 2009;69(3):550-4. [CrossRef]
- Kuroda S, Nishida N, Uzu T, et al. Prevalence of renal artery stenosis in autopsy patients with stroke. Stroke. 2000;31(1):61-5. [CrossRef]
- Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. 1983;51(3):606. [CrossRef]
- Dong H, Mo Y, Liu Y, Luo J, Zhou Y, Huang W. [Impact of simultaneous renal artery and coronary artery stenting on cardiac and renal function in patients with renal artery stenosis and coronary artery disease]. Zhonghua Xin Xue Guan Bing Za Zhi. 2014;42(1):19-24.
- Rzeznik D, Przewlocki T, Kablak-Ziembicka A, et al. Effect of renal artery revascularization on left ventricular hypertrophy, diastolic function, blood pressure, and the one-year outcome. J Vasc Surg. 2011;53(3):692-7. [CrossRef]
- Kawarada O, Kume T, Zen K, et al. Cardiac function response to stenting in atherosclerotic renal artery disease with and without heart failure: results from the Carmel study. ESC Heart Failure. 2019;6(2):319-27. [CrossRef]
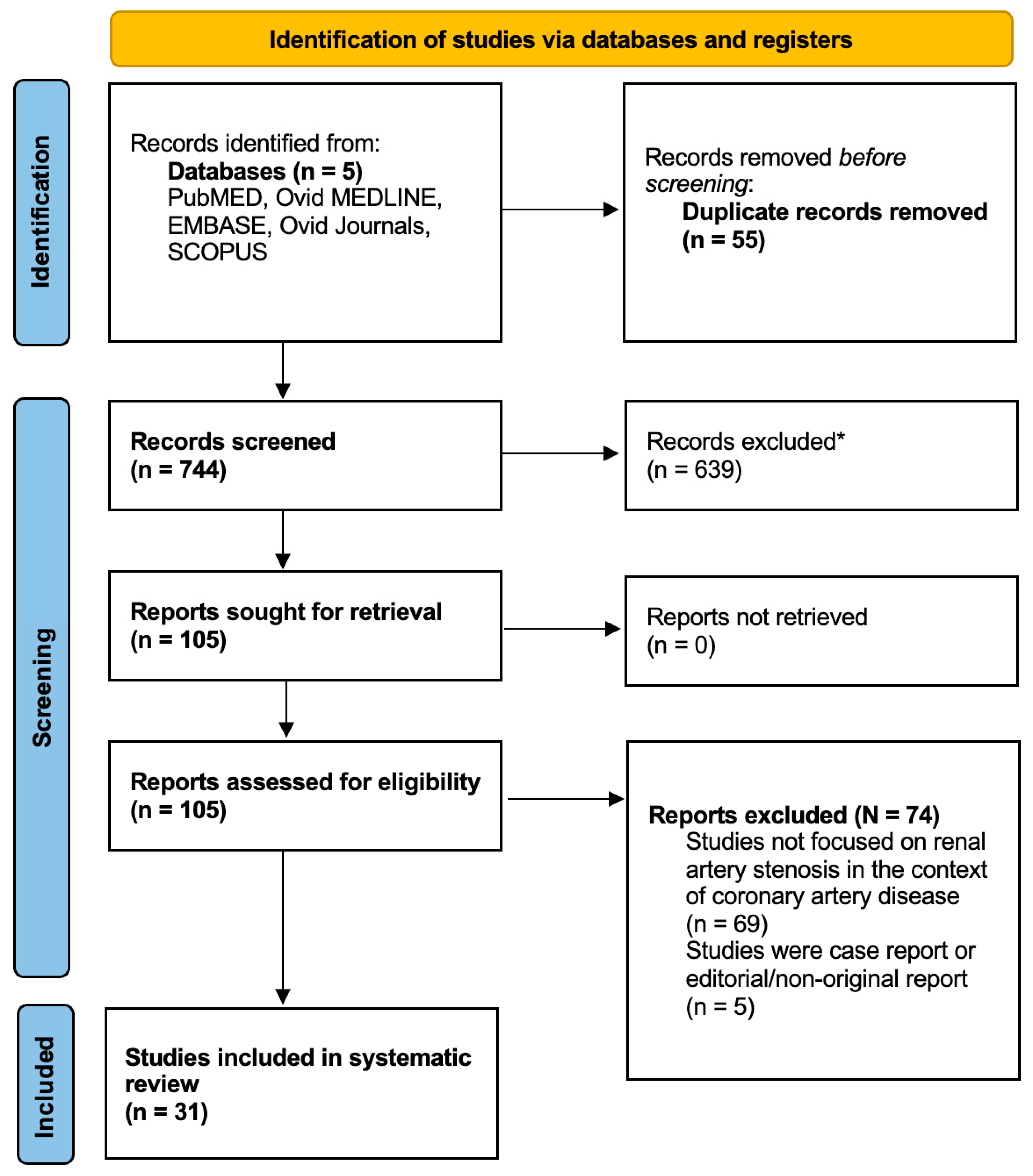
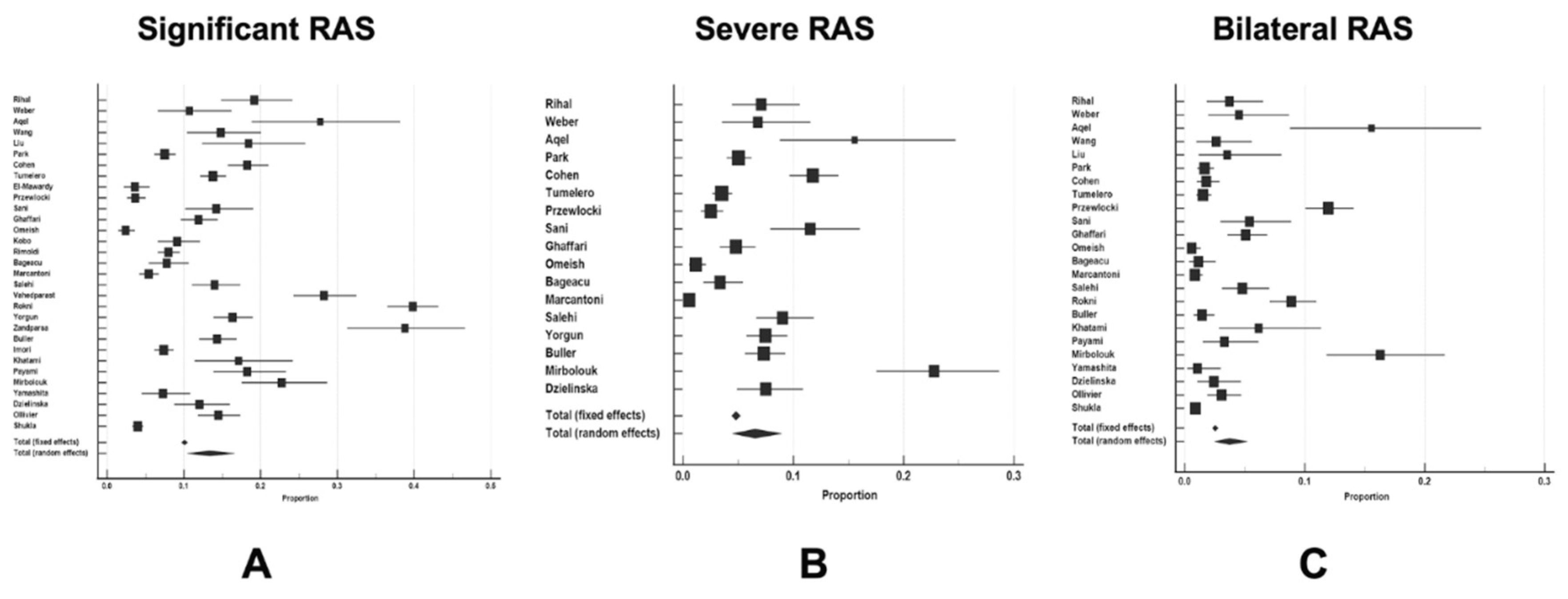
| Authors of the study and year | Total number of patients | Study period | Study location | Multicentric or single-centre study | Study type |
|---|---|---|---|---|---|
| Rihal et al. 2002 | N=300 | July 1998 to March 1999 | Mayo Clinic, Rochester, USA | Single-center | Prospective cohort analysis |
| Weber et al. 2002 | N=177 | - | University Graz, Austria | Single-center | Cohort study |
| Yamashita et al. 2002 | N=289 | April 2000 to October 2000 | Kitami Red Cross Hospital, Japan | Single-center | Cohort study |
| Aqel et al. 2003 | N=542 | February 2001 to November 2001 | Veterans’ Administration (VA) Medical Center, USA | Single-center | Prospective study |
| Wang et al. 2003 | N=230 | - | Queen Mary Hospital, Hong Kong | Single-center | Prospective study |
| Liu et al. 2004 | N=141 | January 2000 to March 2004 | Zhong Da Hospital, Nanjing, PR China | Single-center | Cohort study |
| Park et al. 2004 | N=1459 | March 1998 to July 1999 | Yonsei University Cardiovascular Center, Seoul, South Korea | Single-center | Retrospective cohort study |
| Cohen et al. 2005 | N=843 | September 2000 to May 2002 | Hospital Italiano de Buenos Aires, Argentina | Single-center | Prospective study |
| Dzielinska et al. 2006 | N=333 | - | Institute of Cardiology in Warsaw, Poland | Single-centre | Prospective cohort study |
| Tumelero et al. 2006 | N=1656 | January 2002 to February 2004 | Hospital Sao Vicente de Paulo, Passo Fondo, Brazil | Single-center | Prospective cross-sectional study |
| Ollivier et al. 2008 | N=650 | May 2004 to May 2006 | CHU de Rennes, France | Single-centre | Prospective cohort study |
| El-Mawardy et al. 2008 | N=525 | November 2000 to June 2002 | Ain Shams University Hospital, Cairo, Egypt | Single-center | Cohort study |
| Przewlocki et al. 2008 | N=1036 | Period of 12 months | University Hospital, Krakow, Poland | Single-center | Cohort study |
| Sani et al. 2008 | N=260 | April 2005 to 2006 | Two educational hospitals in Mashhad (Emam Reza & Qaem), Iran | Multicentric | Cross-sectional study |
| Ghaffari et al. 2009 | N=732 | April 2007 to May 2008 | 3 hospitals in Tabriz, Iran | Multicenter | Cross-sectional study |
| Omeish et al. 2009 | N=870 | Januar 2006 to April 2006 | Queen Alia Heart Institute, Amman, Jordan | Single-center | Prospective cross-sectional study |
| Kobo et al. 2010 | N=7500 | 2001 to 2007 | Bnai-Zion Medical Center, Haifa, Israel | Single-center | Cohort study |
| Rimoldi et al. 2010 | N=1504 | 1st of January 2004 to 31st of August 2007 | Swiss Cardiovascular Center Bern, University Hospital Bern, Bern, Switzerland | Single-center | Retrospective study |
| Bageacu et al. 2011 | N=492 | 4 months period | University Hospital of Saint-Erienne, France | Single-center | Prospective study |
| Marcantoni et al. 2011 | N=1298 | April 2007 to March 2008 | The Division of Cardiology, University of Catania, Italy | Single-center | Prospective study |
| Salehi et al. 2011 | N=500 | Period of 12 months from November 2008 | Shaheed Rajeie Cardiovascular Medical, and Research Center, Iran | Single-center | Prospective cohort study |
| Vahedparast et al. 2011 | N=835 | August 2008 to August 2009 | Bent Al-Hoda Hospital od Bushehr University of Medical Science, Iran | Single-center | Prospective cross-sectional study |
| Rokni et al. 2012 | N=18419 | October 2009 to July 2011 | Tehran Heart Center, Iran | Single-center | Retrospective cross-sectional study |
| Yorgun et al. 2012 | N=832 | - | Hacettepe University, Ankara, Turkey | Single-center | Observational study |
| Zandparsa et al. 2012 | N=165 | September 2010 to May 2011 | Tehran University of Medical Sciences, Tehran, Iran | Single-center | Cohort study |
| Shukla et al. 2013 | N=3500 | January 2012 to June 2012 | Civil Hospital, Ahmedabad, India | Single-centre | Prospective cohort sudy |
| Buller et al. 2014 | N=851 | June 2001 to May 2002 | Vancouver Hospital, Canada | Single-center | Prospective cohort study |
| Imori et al. 2014 | N=2571 | September 2010 to July 2011 | Shonan Kamakura General Hospital, Kanagawa, Japan | Single-center | Cross-sectional analysis |
| Khatami et al. 2014 | N=173 | - | Tehran Unviversity of Medical Sciences, Tehran, Iran | Single-center | Cross-sectional study |
| Payami et al. 2016 | N=312 | March 2009 to October 2010 | Emam Hospital, Ahvaz, Iran | Single-center | Cross-sectional study |
| Mirbolouk et al. 2019 | N=247 | May 2015 to June 2016 | Heshmat Heart Hospital, Rasht, Iran | Single-center | Cross-sectional study |
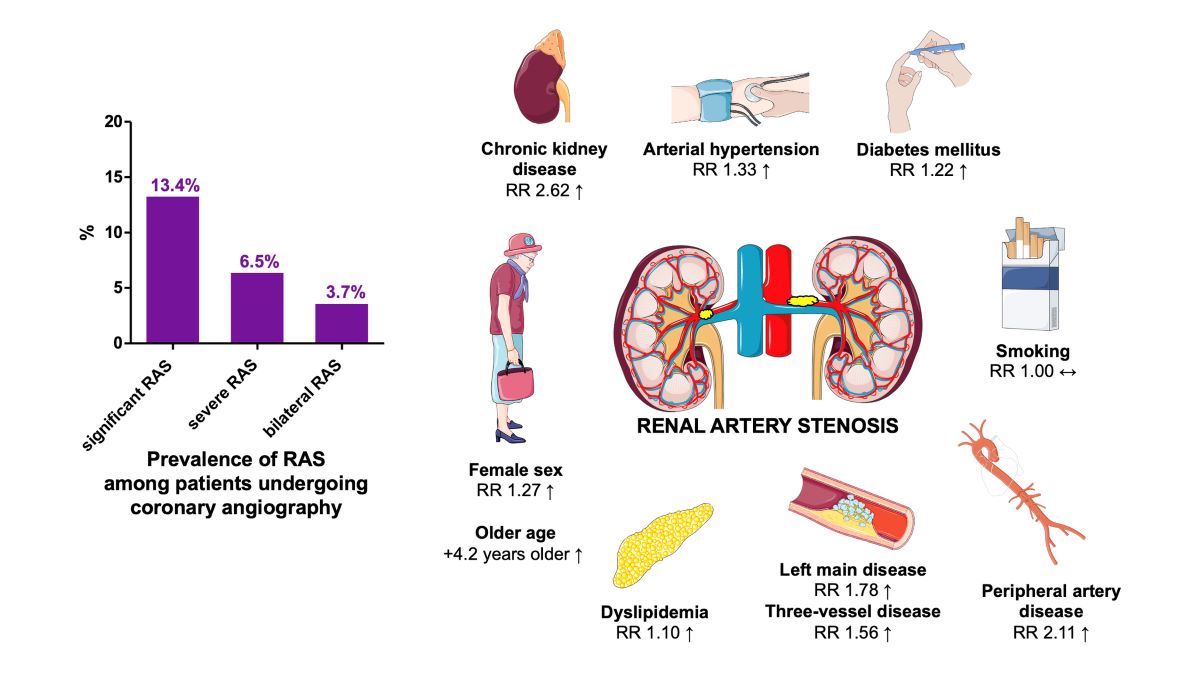
| Variable | Mean ± SD or % (95% CI) |
|---|---|
| Age, mean (years) | 63.2 ± 8.7 |
| Female sex, % | 36.4 (32.4-40.5) |
| Diabetes mellitus, % | 28.7 (25.0-32.5) |
| Arterial hypertension, % | 80.3 (70.3-88.6) |
| Dyslipidemia, % | 61.6 (53.5-69.3) |
| Smoking, % | 38.4 (31.7-45.2) |
| Renal failure, % | 10.9 (7.0-15.6) |
| Peripheral vascular disease, % | 14.9 (6.9-25.2) |
| Carotid artery disease, % | 23.4 (2.9-55.4) |
| Previous myocardial infarction, % | 22.9 (18.7-27.4) |
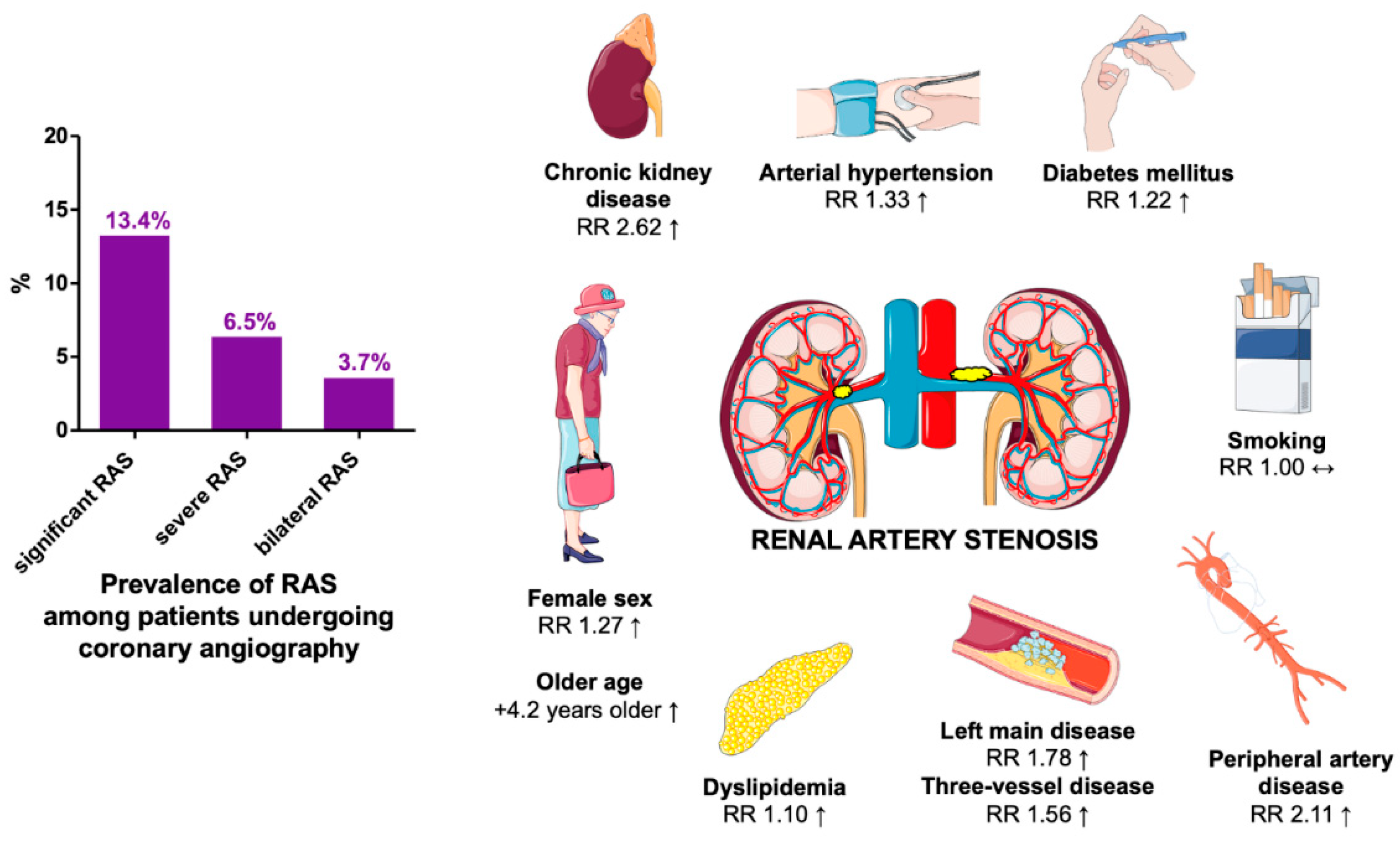
| Variable | Risk ratio (RR) | 95% confidence interval | P-value | Heterogeneity* |
|---|---|---|---|---|
|
Female sex N=27 studies |
1.27 | 1.03 - 1.57 | 0.030 | High I2=92% |
|
Diabetes mellitus N=28 studies |
1.22 | 1.10 - 1.36 | <0.001 | Moderate I2=57% |
|
Arterial hypertension N=19 studies |
1.33 | 1.21 - 1.46 | <0.001 | High I2=94% |
|
Dyslipidemia N=24 studies |
1.10 | 1.06 - 1.14 | <0.001 | Moderate I2=59% |
|
Current smoking N=24 studies |
1.00 | 0.94 - 1.06 | 0.930 | Low I2=26% |
|
Chronic kidney disease N=13 studies |
2.62 | 2.04 - 3.37 | <0.001 | Moderate I2=66% |
|
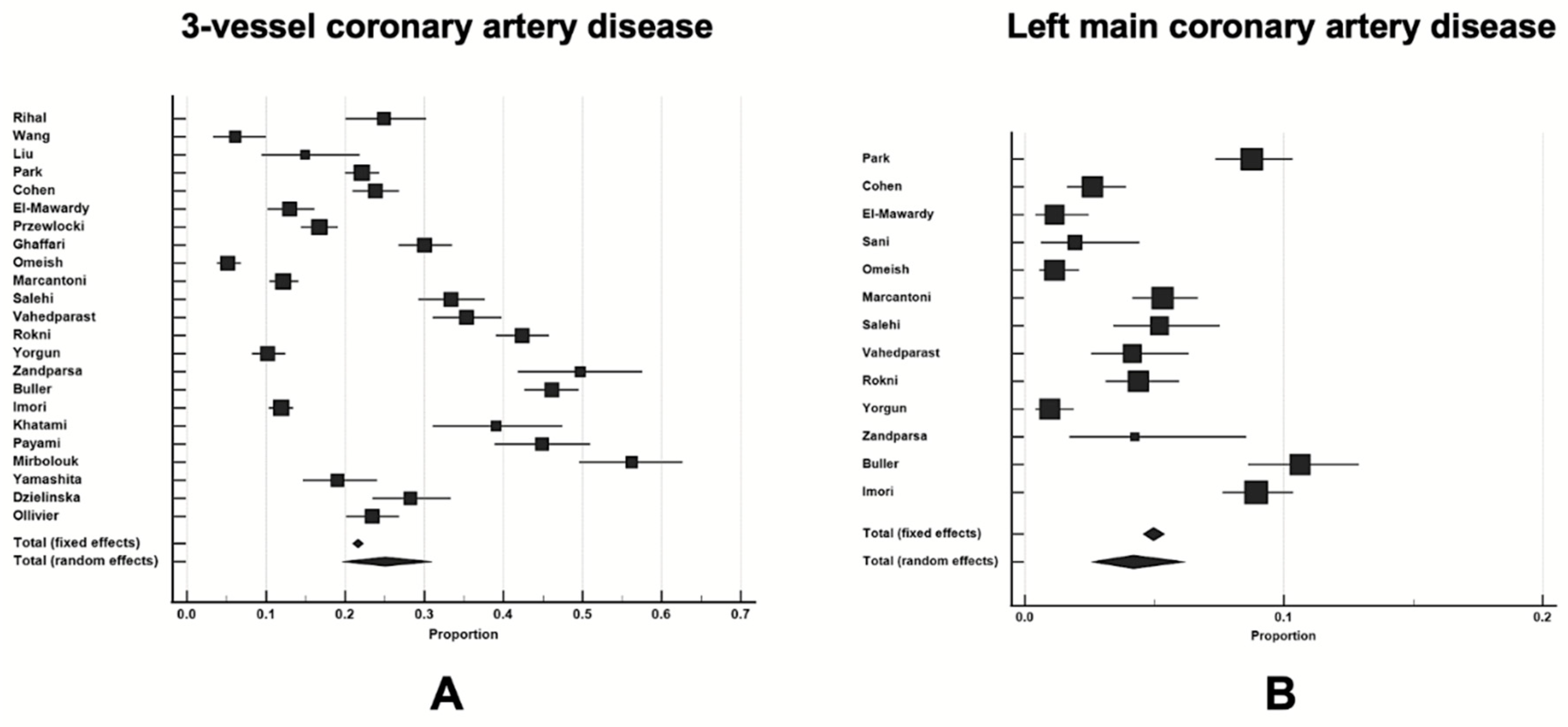
Three-vessel disease N=17 studies |
1.56 | 1.30 - 1.87 | <0.001 | High I2=81% |
|
Left main disease N=10 studies |
1.78 | 1.28 - 2.47 | <0.001 | Moderate I2=52% |
|
Peripheral artery disease N=13 studies |
2.11 | 1.40 - 3.16 | <0.001 | High I2=94% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





