Submitted:
15 August 2024
Posted:
20 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
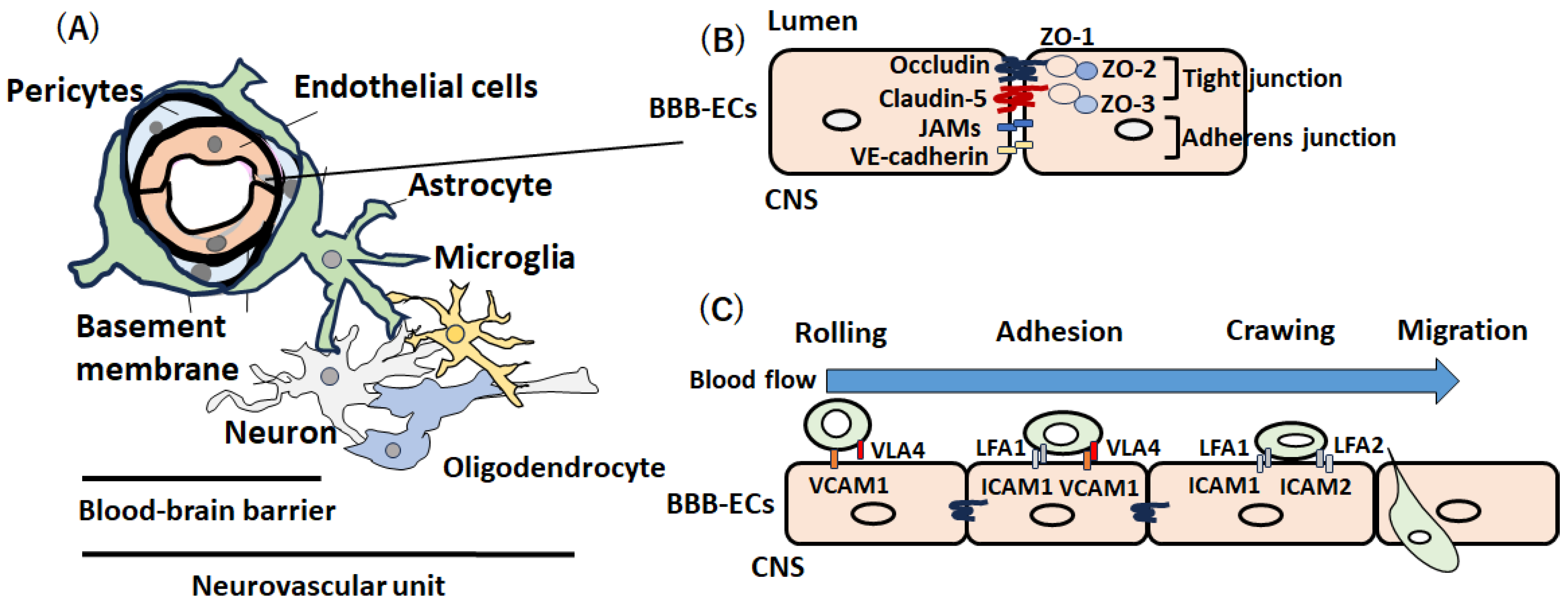
2. Multiple sclerosis
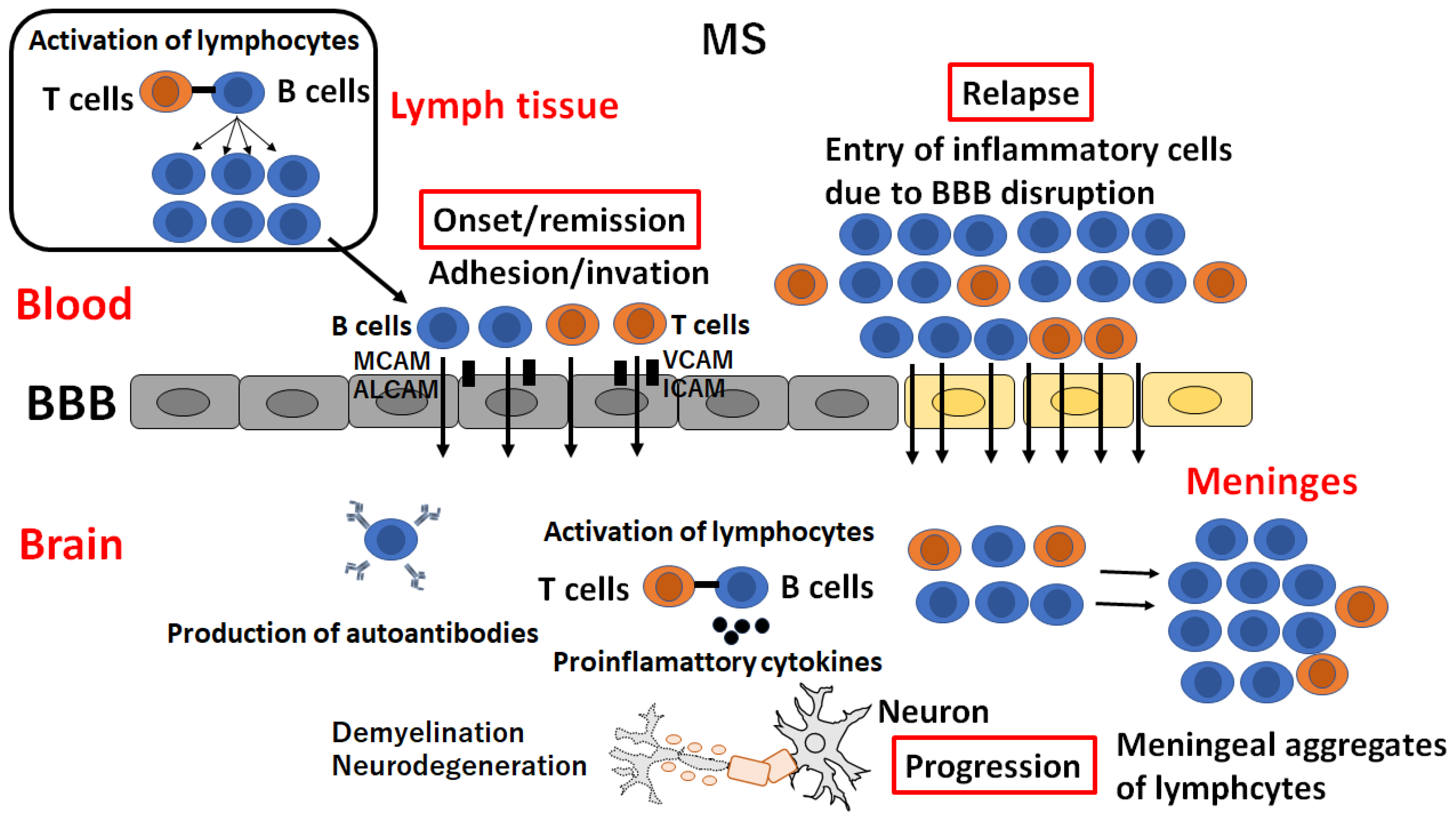
2.1. The BBB Breakdown in Multiple Sclerosis
2.2. Molecular Basis of BBB Disruption in RRMS
2.3. BBB in Progressive MS
2.4. Fluid Biomarkers for BBB Disturbance in MS
2.5. Possible Causes of BBB Disturbance in MS
2.6. Therapies Modulating the BBB in MS
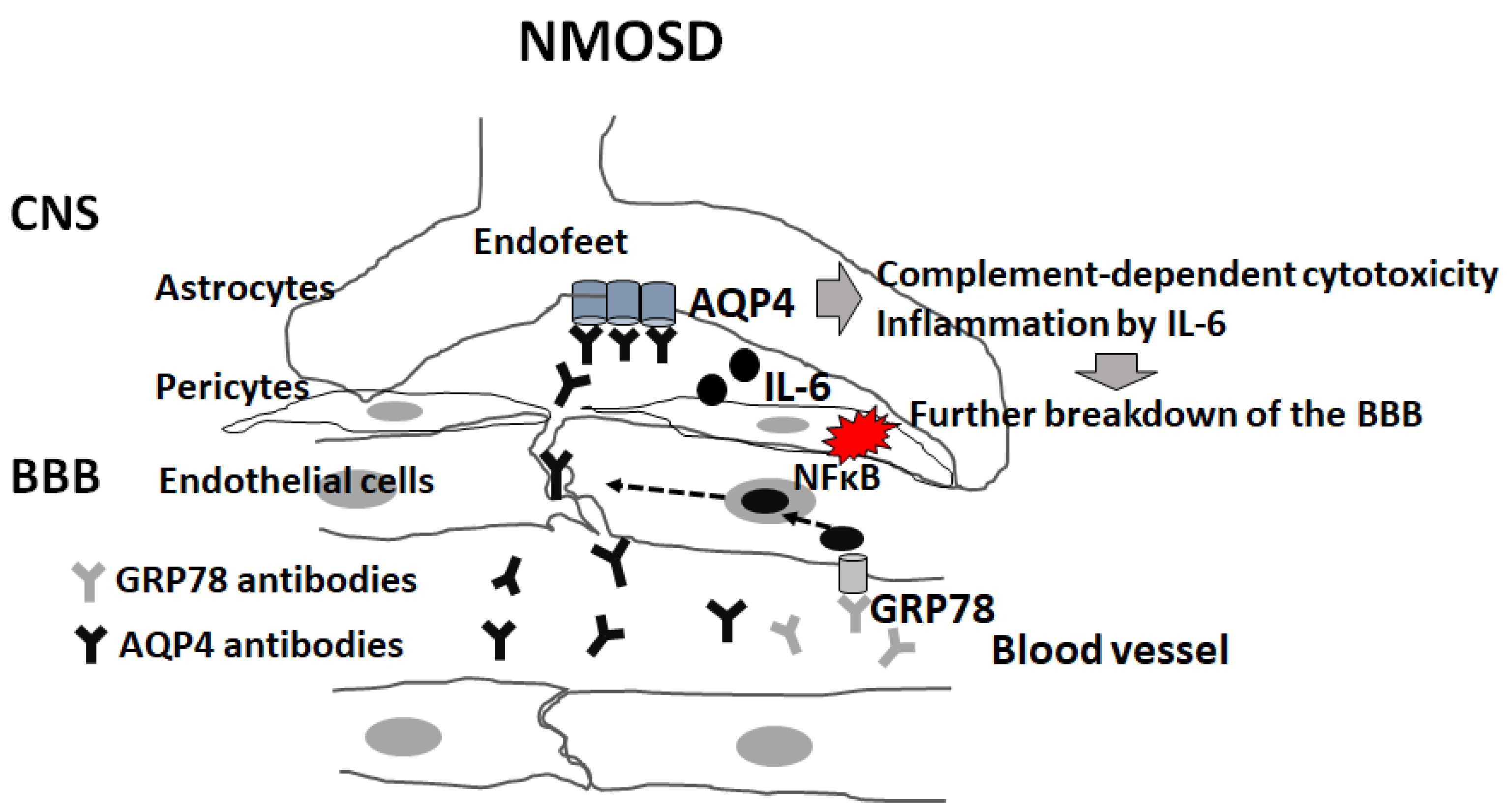
3. Neuromyelitis Optica Spectrum Disorder (NMOSD)
3.1. Pathophysiological Mechanism Underlying NMOSD
3.2. Fluid Biomarkers for BBB Disturbance in NMOSD
3.3. Disruption of BBB in NMOSD
4. Pathophysiological Mechanism and BBB Breakdown in MOGAD
5. NPSLE and AE
5.1. NPSLE and the Blood-Brain Barrier
5.2. AE and BBB
6. Paraneoplastic Neurological Syndromes (PNSs)
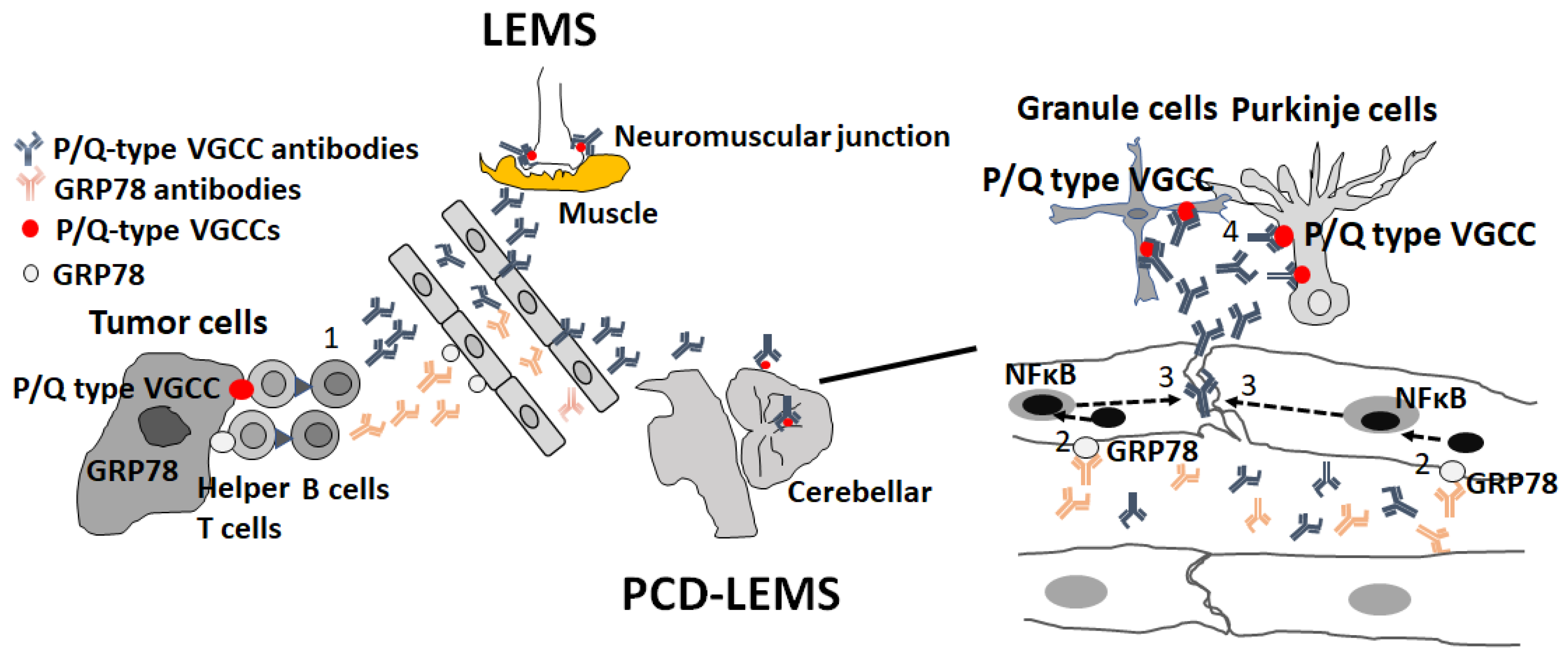
6.1. PNSs and the BBB
6.2. PCD-LEMS
6.3. Paraneoplastic NMOSD
7. Conclusions and Future Direction
Author Contributions
Funding
Provenance and Peer Review
Conflicts of Interest
References
- Schreiner, T.G.; Romanescu, C.; Popescu, B.O. The Blood–Brain Barrier—A Key Player in Multiple Sclerosis Disease Mechanisms. Biomolecules 2022, 12, 538. [Google Scholar] [CrossRef]
- Shimizu, F.; Nishihara, H.; Kanda, T. Blood–brain barrier dysfunction in immuno-mediated neurological diseases. Immunol. Med. 2018, 41, 120–128. [Google Scholar] [CrossRef]
- Bell, A.H.; Miller, S.L.; Castillo-Melendez, M.; Malhotra, A. The Neurovascular Unit: Effects of Brain Insults During the Perinatal Period. Front. Neurosci. 2020, 13, 1452. [Google Scholar] [CrossRef] [PubMed]
- Brandl, S.; Reindl, M. Blood–Brain Barrier Breakdown in Neuroinflammation: Current In Vitro Models. Int. J. Mol. Sci. 2023, 24, 12699. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Chow, B.W.; Gu, C. The Molecular Constituents of the Blood–Brain Barrier. Trends Neurosci. 2015, 38, 598–608. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-Brain Barrier: From Physiology to Disease and Back. Physiol. Rev. 2019, 99, 21–78. [Google Scholar] [CrossRef] [PubMed]
- Zlokovic, B.V. The Blood-Brain Barrier in Health and Chronic Neurodegenerative Disorders. Neuron 2008, 57, 178–201. [Google Scholar] [CrossRef] [PubMed]
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef]
- Brimberg, L.; Mader, S.; Fujieda, Y.; Arinuma, Y.; Kowal, C.; Volpe, B.T.; Diamond, B. Antibodies as Mediators of Brain Pathology. Trends Immunol. 2015, 36, 709–724. [Google Scholar] [CrossRef]
- Chataway, J.; Williams, T.; Li, V.; Marrie, R.A.; Ontaneda, D.; Fox, R.J. Clinical trials for progressive multiple sclerosis: Progress, new lessons learned, and remaining challenges. Lancet Neurol. 2024, 23, 277–301. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, G.G.; Pacheco-Moisés, F.P.; Macías-Islas, M.Á.; Flores-Alvarado, L.J.; Mireles-Ramírez, M.A.; González-Renovato, E.D.; Hernández-Navarro, V.E.; Sánchez-López, A.L.; Alatorre-Jiménez, M.A. Role of the Blood–Brain Barrier in Multiple Sclerosis. Arch. Med. Res. 2014, 45, 687–697. [Google Scholar] [CrossRef]
- Noseworthy, J.H.; Lucchinetti, C.; Rodriguez, M.; Weinshenker, B.G. Multiple sclerosis. N. Engl. J. Med. 2000, 343, 938–952. [Google Scholar] [CrossRef]
- Lublin, F.D.; Reingold, S.C. Defining the clinical course of multiple sclerosis: Results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology 1996, 46, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Mahad, D.H.; Trapp, B.D.; Lassmann, H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 2015, 14, 183–193. [Google Scholar] [CrossRef]
- Ontaneda, D.; Fox, R.J.; Chataway, J. Clinical trials in progressive multiple sclerosis: Lessons learned and future perspectives. Lancet Neurol. 2015, 14, 208–223. [Google Scholar] [CrossRef]
- Dutta, R.; Trapp, B.D. Relapsing and progressive forms of multiple sclerosis: Insights from pathology. Curr. Opin. Neurol. 2014, 27, 271–278. [Google Scholar] [CrossRef]
- Lassmann, H. Pathogenic Mechanisms Associated With Different Clinical Courses of Multiple Sclerosis. Front. Immunol. 2019, 9, 3116. [Google Scholar] [CrossRef]
- Leray, E.; Yaouanq, J.; Le Page, E.; Coustans, M.; Laplaud, D.; Oger, J.; Edan, G. Evidence for a two-stage disability progression in multiple sclerosis. Brain 2010, 133, 1900–1913. [Google Scholar] [CrossRef]
- Alvarez, J.I.; Cayrol, R.; Prat, A. Disruption of central nervous system barriers in multiple sclerosis. Biochim. et Biophys. Acta (BBA) - Mol. Basis Dis. 2010, 1812, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Larochelle, C.; Alvarez, J.I.; Prat, A. How do immune cells overcome the blood-brain barrier in multiple sclerosis? FEBS Lett. 2011, 585, 3770–3780. [Google Scholar] [CrossRef] [PubMed]
- Kirk, J.; Plumb, J.; Mirakhur, M.; McQuaid, S. Tight junctional abnormality in multiple sclerosis white matter affects all calibres of vessel and is associated with blood–brain barrier leakage and active demyelination. J. Pathol. 2003, 201, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Plumb, J.; McQuaid, S.; Mirakhur, M.; Kirk, J. Abnormal Endothelial Tight Junctions in Active Lesions and Normal-appearing White Matter in Multiple Sclerosis. Brain Pathol. 2002, 12, 154–169. [Google Scholar] [CrossRef]
- Waubant, E. Biomarkers Indicative of Blood-Brain Barrier Disruption in Multiple Sclerosis. Dis. Markers 2006, 22, 235–244. [Google Scholar] [CrossRef]
- Leech, S.; Kirk, J.; Plumb, J.; McQuaid, S. Persistent endothelial abnormalities and blood–brain barrier leak in primary and secondary progressive multiple sclerosis. Neuropathol. Appl. Neurobiol. 2007, 33, 86–98. [Google Scholar] [CrossRef]
- Zierfuss, B.; Larochelle, C.; Prat, A. Blood-brain barrier dysfunction in multiple sclerosis: Causes, consequences, and potential effects of therapies. Lancet Neurol. 2024, 23, 95–109. [Google Scholar] [CrossRef]
- Balasa, R.; Barcutean, L.; Mosora, O.; Manu, D. Reviewing the Significance of Blood–Brain Barrier Disruption in Multiple Sclerosis Pathology and Treatment. Int. J. Mol. Sci. 2021, 22, 8370. [Google Scholar] [CrossRef] [PubMed]
- Ifergan, I.; Kebir, H.; Alvarez, J.I.; Marceau, G.; Bernard, M.; Bourbonnière, L.; Poirier, J.; Duquette, P.; Talbot, P.J.; Arbour, N.; Prat, A. Central nervous system recruitment of effector memory CD8+ T lymphocytes during neuroinflammation is dependent on α4 integrin. Brain 2011, 134, 3560–3577. [Google Scholar] [CrossRef]
- Marchetti, L.; Engelhardt, B. Immune cell trafficking across the blood-brain barrier in the absence and presence of neuroinflammation. Vasc. Biol. 2020, 2, H1–H18. [Google Scholar] [CrossRef]
- Mitroulis, I.; Alexaki, V.I.; Kourtzelis, I.; Ziogas, A.; Hajishengallis, G.; Chavakis, T. Leukocyte integrins: Role in leukocyte recruitment and as therapeutic targets in inflammatory disease. Pharmacol. Ther. 2015, 147, 123–135. [Google Scholar] [CrossRef]
- Holman, D.W.; Klein, R.S.; Ransohoff, R.M. The blood–brain barrier, chemokines and multiple sclerosis. Biochim. et Biophys. Acta (BBA) - Mol. Basis Dis. 2010, 1812, 220–230. [Google Scholar] [CrossRef]
- Alvarez, J.I.; Saint-Laurent, O.; Godschalk, A.; Terouz, S.; Briels, C.; Larouche, S.; Bourbonnière, L.; Larochelle, C.; Prat, A. Focal disturbances in the blood–brain barrier are associated with formation of neuroinflammatory lesions. Neurobiol. Dis. 2015, 74, 14–24. [Google Scholar] [CrossRef]
- Miller, D.H.; Soon, D.; Fernando, K.T.; MacManus, D.G.; Barker, G.J.; Yousry, T.A.; Fisher, E.; O’Connor, P.W.; Phillips, J.T.; Polman, C.H.; et al. MRI outcomes in a placebo-controlled trial of natalizumab in relapsing MS. Neurology 2007, 68, 1390–1401. [Google Scholar] [CrossRef]
- Charabati, M.; Zandee, S.; Fournier, A.P.; Tastet, O.; Thai, K.; Zaminpeyma, R.; Lécuyer, M.-A.; Bourbonnière, L.; Larouche, S.; Klement, W.; et al. MCAM+ brain endothelial cells contribute to neuroinflammation by recruiting pathogenic CD4+ T lymphocytes. Brain 2022, 146, 1483–1495. [Google Scholar] [CrossRef]
- Michel, L.; Grasmuck, C.; Charabati, M.; Lécuyer, M.-A.; Zandee, S.; Dhaeze, T.; Alvarez, J.I.; Li, R.; Larouche, S.; Bourbonnière, L.; et al. Activated leukocyte cell adhesion molecule regulates B lymphocyte migration across central nervous system barriers. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Wimmer, I.; Tietz, S.; Nishihara, H.; Deutsch, U.; Sallusto, F.; Gosselet, F.; Lyck, R.; Muller, W.A.; Lassmann, H.; Engelhardt, B. PECAM-1 Stabilizes Blood-Brain Barrier Integrity and Favors Paracellular T-Cell Diapedesis Across the Blood-Brain Barrier During Neuroinflammation. Front. Immunol. 2019, 10, 711. [Google Scholar] [CrossRef] [PubMed]
- Charabati, M.; Grasmuck, C.; Ghannam, S.; Bourbonnière, L.; Fournier, A.P.; Lécuyer, M.A.; Tastet, O.; Kebir, H.; Rébillard, R.M.; Hoornaert, C.; et al. DICAM promotes TH17 lymphocyte trafficking across the blood-brain barrier during autoimmune neuroinflammation. Sci. Transl. Med. 2022, 14, eabj0473. [Google Scholar] [CrossRef]
- Benedict, R.H.B.; Amato, M.P.; DeLuca, J.; Geurts, J.J.G. Cognitive impairment in multiple sclerosis: Clinical management, MRI, and therapeutic avenues. Lancet Neurol. 2020, 19, 860–871. [Google Scholar] [CrossRef]
- Nishihara, H.; Shimizu, F.; Kitagawa, T.; Yamanaka, N.; Akada, J.; Kuramitsu, Y.; Sano, Y.; Takeshita, Y.; Maeda, T.; Abe, M.; et al. Identification of galectin-3 as a possible antibody target for secondary progressive multiple sclerosis. Mult. Scler. J. 2016, 23, 382–394. [Google Scholar] [CrossRef]
- Dumic, J.; Dabelic, S.; Flögel, M. Galectin-3: An open-ended story. Biochim. et Biophys. Acta (BBA) - Gen. Subj. 2006, 1760, 616–635. [Google Scholar] [CrossRef] [PubMed]
- Radosavljevic, G.; Volarevic, V.; Jovanovic, I.; Milovanovic, M.; Pejnovic, N.; Arsenijevic, N.; Hsu, D.K.; Lukic, M.L. The roles of Galectin-3 in autoimmunity and tumor progression. Immunol. Res. 2012, 52, 100–110. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Rosenthal, H.; Cymring, B.; Gratch, D.; Pagano, B.; Xie, B.; Sadiq, S.A. Progressive multiple sclerosis cerebrospinal fluid induces inflammatory demyelination, axonal loss, and astrogliosis in mice. Exp. Neurol. 2014, 261, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Granziera, C.; Wuerfel, J.; Barkhof, F.; Calabrese, M.; De Stefano, N.; Enzinger, C.; Evangelou, N.; Filippi, M.; Geurts, J.J.G.; Reich, D.S.; et al. Quantitative magnetic resonance imaging towards clinical application in multiple sclerosis. Brain 2021, 144, 1296–1311. [Google Scholar] [CrossRef] [PubMed]
- Sivakolundu, D.K.; West, K.L.; Maruthy, G.B.; Zuppichini, M.; Turner, M.P.; Abdelkarim, D.; Zhao, Y.; Nguyen, D.; Spence, J.S.; Lu, H.; et al. Reduced arterial compliance along the cerebrovascular tree predicts cognitive slowing in multiple sclerosis: Evidence for a neurovascular uncoupling hypothesis. Mult. Scler. J. 2019, 26, 1486–1496. [Google Scholar] [CrossRef]
- Spencer, J.I.; Bell, J.S.; DeLuca, G.C. Vascular pathology in multiple sclerosis: Reframing pathogenesis around the blood-brain barrier. J. Neurol. Neurosurg. Psychiatry 2018, 89, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Barro, C.; Benkert, P.; Disanto, G.; Tsagkas, C.; Amann, M.; Naegelin, Y.; Leppert, D.; Gobbi, C.; Granziera, C.; Yaldizli. ; et al. Serum neurofilament as a predictor of disease worsening and brain and spinal cord atrophy in multiple sclerosis. Brain 2018, 141, 2382–2391. [Google Scholar] [CrossRef]
- Uher, T.; McComb, M.; Galkin, S.; Srpova, B.; Oechtering, J.; Barro, C.; Tyblova, M.; Bergsland, N.; Krasensky, J.; Dwyer, M.; et al. Neurofilament levels are associated with blood–brain barrier integrity, lymphocyte extravasation, and risk factors following the first demyelinating event in multiple sclerosis. Mult. Scler. J. 2020, 27, 220–231. [Google Scholar] [CrossRef]
- Uher, T.; McComb, M.; Galkin, S.; Srpova, B.; Oechtering, J.; Barro, C.; Tyblova, M.; Bergsland, N.; Krasensky, J.; Dwyer, M.; et al. Neurofilament levels are associated with blood–brain barrier integrity, lymphocyte extravasation, and risk factors following the first demyelinating event in multiple sclerosis. Mult. Scler. J. 2020, 27, 220–231. [Google Scholar] [CrossRef]
- Sejbaek, T.; Nielsen, H.H.; Penner, N.; Plavina, T.; Mendoza, J.P.; Martin, N.A.; Elkjaer, M.L.; Ravnborg, M.H.; Illes, Z. Dimethyl fumarate decreases neurofilament light chain in CSF and blood of treatment naïve relapsing MS patients. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1324–1330. [Google Scholar] [CrossRef]
- Hyun, J.-W.; Kim, Y.; Kim, G.; Kim, S.-H.; Kim, H.J. Longitudinal analysis of serum neurofilament light chain: A potential therapeutic monitoring biomarker for multiple sclerosis. Mult. Scler. J. 2020, 26, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Abdelhak, A.; Antweiler, K.; Kowarik, M.C.; Senel, M.; Havla, J.; Zettl, U.K.; Kleiter, I.; Skripuletz, T.; Haarmann, A.; Stahmann, A.; et al. Serum glial fibrillary acidic protein and disability progression in progressive multiple sclerosis. Ann. Clin. Transl. Neurol. 2023, 11, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Papa, L.; Ladde, J.G.; O’brien, J.F.; Thundiyil, J.G.; Tesar, J.; Leech, S.; Cassidy, D.D.; Roa, J.; Hunter, C.; Miller, S.; et al. Evaluation of Glial and Neuronal Blood Biomarkers Compared With Clinical Decision Rules in Assessing the Need for Computed Tomography in Patients With Mild Traumatic Brain Injury. JAMA Netw. Open 2022, 5, e221302–e221302. [Google Scholar] [CrossRef] [PubMed]
- Uher, T.; McComb, M.; Galkin, S.; Srpova, B.; Oechtering, J.; Barro, C.; Tyblova, M.; Bergsland, N.; Krasensky, J.; Dwyer, M.; et al. Neurofilament levels are associated with blood–brain barrier integrity, lymphocyte extravasation, and risk factors following the first demyelinating event in multiple sclerosis. Mult. Scler. J. 2020, 27, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, R. The contribution of astrocytes to the neuroinflammatory response in multiple sclerosis and experimental autoimmune encephalomyelitis. Acta Neuropathol. 2019, 137, 757–783. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.; Søndergaard, H.; Oturai, A.; Jensen, P.; Sorensen, P.; Sellebjerg, F.; Börnsen, L. Soluble serum VCAM-1, whole blood mRNA expression and treatment response in natalizumab-treated multiple sclerosis. Mult. Scler. Relat. Disord. 2016, 10, 66–72. [Google Scholar] [CrossRef]
- Wang, D.; Duan, H.; Feng, J.; Xiang, J.; Feng, L.; Liu, D.; Chen, X.; Jing, L.; Liu, Z.; Zhang, D.; et al. Soluble CD146, a cerebrospinal fluid marker for neuroinflammation, promotes blood-brain barrier dysfunction. Theranostics 2020, 10, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Cotsapas, C.; Mitrovic, M. Genome-wide association studies of multiple sclerosis. Clin. Transl. Immunol. 2018, 7, e1018. [Google Scholar] [CrossRef] [PubMed]
- International Multiple Sclerosis Genetics Consortium. Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. Science 2019, 365, eaav7188. [Google Scholar] [CrossRef]
- Bäcker-Koduah, P.; Bellmann-Strobl, J.; Scheel, M.; Wuerfel, J.; Wernecke, K.-D.; Dörr, J.; Brandt, A.U.; Paul, F. Vitamin D and Disease Severity in Multiple Sclerosis—Baseline Data From the Randomized Controlled Trial (EVIDIMS). Front. Neurol. 2020, 11, 129. [Google Scholar] [CrossRef] [PubMed]
- Hupperts, R.; Smolders, J.; Vieth, R.; Holmøy, T.; Marhardt, K.; Schluep, M.; Killestein, J.; Barkhof, F.; Beelke, M.; Grimaldi, L.M.; et al. Randomized trial of daily high-dose vitamin D 3 in patients with RRMS receiving subcutaneous interferon β-1a. Neurology 2019, 93, e1906–e1916. [Google Scholar] [CrossRef]
- Sangha, A.; Quon, M.; Pfeffer, G.; Orton, S.-M. The Role of Vitamin D in Neuroprotection in Multiple Sclerosis: An Update. Nutrients 2023, 15, 2978. [Google Scholar] [CrossRef]
- Takahashi, S.; Maeda, T.; Sano, Y.; Nishihara, H.; Takeshita, Y.; Shimizu, F.; Kanda, T. Active form of vitamin D directly protects the blood–brain barrier in multiple sclerosis. Clin. Exp. Neuroimmunol. 2017, 8, 244–254. [Google Scholar] [CrossRef]
- Bjornevik, K.; Bjornevik, K.; Cortese, M.; Cortese, M.; Healy, B.C.; Healy, B.C.; Kuhle, J.; Kuhle, J.; Mina, M.J.; Mina, M.J.; et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science 2022, 375, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Lanz, T.V.; Brewer, R.C.; Ho, P.P.; Moon, J.-S.; Jude, K.M.; Fernandez, D.; Fernandes, R.A.; Gomez, A.M.; Nadj, G.-S.; Bartley, C.M.; et al. Clonally expanded B cells in multiple sclerosis bind EBV EBNA1 and GlialCAM. Nature 2022, 603, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Soldan, S.S.; Lieberman, P.M. Epstein–Barr virus and multiple sclerosis. Nat. Rev. Microbiol. 2022, 21, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Tengvall, K.; Huang, J.; Hellström, C.; Kammer, P.; Biström, M.; Ayoglu, B.; Bomfim, I.L.; Stridh, P.; Butt, J.; Brenner, N.; et al. Molecular mimicry between Anoctamin 2 and Epstein-Barr virus nuclear antigen 1 associates with multiple sclerosis risk. Proc. Natl. Acad. Sci. 2019, 116, 16955–16960. [Google Scholar] [CrossRef] [PubMed]
- Casiraghi, C.; Dorovini-Zis, K.; Horwitz, M.S. Epstein-Barr virus infection of human brain microvessel endothelial cells: A novel role in multiple sclerosis. J. Neuroimmunol. 2011, 230, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Degelman, M.L.; Herman, K.M. Smoking and multiple sclerosis: A systematic review and meta-analysis using the Bradford Hill criteria for causation. Mult. Scler. Relat. Disord. 2017, 17, 207–216. [Google Scholar] [CrossRef]
- Hawkins, B.T.; Abbruscato, T.J.; Egleton, R.D.; Brown, R.C.; Huber, J.D.; Campos, C.R.; Davis, T.P. Nicotine increases in vivo blood–brain barrier permeability and alters cerebral microvascular tight junction protein distribution. Brain Res. 2004, 1027, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Johansson, E.; Alfredsson, L.; Strid, P.; Kockum, I.; Olsson, T.; Hedström, A.K. Head trauma results in manyfold increased risk of multiple sclerosis in genetically susceptible individuals. J. Neurol. Neurosurg. Psychiatry 2024, 95, 554–560. [Google Scholar] [CrossRef]
- Cash, A.; Theus, M.H. Mechanisms of Blood–Brain Barrier Dysfunction in Traumatic Brain Injury. Int. J. Mol. Sci. 2020, 21, 3344. [Google Scholar] [CrossRef] [PubMed]
- Milligan, N.M.; Newcombe, R.; A Compston, D. A double-blind controlled trial of high dose methylprednisolone in patients with multiple sclerosis: Clinical effects. J. Neurol. Neurosurg. Psychiatry 1987, 50, 511–516. [Google Scholar] [CrossRef]
- Sloka, J.S.; Stefanelli, M. The mechanism of action of methylprednisolone in the treatment of multiple sclerosis. Mult. Scler. J. 2005, 11, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Cáceres, E.M.; A Barrau, M.; Brieva, L.; Espejo, C.; Barberà, N.; Montalban, X. Treatment with methylprednisolone in relapses of multiple sclerosis patients: Immunological evidence of immediate and short-term but not long-lasting effects. Clin. Exp. Immunol. 2002, 127, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Gelati, M.; Corsini, E.; Dufour, A.; Massa, G.; Giombini, S.; Solero, C.L.; Salmaggi, A. High-dose methylprednisolone reduces cytokine-induced adhesion molecules on human brain endothelium. Can. J. Neurol. Sci. 2000, 27, 241–244. [Google Scholar] [CrossRef]
- Xu, J.; Kim, G.-M.; Ahmed, S.H.; Xu, J.; Yan, P.; Xu, X.M.; Hsu, C.Y. Glucocorticoid Receptor-Mediated Suppression of Activator Protein-1 Activation and Matrix Metalloproteinase Expression after Spinal Cord Injury. J. Neurosci. 2001, 21, 92–97. [Google Scholar] [CrossRef]
- Förster, C.; Silwedel, C.; Golenhofen, N.; Burek, M.; Kietz, S.; Mankertz, J.; Drenckhahn, D. Occludin as direct target for glucocorticoid-induced improvement of blood-brain barrier properties in a murine in vitro system. J. Physiol. 2005, 565, 475–486. [Google Scholar] [CrossRef]
- Kraus, J.; Ling, A.K.; Hamm, S.; Voigt, K.; Oschmann, P.; Engelhardt, B. Interferon-β stabilizes barrier characteristics of brain endothelial cells in vitro. Ann. Neurol. 2004, 56, 192–205. [Google Scholar] [CrossRef]
- Kraus, J.; Oschmann, P. The impact of interferon-β treatment on the blood–brain barrier. Drug Discov. Today 2006, 11, 755–762. [Google Scholar] [CrossRef]
- Kuruganti, P.A.; Hinojoza, J.R.; Eaton, M.J.; Ehmann, U.K.; Sobel, R.A. Interferon-β Counteracts Inflammatory Mediator-Induced Effects on Brain Endothelial Cell Tight Junction Molecules—Implications for Multiple Sclerosis. J. Neuropathol. Exp. Neurol. 2002, 61, 710–724. [Google Scholar] [CrossRef]
- McCormack, P.L. Natalizumab: A Review of Its Use in the Management of Relapsing-Remitting Multiple Sclerosis. Drugs 2013, 73, 1463–1481. [Google Scholar] [CrossRef]
- Butzkueven, H.; Kappos, L.; Wiendl, H.; Trojano, M.; Spelman, T.; Chang, I.; Kasliwal, R.; Jaitly, S.; Campbell, N.; Ho, P.-R.; et al. Long-term safety and effectiveness of natalizumab treatment in clinical practice: 10 years of real-world data from the Tysabri Observational Program (TOP). J. Neurol. Neurosurg. Psychiatry 2020, 91, 660–668. [Google Scholar] [CrossRef]
- A Mills, E.; Mao-Draayer, Y. Aging and lymphocyte changes by immunomodulatory therapies impact PML risk in multiple sclerosis patients. Mult. Scler. J. 2018, 24, 1014–1022. [Google Scholar] [CrossRef]
- Cortese, I.; Reich, D.S.; Nath, A. Progressive multifocal leukoencephalopathy and the spectrum of JC virus-related disease. Nat. Rev. Neurol. 2020, 17, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Bresciani, G.; Manai, F.; Davinelli, S.; Tucci, P.; Saso, L.; Amadio, M. Novel potential pharmacological applications of dimethyl fumarate—An overview and update. Front. Pharmacol. 2023, 14, 1264842. [Google Scholar] [CrossRef] [PubMed]
- Mehta, D.; Miller, C.; Arnold, D.L.; Bame, E.; Bar-Or, A.; Gold, R.; Hanna, J.; Kappos, L.; Liu, S.; Matta, A.; et al. Effect of dimethyl fumarate on lymphocytes in RRMS. Neurology 2019, 92, e1724–e1738. [Google Scholar] [CrossRef]
- Hammer, A.; Waschbisch, A.; Kuhbandner, K.; Bayas, A.; Lee, D.; Duscha, A.; Haghikia, A.; Gold, R.; Linker, R.A. The NRF2 pathway as potential biomarker for dimethyl fumarate treatment in multiple sclerosis. Ann. Clin. Transl. Neurol. 2018, 5, 668–676. [Google Scholar] [CrossRef]
- Mills, E.A.; Ogrodnik, M.A.; Plave, A.; Mao-Draayer, Y. Emerging Understanding of the Mechanism of Action for Dimethyl Fumarate in the Treatment of Multiple Sclerosis. Front. Neurol. 2018, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Galloway, D.A.; Williams, J.B.; Moore, C.S. Effects of fumarates on inflammatory human astrocyte responses and oligodendrocyte differentiation. Ann. Clin. Transl. Neurol. 2017, 4, 381–391. [Google Scholar] [CrossRef]
- Hla, T.; Brinkmann, V. Sphingosine 1-phosphate (S1P). Neurology 2011, 76, S3–S8. [Google Scholar] [CrossRef]
- Van Doorn, R.; Nijland, P.G.; Dekker, N.; Witte, M.E.; Lopes-Pinheiro, M.A.; van het Hof, B.; Kooij, G.; Reijerkerk, A.; Dijkstra, C.; Van Van Der Valk, P.; et al. Fingolimod attenuates ceramide-induced blood–brain barrier dysfunction in multiple sclerosis by targeting reactive astrocytes. Acta Neuropathol. 2012, 124, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Hunter, S.F.; Bowen, J.D.; Reder, A.T. The Direct Effects of Fingolimod in the Central Nervous System: Implications for Relapsing Multiple Sclerosis. CNS Drugs 2015, 30, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Spampinato, S.F.; Obermeier, B.; Cotleur, A.; Love, A.; Takeshita, Y.; Sano, Y.; Kanda, T.; Ransohoff, R.M. Sphingosine 1 Phosphate at the Blood Brain Barrier: Can the Modulation of S1P Receptor 1 Influence the Response of Endothelial Cells and Astrocytes to Inflammatory Stimuli? PLoS ONE 2015, 10, e0133392. [Google Scholar] [CrossRef]
- Prager, B.; Spampinato, S.F.; Ransohoff, R.M. Sphingosine 1-phosphate signaling at the blood–brain barrier. Trends Mol. Med. 2015, 21, 354–363. [Google Scholar] [CrossRef]
- Nishihara, H.; Shimizu, F.; Sano, Y.; Takeshita, Y.; Maeda, T.; Abe, M.; Koga, M.; Kanda, T. Fingolimod Prevents Blood-Brain Barrier Disruption Induced by the Sera from Patients with Multiple Sclerosis. PLoS ONE 2015, 10, e0121488–e0121488. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Hartung, H.P. Mechanism of Action of Oral Fingolimod (FTY720) in Multiple Sclerosis. Clin. Neuropharmacol. 2010, 33, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.A.; Schmid, C.; Zurbruegg, S.; Jivkov, M.; Doelemeyer, A.; Theil, D.; Dubost, V.; Beckmann, N. Fingolimod inhibits brain atrophy and promotes brain-derived neurotrophic factor in an animal model of multiple sclerosis. J. Neuroimmunol. 2018, 318, 103–113. [Google Scholar] [CrossRef]
- Miron, V.E.; Jung, C.G.; Kim, H.J.; Kennedy, T.E.; Soliven, B.; Antel, J.P. FTY720 modulates human oligodendrocyte progenitor process extension and survival. Ann. Neurol. 2008, 63, 61–71. [Google Scholar] [CrossRef]
- Sorensen, S.D.; Nicole, O.; Peavy, R.D.; Montoya, L.M.; Lee, C.J.; Murphy, T.J.; Traynelis, S.F.; Hepler, J.R. Common Signaling Pathways Link Activation of Murine PAR-1, LPA, and S1P Receptors to Proliferation of Astrocytes. Mol. Pharmacol. 2003, 64, 1199–1209. [Google Scholar] [CrossRef]
- Noda, H.; Takeuchi, H.; Mizuno, T.; Suzumura, A. Fingolimod phosphate promotes the neuroprotective effects of microglia. J. Neuroimmunol. 2013, 256, 13–18. [Google Scholar] [CrossRef]
- Jackson, S.J.; Giovannoni, G.; Baker, D. Fingolimod modulates microglial activation to augment markers of remyelination. J. Neuroinflammation 2011, 8, 76–76. [Google Scholar] [CrossRef] [PubMed]
- Rammohan, K.; Coyle, P.K.; Sylvester, E.; Galazka, A.; Dangond, F.; Grosso, M.; Leist, T.P. The Development of Cladribine Tablets for the Treatment of Multiple Sclerosis: A Comprehensive Review. Drugs 2020, 80, 1901–1928. [Google Scholar] [CrossRef]
- Leist, T.P.; Weissert, R. Cladribine. Clin. Neuropharmacol. 2011, 34, 28–35. [Google Scholar] [CrossRef]
- Mitosek-Szewczyk, K.; Stelmasiak, Z.; Bartosik-Psujek, H.; Belniak, E. Impact of cladribine on soluble adhesion molecules in multiple sclerosis. Acta Neurol. Scand. 2010, 122, 409–413. [Google Scholar] [CrossRef]
- Siriratnam, P.; Huda, S.; Butzkueven, H.; van der Walt, A.; Jokubaitis, V.; Monif, M. A comprehensive review of the advances in neuromyelitis optica spectrum disorder. Autoimmun. Rev. 2023, 22, 103465. [Google Scholar] [CrossRef] [PubMed]
- Wingerchuk, D.M.; Banwell, B.; Bennett, J.L.; Cabre, P.; Carroll, W.; Chitnis, T.; De Seze, J.; Fujihara, K.; Greenberg, B.; Jacob, A.; et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015, 85, 177–189. [Google Scholar] [CrossRef]
- Hamid, S.H.; Elsone, L.; Mutch, K.; Solomon, T.; Jacob, A. The impact of 2015 neuromyelitis optica spectrum disorders criteria on diagnostic rates. Mult. Scler. J. 2016, 23, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Kermode, A.G.; Hu, X.; Qiu, W. Risk of relapse in patients with neuromyelitis optica spectrum disorder: Recognition and preventive strategy. Mult. Scler. Relat. Disord. 2020, 46, 102522. [Google Scholar] [CrossRef]
- Contentti, E.C.; Correale, J. Neuromyelitis optica spectrum disorders: From pathophysiology to therapeutic strategies. J. Neuroinflammation 2021, 18, 1–18. [Google Scholar] [CrossRef]
- Chihara, N.; Aranami, T.; Oki, S.; Matsuoka, T.; Nakamura, M.; Kishida, H.; Yokoyama, K.; Kuroiwa, Y.; Hattori, N.; Okamoto, T.; et al. Plasmablasts as Migratory IgG-Producing Cells in the Pathogenesis of Neuromyelitis Optica. PLoS ONE 2013, 8, e83036. [Google Scholar] [CrossRef]
- Chihara, N.; Aranami, T.; Sato, W.; Miyazaki, Y.; Miyake, S.; Okamoto, T.; Ogawa, M.; Toda, T.; Yamamura, T. Interleukin 6 signaling promotes anti-aquaporin 4 autoantibody production from plasmablasts in neuromyelitis optica. Proc. Natl. Acad. Sci. USA 2011, 108, 3701–3706. [Google Scholar] [CrossRef]
- Papadopoulos, M.C.; Verkman, A. Aquaporin 4 and neuromyelitis optica. Lancet Neurol. 2012, 11, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Crane, J.M.; Lam, C.; Rossi, A.; Gupta, T.; Bennett, J.L.; Verkman, A.S. Binding affinity and specificity of neuromyelitis optica autoantibodies to aquaporin-4 M1/M23 isoforms and orthogonal arrays. J. Biol. Chem. 2011, 286, 16516–16524. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, M.C.; Bennett, J.L.; Verkman, A.S. Treatment of neuromyelitis optica: State-of-the-art and emerging therapies. Nat. Rev. Neurol. 2014, 10, 493–506. [Google Scholar] [CrossRef]
- Lucchinetti, C.F.; Guo, Y.; Popescu, B.F.G.; Fujihara, K.; Itoyama, Y.; Misu, T. The Pathology of an Autoimmune Astrocytopathy: Lessons Learned from Neuromyelitis Optica. Brain Pathol. 2013, 24, 83–97. [Google Scholar] [CrossRef]
- Linhares, U.C.; Schiavoni, P.B.; Barros, P.O.; Kasahara, T.M.; Teixeira, B.; Ferreira, T.B.; Alvarenga, R.; Hygino, J.; Vieira, M.M.M.; Bittencourt, V.C.B.; et al. The Ex Vivo Production of IL-6 and IL-21 by CD4+ T Cells is Directly Associated with Neurological Disability in Neuromyelitis Optica Patients. J. Clin. Immunol. 2012, 33, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Li, X.; Xia, J. Th17 cells in neuromyelitis optica spectrum disorder: A review. Int. J. Neurosci. 2015, 126, 1051–1060. [Google Scholar] [CrossRef]
- Tomizawa, Y.; Yokoyama, K.; Saiki, S.; Takahashi, T.; Matsuoka, J.; Hattori, N. Blood—Brain Barrier Disruption is More Severe in Neuromyelitis Optica than in Multiple Sclerosis and Correlates with Clinical Disability. J. Int. Med Res. 2012, 40, 1483–1491. [Google Scholar] [CrossRef] [PubMed]
- You, X.; Yan, L.; Li, X.; Pang, Y.; Guo, X.; Ye, J.; Hu, H. Disruption of blood-brain barrier integrity associated with brain lesions in Chinese neuromyelitis optica spectrum disorder patients. Mult. Scler. Relat. Disord. 2018, 27, 254–259. [Google Scholar] [CrossRef]
- Rodin, R.E.; Chitnis, T. Soluble biomarkers for Neuromyelitis Optica Spectrum Disorders: A mini review. Front. Neurol. 2024, 15, 1415535. [Google Scholar] [CrossRef]
- Vaknin-Dembinsky, A.; Brill, L.; Orpaz, N.; Abramsky, O.; Karussis, D. Preferential increase of B-cell activating factor in the cerebrospinal fluid of neuromyelitis optica in a white population. Mult. Scler. J. 2010, 16, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, K.; Sato, D.K.; Nakashima, I.; Ogawa, R.; Akaishi, T.; Takai, Y.; Nishiyama, S.; Takahashi, T.; Misu, T.; Kuroda, H.; et al. CSF cytokine profile in MOG-IgG+ neurological disease is similar to AQP4-IgG+ NMOSD but distinct from MS: A cross-sectional study and potential therapeutic implications. J. Neurol. Neurosurg. Psychiatry 2018, 89, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, J.; Wang, Z.; Wang, Y.; Zheng, D.; Wang, H.; Peng, Y. The CSF Levels of Neutrophil-Related Chemokines in Patients with Neuromyelitis Optica. Ann. Clin. Transl. Neurol. 2020, 7, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, T.; Tateishi, T.; Isobe, N.; Yonekawa, T.; Yamasaki, R.; Matsuse, D.; Murai, H.; Kira, J.-I. Characteristic Cerebrospinal Fluid Cytokine/Chemokine Profiles in Neuromyelitis Optica, Relapsing Remitting or Primary Progressive Multiple Sclerosis. PLoS ONE 2013, 8, e61835. [Google Scholar] [CrossRef] [PubMed]
- Grebenciucova, E.; VanHaerents, S. Interleukin 6: At the interface of human health and disease. Front. Immunol. 2023, 14, 1255533. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, K.; Bennett, J.L.; de Seze, J.; Haramura, M.; Kleiter, I.; Weinshenker, B.G.; Kang, D.; Mughal, T.; Yamamura, T. Interleukin-6 in neuromyelitis optica spectrum disorder pathophysiology. Neurol. - Neuroimmunol. Neuroinflammation 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Uzawa, A.; Mori, M.; Ito, M.; Uchida, T.; Hayakawa, S.; Masuda, S.; Kuwabara, S. Markedly increased CSF interleukin-6 levels in neuromyelitis optica, but not in multiple sclerosis. J. Neurol. 2009, 256, 2082–2084. [Google Scholar] [CrossRef]
- Uzawa, A.; Mori, M.; Arai, K.; Sato, Y.; Hayakawa, S.; Masuda, S.; Taniguchi, J.; Kuwabara, S. Cytokine and chemokine profiles in neuromyelitis optica: Significance of interleukin-6. Mult. Scler. J. 2010, 16, 1443–1452. [Google Scholar] [CrossRef]
- Misu, T.; Takano, R.; Fujihara, K.; Takahashi, T.; Sato, S.; Itoyama, Y. Marked increase in cerebrospinal fluid glial fibrillar acidic protein in neuromyelitis optica: An astrocytic damage marker. J. Neurol. Neurosurg. Psychiatry 2009, 80, 575–577. [Google Scholar] [CrossRef]
- Aktas, O.; Smith, M.A.; Marignier, R.; Kim, H.J.; Weinshenker, B.G.; Pittock, S.J.; Wingerchuk, D.M.; Cutter, G.R.; Green, A.J.; Mealy, M.A.; et al. Serum Glial Fibrillary Acidic Protein: A Neuromyelitis Optica Spectrum Disorder Biomarker. Ann. Neurol. 2021, 89, 895–910. [Google Scholar] [CrossRef]
- Aktas, O.; Hartung, H.-P.; A Smith, M.; A Rees, W.; Fujihara, K.; Paul, F.; Marignier, R.; Bennett, J.L.; Kim, H.J.; Weinshenker, B.G.; et al. Serum neurofilament light chain levels at attack predict post-attack disability worsening and are mitigated by inebilizumab: Analysis of four potential biomarkers in neuromyelitis optica spectrum disorder. J. Neurol. Neurosurg. Psychiatry 2023, 94, 757–768. [Google Scholar] [CrossRef]
- Kim, S.-M.; Waters, P.; Vincent, A.; Go, M.J.; Park, K.S.; Sung, J.-J.; Lee, K.-W. Cerebrospinal fluid/serum gradient of IgG is associated with disability at acute attacks of neuromyelitis optica. J. Neurol. 2011, 258, 2176–2180. [Google Scholar] [CrossRef] [PubMed]
- Jarius, S.; Franciotta, D.; Paul, F.; Ruprecht, K.; Bergamaschi, R.; Rommer, P.S.; Reuss, R.; Probst, C.; Kristoferitsch, W.; Wandinger, K.P.; et al. Cerebrospinal fluid antibodies to aquaporin-4 in neuromyelitis optica and related disorders: Frequency, origin, and diagnostic relevance. J. Neuroinflamm. 2010, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Kowarik, M.C.; Dzieciatkowska, M.; Wemlinger, S.; Ritchie, A.M.; Hemmer, B.; Owens, G.P.; Bennett, J.L. The cerebrospinal fluid immunoglobulin transcriptome and proteome in neuromyelitis optica reveals central nervous system-specific B cell populations. J. Neuroinflammation 2015, 12, 1–8. [Google Scholar] [CrossRef]
- Bradl, M.; Misu, T.; Takahashi, T.; Watanabe, M.; Mader, S.; Reindl, M.; Adzemovic, M.; Bauer, J.; Berger, T.; Fujihara, K.; et al. Neuromyelitis optica: Pathogenicity of patient immunoglobulin in vivo. Ann. Neurol. 2009, 66, 630–643. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Fischer, M.-T.; Bauer, J.; Felts, P.A.; Smith, K.J.; Misu, T.; Fujihara, K.; Bradl, M.; Lassmann, H. Inflammation induced by innate immunity in the central nervous system leads to primary astrocyte dysfunction followed by demyelination. Acta Neuropathol. 2010, 120, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Jitprapaikulsan, J.; Fryer, J.P.; Majed, M.; Smith, C.Y.; Jenkins, S.M.; Cabre, P.; Hinson, S.R.; Weinshenker, B.G.; Mandrekar, J.; Chen, J.J.; et al. Clinical utility of AQP4-IgG titers and measures of complement-mediated cell killing in NMOSD. Neurol. - Neuroimmunol. Neuroinflammation 2020, 7. [Google Scholar] [CrossRef]
- Schmetzer, O.; Lakin, E.; Roediger, B.; Duchow, A.; Asseyer, S.; Paul, F.; Siebert, N. Anti-aquaporin 4 IgG Is Not Associated With Any Clinical Disease Characteristics in Neuromyelitis Optica Spectrum Disorder. Front. Neurol. 2021, 12. [Google Scholar] [CrossRef]
- Shimizu, F.; Ransohoff, R.M. GRP78 autoantibodies initiate the breakdown of the blood-brain barrier in neuromyelitis optica. Oncotarget 2017, 8, 106175–106176. [Google Scholar] [CrossRef]
- Kim, H.J.; Paul, F.; Lana-Peixoto, M.A.; Tenembaum, S.; Asgari, N.; Palace, J.; Klawiter, E.C.; Sato, D.K.; de Seze, J.; Wuerfel, J.; et al. MRI characteristics of neuromyelitis optica spectrum disorder: An international update. Neurology 2015, 84, 1165–1173. [Google Scholar] [CrossRef]
- Clarke, L.; Arnett, S.; Bukhari, W.; Khalilidehkordi, E.; Sanchez, S.J.; O’Gorman, C.; Sun, J.; Prain, K.M.; Woodhall, M.; Silvestrini, R.; et al. MRI Patterns Distinguish AQP4 Antibody Positive Neuromyelitis Optica Spectrum Disorder From Multiple Sclerosis. Front. Neurol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, F.; Sano, Y.; Takahashi, T.; Haruki, H.; Saito, K.; Koga, M.; Kanda, T. Sera from neuromyelitis optica patients disrupt the blood–brain barrier. J. Neurol. Neurosurg. Psychiatry 2011, 83, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Tasaki, A.; Shimizu, F.; Sano, Y.; Fujisawa, M.; Takahashi, T.; Haruki, H.; Abe, M.; Koga, M.; Kanda, T. Autocrine MMP-2/9 secretion increases the BBB permeability in neuromyelitis optica. J. Neurol. Neurosurg. Psychiatry 2014, 85, 419–430. [Google Scholar] [CrossRef]
- Shimizu, F.; Nishihara, H.; Sano, Y.; Takeshita, Y.; Takahashi, S.; Maeda, T.; Takahashi, T.; Abe, M.; Koga, M.; Kanda, T. Markedly Increased IP-10 Production by Blood-Brain Barrier in Neuromyelitis Optica. PLoS ONE 2015, 10, e0122000. [Google Scholar] [CrossRef]
- Shimizu, F.; Schaller, K.L.; Owens, G.P.; Cotleur, A.C.; Kellner, D.; Takeshita, Y.; Obermeier, B.; Kryzer, T.J.; Sano, Y.; Kanda, T.; et al. Glucose-regulated protein 78 autoantibody associates with blood-brain barrier disruption in neuromyelitis optica. Sci. Transl. Med. 2017, 9, 397. [Google Scholar] [CrossRef]
- Shimizu, F.; Takeshita, Y.; Hamamoto, Y.; Nishihara, H.; Sano, Y.; Honda, M.; Sato, R.; Maeda, T.; Takahashi, T.; Fujikawa, S.; et al. GRP 78 antibodies are associated with clinical phenotype in neuromyelitis optica. Ann. Clin. Transl. Neurol. 2019, 6, 2079–2087. [Google Scholar] [CrossRef]
- Bläss, S.; Union, A.; Raymackers, J.; Schumann, F.; Ungethüm, U.; Müller-Steinbach, S.; De Keyser, F.; Engel, J.M.; Burmester, G.R. The stress protein BiP is overexpressed and is a major B and T cell target in rheumatoid arthritis. Arthritis Rheum 2001, 44, 761–771. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Chang, H.; Wang, H.; Xu, W.; Cong, H.; Zhang, X.; Liu, J.; Yin, L. NMO-IgG Induce Interleukin-6 Release via Activation of the NF-κB Signaling Pathway in Astrocytes. Neuroscience 2022, 496, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Chang, H.; Xu, W.; Wei, Y.; Wang, Y.; Yin, L.; Zhang, X. Effect of NMO-IgG on the interleukin-6 cascade in astrocytes via activation of the JAK/STAT3 signaling pathway. Life Sci. 2020, 258, 118217. [Google Scholar] [CrossRef]
- Takeshita, Y.; Obermeier, B.; Cotleur, A.C.; Spampinato, S.F.; Shimizu, F.; Yamamoto, E.; Sano, Y.; Kryzer, T.J.; Lennon, V.A.; Kanda, T.; et al. Effects of neuromyelitis optica–IgG at the blood–brain barrier in vitro. Neurol. - Neuroimmunol. Neuroinflammation 2017, 4, e311. [Google Scholar] [CrossRef]
- Takeshita, Y.; Fujikawa, S.; Serizawa, K.; Fujisawa, M.; Matsuo, K.; Nemoto, J.; Shimizu, F.; Sano, Y.; Tomizawa-Shinohara, H.; Miyake, S.; et al. New BBB Model Reveals That IL-6 Blockade Suppressed the BBB Disorder, Preventing Onset of NMOSD. Neurol.-Neuroimmunol. Neuroinflammation 2021, 8. [Google Scholar] [CrossRef]
- Banwell, B.; Bennett, J.L.; Marignier, R.; Kim, H.J.; Brilot, F.; Flanagan, E.P.; Ramanathan, S.; Waters, P.; Tenembaum, S.; Graves, J.S.; et al. Diagnosis of myelin oligodendrocyte glycoprotein antibody-associated disease: International MOGAD Panel proposed criteria. Lancet Neurol. 2023, 22, 268–282. [Google Scholar] [CrossRef] [PubMed]
- Marignier, R.; Hacohen, Y.; Cobo-Calvo, A.; Pröbstel, A.-K.; Aktas, O.; Alexopoulos, H.; Amato, M.-P.; Asgari, N.; Banwell, B.; Bennett, J.; et al. Myelin-oligodendrocyte glycoprotein antibody-associated disease. Lancet Neurol. 2021, 20, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Kezuka, T.; Ishikawa, H.; Tanaka, M.; Sakimura, K.; Abe, M.; Kawamura, M. Pathogenesis, Clinical Features, and Treatment of Patients with Myelin Oligodendrocyte Glycoprotein (MOG) Autoantibody-Associated Disorders Focusing on Optic Neuritis with Consideration of Autoantibody-Binding Sites: A Review. Int. J. Mol. Sci. 2023, 24, 13368. [Google Scholar] [CrossRef] [PubMed]
- Ambrosius, W.; Michalak, S.; Kozubski, W.; Kalinowska, A. Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease: Current Insights into the Disease Pathophysiology, Diagnosis and Management. Int. J. Mol. Sci. 2020, 22, 100. [Google Scholar] [CrossRef]
- Wang, J.J.; Jaunmuktane, Z.; Mummery, C.; Brandner, S.; Leary, S.; Trip, S.A. Inflammatory demyelination without astrocyte loss in MOG antibody–positive NMOSD. Neurology 2016, 87, 229–231. [Google Scholar] [CrossRef]
- Zhou, L.; Huang, Y.; Li, H.; Fan, J.; Zhangbao, J.; Yu, H.; Li, Y.; Lu, J.; Zhao, C.; Lu, C.; et al. MOG-antibody associated demyelinating disease of the CNS: A clinical and pathological study in Chinese Han patients. J. Neuroimmunol. 2017, 305, 19–28. [Google Scholar] [CrossRef]
- Körtvélyessy, P.; Breu, M.; Pawlitzki, M.; Metz, I.; Heinze, H.-J.; Matzke, M.; et al. ADEM-like presentation, anti-MOG antibodies, and MS pathology: Two case reports. Neurol. Neuroimmunol Neuroinflamm 2017, 4, e335. [Google Scholar] [CrossRef]
- Höftberger, R.; Guo, Y.; Flanagan, E.P.; Lopez-Chiriboga, A.S.; Endmayr, V.; Hochmeister, S.; Joldic, D.; Pittock, S.J.; Tillema, J.M.; Gorman, M.; et al. The pathology of central nervous system inflammatory demyelinating disease accompanying myelin oligodendrocyte glycoprotein autoantibody. Acta Neuropathol. 2020, 139, 875–892. [Google Scholar] [CrossRef]
- Machado-Santos, J.; Saji, E.; Tröscher, A.R.; Paunovic, M.; Liblau, R.; Gabriely, G.; Bien, C.G.; Bauer, J.; Lassmann, H. The compartmentalized inflammatory response in the multiple sclerosis brain is composed of tissue-resident CD8+ T lymphocytes and B cells. Brain 2018, 141, 2066–2082. [Google Scholar] [CrossRef]
- Takai, Y.; Misu, T.; Kaneko, K.; Chihara, N.; Narikawa, K.; Tsuchida, S.; Nishida, H.; Komori, T.; Seki, M.; Komatsu, T.; et al. Myelin oligodendrocyte glycoprotein antibody-associated disease: An immunopathological study. Brain 2020, 143, 1431–1446. [Google Scholar] [CrossRef] [PubMed]
- Jarius, S.; Pellkofer, H.; Siebert, N.; Korporal-Kuhnke, M.; Hümmert, M.W.; Ringelstein, M.; Rommer, P.S.; Ayzenberg, I.; Ruprecht, K.; Klotz, L.; et al. Cerebrospinal fluid findings in patients with myelin oligodendrocyte glycoprotein (MOG) antibodies. Part 1: Results from 163 lumbar punctures in 100 adult patients. J. Neuroinflammation 2020, 17, 1–26. [Google Scholar] [CrossRef] [PubMed]
- in cooperation with the Neuromyelitis Optica Study Group (NEMOS); Jarius, S. ; Ruprecht, K.; Kleiter, I.; Borisow, N.; Asgari, N.; Pitarokoili, K.; Pache, F.; Stich, O.; Beume, L.-A.; et al. MOG-IgG in NMO and related disorders: A multicenter study of 50 patients. Part 2: Epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J. Neuroinflammation 2016, 13, 1–45. [Google Scholar] [CrossRef]
- Tanaka, S.; Hashimoto, B.; Izaki, S.; Oji, S.; Fukaura, H.; Nomura, K. Clinical and immunological differences between MOG associated disease and anti AQP4 antibody-positive neuromyelitis optica spectrum disorders: Blood-brain barrier breakdown and peripheral plasmablasts. Mult. Scler. Relat. Disord. 2020, 41, 102005. [Google Scholar] [CrossRef]
- Kaneko, K.; Sato, D.K.; Nakashima, I.; Ogawa, R.; Akaishi, T.; Takai, Y.; Nishiyama, S.; Takahashi, T.; Misu, T.; Kuroda, H.; et al. CSF cytokine profile in MOG-IgG+ neurological disease is similar to AQP4-IgG+ NMOSD but distinct from MS: A cross-sectional study and potential therapeutic implications. J. Neurol. Neurosurg. Psychiatry 2018, 89, 927–936. [Google Scholar] [CrossRef]
- Kothur, K.; Wienholt, L.; Tantsis, E.M.; Earl, J.; Bandodkar, S.; Prelog, K.; Tea, F.; Ramanathan, S.; Brilot, F.; Dale, R.C. B Cell, Th17, and Neutrophil Related Cerebrospinal Fluid Cytokine/Chemokines Are Elevated in MOG Antibody Associated Demyelination. PLoS ONE 2016, 11, e0149411. [Google Scholar] [CrossRef] [PubMed]
- Goverman, J. Autoimmune T cell responses in the central nervous system. Nat. Rev. Immunol. 2009, 9, 393–407. [Google Scholar] [CrossRef]
- Corbali, O.; Chitnis, T. Pathophysiology of myelin oligodendrocyte glycoprotein antibody disease. Front. Neurol. 2023, 14, 1137998. [Google Scholar] [CrossRef]
- Spadaro, M.; Winklmeier, S.; Beltrán, E.; Macrini, C.; Höftberger, R.; Schuh, E.; Thaler, F.S.; Gerdes, L.A.; Laurent, S.; Gerhards, R.; et al. Pathogenicity of human antibodies against myelin oligodendrocyte glycoprotein. Ann. Neurol. 2018, 84, 315–328. [Google Scholar] [CrossRef]
- Shimizu, F.; Ogawa, R.; Mizukami, Y.; Watanabe, K.; Hara, K.; Kadono, C.; Takahashi, T.; Misu, T.; Takeshita, Y.; Sano, Y.; et al. GRP78 Antibodies Are Associated With Blood-Brain Barrier Breakdown in Anti–Myelin Oligodendrocyte Glycoprotein Antibody–Associated Disorder. Neurol. - Neuroimmunol. Neuroinflammation 2021, 9. [Google Scholar] [CrossRef]
- Liu, Y.; Tu, Z.; Zhang, X.; Du, K.; Xie, Z.; Lin, Z. Pathogenesis and treatment of neuropsychiatric systemic lupus erythematosus: A review. Front. Cell Dev. Biol. 2022, 10, 998328. [Google Scholar] [CrossRef] [PubMed]
- Stock, A.D.; Gelb, S.; Pasternak, O.; Ben-Zvi, A.; Putterman, C. The blood brain barrier and neuropsychiatric lupus: New perspectives in light of advances in understanding the neuroimmune interface. Autoimmun. Rev. 2017, 16, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Brimberg, L.; Mader, S.; Fujieda, Y.; Arinuma, Y.; Kowal, C.; Volpe, B.T.; Diamond, B. Antibodies as Mediators of Brain Pathology. Trends Immunol. 2015, 36, 709–724. [Google Scholar] [CrossRef] [PubMed]
- Govoni, M.; Bortoluzzi, A.; Padovan, M.; Silvagni, E.; Borrelli, M.; Donelli, F.; Ceruti, S.; Trotta, F. The diagnosis and clinical management of the neuropsychiatric manifestations of lupus. J. Autoimmun. 2016, 74, 41–72. [Google Scholar] [CrossRef] [PubMed]
- Kivity, S.; Agmon-Levin, N.; Zandman-Goddard, G.; Chapman, J.; Shoenfeld, Y. Neuropsychiatric lupus: A mosaic of clinical presentations. BMC Med. 2015, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Stock, A.D.; Wen, J.; Putterman, C. Neuropsychiatric Lupus, the Blood Brain Barrier, and the TWEAK/Fn14 Pathway. Front. Immunol. 2013, 4, 484. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-Y.; Lin, J.; Lu, X.-Y.; Zhao, X.-Y. Expression of S100B protein levels in serum and cerebrospinal fluid with different forms of neuropsychiatric systemic lupus erythematosus. Clin. Rheumatol. 2007, 27, 353–357. [Google Scholar] [CrossRef]
- Gris, J.-C.; Nobile, B.; Bouvier, S. Neuropsychiatric presentations of antiphospholipid antibodies. Thromb. Res. 2015, 135, S56–S59. [Google Scholar] [CrossRef]
- Fleetwood, T.; Cantello, R.; Comi, C. Antiphospholipid Syndrome and the Neurologist: From Pathogenesis to Therapy. Front. Neurol. 2018, 9, 1001. [Google Scholar] [CrossRef]
- Brimberg, L.; Mader, S.; Fujieda, Y.; Arinuma, Y.; Kowal, C.; Volpe, B.T.; Diamond, B. Antibodies as Mediators of Brain Pathology. Trends Immunol. 2015, 36, 709–724. [Google Scholar] [CrossRef]
- Mader, S.; Brimberg, L.; Diamond, B. The Role of Brain-Reactive Autoantibodies in Brain Pathology and Cognitive Impairment. Front. Immunol. 2017, 8, 1101–1101. [Google Scholar] [CrossRef] [PubMed]
- Yoshio, T.; Okamoto, H.; Hirohata, S.; Minota, S. IgG anti–NR2 glutamate receptor autoantibodies from patients with systemic lupus erythematosus activate endothelial cells. Arthritis Rheum. 2012, 65, 457–463. [Google Scholar] [CrossRef]
- Matus, S.; Burgos, P.V.; Bravo-Zehnder, M.; Kraft, R.; Porras, O.H.; Farías, P.; Barros, L.F.; Torrealba, F.; Massardo, L.; Jacobelli, S.; et al. Antiribosomal-P autoantibodies from psychiatric lupus target a novel neuronal surface protein causing calcium influx and apoptosis. J. Exp. Med. 2007, 204, 3221–3234. [Google Scholar] [CrossRef]
- DeGiorgio, L.A.; Konstantinov, K.N.; Lee, S.C.; Hardin, J.A.; Volpe, B.T.; Diamond, B. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat. Med. 2001, 7, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Kowal, C.; DeGiorgio, L.A.; Lee, J.Y.; Edgar, M.A.; Huerta, P.T.; Volpe, B.T.; Diamond, B. Human lupus autoantibodies against NMDA receptors mediate cognitive impairment. Proc. Natl. Acad. Sci. 2006, 103, 19854–19859. [Google Scholar] [CrossRef]
- Kowal, C.; DeGiorgio, L.A.; Nakaoka, T.; Hetherington, H.; Huerta, P.T.; et al. Cognition and immunity; antibody impairs memory. Immunity 2004, 21, 179–188. [Google Scholar] [CrossRef]
- Conti, F.; Alessandri, C.; Bompane, D.; Bombardieri, M.; Spinelli, F.R.; Rusconi, A.C.; Valesini, G. Autoantibody profile in systemic lupus erythematosus with psychiatric manifestations: A role for anti-endothelial-cell antibodies. Arthritis Res. Ther. 2004, 6, R366–R372. [Google Scholar] [CrossRef]
- Carvalho, D.; Savage, C.O.S.; Isenberg, D.; Pearson, J.D. IgG anti-endothelial cell autoantibodies from patients with systemic lupus erythematosus or systemic vasculitis stimulate the release of two endothelial cell-derived mediators, which enhance adhesion molecule expression and leukocyte adhesion in an autocrine manner. Arthritis Rheum. 1999, 42, 631–640. [Google Scholar] [CrossRef]
- Cieślik, P.; Semik-Grabarczyk, E.; Hrycek, A.; Holecki, M. The impact of anti-endothelial cell antibodies (AECAs) on the development of blood vessel damage in patients with systemic lupus erythematosus: The preliminary study. Rheumatol. Int. 2022, 42, 791–801. [Google Scholar] [CrossRef]
- Renaudineau, Y.; Dugué, C.; Dueymes, M.; Youinou, P. Antiendothelial cell antibodies in systemic lupus erythematosus. Autoimmun. Rev. 2002, 1, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Matsueda, Y.; Arinuma, Y.; Nagai, T.; Hirohata, S. Elevation of serum anti–glucose-regulated protein 78 antibodies in neuropsychiatric systemic lupus erythematosus. Lupus Sci. Med. 2018, 5, e000281. [Google Scholar] [CrossRef] [PubMed]
- Graus, F.; Titulaer, M.J.; Balu, R.; Benseler, S.; Bien, C.G.; Cellucci, T.; Cortese, I.; Dale, R.C.; Gelfand, J.M.; Geschwind, M.; et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016, 15, 391–404. [Google Scholar] [CrossRef]
- Tanaka, K.; Kawamura, M.; Sakimura, K.; Kato, N. Significance of Autoantibodies in Autoimmune Encephalitis in Relation to Antigen Localization: An Outline of Frequently Reported Autoantibodies with a Non-Systematic Review. Int. J. Mol. Sci. 2020, 21, 4941. [Google Scholar] [CrossRef] [PubMed]
- Shan, W.; Yang, H.; Wang, Q. Neuronal Surface Antibody-Medicated Autoimmune Encephalitis (Limbic Encephalitis) in China: A Multiple-Center, Retrospective Study. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef]
- Dalmau, J.; Armangué, T.; Planagumà, J.; Radosevic, M.; Mannara, F.; Leypoldt, F.; Geis, C.; Lancaster, E.; Titulaer, M.J.; Rosenfeld, M.R.; et al. An update on anti-NMDA receptor encephalitis for neurologists and psychiatrists: Mechanisms and models. Lancet Neurol. 2019, 18, 1045–1057. [Google Scholar] [CrossRef]
- Dalmau, J.; Gleichman, A.J.; Hughes, E.G.; Rossi, J.E.; Peng, X.; Lai, M.; Dessain, S.K.; Rosenfeld, M.R.; Balice-Gordon, R.; Lynch, D.R. Anti-NMDA-receptor encephalitis: Case series and analysis of the effects of antibodies. Lancet Neurol. 2008, 7, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Hughes, E.G.; Peng, X.; Gleichman, A.J.; Lai, M.; Zhou, L.; Tsou, R.; Parsons, T.D.; Lynch, D.R.; Dalmau, J.; Balice-Gordon, R.J. Cellular and Synaptic Mechanisms of Anti-NMDA Receptor Encephalitis. J. Neurosci. 2010, 30, 5866–5875. [Google Scholar] [CrossRef]
- Hughes, E.G.; Peng, X.; Gleichman, A.J.; Lai, M.; Zhou, L.; Tsou, R.; Parsons, T.D.; Lynch, D.R.; Dalmau, J.; Balice-Gordon, R.J. Cellular and Synaptic Mechanisms of Anti-NMDA Receptor Encephalitis. J. Neurosci. 2010, 30, 5866–5875. [Google Scholar] [CrossRef]
- Abe, M.; Fukaya, M.; Yagi, T.; Mishina, M.; Watanabe, M.; Sakimura, K. NMDA Receptor GluRϵ/NR2 Subunits Are Essential for Postsynaptic Localization and Protein Stability of GluRζ1/NR1 Subunit. J. Neurosci. 2004, 24, 7292–7304. [Google Scholar] [CrossRef]
- Zhang, Q.; Tanaka, K.; Sun, P.; Nakata, M.; Yamamoto, R.; Sakimura, K.; Matsui, M.; Kato, N. Suppression of synaptic plasticity by cerebrospinal fluid from anti-NMDA receptor encephalitis patients. Neurobiol. Dis. 2011, 45, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Camdessanché, J.-P.; Streichenberger, N.; Cavillon, G.; Rogemond, V.; Jousserand, G.; Honnorat, J.; Convers, P.; Antoine, J.-C. Brain immunohistopathological study in a patient with anti-NMDAR encephalitis. Eur. J. Neurol. 2010, 18, 929–931. [Google Scholar] [CrossRef]
- Dalmau, J.; Tüzün, E.; Wu, H.; Masjuan, J.; Rossi, J.E.; Voloschin, A.; Baehring, J.M.; Shimazaki, H.; Koide, R.; King, D.; et al. Paraneoplastic anti–N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann. Neurol. 2007, 61, 25–36. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, Y.; Cao, X.; Li, J.; Liao, X.; Wei, J.; Huang, W. The Clinical Features and Prognosis of Anti-NMDAR Encephalitis Depends on Blood Brain Barrier Integrity. Mult. Scler. Relat. Disord. 2020, 47, 102604. [Google Scholar] [CrossRef] [PubMed]
- Mao, F.; Huang, F.; Nong, W.; Lao, D.; Gong, Z.; Huang, W. N-methyl-D-aspartic acid increases tight junction protein destruction in brain endothelial cell via caveolin-1-associated ERK1/2 signaling. Toxicology 2022, 470, 153139. [Google Scholar] [CrossRef]
- Gong, Z.; Lao, D.; Wu, Y.; Li, T.; Lv, S.; Mo, X.; Huang, W. Inhibiting PI3K/Akt-Signaling Pathway Improves Neurobehavior Changes in Anti-NMDAR Encephalitis Mice by Ameliorating Blood–Brain Barrier Disruption and Neuronal Damage. Cell. Mol. Neurobiol. 2023, 43, 3623–3637. [Google Scholar] [CrossRef]
- Hampe, C.S.; Mitoma, H. A Breakdown of Immune Tolerance in the Cerebellum. Brain Sci. 2022, 12, 328. [Google Scholar] [CrossRef]
- Mitoma, H.; Buffo, A.; Gelfo, F.; Guell, X.; Fucà, E.; Kakei, S.; Schmahmann, J.D. Consensus Paper. Cerebellar Reserve: From Cerebellar Physiology to Cerebellar Disorders. Cerebellum.
- Yshii, L.; Bost, C.; Liblau, R. Immunological Bases of Paraneoplastic Cerebellar Degeneration and Therapeutic Implications. Front. Immunol. 2020, 11, 991. [Google Scholar] [CrossRef] [PubMed]
- Furneaux, H.M.; Rosenblum, M.K.; Dalmau, J.; Wong, E.; Woodruff, P.; Graus, F.; Posner, J.B. Selective Expression of Purkinje-Cell Antigens in Tumor Tissue from Patients with Paraneoplastic Cerebellar Degeneration. New Engl. J. Med. 1990, 322, 1844–1851. [Google Scholar] [CrossRef] [PubMed]
- Graus, F.; Vogrig, A.; Muñiz-Castrillo, S.; Antoine, J.-C.G.; Desestret, V.; Dubey, D.; Giometto, B.; Irani, S.R.; Joubert, B.; Leypoldt, F.; et al. Updated Diagnostic Criteria for Paraneoplastic Neurologic Syndromes. Neurol.-Neuroimmunol. Neuroinflammation 2021, 8. [Google Scholar] [CrossRef]
- Hadjivassiliou, M.; Manto, M.; Mitoma, H. Rare Etiologies in Immune-Mediated Cerebellar Ataxias: Diagnostic Challenges. Brain Sci. 2022, 12, 1165. [Google Scholar] [CrossRef]
- Flanagan, E.P. Paraneoplastic Disorders of the Nervous System. Contin. Lifelong Learn. Neurol. 2020, 26, 1602–1628. [Google Scholar] [CrossRef]
- Dalmau, J.; Rosenfeld, M.R. Paraneoplastic syndromes of the CNS. Lancet Neurol. 2008, 7, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Peterson, K.; Rosenblum, M.K.; Kotanides, H.; Posner, J.B. Paraneoplastic cerebellar degeneration: I. A clinical analysis of 55 anti-Yo antibody-positive patients. Neurology 2011, 77, 1690–1690. [Google Scholar] [CrossRef]
- Small, M.; Treilleux, I.; Couillault, C.; Pissaloux, D.; Picard, G.; Paindavoine, S.; Attignon, V.; Wang, Q.; Rogemond, V.; Lay, S.; et al. Genetic alterations and tumor immune attack in Yo paraneoplastic cerebellar degeneration. Acta Neuropathol. 2018, 135, 569–579. [Google Scholar] [CrossRef]
- Titulaer, M.J.; Lang, B.; Verschuuren, J.J. Lambert–Eaton myasthenic syndrome: From clinical characteristics to therapeutic strategies. Lancet Neurol. 2011, 10, 1098–1107. [Google Scholar] [CrossRef]
- Hülsbrink, R.; Hashemolhosseini, S. Lambert-Eaton myasthenic syndrome - diagnosis, pathogenesis and therapy. Clin. Neurophysiol. 2014, 125, 2328–2336. [Google Scholar] [CrossRef]
- Fukuda, T.; Motomura, M.; Nakao, Y.; Shiraishi, H.; Yoshimura, T.; Iwanaga, K.; Tsujihata, M.; Dosaka-Akita, H.; Eguchi, K. Reduction of P/Q-type calcium channels in the postmortem cerebellum of paraneoplastic cerebellar degeneration with Lambert-Eaton myasthenic syndrome. Ann. Neurol. 2002, 53, 21–28. [Google Scholar] [CrossRef]
- Shimizu, F.; Takeshita, Y.; Sano, Y.; Hamamoto, Y.; Shiraishi, H.; Sato, T.; Yoshimura, S.; Maeda, T.; Fujikawa, S.; Nishihara, H.; et al. GRP78 antibodies damage the blood–brain barrier and relate to cerebellar degeneration in Lambert-Eaton myasthenic syndrome. Brain 2019, 142, 2253–2264. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S. Glucose-regulated proteins in cancer: Molecular mechanisms and therapeutic potential. Nat. Rev. Cancer 2014, 14, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, C.G.; Rasmussen, N.; Laenkholm, A.-V.; Ditzel, H.J. Phage Display–Derived Human Monoclonal Antibodies Isolated by Binding to the Surface of Live Primary Breast Cancer Cells Recognize GRP78. Cancer Res. 2007, 67, 9507–9517. [Google Scholar] [CrossRef]
- Mintz, P.J.; Kim, J.; Do, K.A.; et al. Fingerprinting the circulating repertoire of antibodies from cancer patients. Nat. Biotechnol. 2003, 21, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Amaresan, R.; Gopal, U. Cell surface GRP78: A potential mechanism of therapeutic resistant tumors. Cancer Cell Int. 2023, 23, 100. [Google Scholar] [CrossRef]
- Tanigawa; Tsunemi, S. ; Nakanishi, T.; Fujita, Y.; Bouras, G.; Miyamoto, Y.; Miyamoto, A.; Nomura, E.; Takubo, T.; Tanigawa, N. Proteomics-based identification of a tumor-associated antigen and its corresponding autoantibody in gastric cancer. Oncol. Rep. 2010, 23, 949–956. [Google Scholar] [CrossRef]
- Raiter, A.; Vilkin, A.; Gingold, R.; Levi, Z.; Halpern, M.; Niv, Y.; Hardy, B. The Presence of Anti-GRP78 Antibodies in the Serum of Patients with Colorectal Carcinoma: A Potential Biomarker for Early Cancer Detection. Int. J. Biol. Markers 2014, 29, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gronow, M.; Pizzo, S.V. Physiological Roles of the Autoantibodies to the 78-Kilodalton Glucose-Regulated Protein (GRP78) in Cancer and Autoimmune Diseases. Biomedicines 2022, 10, 1222. [Google Scholar] [CrossRef]
- Shahmohammadi, S.; Doosti, R.; Shahmohammadi, A.; Azimi, A.; Sahraian, M.A.; Fattahi, M.-R.; Moghadasi, A.N. Neuromyelitis optica spectrum disorder (NMOSD) associated with cancer: A systematic review. Mult. Scler. Relat. Disord. 2021, 56, 103227. [Google Scholar] [CrossRef]
- Srichawla, B.S.; Doshi, K.; Cheraghi, S.N.; Sivakumar, S. The temporal relationship of paraneoplastic aquaporin-4-IgG seropositive neuromyelitis optica spectrum disorder (NMOSD) and breast cancer: A systematic review and meta-analysis. Neurol. Sci. 2023, 44, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Wang, X.; Duan, X.; Gao, H.; Ji, X.; Xiao, X.; Zhu, F.; Xue, Q. Neuromyelitis optica spectrum disorders associated with AQP4-positive-cancer—A case series. Front. Neurol. 2022, 13, 1071519. [Google Scholar] [CrossRef]
- Minomo, S.; Ichijo, M.; Shimizu, F.; Sato, R.; Kanda, T.; Takai, Y.; Misu, T.; Sakurai, Y.; Amino, T.; Kamata, T. Paraneoplastic Neuromyelitis Optica Spectrum Disorder Related to Glucose-regulated Protein 78 (GRP78) Autoantibodies in a Patient with Lynch Syndrome-associated Colorectal Cancer. Intern. Med. 2023, 62, 1653–1657. [Google Scholar] [CrossRef]
| DMT | Effects on the BBB-ECs | Cross the BBB? |
|---|---|---|
| Steroid | ↑Tight junction (occludin, claudin-5) ↑BBB function ↑TIMP-1 ↓MMP-9 |
Yes |
| Interferon-β | ↑BBB function ↓VCAM-1, ICAM-1, E selectin ↓Trans-endothelial migration of lymphocyte |
Yes, slightly |
| Anti-α4 integrin Ab Natalizumab |
↓↓Trans-endothelial migration of lymphocyte (Block the interaction between α4 integrin and VCAM1) |
None |
| Anti-CD20 Abs Ofatumumab, Ocrelizumab, Rituximab, |
Indirect effect due to depletion of B cells ↓proinflammatory cytokines ↓complement activity ↓autoantibody |
None |
| Fingolimod | ↓S1P1/S1P3 on the surface of BMECs ↓Trans-endothelial migration of lymphocyte ↓VCAM-1 ↑Tight junction (claudin-5) |
Yes |
| Dimethyl fumarate | ↓Trans-endothelial migration of lymphocyte ↓VCAM-1, ICAM-1 ↑BBB function via Nrf2-activity |
Yes |
| Cladribine | ↓ICAM-1, E-selectin ↓MMP-2, MMP-9 ↓CXCL8, CCL5 |
Yes |
| Anti-IL6 Ab Satralizumab |
↑BBB function ↓CCL2, CXCL8 ↓Trans-endothelial migration of lymphocyte |
None |
| Anti-CD19 Ab Inebilizumab |
Indirect effect due to depletion of B cells ↓proinflammatory cytokines ↓complement activity ↓autoantibody |
None |
| Anti-complement Ab Eculizumab Ravulizumab |
Unknown | Unknown |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





