Submitted:
15 August 2024
Posted:
16 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments
2.3. Methods
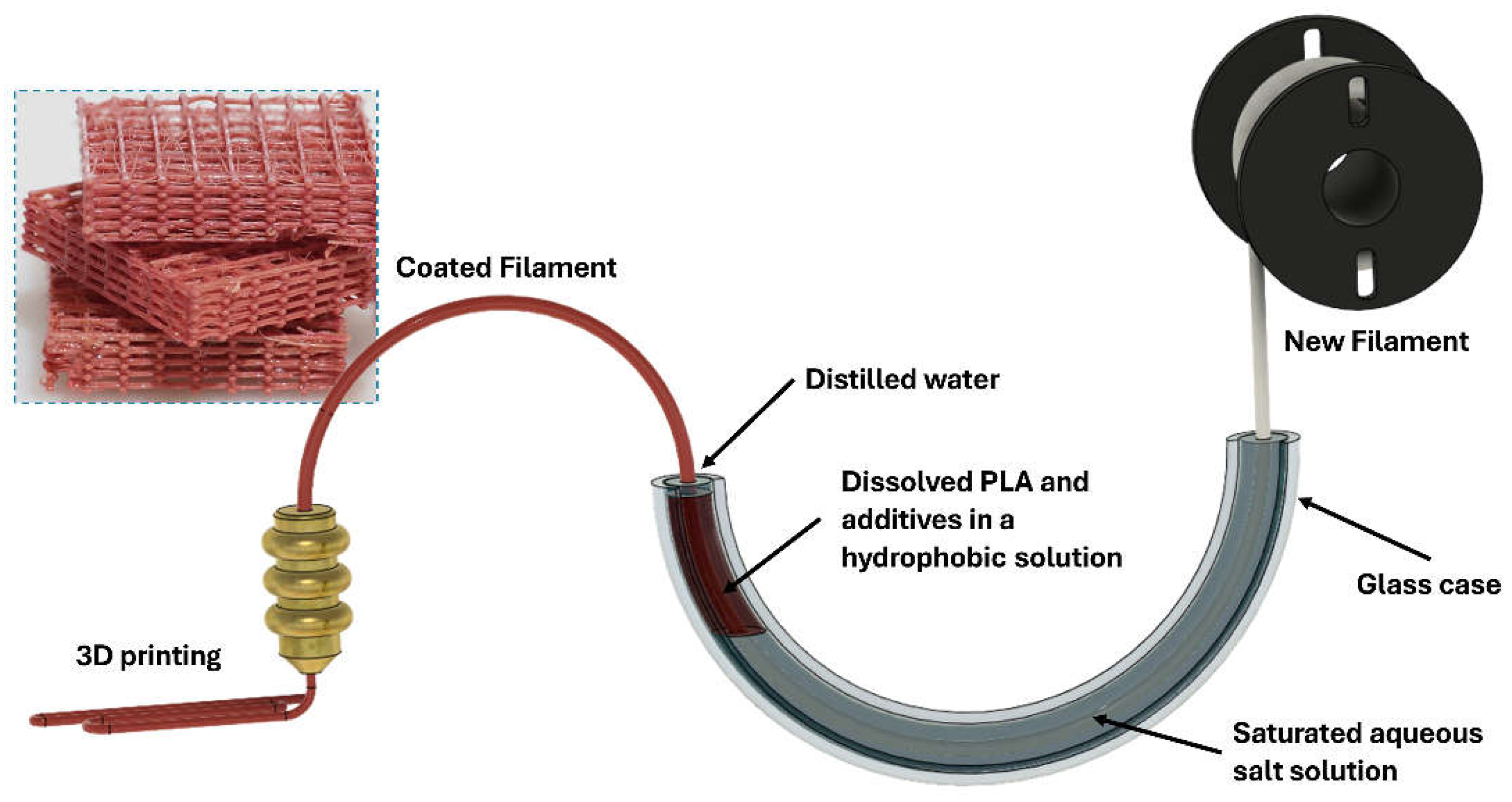
2.3.1. Coating Solution Preparation
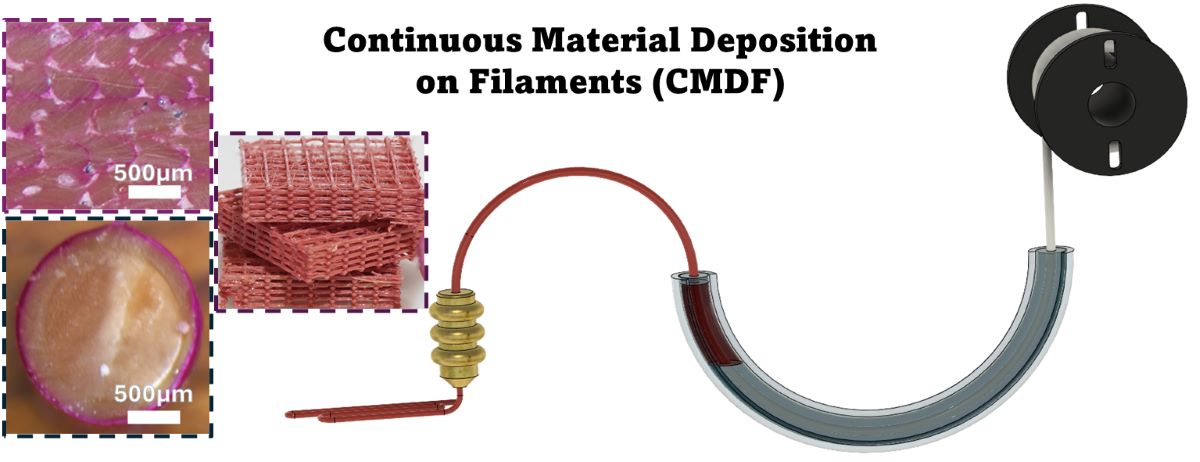
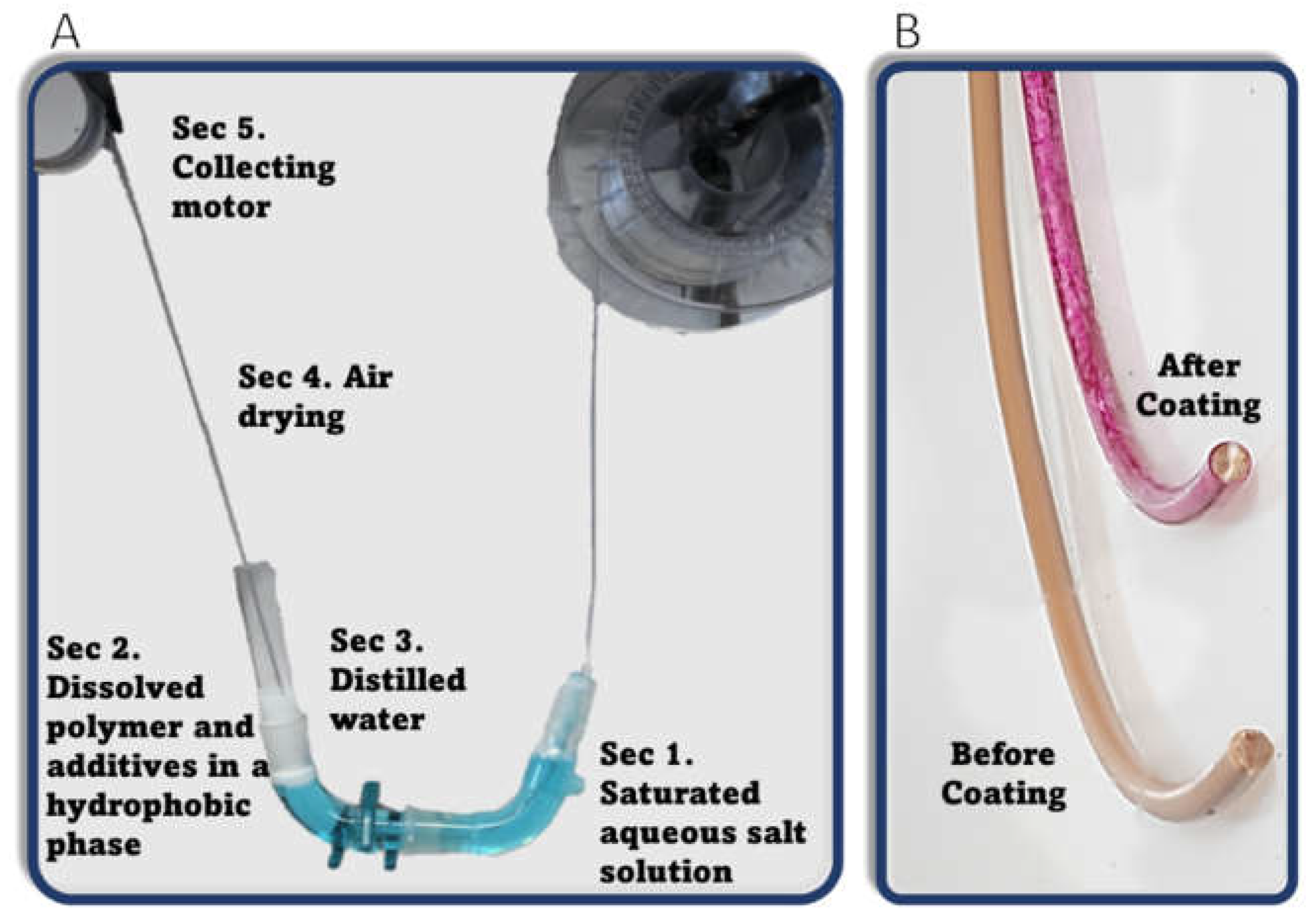
2.3.2. Coating Setup
2.3.3. FDM Printing
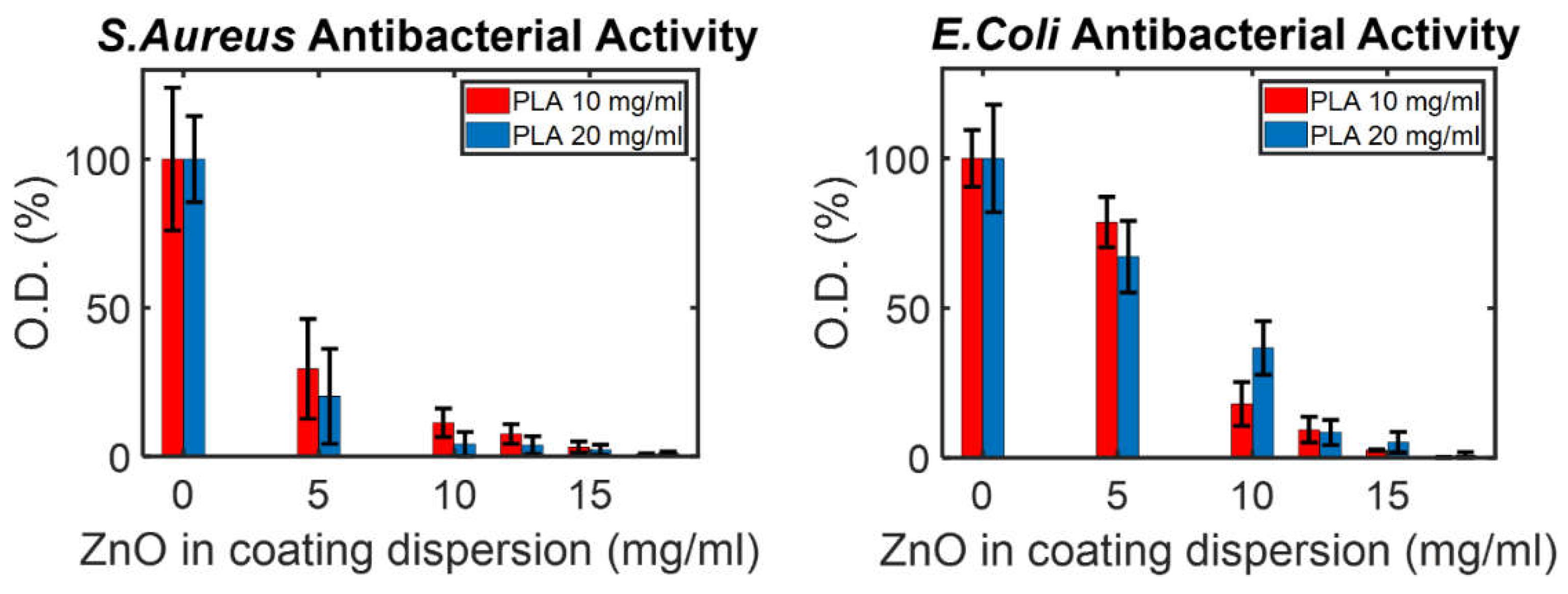
2.3.4. Antibacterial Tests
2.3.5. Leaching of Zn(II) and Presence of Zn(II) in Samples
2.3.6. Tensile Testing
2.3.7. Cip release and Entrapment
3. Results and Discussion
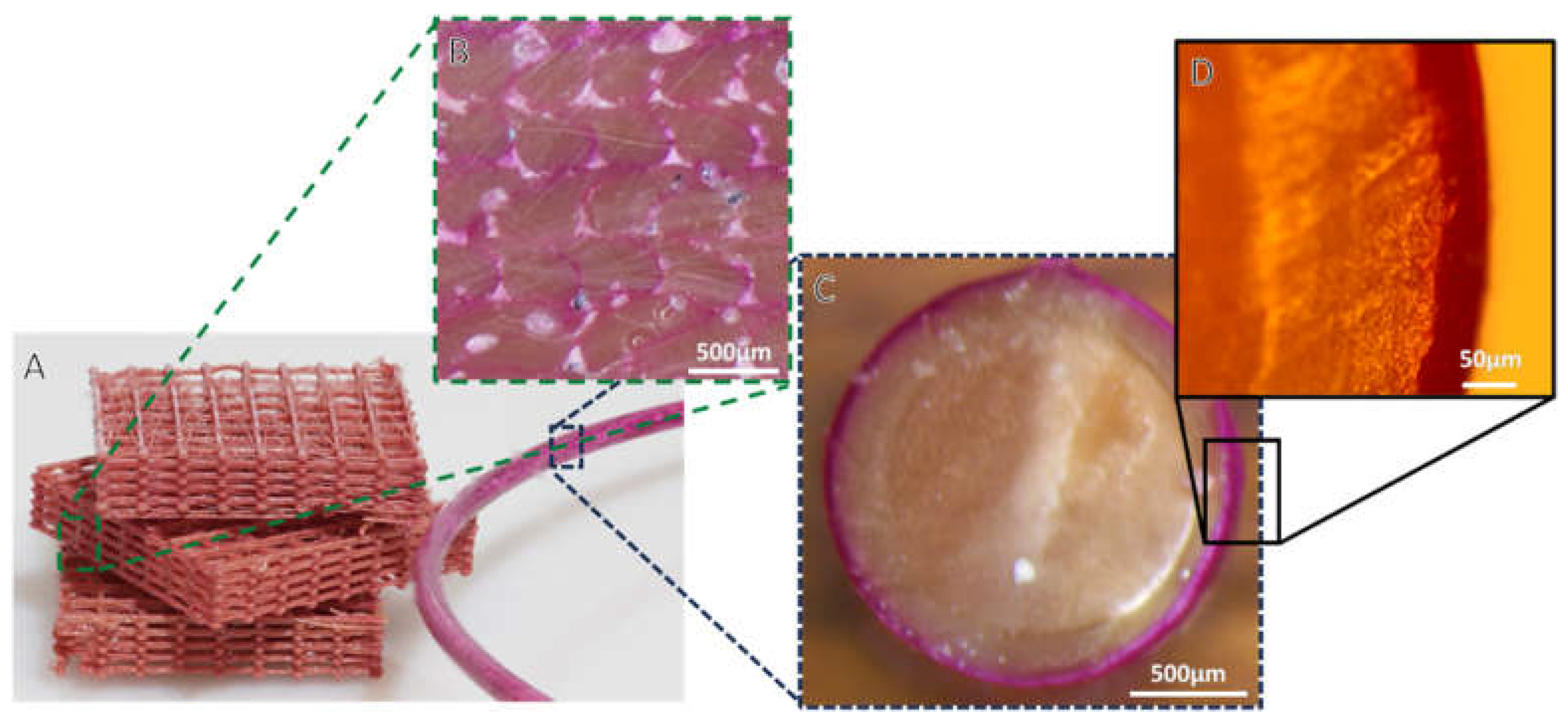
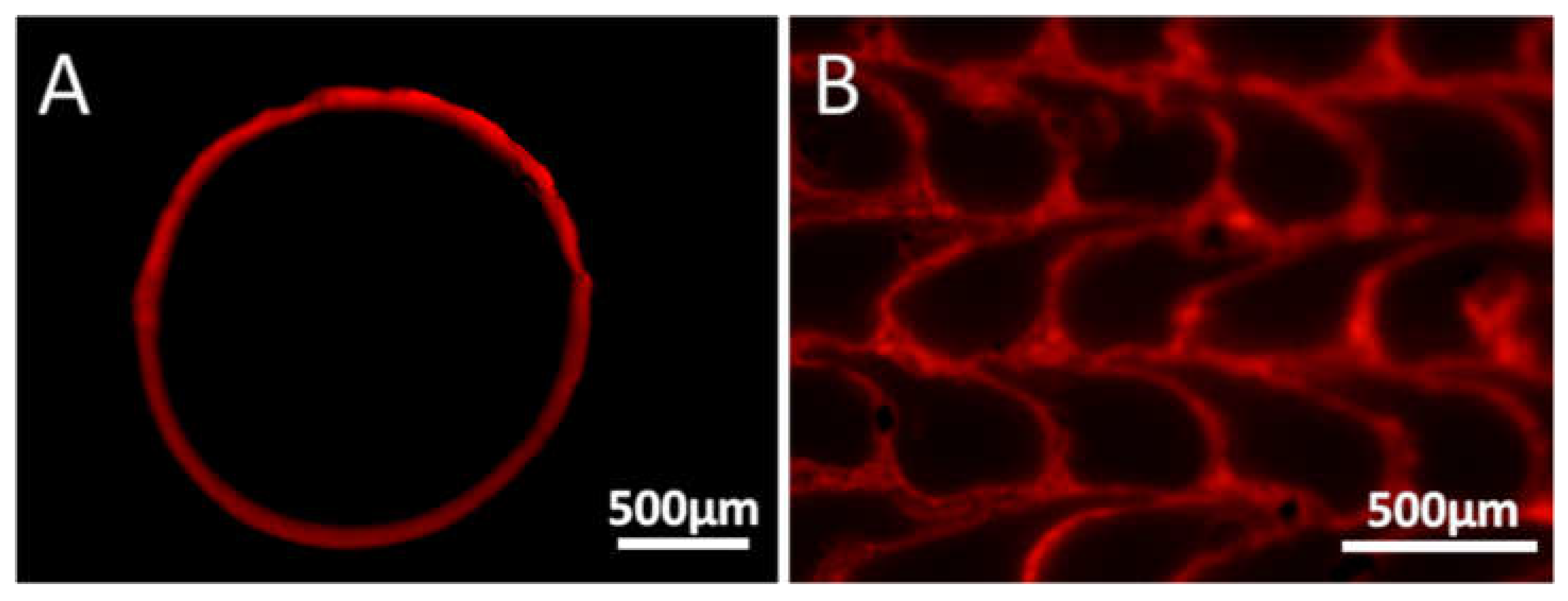
3.1. Rhodamine B Coating
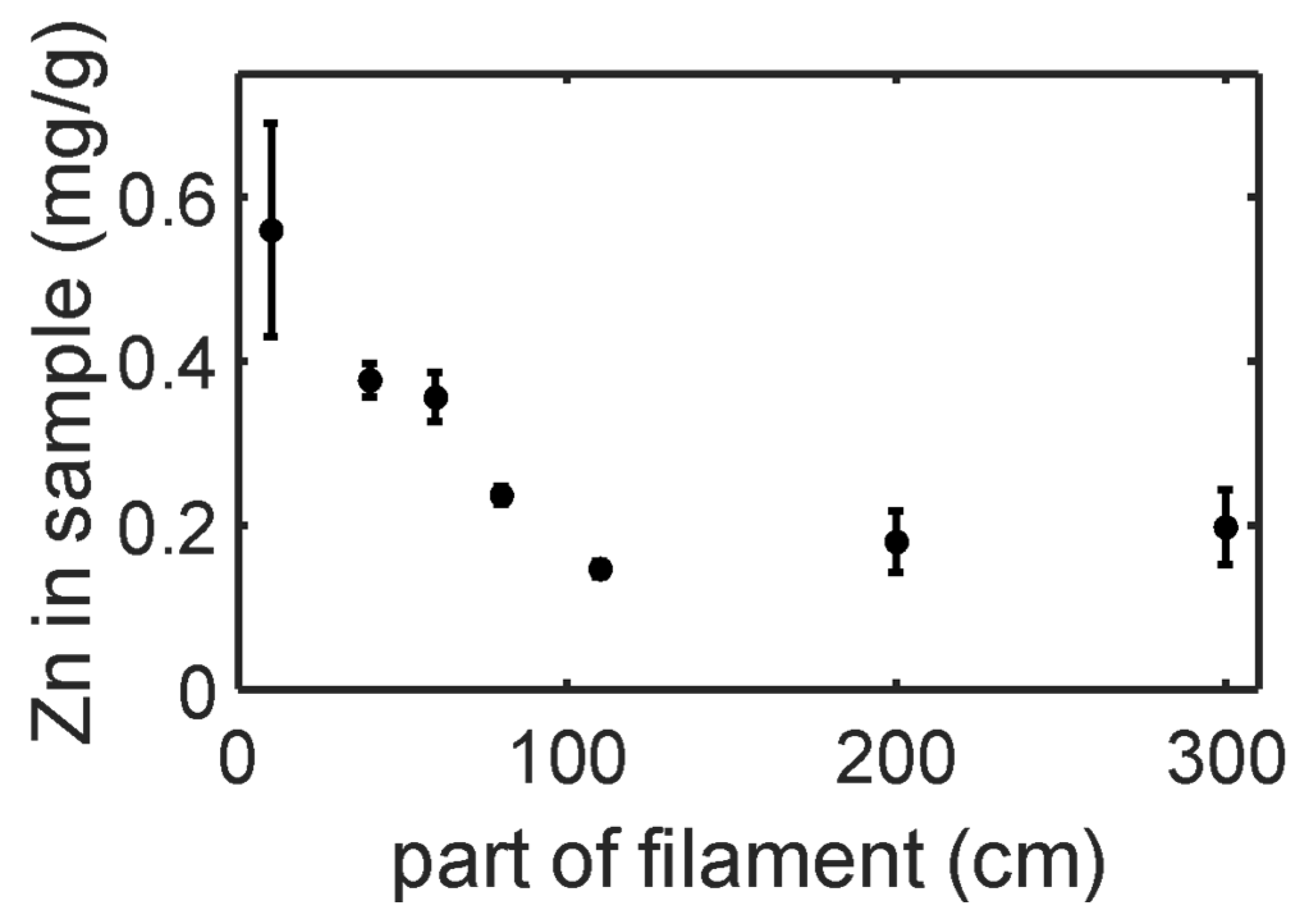
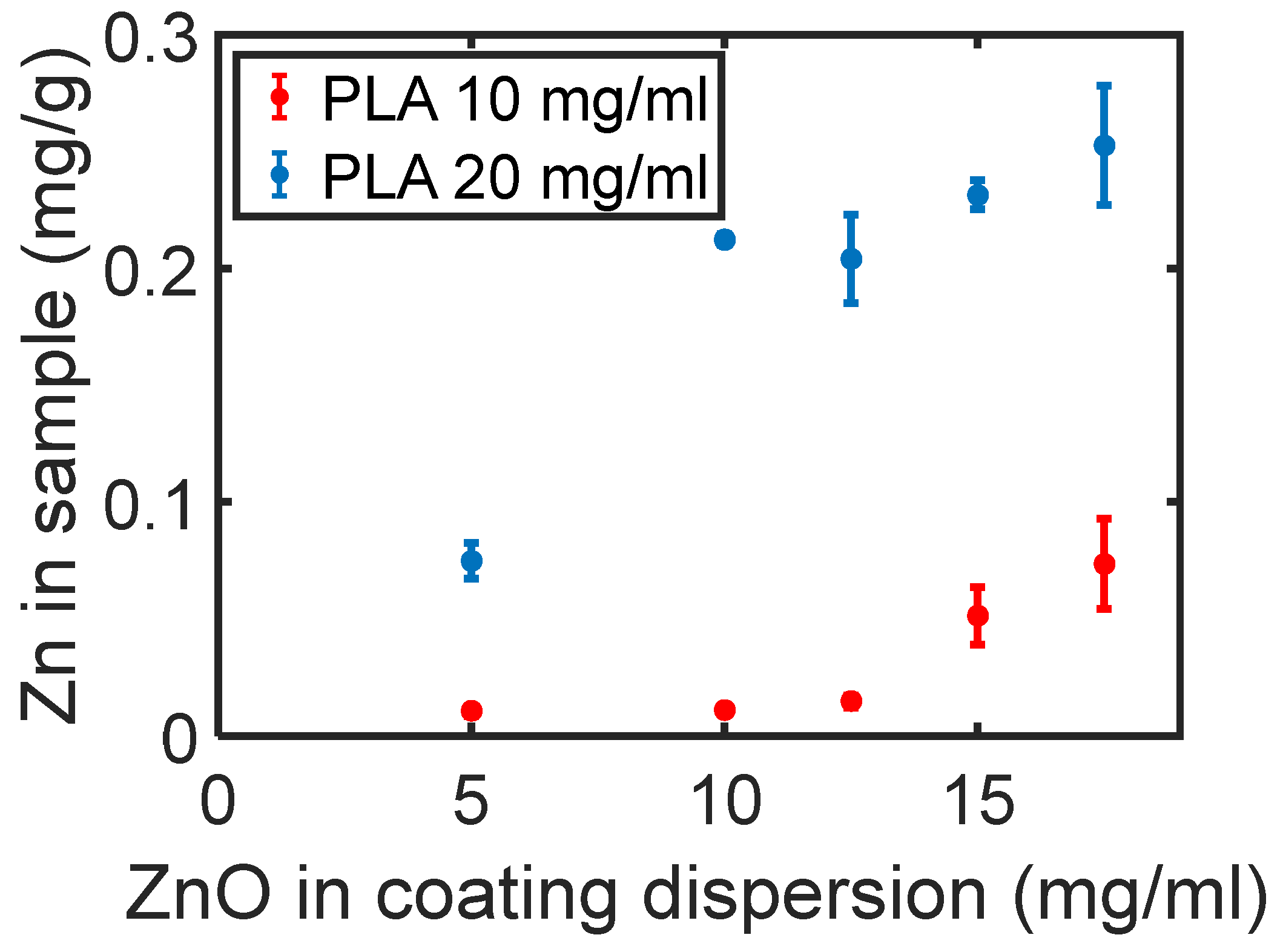
3.2. ZnO NP Coating
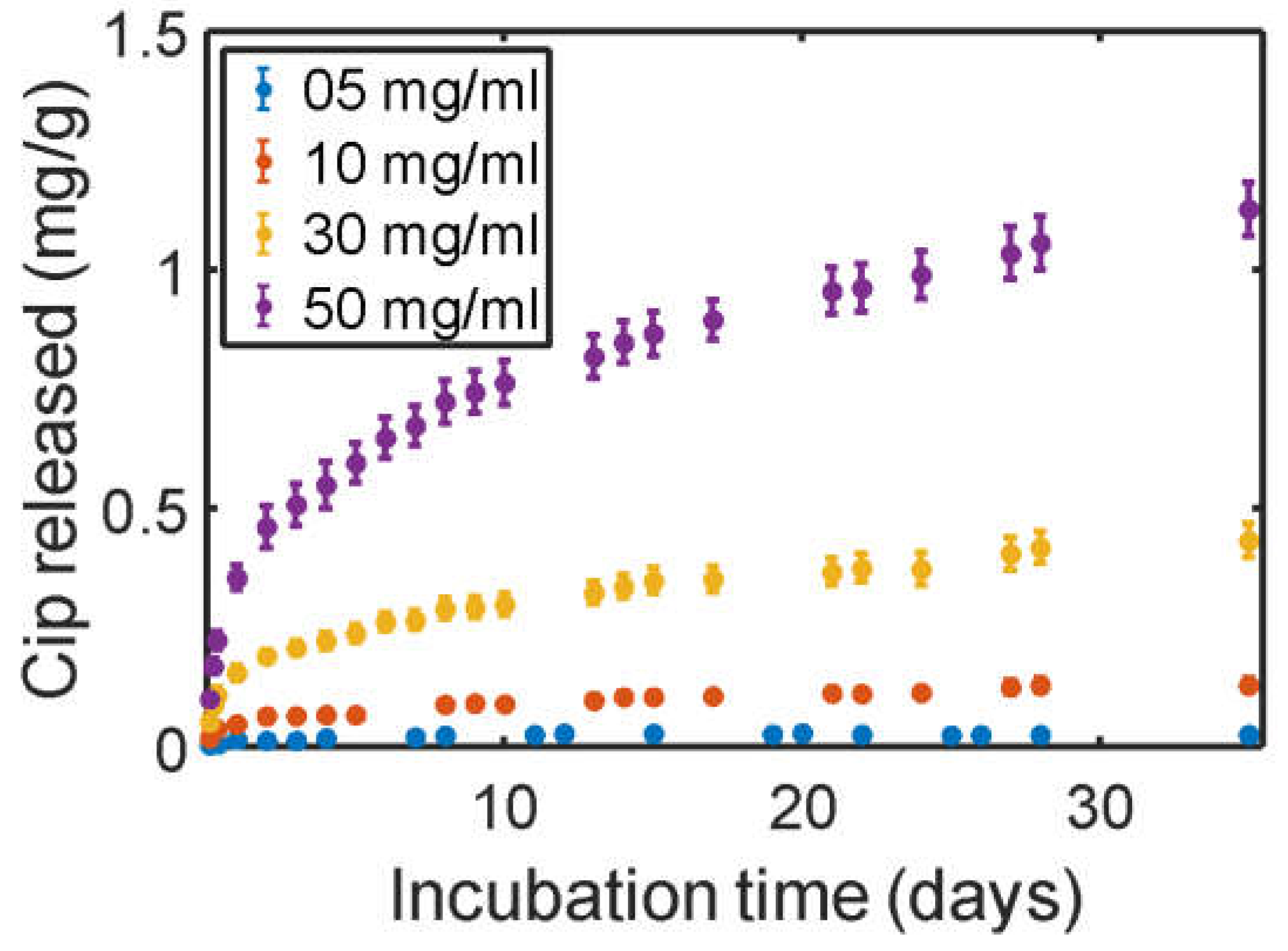
3.3. Cip Coating
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cano-Vicent, A.; Tambuwala, M.M.; Hassan, S.S.; Barh, D.; Aljabali, A.A.A.; Birkett, M.; Arjunan, A.; Serrano-Aroca, A. Fused deposition modelling: Current status, methodology, applications and future prospects. Additive Manufacturing 2021, 47, 102378. [Google Scholar] [CrossRef]
- Khalid, G.M.; Billa, N. Solid Dispersion Formulations by FDM 3D Printing—A Review. Pharmaceutics 2022, 14, 690. [Google Scholar] [CrossRef]
- Dey, A.; Roan Eagle, I.N.; Yodo, N. A Review on Filament Materials for Fused Filament Fabrication. Journal of Manufacturing and Materials Processing 2021, 5, 69. [Google Scholar] [CrossRef]
- Ahmad, M.N.; Ishak, M.R.; Mohammad Taha, M.; Mustapha, F.; Leman, Z. A Review of Natural Fiber-Based Filaments for 3D Printing: Filament Fabrication and Characterization. Materials 2023, 16, 4052. [Google Scholar] [CrossRef] [PubMed]
- Rupp, H.; Binder, W.H. 3D Printing of Solvent-Free Supramolecular Polymers. Frontiers in Chemistry 2021, 9, 771974. [Google Scholar] [CrossRef] [PubMed]
- Heidari-Rarani, M.; Rafiee-Afarani, M.; Zahedi, A.M. Mechanical characterization of FDM 3D printing of continuous carbon fiber reinforced PLA composites. Composites Part B-Engineering 2019, 175, 107147. [Google Scholar] [CrossRef]
- Wickramasinghe, S.; Do, T.; Tran, P. FDM-Based 3D Printing of Polymer and Associated Composite: A Review on Mechanical Properties, Defects and Treatments. Polymers 2020, 12, 1529. [Google Scholar] [CrossRef] [PubMed]
- Andreu, A.; Kim, S.; Dittus, J.; Friedmann, M.; Fleischer, J.; Yoon, Y.J. Hybrid material extrusion 3D printing to strengthen interlayer adhesion through hot rolling. Additive Manufacturing 2022, 55, 102773. [Google Scholar] [CrossRef]
- Lee, J.E.; Park, S.J.; Son, Y.; Park, K.; Park, S.H. Mechanical reinforcement of additive-manufactured constructs using in situ auxiliary heating process. Additive Manufacturing 2021, 43, 101995. [Google Scholar] [CrossRef]
- Prajapati, H.; Salvi, S.S.; Ravoori, D.; Qasaimeh, M.; Adnan, A.; Jain, A. Improved print quality in fused filament fabrication through localized dispensing of hot air around the deposited filament. Additive Manufacturing 2021, 40, 101917. [Google Scholar] [CrossRef]
- Sabyrov, N.; Abilgaziyev, A.; Ali, M.H. Enhancing interlayer bonding strength of FDM 3D printing technology by diode laser-assisted system. International Journal of Advanced Manufacturing Technology 2020, 108, 603–611. [Google Scholar] [CrossRef]
- Han, P.; Tofangchi, A.; Deshpande, A.; Zhang, S.H.; Hsu, K. An approach to improve interface healing in FFF-3D printed Ultem 1010 using laser pre-deposition heating. In Proceedings of the 47th SME North American Manufacturing Research Conference (NAMRC), Penn Univ, Behrend Coll, Erie, PA, 10--14 Jun 2019; pp. 672–677. [Google Scholar]
- Ravi, A.K.; Deshpande, A.; Hsu, K.H. An in-process laser localized pre-deposition heating approach to inter-layer bond strengthening in extrusion based polymer additive manufacturing. Journal of Manufacturing Processes 2016, 24, 179–185. [Google Scholar] [CrossRef]
- Elumalai, A.; Nayak, Y.; Ganapathy, A.K.; Chen, D.; Tappa, K.; Jammalamadaka, U.; Bishop, G.; Ballard, D.H. Reverse Engineering and 3D Printing of Medical Devices for Drug Delivery and Drug-Embedded Anatomic Implants. Polymers 2023, 15, 4306. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, N.; Ghosh, C.; Sharma, V.; Roy, P.; Kumar, P. Investigations on effect of pore architectures of additively manufactured novel hydroxyapatite coated PLA/Al2O3 composite scaffold for bone tissue engineering. Rapid Prototyping Journal 2023, 29, 1061–1079. [Google Scholar] [CrossRef]
- Daroonparvar, M.; Bakhsheshi-Rad, H.R.; Saberi, A.; Razzaghi, M.; Kasar, A.K.; Ramakrishna, S.; Menezes, P.L.; Misra, M.; Ismail, A.F.; Sharif, S.; et al. Surface modification of magnesium alloys using thermal and solid-state cold spray processes: Challenges and latest progresses. Journal of Magnesium and Alloys 2022, 10, 2025–2061. [Google Scholar] [CrossRef]
- Tak, J.; Kang, D.-G.; Choi, J. A lightweight waveguide horn antenna made via 3D printing and conductive spray coating. Microwave and Optical Technology Letters 2017, 59, 727–729. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Xiang, G.; Liu, H.; He, J. Hybrid Additive Manufacturing Method for Selective Plating of Freeform Circuitry on 3D Printed Plastic Structure. Advanced Materials Technologies 2019, 4, 1800529. [Google Scholar] [CrossRef]
- Park, S.; Kim, S.; Park, S.; Lee, J.; Kim, H.; Kim, M. Recent Progress in Development and Applications of Ionic Polymer–Metal Composite. Micromachines 2022, 13, 1290. [Google Scholar] [CrossRef] [PubMed]
- Domsta, V.; Seidlitz, A. 3D-Printing of Drug-Eluting Implants: An Overview of the Current Developments Described in the Literature. Molecules 2021, 26, 4066. [Google Scholar] [CrossRef]
- Auriemma, G.; Tommasino, C.; Falcone, G.; Esposito, T.; Sardo, C.; Aquino, R.P. Additive Manufacturing Strategies for Personalized Drug Delivery Systems and Medical Devices: Fused Filament Fabrication and Semi Solid Extrusion. Molecules 2022, 27, 2784. [Google Scholar] [CrossRef]
- Qamar, N.; Abbas, N.; Irfan, M.; Hussain, A.; Arshad, M.S.; Latif, S.; Mehmood, F.; Ghori, M.U. Personalized 3D printed ciprofloxacin impregnated meshes for the management of hernia. Journal of Drug Delivery Science and Technology 2019, 53, 101164. [Google Scholar] [CrossRef]
- Yang, W.-W.; Pierstorff, E. Reservoir-Based Polymer Drug Delivery Systems. SLAS Technology 2012, 17, 50–58. [Google Scholar] [CrossRef]
- Shi, S.; Chen, Y.; Jing, J.; Yang, L. Preparation and 3D-printing of highly conductive polylactic acid/carbon nanotube nanocomposites via local enrichment strategy. RSC Adv 2019, 9, 29980–29986. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Gu, H.; Cui, C.; Hao, J. Improved mechanical properties of 3D-printed SiC/PLA composite parts by microwave heating. Journal of Materials Research 2019, 34, 3412–3419. [Google Scholar] [CrossRef]
- Farto-Vaamonde, X.; Auriemma, G.; Aquino, R.P.; Concheiro, A.; Alvarez-Lorenzo, C. Post-manufacture loading of filaments and 3D printed PLA scaffolds with prednisolone and dexamethasone for tissue regeneration applications. European Journal of Pharmaceutics and Biopharmaceutics 2019, 141, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Farto-Vaamonde, X.; Diaz-Gomez, L.; Parga, A.; Otero, A.; Concheiro, A.; Alvarez-Lorenzo, C. Perimeter and carvacrol-loading regulate angiogenesis and biofilm growth in 3D printed PLA scaffolds. Journal of Controlled Release 2022, 352, 776–792. [Google Scholar] [CrossRef]
- Francis, V.; Jain, P.K. A filament modification approach for in situ ABS/OMMT nanocomposite development in extrusion-based 3D printing. Journal of the Brazilian Society of Mechanical Sciences and Engineering 2018, 40, 1–13. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Xie, M.; Zhou, Z.; Xu, H. A surface modification of polylactic acid composites for additive manufacturing with modified chopped carbon fiber and modified nano-hydroxyapatite. Polymer Composites 2022, 43, 7859–7870. [Google Scholar] [CrossRef]
- Sweeney, C.B.; Lackey, B.A.; Pospisil, M.J.; Achee, T.C.; Hicks, V.K.; Moran, A.G.; Teipel, B.R.; Saed, M.A.; Green, M.J. Welding of 3D-printed carbon nanotube-polymer composites by locally induced microwave heating. Science Advances 2017, 3, e1700262. [Google Scholar] [CrossRef]
- Sweeney, C.B.; Burnette, M.L.; Pospisil, M.J.; Shah, S.A.; Anas, M.; Teipel, B.R.; Zahner, B.S.; Staack, D.; Green, M.J. Dielectric Barrier Discharge Applicator for Heating Carbon Nanotube-Loaded Interfaces and Enhancing 3D-Printed Bond Strength. Nano Letters 2020, 20, 2310–2315. [Google Scholar] [CrossRef] [PubMed]
- Shinde, V.V.; Taylor, G.; Celestine, A.D.N.; Beckingham, B.S. Fused Filament Fabrication 3D Printing of Self-Healing High-Impact Polystyrene Thermoplastic Polymer Composites Utilizing Eco-friendly Solvent-Filled Microcapsules. Acs Applied Polymer Materials 2022, 4, 3324–3332. [Google Scholar] [CrossRef]
- Geuli, O.; Lewinstein, I.; Mandler, D. Composition-Tailoring of ZnO-Hydroxyapatite Nanocomposite as Bioactive and Antibacterial Coating. Acs Applied Nano Materials 2019, 2, 2946–2957. [Google Scholar] [CrossRef]
- Naim, G.; Bruchiel-Spanier, N.; Betsis, S.; Eliaz, N.; Mandler, D. Vat Polymerization by Three-Dimensional Printing and Curing of Antibacterial Zinc Oxide Nanoparticles Embedded in Poly(ethylene glycol) Diacrylate for Biomedical Applications. Polymers 2023, 15, 3586. [Google Scholar] [CrossRef] [PubMed]
- Sabel, C.E.; Neureuther, J.M.; Siemann, S. A spectrophotometric method for the determination of zinc, copper, and cobalt ions in metalloproteins using Zincon. Analytical Biochemistry 2010, 397, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Slavin, Y.N.; Asnis, J.; Hafeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. Journal of Nanobiotechnology 2017, 15, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Czyzowska, A.; Barbasz, A. A review: Zinc oxide nanoparticles - friends or enemies? International Journal of Environmental Health Research 2022, 32, 885–901. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Li, H.; Zhang, T.; Song, B.; Wang, X.; Gu, Z. Recent Advances in ZnO Nanomaterial-Mediated Biological Applications and Action Mechanisms. Nanomaterials 2023, 13, 1500. [Google Scholar] [CrossRef] [PubMed]
- Mendes, C.R.; Dilarri, G.; Forsan, C.F.; Sapata, V.D.M.R.; Lopes, P.R.M.; De Moraes, P.B.; Montagnolli, R.N.; Ferreira, H.; Bidoia, E.D. Antibacterial action and target mechanisms of zinc oxide nanoparticles against bacterial pathogens. Scientific Reports 2022, 12, 2658. [Google Scholar] [CrossRef]
- Kloskowski, T.; Gurtowska, N.; Drewa, T. Does ciprofloxacin have an obverse and a reverse? Pulmonary Pharmacology & Therapeutics 2010, 23, 373–375. [Google Scholar] [CrossRef]
- Zhang, G.F.; Liu, X.F.; Zhang, S.; Pan, B.F.; Liu, M.L. Ciprofloxacin derivatives and their antibacterial activities. European Journal of Medicinal Chemistry 2018, 146, 599–612. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




