Submitted:
15 August 2024
Posted:
16 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Ascertainment of Exposure and Adverse Effects on Kidneys
2.3. Normalization of Excretion Rate of Cd and NAG
2.4. Mediation Analysis
2.5. Statistical Analysis
3. Results
3.1. Study Subjects
3.2. Moderate-to-Strong Association of ENAG/Ccr with ECd/Ccr
3.3.Qunatification of Effects of Cadmium and Tubular Injury on eGFR
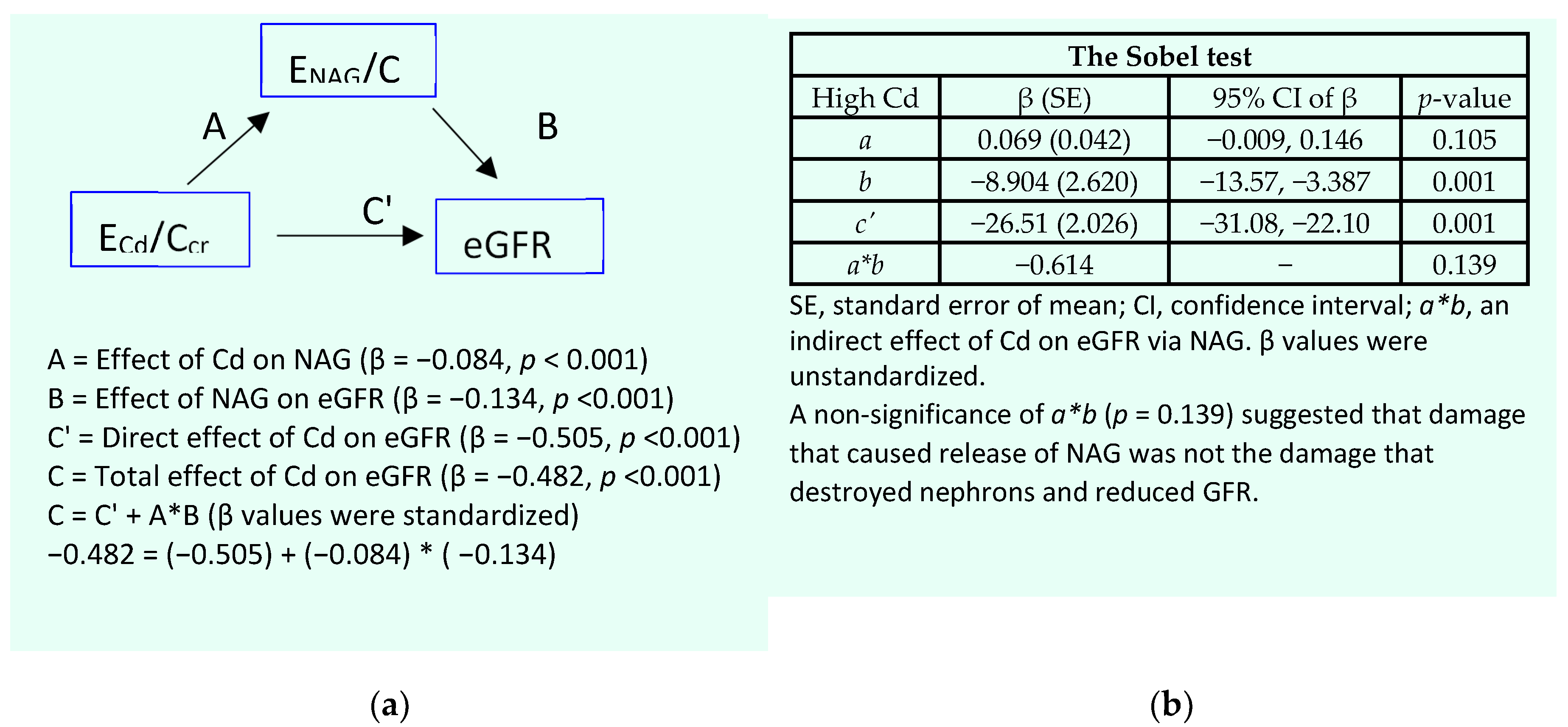
3.4. Mediation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foreman, K.J.; Marquez, N.; Dolgert, A.; Fukutaki, K.; Fullman, N.; McGaughey, M.; Pletcher, M.A.; Smith, A.E.; Tang, K.; Yuan, C.W.; et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: Reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet 2018, 392, 2052–2090. [Google Scholar] [CrossRef]
- GBD 2021 Forecasting Collaborators. Burden of disease scenarios for 204 countries and territories, 2022–2050: A forecasting analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2204–2256. [Google Scholar]
- Murton M, Goff-Leggett D, Bobrowska A, Garcia Sanchez JJ, James G, Wittbrodt E, Nolan S, Sörstadius E, Pecoits-Filho, R. ; Tuttle, K. Burden of Chronic Kidney Disease by KDIGO Categories of Glomerular Filtration Rate and Albuminuria: A Systematic Review. Adv. Ther. 2021, 38, 180–200. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Jafar, T.H.; Nitsch, D.; Neuen, B.L.; Perkovic, V. Chronic kidney disease. Lancet 2021, 398, 786–802. [Google Scholar] [CrossRef] [PubMed]
- Farrell, D.R.; Vassalotti, J.A. Screening, identifying, and treating chronic kidney disease: why, who, when, how, and what? BMC Nephrol. 2024, 25, 34. [Google Scholar]
- Satarug, S.; Vesey, D.A.; Gobe, G.C.; Phelps, K.R. Estimation of health risks associated with dietary cadmium exposure. Arch. Toxicol. 2023, 97, 329–358. [Google Scholar] [PubMed]
- Fechner, C.; Hackethal, C.; Höpfner, T.; Dietrich, J.; Bloch, D.; Lindtner, O.; Sarvan, I. Results of the BfR MEAL Study: In Germany, mercury is mostly contained in fish and seafood while cadmium, lead, and nickel are present in a broad spectrum of foods. Food Chem. X 2022, 14, 100326. [Google Scholar] [CrossRef]
- Watanabe, T.; Kataoka, Y.; Hayashi, K.; Matsuda, R.; Uneyama, C. Dietary exposure of the Japanese general population to elements: Total diet study 2013–2018. Food Saf. 2022, 10, 83–101. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.T.; Jandev, V.; Petroni, M.; Atallah-Yunes, N.; Bendinskas, K.; Brann, L.S.; Heffernan, K.; Larsen, D.A.; MacKenzie, J.A.; Palmer, C.D.; et al. Airborne levels of cadmium are correlated with urinary cadmium concentrations among young children living in the New York state city of Syracuse, USA. Environ. Res. 2023, 223, 115450. [Google Scholar] [PubMed]
- Almerud, P.; Zamaratskaia, G.; Lindroos, A.K.; Bjermo, H.; Andersson, E.M.; Lundh, T.; Ankarberg, E.H.; Lignell, S. Cadmium, total mercury, and lead in blood and associations with diet, sociodemographic factors, and smoking in Swedish adolescents. Environ Res. 2021, 197, 110991. [Google Scholar] [CrossRef] [PubMed]
- Pappas, R.S.; Fresquez, M.R.; Watson, C.H. Cigarette smoke cadmium breakthrough from traditional filters: implications for exposure. J. Anal. Toxicol. 2015, 39, 45–51. [Google Scholar] [CrossRef] [PubMed]
- JECFA. Summary and Conclusions. In Proceedings of the Joint FAO/WHO Expert Committee on Food Additives and Contaminants, Seventy-Third Meeting, Geneva, Switzerland, 8–17 June 2010; JECFA/73/SC. Food and Agriculture Organization of the United Nations/World Health Organization: Geneva, Switzerland, 2011. Available online: https://apps.who.int/iris/handle/10665/44521 (accessed on 12 August 2024).
- Satarug, S.; Vesey, D.A.; Ruangyuttikarn, W.; Nishijo, M.; Gobe, G.C.; Phelps, K.R. The Source and Pathophysiologic Significance of Excreted Cadmium. Toxics 2019, 7, 55. [Google Scholar] [CrossRef]
- Doccioli, C.; Sera, F.; Francavilla, A.; Cupisti, A.; Biggeri, A. Association of cadmium environmental exposure with chronic kidney disease: A systematic review and meta-analysis. Sci. Total Environ. 2024, 906, 167165. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, Y.; Nomiyama, T.; Kumagai, N.; Dekio, F.; Uemura, T.; Takebayashi, T.; Nishiwaki, Y.; Matsumoto, Y.; Sano, Y.; Hosoda, K.; et al. Uptake of cadmium in meals from the digestive tract of young non-smoking Japanese female volunteers. J. Occup. Health 2003, 45, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, H.; Oguma, E.; Sasaki, S.; Miyamoto, K.; Ikeda, Y.; Machida, M.; Kayama, F. Comprehensive study of the effects of age, iron deficiency, diabetes mellitus, and cadmium burden on dietary cadmium absorption in cadmium-exposed female Japanese farmers. Toxicol. Appl. Pharmacol. 2004, 196, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Li, C.; Zhao, D.; Huang, L. Associations of micronutrients exposure with cadmium body burden among population: A systematic review. Ecotoxicol. Environ. Saf. 2023, 256, 114878. [Google Scholar]
- Satarug, S.; Baker, J.R.; Reilly, P.E.; Moore, M.R.; Williams, D.J. Cadmium levels in the lung, liver, kidney cortex, and urine samples from Australians without occupational exposure to metals. Arch. Environ. Health 2002, 57, 69–77. [Google Scholar] [CrossRef]
- Akerstrom, M.; Barregard, L.; Lundh, T.; Sallsten, G. The relationship between cadmium in kidney and cadmium in urine and blood in an environmentally exposed population. Toxicol. Appl. Pharmacol. 2013, 268, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Barregard, L.; Sallsten, G.; Lundh, T.; Mölne, J. Low-level exposure to lead, cadmium and mercury, and histopathological findings in kidney biopsies. Environ. Res. 2022, 211, 113119. [Google Scholar] [CrossRef]
- Thévenod, F.; Lee, W.K.; Garrick, M.D. Iron and cadmium entry into renal mitochondria: Physiological and toxicological implications. Front. Cell Dev. Biol. 2020, 8, 848. [Google Scholar] [CrossRef]
- Ning, B.; Guo, C.; Kong, A.; Li, K.; Xie, Y.; Shi, H.; Gu, J. Calcium signaling mediates cell death and crosstalk with autophagy in kidney disease. Cells 2021, 10, 3204. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Guo, C.; Ruan, J.; Ning, B.; Wong, C.K.-C.; Shi, H.; Gu, J. Cadmium disrupted ER Ca2+ homeostasis by inhibiting SERCA2 expression and activity to induce apoptosis in renal proximal tubular cells. Int. J. Mol. Sci. 2023, 24, 5979. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.T.; Liu, T.B.; Li, Y.; Wang, Z.Y.; Lian, C.Y.; Wang, L. HO-1 activation contributes to cadmium-induced ferroptosis in renal tubular epithelial cells via increasing the labile iron pool and promoting mitochondrial ROS generation. Chem. Biol. Interact. 2024, 399, 111152. [Google Scholar]
- Satarug, S. Is Chronic Kidney Disease Due to Cadmium Exposure Inevitable and Can It Be Reversed? Biomedicines 2024, 12, 718. [Google Scholar] [CrossRef] [PubMed]
- Phelps, K.R.; Gosmanova, E.O. A generic method for analysis of plasma concentrations. Clin. Nephrol. 2020, 94, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Vesey, D.A.; Gobe, G.C.; Đorđević, A.B. The Validity of Benchmark Dose Limit Analysis for Estimating Permissible Accumulation of Cadmium. Int. J. Environ. Res. Public Health 2022, 19, 15697. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Đorđević, A.B.; Yimthiang, S.; Vesey, D.A.; Gobe, G.C. The NOAEL Equivalent of Environmental Cadmium Exposure Associated with GFR Reduction and Chronic Kidney Disease. Toxics 2022, 10, 614. [Google Scholar] [CrossRef]
- Suwatvitayakorn, P.; Ko, M.S.; Kim, K.W.; Chanpiwat, P. Human health risk assessment of cadmium exposure through rice consumption in cadmium-contaminated areas of the Mae Tao sub-district, Tak, Thailand. Environ. Geochem. Health 2020, 42, 2331–2344. [Google Scholar] [CrossRef] [PubMed]
- Swaddiwudhipong, W.; Nguntra, P.; Kaewnate, Y.; Mahasakpan, P.; Limpatanachote, P.; Aunjai, T.; Jeekeeree, W.; Punta, B.; Funkhiew, T.; Phopueng, I. Human health effects from cadmium exposure: Comparison between persons living in cad-mium-contaminated and non-contaminated areas in northwestern Thailand. Southeast Asian J. Trop. Med. Publ. Health 2015, 46, 133–142. [Google Scholar]
- Satarug, S.; Swaddiwudhipong, W.; Ruangyuttikarn, W.; Nishijo, M.; Ruiz, P. Modeling cadmium exposures in low- and high-exposure areas in Thailand. Environ. Health Perspect. 2013, 121, 531–536. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Scmid, C.H.; Zhang, Y.; Castro, A.F., III; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- White, C.A.; Allen, C.M.; Akbari, A.; Collier, C.P.; Holland, D.C.; Day, A.G.; Knoll, G.A. Comparison of the new and traditional CKD-EPI GFR estimation equations with urinary inulin clearance: A study of equation performance. Clin. Chim. Acta 2019, 488, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Hornung, R.W.; Reed, L.D. Estimation of average concentration in the presence of nondetectable values. Appl. Occup. Environ. Hyg. 1990, 5, 46–51. [Google Scholar] [CrossRef]
- Spencer, K. Analytical reviews in clinical biochemistry: The estimation of creatinine. Ann. Clin. Biochem. 1985, 23, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Preacher, K.J. Advances in mediation analysis: a survey and synthesis of new developments. Annu. Rev. Psychol. 2015, 66, 825–52. [Google Scholar] [CrossRef] [PubMed]
- MacKinnon, D.P.; Warsi, G.; Dwyer, J.H. A simulation study of mediated effect measures. Multiv. Behav. Res. 1995, 30, 41–62. [Google Scholar] [CrossRef] [PubMed]
- Preacher, K.J.; Hayes, A.F. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav. Res. Meth. Instrum. Comput. 2004, 36, 717–731. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.K.; Hodgson, S.; Nieuwenhuijsen, M.; Jarup, L. Early kidney damage in a population exposed to cadmium and other heavy metals. Environ. Health Perspect. 2009, 117, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Moffett, D.B.; Mumtaz, M.M.; Sullivan, D.W., Jr.; Whittaker, M.H. Chapter 13, General Considerations of Dose-Effect and Dose-Response Relationships. In Handbook on the Toxicology of Metals, 5th ed.; Volume I: General, Considerations, Nordberg, G., Costa, M., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 299–317. [Google Scholar]
- Liu, C.; Li, Y.; Zhu, C.; Dong, Z.; Zhang, K.; Zhao, Y.; Xu, Y. Benchmark dose for cadmium exposure and elevated N-acetyl-β-D-glucosaminidase: A meta-analysis. Environ. Sci. Pollut. Res. Int. 2016, 23, 20528–20538. [Google Scholar] [CrossRef] [PubMed]
- Price, R.G. Measurement of N-acetyl-beta-glucosaminidase and its isoenzymes in urine: Methods and clinical applications. Eur. J. Clin. Chem. Clin. Biochem. 1992, 30, 693–705. [Google Scholar] [PubMed]
- Pócsi, I.; Dockrell, M.E.; Price, R.G. Nephrotoxic biomarkers with specific indications for metallic pollutants: Implications for environmental health. Biomark. Insights 2022, 17, 11772719221111882. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Taylor, A.W.; Riley. , M.; Byles., J.; Liu, J.; Noakes, M. Association between dietary patterns, cadmium intake and chronic kidney disease among adults. Clin. Nutr. 2018, 37, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Suwazono, Y.; Sand, S.; Vahter, M.; Filipsson, A.F.; Skerfving, S.; Lidfeldt, J.; Akesson, A. Benchmark dose for cadmium-induced renal effects in humans. Environ. Health Perspect. 2006, 114, 1072–1076. [Google Scholar] [CrossRef] [PubMed]
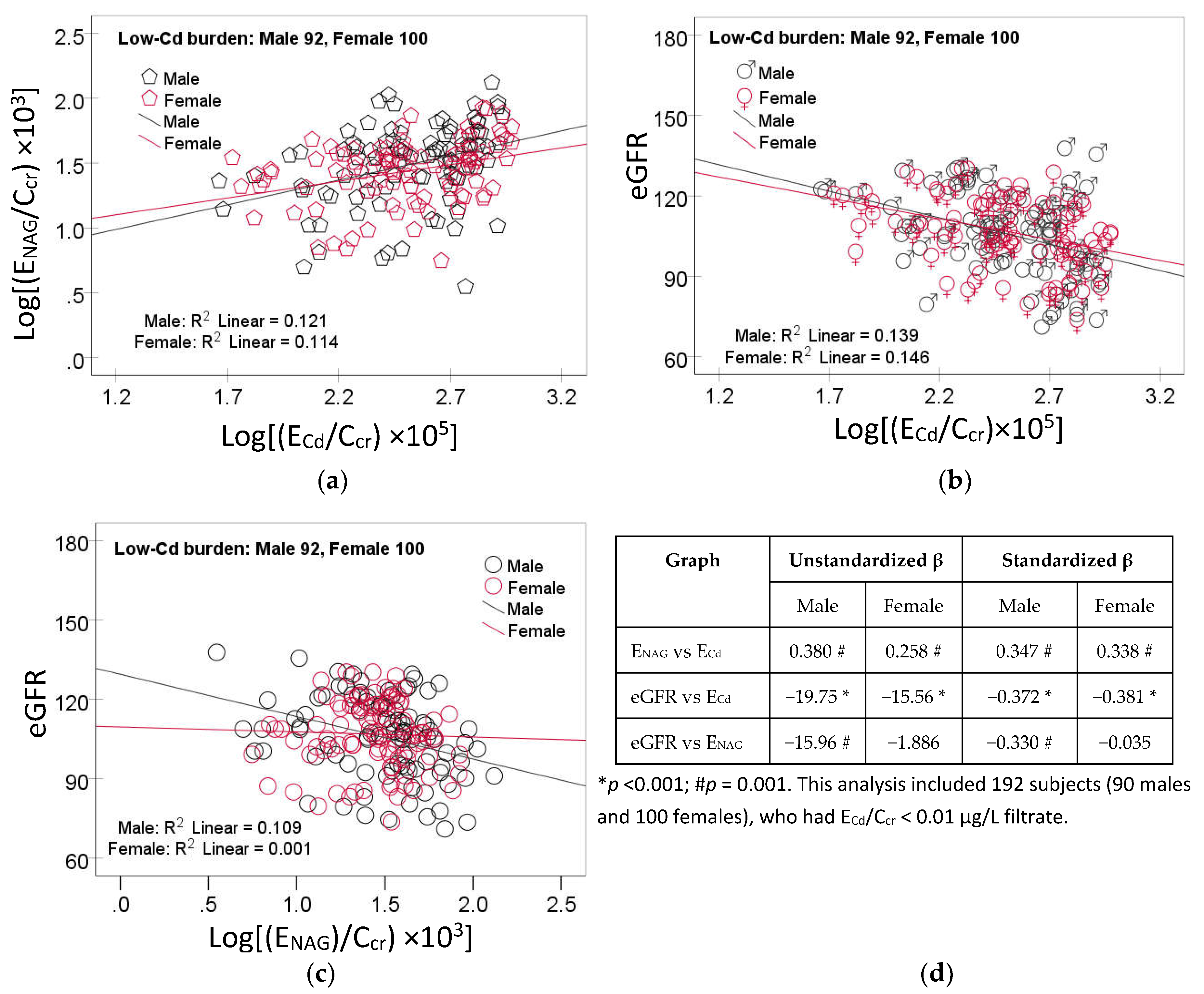
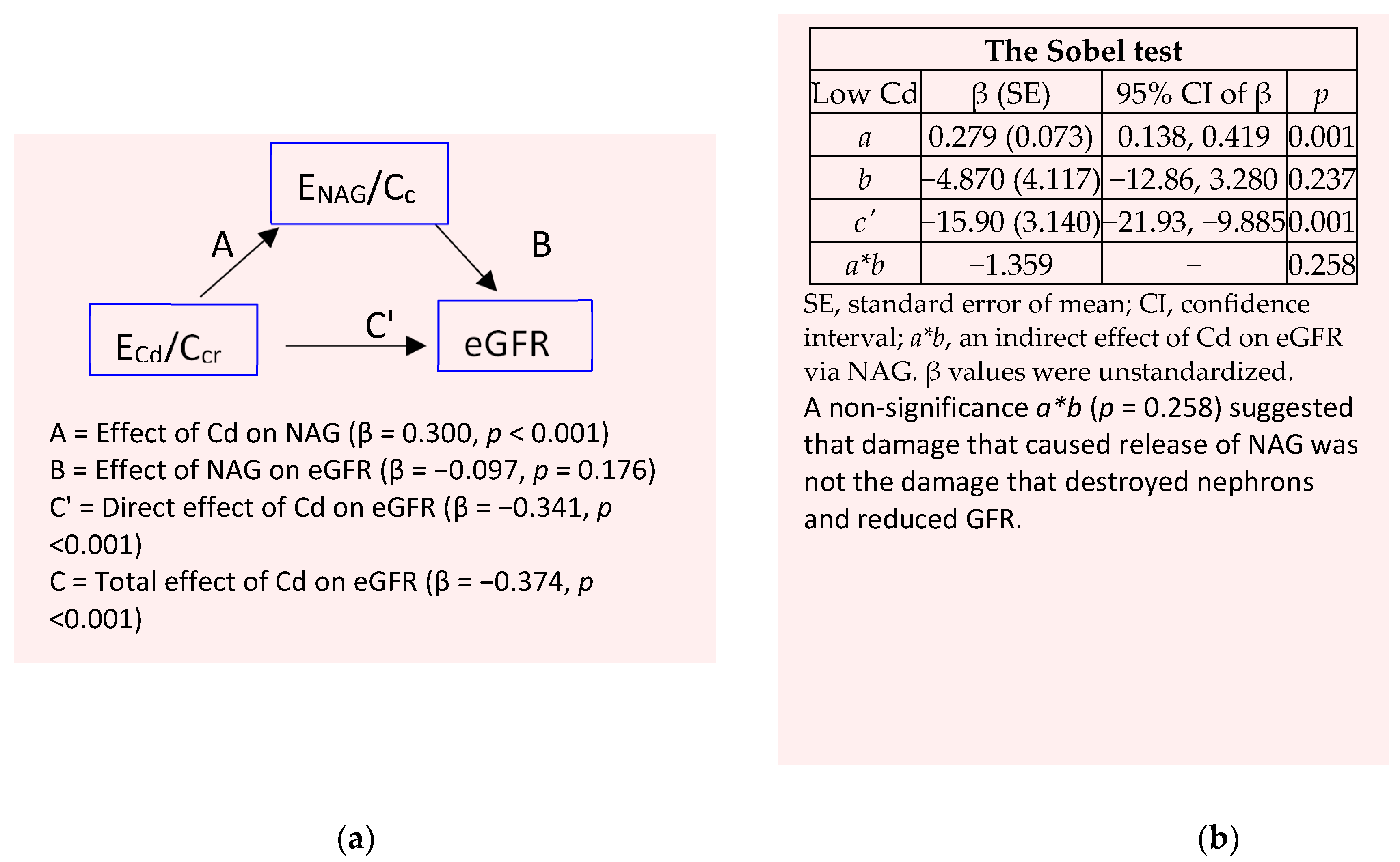
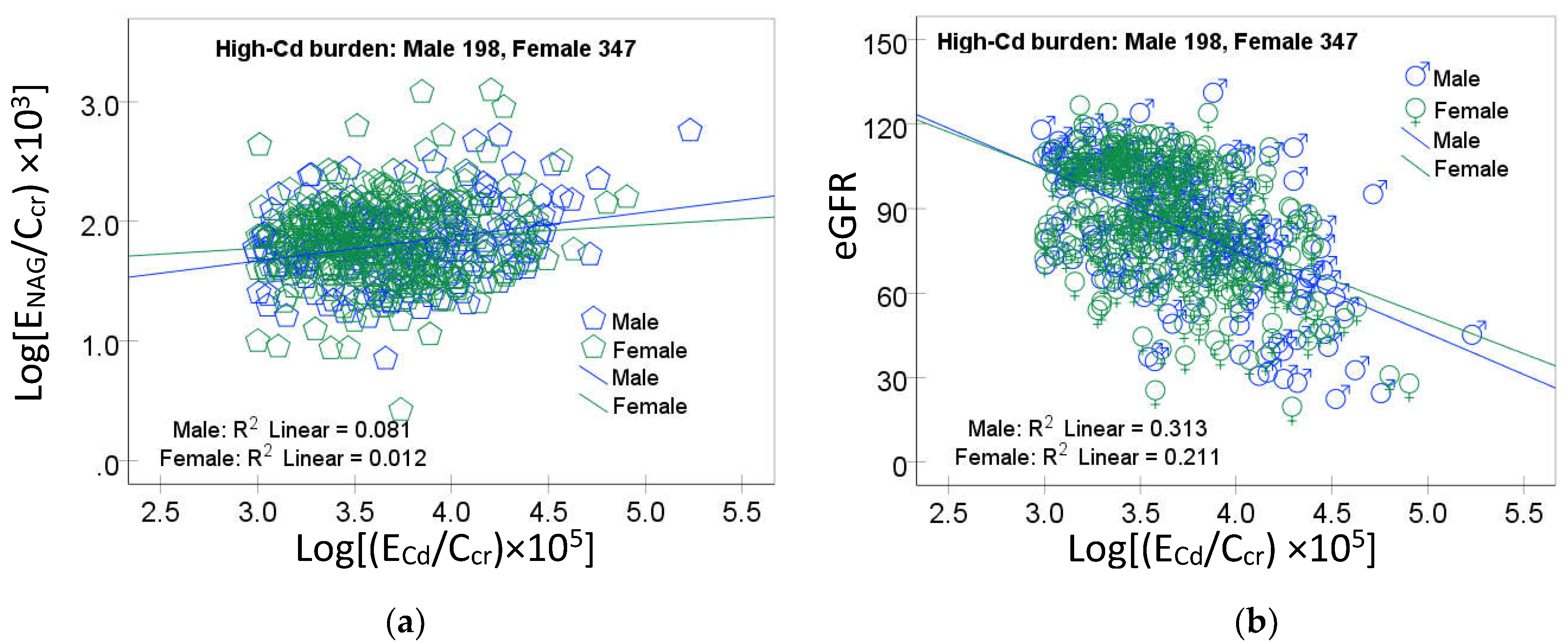
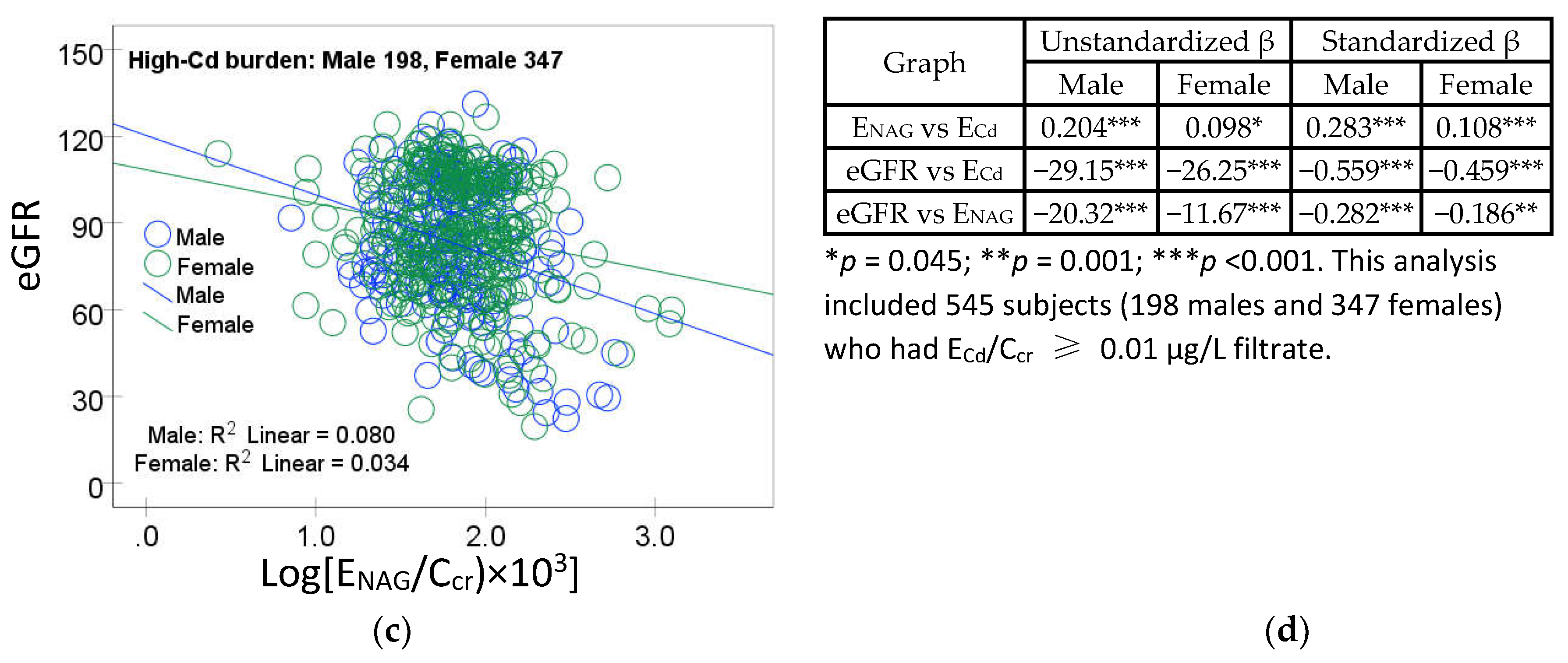
| Parameters | All, n 737 | Low-Cd burden a | High-Cd burden | ||
|---|---|---|---|---|---|
| Males, n 92 | Females, n 100 | Males, n 198 | Females, n 347 | ||
| Age, years | 48.1 (11.0) | 35.8 (10.2) | 42.1 (9.1) *** | 52.8 (11.3) | 50.4 (8.2) |
| Age range, years | 16−87 | 16−87 | 23−60 | 30−87 | 33−84 |
| BMI, kg/m2 | 23.2 (3.8) | 23.3 (3.4) | 23.4 (3.8) | 22.0 (3.3) | 23.7 (4.1) *** |
| % Female | 60.7 | − | 52.0 | − | 63.7 |
| % Smoking | 42.7 | 43.5 | 1.0 *** | 81.8 | 32.3*** |
| % Hypertension | 32.2 | 27.0 | 15.8 | 30.9 | 39.2 * |
| eGFR a, mL/min/1.73 m2 | 91 (22) | 106 (16) | 107 (13) | 83 (22) | 87 (21) * |
| % eGFR ≤ 60 mL/min/1.73 m2 | 9.1 | 0 | 0 | 15.2 | 10.7 |
| [cr]p, mg/dL | 0.88 (0.28) | 0.92 (0.12) | 0.67 (0.10) *** | 1.08 (0.34) | 0.82 (0.23) *** |
| [cr]u, mg/dL | 110.2 (73.8) | 89.8 (80.3) | 70.3 (58.3) | 135.6 (65,2) | 112.6 (74.5) *** |
| [Cd]u, µg/L | 6.53 (11.71) | 0.39 (0.48) | 0.44 (0.57) | 10.7 (18.7) | 7.52 (7.82) |
| Normalized to Ecr (Ex/Ecr) b | |||||
| ECd/Ecr, µg/g creatinine | 3.72 (7.50) | 0.42 (0.26) | 0.50 (0.32) | 6.01 (10.4) | 4.21 (6.96) * |
| ENAG/Ecr, units/g creatinine | 3.65 (3.98) | 3.91 (2.45) | 4.21 (2.41) | 3.91 (4.43) | 3.28 (4.36) |
| Normalized to Ccr, (Ex/Ccr) c | |||||
| (ECd/Ccr) × 100, µg/L filtrate | 5.67 (9.89) | 0.39 (0.22) | 0.40 (0.24) | 9.08 (14.7) | 6.64 (7.90) |
| (ENAG/Ccr) × 100, µg/L filtrate | 7.70 (9.56) | 3.85 (2.46) | 3.14 (1.62) | 8.59 (7.89) | 9.53 (12.0) |
| Independent Variables/ Factors |
Log[(ENAG/Ccr)×103], U/L filtrate | |||||||
|---|---|---|---|---|---|---|---|---|
| Males, n = 277 |
Females, n = 427 |
Low-Cd burden a, n = 186 |
High-Cd burden, n = 538 |
|||||
| β | p | β | p | β | p | β | p | |
| Age, years | −0.012 | 0.892 | −0.170 | 0.003 | −0.094 | 0.419 | −0.124 | 0.026 |
| BMI, kg/m2 | 0.002 | 0.974 | 0.132 | 0.003 | −0.008 | 0.924 | 0.087 | 0.063 |
| Log2[(ECd/Ccr) × 105], µg/L filtrate | 0.447 | <0.001 | 0.394 | <0.001 | 0.287 | 0.001 | 0.145 | 0.004 |
| eGFR, mL/min/1.73 m2 | −0.127 | 0.127 | −0.178 | 0.002 | −0.132 | 0.205 | −0.223 | <0.001 |
| Hypertension | 0.167 | 0.002 | 0.169 | <0.001 | 0.180 | 0.022 | 0.158 | <0.001 |
| Smoking | −0.037 | 0.496 | 0.111 | 0.016 | −0.055 | 0.517 | 0.045 | 0.356 |
| Gender | − | − | − | − | −0.061 | 0.511 | 0.045 | 0.343 |
| Adjusted R2 | 0.293 | <0.001 | 0.266 | <0.001 | 0.114 | <0.001 | 0.090 | <0.001 |
| Independent Variables/Factors |
Low eGFR | ||||
|---|---|---|---|---|---|
| β Coefficients | POR | 95% CI | p | ||
| (SE) | Lower | Upper | |||
| Age, years | 0.156 (0.022) | 1.168 | 1.118 | 1.221 | <0.001 |
| BMI, kg/m2 | 0.104 (0.050) | 1.109 | 1.006 | 1.222 | 0.037 |
| Log2[(ECd/Ccr) × 105], µg/L filtrate | 0.998 (0.164) | 2.714 | 1.967 | 3.744 | <0.001 |
| Gender | −0.389 (0.429) | 0.678 | 0.292 | 1.572 | 0.365 |
| Hypertension | −0.707 (0.387) | 0.493 | 0.231 | 1.052 | 0.068 |
| Smoking | 0.268 (0.414) | 1.307 | 0.581 | 2.945 | 0.517 |
| Tubular injury a | |||||
| Minimal | Referent | ||||
| Mild | −0.529 (0.754) | 0.589 | 0.134 | 2.581 | 0.483 |
| Moderate | 0.218 (0.694) | 1.244 | 0.320 | 4.843 | 0.753 |
| Severe | 1.570 (0.648) | 4.804 | 1.350 | 17.09 | 0.015 |
| Independent Variables/ Factors |
eGFR, mL/min/1.73 m2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Males, n = 277 |
Females, n = 427 |
Low-Cd burden a, n = 186 |
High-Cd burden, n = 538 |
|||||
| η2 | p | η2 | p | η2 | p | η2 | p | |
| Age, years | 0.340 | <0.001 | 0.249 | <0.001 | 0.380 | <0.001 | 0.300 | <0.001 |
| BMI, kg/m2 | 0.001 | 0.637 | 0.004 | 0.228 | 0.002 | 0.632 | 0.010 | 0.024 |
| Log2[(ECd/Ccr) × 105], µg/L filtrate | 0.081 | <0.001 | 0.114 | <0.001 | 0.000335 | 0.828 | 0.150 | <0.001 |
| Smoking | 0.002 | 0.434 | 0.000171 | 0.792 | 0.000407 | 0.811 | 0.000248 | 0.724 |
| Hypertension | 0.016 | 0.044 | 0.002 | 0.420 | 0.000012 | 0.968 | 0.004 | 0.167 |
| ENAG/Ccr quartiles | 0.015 | 0.275 | 0.034 | 0.003 | 0.018 | 0.460 | 0.013 | 0.085 |
| Gender | − | − | − | − | 0.051 | 0.007 | 0.003 | 0.256 |
| Smoking × Hypertension | − | − | 0.022 | 0.003 | 0.013 | 0.176 | 0.004 | 0.147 |
| Smoking × Hypertension × ENAG/Ccr quartile | − | − | − | − | 0.059 | 0.014 | − | − |
| Adjusted R2 | 0.633 | <0.001 | 0.440 | <0.001 | 0.494 | <0.001 | 0.493 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




