1. Introduction
First described in 1729 by the Italian priest and biologist Pier Antonio Micheli, the fungus
Aspergillus, from the Latin
aspergere (to spread), received its name because it resembles an aspergillum, a device used in churches to sprinkle holy water [
1].
Aspergillus is a filamentous fungus, ubiquitous in nature, and can be found in soil, dust, plants and even in hospital ventilation systems [
2]. For example, they are important microorganisms that produce food, medicines, and enzymes [
3]. However, not all species are beneficial; some can cause human illness. Its spores remain suspended for long periods and can remain viable for months in a dry environment [
4,
5]. Inhalation of these
Aspergillus spores can cause an opportunistic infection known as aspergillosis, which is still frequently underdiagnosed [
6]. Theodor Sluyter reported the first case of aspergillosis in humans in 1847 [
7].
There are different clinical presentations of aspergillosis that can range from allergic-type disease (non-invasive) to generalized forms, leading to the risk of death (invasive form) [
9]. Allergic bronchopulmonary aspergillosis (ABPA) and allergic fungal rhinosinusitis are the non-invasive forms, while chronic pulmonary aspergillosis and invasive pulmonary aspergillosis (IPA) comprise the invasive forms of the disease [
8,
9].
The manifestation of each clinical type depends on the fungus’s virulence and the immunological host response [
10]. In immunocompetent patients, inhalation of
Aspergillus spores rarely causes illness, as they are eliminated from the body due to the coordinated action between the innate and adaptive responses of the immune system [
2,
11]. However, the infection can spread through vascular invasion to other organs [
10,
12], principally in immunocompromised patients. The dissemination of
Aspergillus may affect the nose, paranasal sinuses, and the oral cavity, which makes this infection of particular interest to dentistry [
5,
13].
Fungal sinusitis is the main manifestation of
Aspergillus infection in the maxillofacial region in immunocompromised patients [
14]. Other forms of infection include oral and maxillofacial lesions with nonspecific radiographic presentation, such as radiolucent lesions in the periapex after extraction or endodontics and ulcerations on the palate or gums [
5,
15]. While sinus aspergillosis lesions can be radiographically like viral or bacterial rhinosinusitis or even neoplasms [
16], ulcerated lesions can be confused with infections caused by other filamentous fungi, such as various
Zygomycetes (oral mucormycosis) [
17]. These overlapping clinical findings can hinder the differential diagnosis of aspergillosis cases, delaying its early identification in the oral medicine routine [
14].
Although
Aspergillus spores are constantly inhaled, disease is rarely observed in immunocompetent hosts. On the other hand, immunosuppressed patients (corticosteroid therapy) or critically ill patients are strongly associated with high mortality and morbidity rates due to aspergillosis [
18] and, therefore, early diagnosis is important to provide a better prognosis through an assertive therapeutic intervention [
14]. Histopathological analysis and culture of microorganisms are currently the gold standard for diagnosis [
9]. However, culture is a time-consuming examination, and histopathological analysis is only suggestive due to the microscopic similarity between the different fungal organisms. In addition, hyphae are not always visible on histopathological examination, and other fungal infections, such as fusariosis and mucormycosis, may have histopathological features superimposed on aspergillosis [
9,
19].
Therefore, it is necessary to develop a tool that helps in the rapid and reliable diagnosis of aspergillosis. The Polymerase Chain Reaction (PCR) may be a potential tool for early and assertive diagnosis since its benefits are already scientifically proven [
12]. However, due to the lack of a standardized technique for
Aspergillus, PCR is not yet widely used as a diagnostic method for aspergillosis [
10,
20]. In the present study, we aimed to standardize PCR assays for the detection of
Aspergillus fumigatus,
Aspergillus flavus and
Aspergillus niger, the main species responsible for human infections [
9,
10] and evaluate the reliability of this technique for the rapid diagnosis of aspergillosis.
2. Materials and Methods
This study was approved by the Research Ethics Committee of the Universidade Federal de Minas Gerais (CAAE: 74144623.4.0000.5149) in accordance with the principles of the 1964 Helsinki Declaration and its later amendments.
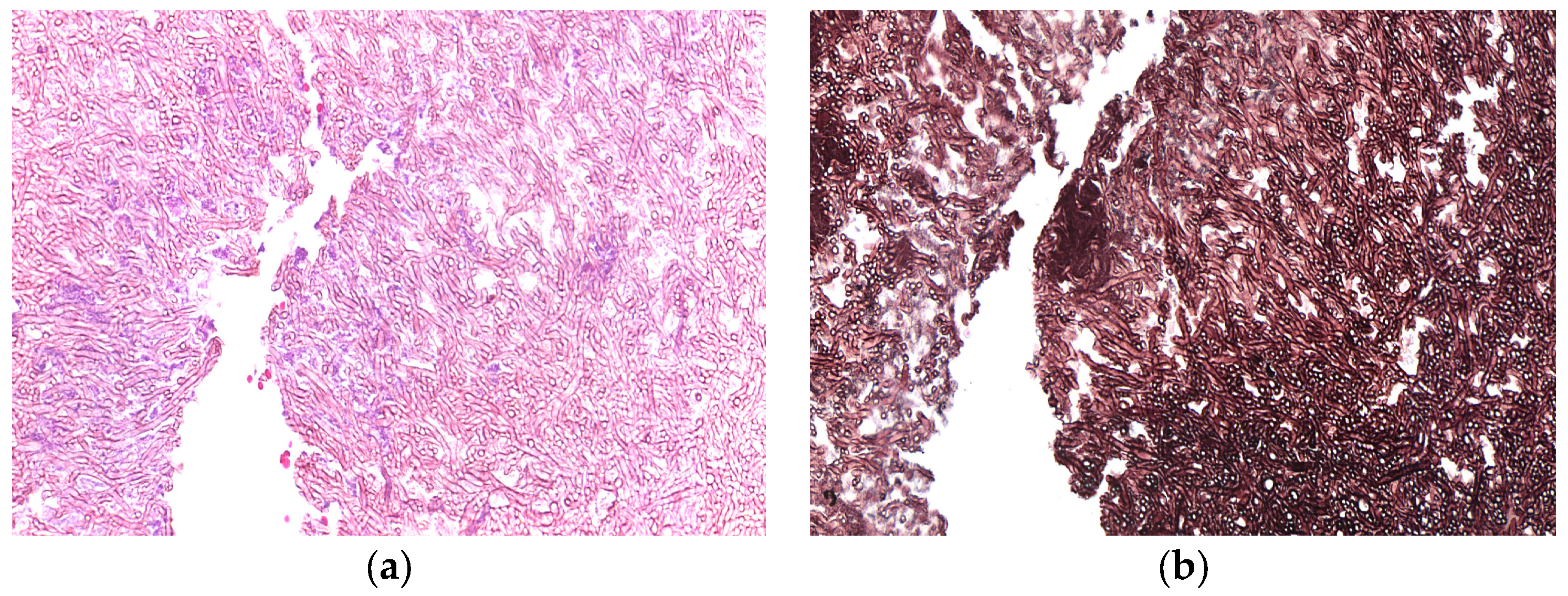
Digital records from the Oral and Maxillofacial Pathology Laboratory of the School of Dentistry of the Universidade Federal de Minas Gerais (UFMG) and Universidade Federal do Rio de Janeiro (UFRJ) were retrospectively accessed for all suspected cases of aspergillosis between 2000 and 2023. Six samples were analysed in the present study, all from male patients aged between 52 and 79 years old. For each case, clinical data and the corresponding FFPE samples were retrieved. The case 5 and 6 were obtained from patients with a history of frontal bone and zygomatic bone fractures, respectively. The microscopic aspects of all cases were reviewed by two Oral and Maxillofacial pathologists (RSG and MJRGS (
Figure 1). Fungal cultures of each species were used as positive controls. The Mycology Laboratory of the Institute of Biological Sciences at UFMG provided us with fungal cultures of
A. fumigatus and
A. niger, while the species
A. flavus was made available by the Faculty of Veterinary Medicine at UFMG, which were cultivated in medium own for seven days. Inflammatory fibrous hyperplasia sample was used as the negative control.
The fungal genomic DNA (gDNA) extraction from FFPE samples was performed using the commercial kits QIAamp DNA Blood Mini Kit (QIAGEN, Hilden, Germany) and DNeasy Blood & Tissue Kit (QIAGEN, Hilden, Germany), following the manufacturer's protocol with few adaptations. The first two 5µm-thick sections were discarded, and subsequently, ten tissue sections were collected in 1.5ml tubes and deparaffinized with 320µl of Deparaffinization solution (QIAGEN, Hilden, Germany). After adding 360µl of ATL buffer, the samples were left at -20 ºC for a minimum of 10 minutes. Three stainless steel beads were added to the tubes and vortexed for 10 minutes to macerate the tissue, disrupting the cell wall of the filamentous fungi. After removing the beads, 40µl of Proteinase K (QIAamp DNA Blood Mini Kit) were added and incubated overnight at 56°C. Then, 400µl of AL buffer were added and vortexed for another 10 minutes, followed by incubation for 1 hour at 70 ºC. The entire clear phase was transferred to a new 1.5ml tube where 400 µl of 100% alcohol were added. Subsequent steps followed the manufacturer's protocol. gDNA extraction from purified fungal culture samples, used as a positive control, followed a similar protocol. Briefly, culture plates were scraped with a plastic rod and washed with sterile phosphate buffered solution (PBS). The mycelia were collected in 15ml tubes and centrifuged to obtain a pellet. The pellet was redistributed into 1.5ml tubes and conditioned at -20 ºC until DNA extraction.
PCR reactions were performed following standard procedures using the MyTaq HS Red Mix, 2x (Bioline Reagents, London, UK) reagent. Optimal annealing temperatures were determined by gradient PCR and were settled at 57.8°C for all the primer sets. Cycling conditions were optimized as follows: 3 min - 95 ºC for initial denaturation, followed by 60 cycles (95ºC – 15s, 57.8°C – 15 s, 72°C – 30s), and 10 minutes at 72 ºC for final extension. 300ng of fungal gDNA was used to test the specificity of each primer set in a 25µl-PCR reaction and also served as positive control in all the experiments. For the analysis of the FFPE samples, an input of 100-200ng of gDNA was used in the reactions. gDNA extacted from Fibrous hyperplasia samples were used as negative controls. The primer sets used in the present study were kindly designed by Professor Dr. Leonel Mendoza from the University of Michigan, finding unique internal transcribed spacer (ITS) regions of these three fungi (
Table 1). 1µl of each primer (F and R respective of each species) at 10µM concentration were added to the PCR tubes. In order to minimize the risk of cross-contamination, the reactions for each species were performed separately. PCR products were loaded into a 1.5% agarose gel electrophoresis and inspected under UV light.
3. Results
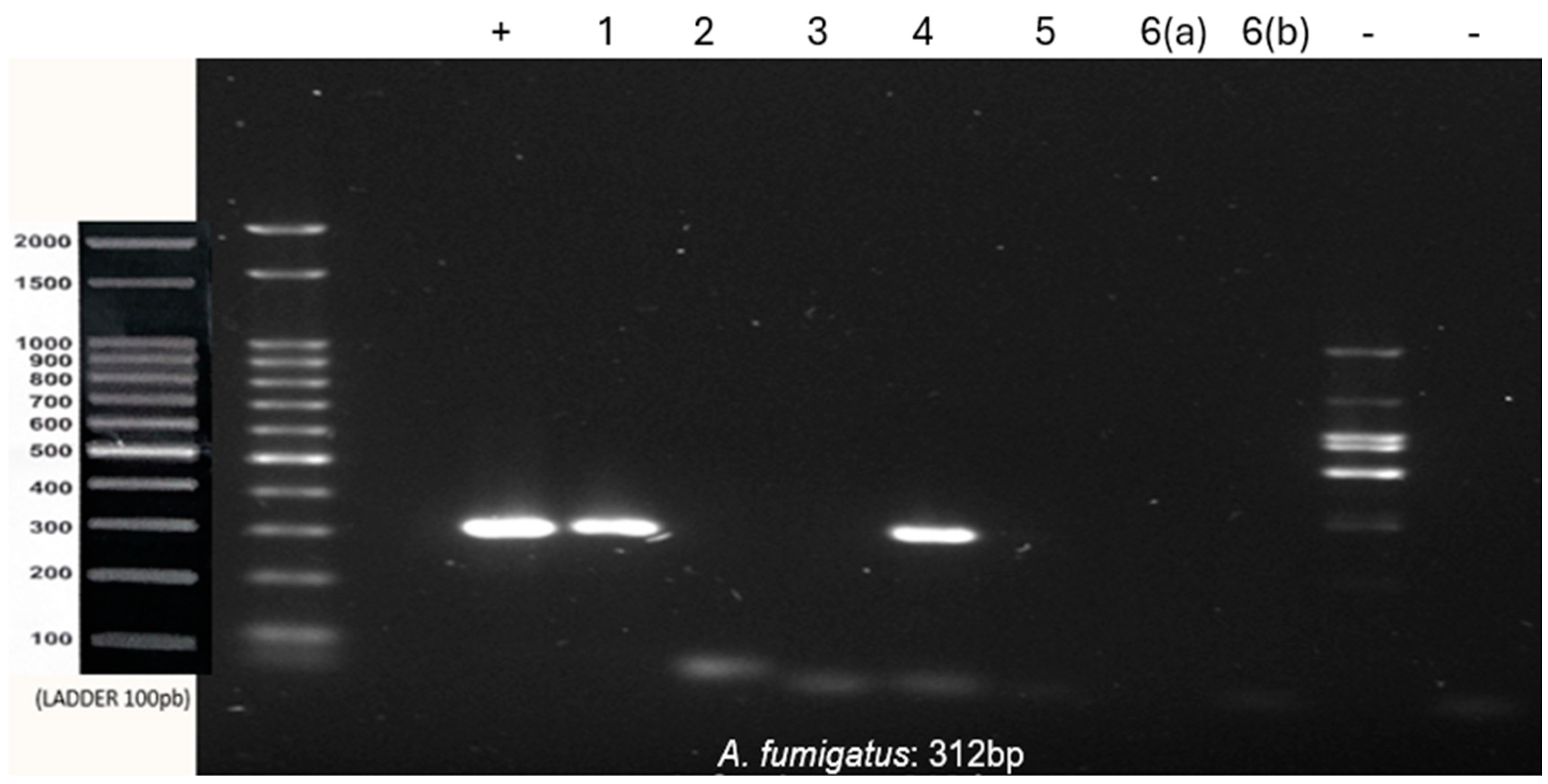
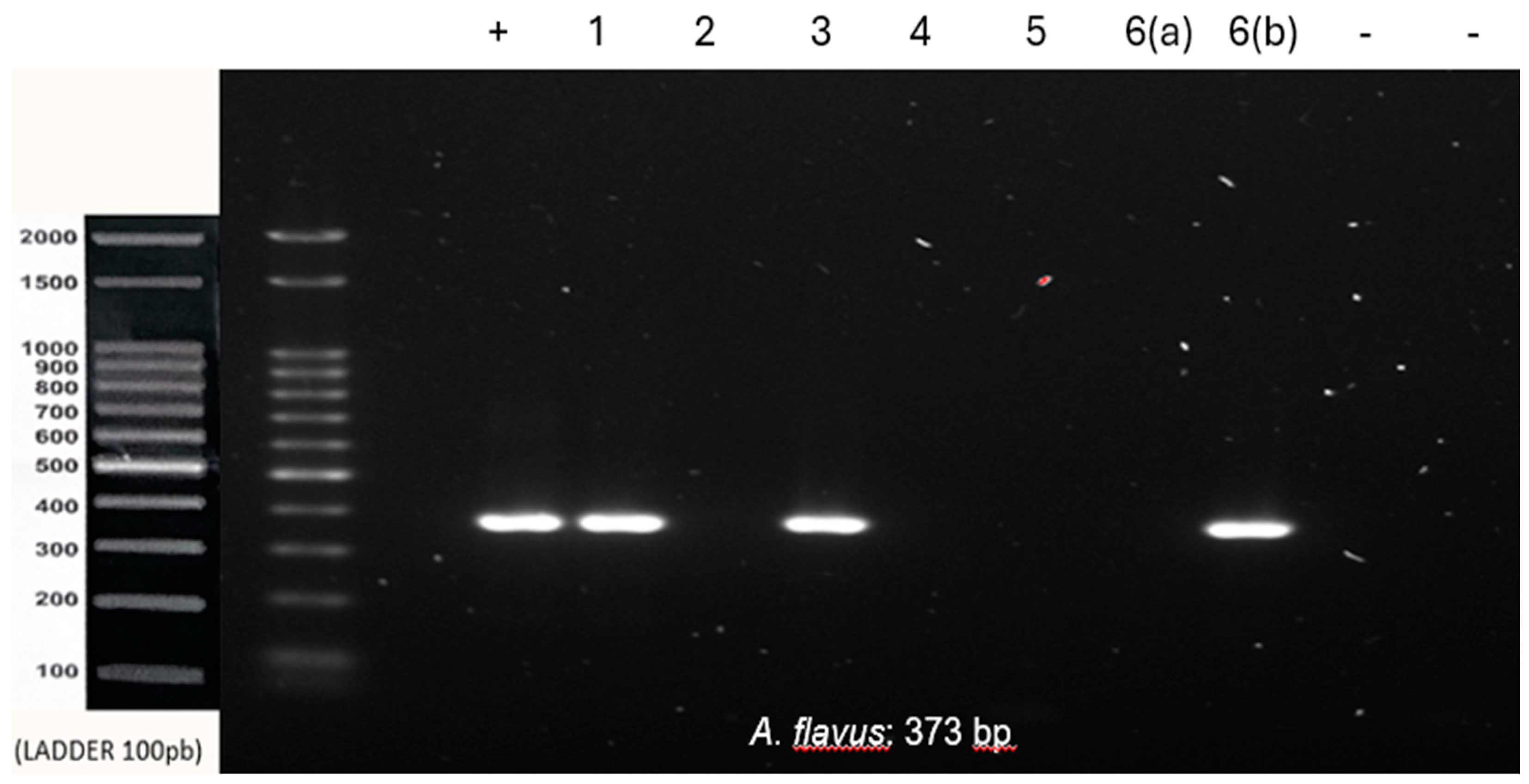
Co-infection with
A. fumigatus and
A. flavus was observed in samples 1 and 4. Samples 2, 3 and 6 were positive for
A. flavus, and sample 5 was negative for all tested species (
Figure 2 and
Figure 3).
All samples tested negative for A. niger. The inflammatory fibrous hyperplasia samples were negative for all three species tested, thus reinforcing the specificity of the primers and the lack of contamination during the reaction’s setup. All results are summarized in
Table 2.
4. Discussion
PCR has been increasingly consolidated as a potential diagnostic tool for several infectious diseases, being faster than traditional methods and providing good sensitivity and specificity [
23]. Early diagnosis of aspergillosis is still highly challenging, though. Its signs and symptoms are nonspecific, and the gold standard test, which is culture, can take a long time to provide a definitive and assertive result. The second most commonly used diagnostic tool in these cases is histopathological analysis, which has significant limitations in terms of specificity and sensitivity, since the arrangement of hyphae may resemble other fungal infections, thus requiring deep knowledge from the pathologist to establish the differential diagnosis [
19,
24].
With the advance of the SARS-CoV-2 virus and the possibility of co-infection with aspergillosis, mortality and morbidity rates, associated with fungal infections, have increased from 2019 [
9,
19]. This scenario justifies the importance of obtaining a rapid and accurate diagnosis, capable of optimizing and adequately directing the therapeutic management of these patients to minimize the risks that this infection offers.
Aspergillosis is also of dental interest since filamentous fungi, such as Aspergillus, have been isolated in root canals of teeth with pulp necrosis and apical periodontitis. Such endodontic conditions in upper posterior teeth are strongly associated with maxillary sinusitis [
15].
Despite advances in molecular studies in recent times as a diagnostic tool for many diseases, its application to aspergillosis is still incipient and restricted [
23]. Its major limitation is the lack of standardization to ensure reproducibility, specificity, and sensitivity [
10,
20].
The main changes made in the sample processing protocol and in the traditional sequence of gDNA extraction kits commonly used in molecular biology were: freezing and agitation with 3 mm diameter beads, to promote disruption of the fungal cell wall composed mainly of chitin; increase in the concentrations of the reagents used in the protocol, for example, 40μl of proteinase K instead of 20μl as suggested by the protocol of the manufacturer of the kit used. Thus, we sought to associate a chemical and mechanical method to obtain the highest possible concentration of gDNA from the samples.
Of the more than 200 existing species of
Aspergillus, we selected the three most important in the human pathological context [
22]. We searched for specific ITS regions of each species, which resulted in three primers that allowed us to identify Aspergillus in the selected samples. Testing these primers in positive and negative control samples ensured the specificity of the primers for the sequences of interest.
The study also allowed us to observe the possibility of co-infection of different species in the same lesion, which may have a significant clinical impact, given that distinct species may exhibit variable susceptibilities to antifungal drugs. For example,
A. flavus is more resistant to antifungal drugs than other previously known species [
22]. Although our study shows that PCR can be an auxiliary tool in diagnosing aspergillosis, the low number of samples and the fact that none of the cases had the diagnosis confirmed by culture are some limiting factors that should be considered. Finally, information regarding patient management and treatment was not available because of the retrospective nature of our study. Therefore, further prospective investigations using a more significant number of samples are needed to validate the results presented here and better clarify the role of PCR in the clinical management of the disease. In addition, the comparative analysis of PCR to other aspergillosis diagnostic tests is essential to evaluate the accuracy of the standardized protocol.
5. Conclusions
In conclusion, we show in the present study that clinical/pathological diagnosis of aspergillosis can be enriched by PCR, especially in challenging cases or cases where the patient is resistant to first-choice treatment. We also show that co-infection of different Aspergillus species can occur during the oral manifestation of the disease. Further studies with a higher sample size in which cell culture could be employed as a comparative diagnostic method are vital to calculating PCR's accuracy in diagnosing aspergillosis.
Author Contributions
Thaís Ellen Chaves Gomes: Conceptualization; investigation; methodology; writing – original draft; writing – review and editing; data curation. Victor Coutinho Bastos: Investigation; data curation; methodology; writing – review. Douglas Boniek: Data curation; methodology and writing – review. Fernanda Faria Rocha: Writing – review and methodology. Roberta Rayra Martins Chaves: Conceptualization; investigation; writing – review and editing. Ricardo Santiago Gomez: Conceptualization; investigations; methodology; formal analysis; project administration; writing – review and editing.
Funding
This research was supported by Fundação de Amparo à Pesquisa de Minas Gerais (FAPEMIG) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brasil. RSG is a CNPq research fellow.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Universidade Federal de Minas Gerais (protocol code 74144623.4.0000.5149 and date of approval 10/30/2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Acknowledgments
The authors would like to thank the Image Acquisition and Processing Center (CAPI-ICB/UFMG) for the slide digitization service. To Prof. Kelly Moura Keller from the mycology and mycotoxins laboratory at the UFMG veterinary school for the Aspergillus flavus culture and to Prof. Dr. Leonel Mendoza from the University of Michigan for the primer design used.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Baker SE, Bennett JW. An Overview of the Genus Aspergillus. 2007 Dec 7;3–13.
- Paulussen C, Hallsworth JE, Álvarez-Pérez S, Nierman WC, Hamill PG, Blain D, et al. Ecology of aspergillosis: insights into the pathogenic potency of Aspergillus fumigatus and some other Aspergillus species. Microbial Biotechnology [Internet]. 2016 Jun 7;10(2):296–322. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5328810/. [CrossRef]
- Batista PP. Caracterização de linhagens do grupo Aspergillus flavus baseada em marcadores de DNA [Internet]. repositorio.ufpe.br. 2007. Available from: https://repositorio.ufpe.br/handle/123456789/6454.
- Egger M, Jenks JD, Hoenigl M, Prattes J. Blood Aspergillus PCR: The Good, the Bad, and the Ugly. Journal of Fungi [Internet]. 2020 Jan 27 [cited 2021 Feb 17];6(1). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7151127/.
- Telles DR, Karki N, Marshall MW. Oral Fungal Infections: Diagnosis and Management. Dental Clinics of North America [Internet]. 2017 Apr 1;61(2):319–49. Available from: https://www.sciencedirect.com/science/article/abs/pii/S0011853216301380.
- Latgé JP, Chamilos G. Aspergillus fumigatus and Aspergillosis in 2019. Clinical Microbiology Reviews [Internet]. 2019 Nov 13;33(1). Available from: https://cmr.asm.org/content/cmr/12/2/310.full.pdf. [CrossRef]
- Knoke M, Bernhardt H, Schwesinger G. [Early description of a pulmonary aspergillosis 1847 from Greifswald]. Mycoses [Internet]. 2003;46 Suppl 1:37–41. Available from: https://pubmed.ncbi.nlm.nih.gov/12955852/.
- Bongomin F, Harris C, Foden P, Kosmidis C, Denning DW. Innate and Adaptive Immune Defects in Chronic Pulmonary Aspergillosis. Journal of Fungi. 2017 May 29;3(2):26. [CrossRef]
- Cadena J, Thompson GR, Patterson TF. Aspergillosis. Infectious Disease Clinics of North America. 2021 Jun;35(2):415–34.
- Elahe Sasani, Farzad Pakdel, Sadegh Khodavaisy, Salehi M, Salami A, Sohrabi M, et al. Mixed Aspergillosis and Mucormycosis Infections in Patients with COVID-19: Case Series and Literature Review. Mycopathologia. 2024 Jan 17;189(1). [CrossRef]
- Carvalho-Pereira J, Fernandes F, Araújo R, Springer J, Loeffler J, Buitrago MJ, et al. Multiplex PCR Based Strategy for Detection of Fungal Pathogen DNA in Patients with Suspected Invasive Fungal Infections. Journal of Fungi. 2020 Nov 23;6(4):308. [CrossRef]
- Lortholary O, Gangneux JP ., Sitbon K, Lebeau B, de Monbrison F, Le Strat Y, et al. Epidemiological trends in invasive aspergillosis in France: the SAIF network (2005–2007). Clinical Microbiology and Infection [Internet]. 2011 Dec;17(12):1882–9. Available from: http://onlinelibrary.wiley.com/doi/10.1111/j.1469-0691.2011.03548.x/full. [CrossRef]
- Migott GB, Santos FM dos, Pagnussat LR, Barbosa B, Barbosa GDL, Hahn SR. Perfil Clínico e Epidemiológico de Pacientes com Suspeita de Aspergilose Pulmonar em Hospital do Estado Rio Grande do Sul, Brasil. Revista de Epidemiologia e Controle de Infecção. 2017 Jan 16;7(1).
- Rai D, Shukla D, Bhola ND. Aspergillosis of Maxillary Sinus’s Diagnosis, Management, and Association With COVID-19: A Case Report. Cureus. 2022 Oct 11; 14(10).
- Gomes CC, Pinto LCC, Victor FL, da Silva EAB, Ribeiro A de A, Sarquis MI de M, et al. Aspergillus in endodontic infection near the maxillary sinus. Brazilian Journal of Otorhinolaryngology. 2015 Sep;81(5):527–32. [CrossRef]
- Sousa C, Romulo Antonio Pasini, Alessandro Pasqualotto, Marchiori E, Altmayer S, Irion K, et al. Imaging Findings in Aspergillosis: From Head to Toe. Mycopathologia. 2023 Jun 28;188(5):623–41. [CrossRef]
- Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America [Internet]. 2008 Feb 1;46(3):327–60. Available from: https://pubmed.ncbi.nlm.nih.gov/18177225/.
- Caggiano G, Apollonio F, Consiglio M, Gasparre V, Trerotoli P, Diella G, et al. Tendency in Pulmonary Aspergillosis Investigation during the COVID-19 Era: What Is Changing? International Journal of Environmental Research and Public Health [Internet]. 2022 Jun 9 [cited 2024 Jun 12];19(12):7079. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9222563/.
- Steinbach WJ. Are We There Yet? Recent Progress in the Molecular Diagnosis and Novel Antifungal Targeting of Aspergillus fumigatus and Invasive Aspergillosis. Goldman WE, editor. PLoS Pathogens. 2013 Oct 24;9(10):e1003642.
- O’Gorman CM, Fuller HT, Dyer PS. Discovery of a sexual cycle in the opportunistic fungal pathogen Aspergillus fumigatus. Nature. 2009 Jan;457(7228):471–4. [CrossRef]
- S. Arunmozhi Balajee, Kano R, Baddley JW, Moser SA, Marr KA, Alexander BD, et al. Molecular Identification of Aspergillus Species Collected for the Transplant-Associated Infection Surveillance Network. Journal of Clinical Microbiology. 2009 Oct 1;47(10):3138–41. [CrossRef]
- Betil Ozhak-Baysan, Alastruey-Izquierdo A, Saba R, Dilara Ogunc, Gozde Ongut, Aysen Timuragaoglu, et al. Aspergillus alliaceusandAspergillus flavusco-infection in an acute myeloid leukemia patient. Medical Mycology [Internet]. 2010 Nov 1 [cited 2024 Mar 14];48(7):995–9. Available from: https://academic.oup.com/mmy/article/48/7/995/1056456. [CrossRef]
- Yang S, Rothman RE. PCR-based Diagnostics for Infectious diseases: uses, limitations, and Future Applications in acute-care Settings. The Lancet Infectious Diseases. 2004 Jun;4(6):337–48. [CrossRef]
- Abate MS, Battle LR, Emerson AN, Gardner JM, Shalin SC. Dermatologic Urgencies and Emergencies: What Every Pathologist Should Know. Archives of Pathology & Laboratory Medicine. 2019 Aug;143(8):919–42. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).