1. Introduction
Gabapentin (GBP) is a pharmaceutical compound that functions as a gamma-aminobutyric acid (GABA) analog. The FDA has approved it for treating partial seizures and postherpetic neuralgias [
1,
2]. However, its additional properties as an anticonvulsant, antinociceptive, anxiolytic, and neuroprotective agent also lead to its use off-label. Specifically, doctors commonly prescribe it to manage chronic neuropathic pain [
1,
2]. The pharmacological mechanism of action relies on the rapid penetration of GBP into cerebral cells [
3]. GBP modulates the activity of the glutamic acid decarboxylase (GAD) and branched-chain amino acid transaminase enzymes [
4]. The cellular mechanisms underlying GBP's action encompass a variety of processes. Its transportation across various membrane barriers is facilitated by a specific amino acid transporter that competes with leucine, isoleucine, valine (BCAAs), and phenylalanine. Additionally, GBP leads to an elevated concentration of GABA in the brain and an increased rate of GABA synthesis. Furthermore, GBP interacts with voltage-sensitive Ca2+ and Na+ channels, as well as other neurotransmitters like serotonin [
4].
The literature extensively elucidates the significant correlation between GABA and stress [
5,
6,
7]. Researchers have observed GABAergic connections on corticotropin-releasing hormone (CRH) neurons located inside the paraventricular nucleus of the hypothalamus (PVN). These neurons play a crucial role in regulating the activity of the hypothalamic-pituitary-adrenal (HPA) axis. Consequently, it appears that GABA exerts a significant regulatory influence over the activity of the hypothalamic-pituitary-adrenal (HPA) axis [
6]. Additionally, it has been suggested that gamma-aminobutyric acid (GABA) is an important part of stress reactions. It does this by directly controlling the hypothalamic-pituitary-adrenal (HPA) axis and also by causing a lot of biochemical adaptability [
5]. Recent research has determined that both phasic and tonic GABAergic inhibition regulate the activity of HPA regulators, including CRH and gonadotropin-releasing hormone (GnRH). Synaptic and extrasynaptic GABA-A receptors (GABAARs) mediate this inhibition. The tonic phase appears to exhibit heightened stimulation during stressful conditions, facilitated by the δ-subunit of GABAARs. This is probably because the ε-subunit is in the best place to control communication between the stress and reproductive axes, especially when neurosteroids from stress are present [
8].
Two separate studies [
9,
10] have revealed a correlation between the introduction of GBP and the suppression of the typical stress response that occurs during the perioperative and postoperative periods. The peripheral antinociceptive action of GBP and its activation of supraspinal brain areas involved in pain signal processing are responsible for this suppression. Additionally, studies have found that the initiation of GBP induces drowsiness [
9,
10,
11].
This case report details the presence of hypocortisolemia, a disorder defined by abnormally low levels of adrenocorticotropic hormone (ACTH), which required the initiation of replacement therapy. The condition occurred transiently during a period of high stress connected to surgery, in a patient who had previously undergone prolonged GBP administration in order to avoid postoperative pain. Furthermore, a comprehensive literature analysis is conducted to evaluate the primary pathophysiological, pharmacological, and clinical components of this uncommon case.
2. Case Report
At the age of 15, a 17-year-old male, standing at 1.80 meters and weighing 118 kilograms, received a diagnosis of left femur osteosarcoma. The EURAMOS protocol [
12] guided his treatment, which involved tumor excision and implantation of a megaprosthesis for limb salvage [
13]. However, he later presented it to our department for a revision of the megaprosthesis. Two months after the initial megaprosthesis placement, a Staphylococcus hominis infection necessitated this revision.
Before deciding to proceed with the revision, we performed surgical debridement, which involved the removal of biofilm and the culturing of specimens. Staphylococcus hominis was identified two days after collecting the samples. As a result, the megaprosthesis was removed and replaced with an antibiotic-loaded cement spacer. Tranexamic acid was used as a hemostatic agent during the procedure. A month later, a second-look operation with surgical debridement and a spacer change was performed.
During the period in which the spacer was in place, the patient experienced a massive pulmonary embolism. An interventional radiologist treated this condition by performing a thrombectomy and administering a therapeutic dose of low molecular weight heparin (LMWH). There was also a suspected deep vein thrombosis of the left common iliac vein. The patient underwent readmission for the implantation of a new prosthesis almost a year after the removal of the first megaprosthesis. Vacuum-assisted closure (VAC) was used to help repair and heal a large, skin-deficient area. This procedure was successful, and the patient spent one day in the Intensive Care Unit (ICU).
Given the patient's complex medical history, an extensive laboratory investigation was conducted before his transfer to a regular ward. The results showed that the patient had a morning cortisol plasma level of 1.18 μg/dl, which was below the normal range (normal range: 6.20–23 μg/dl). However, all other hormone levels, including thyroid hormones, fell within the normal range.
Promptly following this discovery, the patient began hydrocortisone replacement therapy, starting with a 100-mg bolus, followed by 200 mg over the course of 24 hours. The hydrocortisone replacement therapy was then tapered over several days, with cortisol levels closely monitored until they returned to the normal range. Subsequently, during the post-operative period following the placement of the new prosthesis, the patient developed a significant subcutaneous fluid collection. It was unclear whether this collection was a hematoma or had a microbial origin. Clinical findings, including the mass edema, the redness, and the high temperature of the affected area, led to the decision to perform surgical debridement.
Before the debridement, the patient received preoperative preparation with hydrocortisone, and cortisol levels were monitored and adjusted as necessary during the process. ACTH levels were also closely monitored. The patient underwent another surgical debridement for a new subcutaneous collection, which revealed the presence of Staphylococcus xylosus in one of the culture samples. The VAC was removed, and hydrocortisone treatment was modified in response to cortisol level changes.
After the second debridement, the subcutaneous collection gradually improved, and follow-up assessments showed no signs of infection. Eight months later, according to the clinical, laboratory, and imaging findings, no signs of inflammation were present, and the patient successfully resumed his normal daily activities.
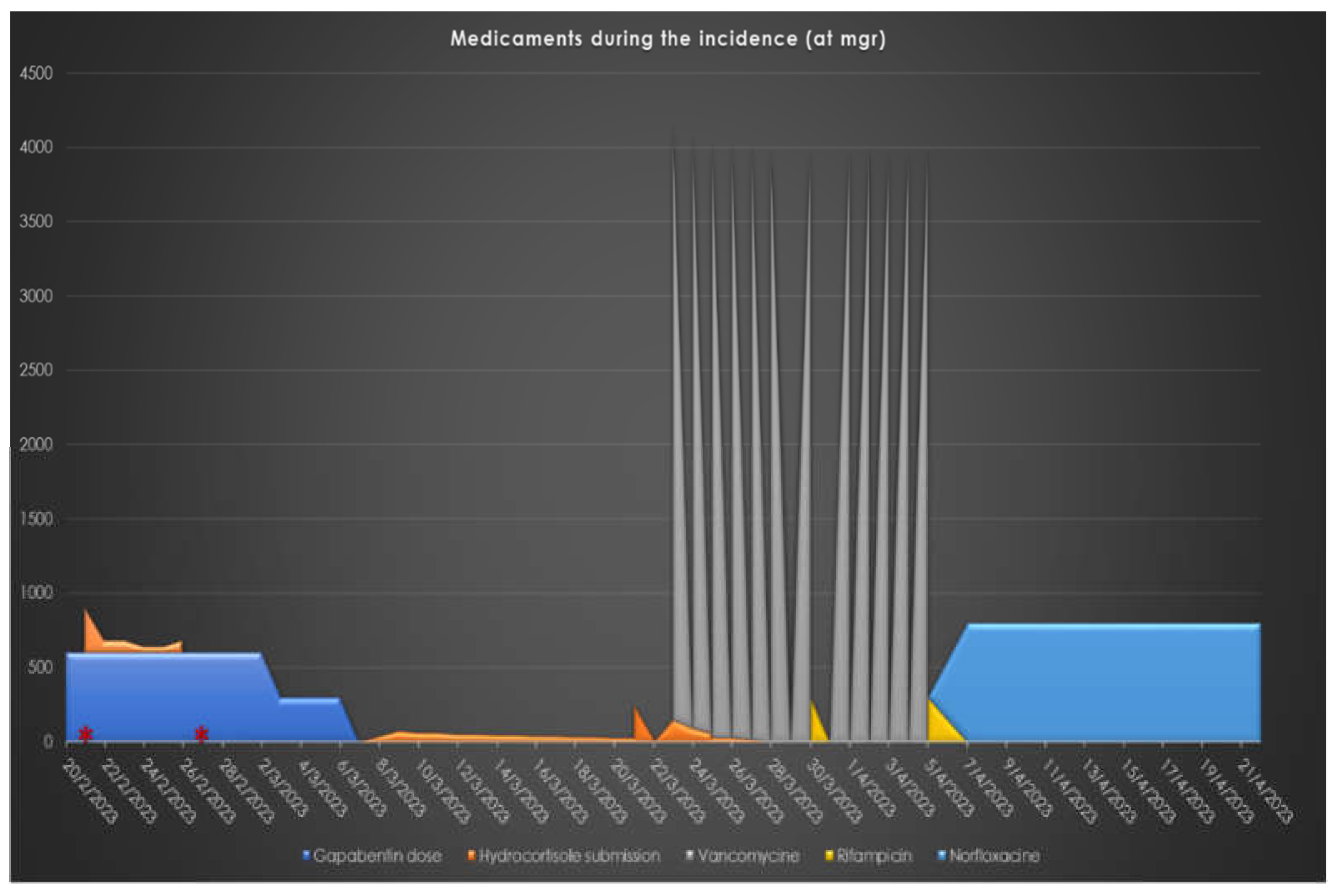
It is noteworthy that gabapentin was initially administered as a prophylactic analgesic treatment before tumor excision and megaprosthesis implantation, with doses varying between 300 mg once, twice, or three times a day, depending on the patient's pain levels at different times, resulting in almost 6 months of administration with breaks inside this period. During the hospitalization for the prosthesis’s revision, the patient received gabapentin at a dosage of 300 mg twice daily. Following the detection of adrenal insufficiency, the gabapentin dosage was reduced to 300 mg once daily and later discontinued after the second adrenal insufficiency diagnosis. During the periods when adrenal insufficiency was detected, the patient was under gabapentin, antibiotic chemoprophylaxis, and LMWH anticoagulant treatment at therapeutic doses.
As for antibiotic chemoprophylaxis, the patient received vancomycin starting from the first pre-operative day at a dosage of 500 mg four times daily intravenously. The same antibiotic scheme was used as chemoprophylaxis before the removal of the spacer and the placement of the new endoprosthesis, along with cefepime at 2 grams three times daily for two days. After the first debridement of the second megaprosthesis, vancomycin was administered at an increased dosage of 550 mg four times daily. Following the second debridement, the antibiotic regimen was further escalated to vancomycin at 1 gram four times daily, in combination with rifampicin at 600 mg once daily, for 16 days. The patient was discharged and continued to take norfloxacin at a dosage of 400 mg twice daily orally for 14 days as an outpatient. It's important to note that vancomycin levels were consistently maintained at the lowest therapeutic levels when assessed through immunofluorescence at all times the glycopeptide was administered. The levels of vancomycin were estimated according to the hospital’s protocol for vancomycin monitoring every 3 days. The antibiotic regimen was decided after detailed discussion with the pediatric infectious diseases department against an invasive staphylococcal infection in an immunosuppressed patient.
A detailed investigation was conducted on an outpatient basis with CT and MRI, ruling out any other pathology that could be implicated in the studied event. The patient is in good condition and has returned to his daily activities.
Figure 1 represents the administration at mgs regarding the medication related to the presented case, and
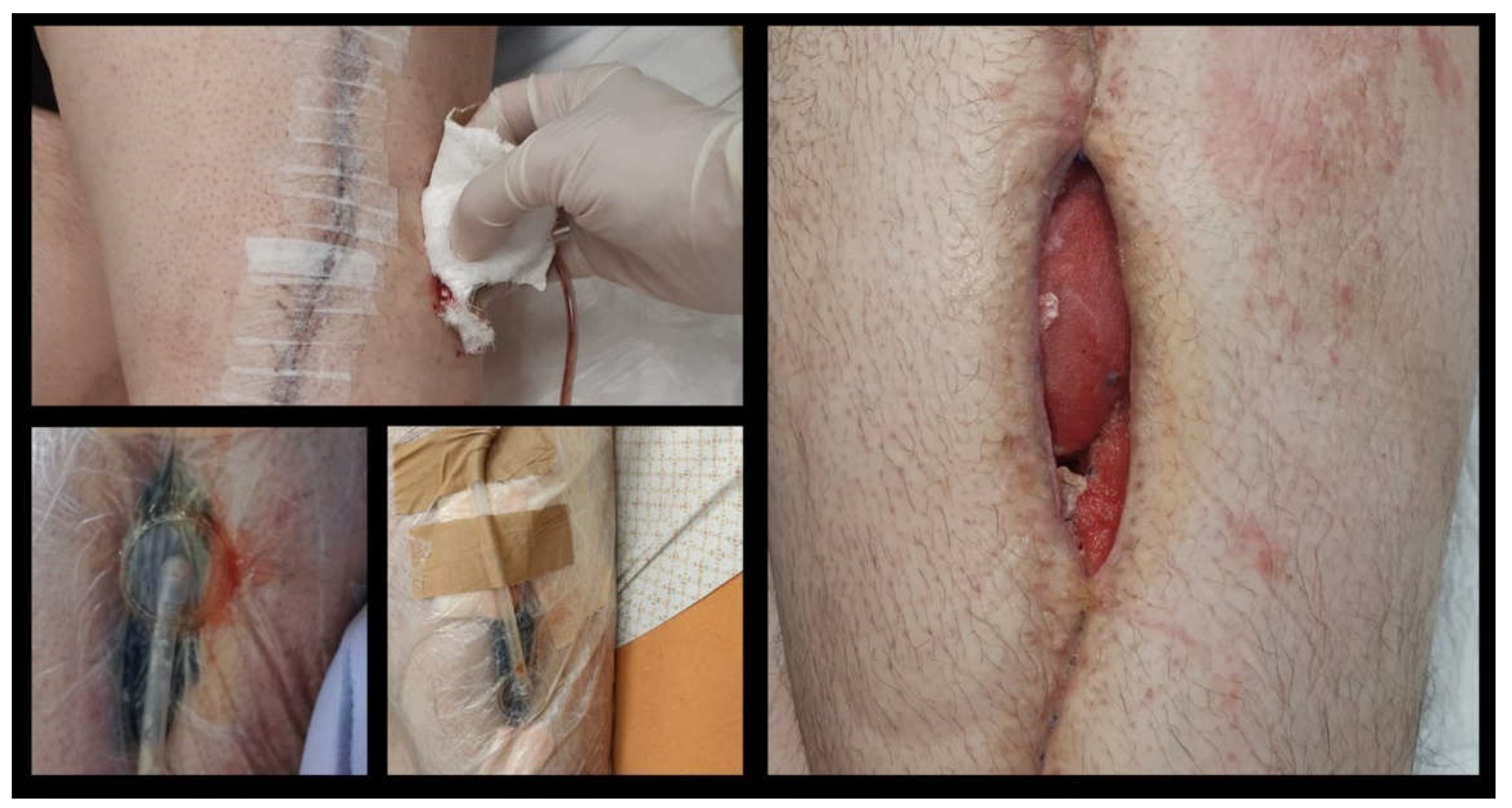
Figure 2 depicts the clinical images indicating wound infection.
3. Review
3.1. Gabapentin’s Pharmacological Profile, the HPA Axis and stress response
GBP, a GABA analog, is recognized for its multifaceted pharmacological actions, including antinociceptive, anxiolytic, and neuroprotective effects. The intricate mechanisms of GBP action involve its ability to access brain cells, modulate enzymes like GAD, and interact with neurotransmitters, particularly serotonin. This multi-faceted pharmacological profile suggests its potential impact on various physiological systems, including the HPA axis [
3,
4,
14,
15,
16]. Interestingly, GABAergic synapses' role in regulating the HPA axis is well-established, and GBP, as a GABA analog, might influence this axis through several mechanisms [
3,
4,
6,
7,
14,
15,
16,
17,
18].
The connection between GABA and stress is a crucial theme in this context. GABA's role in controlling the activity of the HPA axis and stress responses is well-documented, with GABAergic synapses affecting key regulators of the axis, such as CRH neurons [
4,
16,
19,
20]. Moreover, the dichotomy between phasic and tonic GABAergic inhibition, mediated by different GABAARs, adds another layer of complexity to HPA axis regulation under stress conditions. Specifically, the δ-subunit of GABAARs appears to play a pivotal role in mediating stress-related neurosteroid responses [
3,
5,
16,
21].
3.2. Gabapentin and Cortisol Levels in Clinical Settings
Several studies provide valuable insights into the complex relationship between GBP and cortisol levels. Karbić et al. (2014) [
10] investigated the impact of GBP on plasma cortisol levels and immune status in hysterectomized women, revealing a significant decrease in cortisol levels following GBP administration. In detail, the effects of GBP on cortisol levels were examined in a group of 60 women undergoing abdominal hysterectomy. These patients were randomly assigned to receive either 600 mg of GBP or a placebo one hour before surgery. The results demonstrated that GBP significantly reduced plasma cortisol levels 24 hours postoperatively compared to the placebo group. This reduction in cortisol indicates that GBP can suppress the HPA axis's typical stress response, likely contributing to the observed decrease in postoperative pain [
22]. Additionally, GBP was found to reduce catecholamine levels, including epinephrine and norepinephrine, which are also involved in the body’s stress response. This dual effect on both cortisol and catecholamines underscores GBP’s potential role in mitigating surgical stress [
23,
24]. Further supporting this, Hudec and Griffin (2020) [
25] explored the effects of GBP on stress markers in a veterinary context, finding that pre-appointment administration of GBP to cats resulted in a slight but not statistically significant reduction in cortisol levels. This suggests that GBP’s impact on cortisol may be modest and potentially related more to its sedative properties than direct suppression of the HPA axis [
25]. However, this effect, though minor in this study, aligns with other findings that indicate GBP may exert a calming effect, reducing stress-induced hormonal responses. Contrasting these findings is a case reported by Alam and Srinivasan [
9], where a 56-year-old patient undergoing total knee replacement and receiving GBP as part of postoperative pain management displayed significantly low cortisol levels on the first and fourth postoperative days. Despite these low levels, the patient’s adrenal response to a Short Synacthen Test was normal, suggesting that GBP was likely suppressing the stress response rather than causing primary adrenal insufficiency [
6,
19,
24]. This case highlights the variability in how GBP can affect cortisol levels, particularly under conditions of surgical stress.
When comparing these findings to the case report presented in this manuscript, we observe a similar pattern where GBP administration was associated with significantly altered cortisol levels, necessitating adrenal replacement therapy. Our case present a young patient with osteosarcoma who, after receiving GBP, developed transient adrenal insufficiency during a period of high surgical stress. This aligns with the broader literature, suggesting that GBP can suppress the HPA axis’s response to stress, potentially leading to clinically significant reductions in cortisol that may require intervention. Unlike the patient in Alam and Srinivasan's report, where cortisol suppression did not lead to adrenal insufficiency, the patient in this case report did develop hypocortisolemia severe enough to warrant treatment. This emphasizes the need for careful monitoring of cortisol levels in patients receiving GBP, particularly in high-stress surgical contexts.
To our knowledge, this is the 3rd clinical case underlying the GBP’s effects on cortisol levels are complex and context dependent. According to the existing literature, GBP can effectively reduce the body’s stress response by lowering cortisol and catecholamine levels, this effect can vary widely among individuals [
5,
6,
8,
10,
17,
19,
20]. In some cases, such as the one described in this manuscript, GBP may lead to significant adrenal insufficiency, particularly in the perioperative setting. These findings highlight the importance of personalized patient care, with a need for close monitoring of adrenal function when administering GBP, especially to patients undergoing major surgeries or those with underlying vulnerabilities to stress responses [
23,
24,
25].
3.3. Clinical Implications of Gabapentin-Induced Adrenal Insufficiency
The potential of GBP to induce adrenal insufficiency, particularly in high-stress settings such as surgery, carries significant clinical implications [
26,
27]. The ability of GBP to suppress HPA axis and subsequently reduce cortisol levels can be both beneficial and risky, depending on the patient's overall health status and the context of GBP administration [
4,
7,
10,
14].
The case report presented in this manuscript exemplifies the serious consequences that can arise from GBP-induced cortisol suppression. In this case, a young patient with osteosarcoma experienced transient adrenal insufficiency during a high-stress surgical period after prolonged GBP administration. This necessitated adrenal replacement therapy, underscoring the need for careful monitoring and potential intervention when using GBP in similar clinical scenarios.
Supporting this observation, the study by Karbić et al. (2014) [
10] provides robust evidence of GBP's capacity to alter cortisol levels and modulate immune responses in the perioperative setting [
11]. In their study involving women undergoing abdominal hysterectomy, GBP significantly reduced plasma cortisol levels 24 hours after surgery, compared to a placebo group. This reduction in cortisol was correlated with a decrease in pain scores and catecholamine levels, suggesting that GBP effectively dampens the physiological stress response associated with surgery. However, the study also highlights potential risks, noting that such suppression of cortisol and catecholamines could impair the body's ability to respond to acute stressors, such as surgery or infection, potentially leading to complications [
10,
11,
15].
Moreover, our findings along with the findings of the aforementioned studies introduce the broader implications of GBP-induced immunomodulation [
3,
6,
10,
11]. By suppressing cortisol and catecholamine levels, GBP can influence immune function, as these hormones play a crucial role in regulating inflammation and immune responses [
23,
24]. The study found that GBP altered the proportions of various lymphocyte subsets, potentially impacting the body’s ability to mount an effective immune response postoperatively [
10,
23,
24,
28]. This is particularly relevant in surgical patients, who are at increased risk for infections and other complications when immune function is compromised.
The clinical implications of these findings are clear: healthcare providers must weigh the benefits of GBP in controlling pain and reducing stress responses against the potential for inducing adrenal insufficiency and compromising immune function. This is especially critical in patients undergoing major surgeries or those with pre-existing conditions that may predispose them to adrenal insufficiency. Regular monitoring of cortisol levels and immune markers in such patients is advisable to detect and address any adverse effects promptly. While GBP is a valuable tool in perioperative pain management, its impact on the HPA axis and immune function necessitates a cautious approach, particularly in high-risk patients. Further research is needed to develop guidelines that balance the analgesic and stress-reducing benefits of GBP with the need to maintain adequate adrenal and immune function, ensuring patient safety and optimal outcomes in the surgical setting.
3.4. Gabapentin-Induced Hypocortisolemia and Infection Risk
The connection between gabapentin-induced hypocortisolemia and subsequent infection in this case report cannot be overlooked. The initial administration of GBP raised questions about its role in modulating the patient's stress response, as it led to a profound reduction in cortisol levels. The subsequent infection with
Staphylococcus hominis, which occurred two months after megaprosthesis implantation, may have been facilitated by this compromised stress response, as cortisol is a crucial component of the body's immune defenses. Hypocortisolemia resulting from GBP might have contributed to the patient's increased susceptibility to infection [
28]. It is well known that glucocorticoids affect both the innate and adaptive immune systems through a variety of mechanisms [
29]. Thus, the reduction of these adaptations, in combination with the stress provoked by the surgical procedure and the fact that the natural barrier against pathogens had been injured due to the extensive surgical incision, put the patient at a disadvantage against potential invaders and led to infection [
5,
23,
28,
29].
This case vividly illustrates how the complex interplay between GBP and cortisol can have substantial clinical implications, particularly in the context of major surgical procedures, as it may affect a patient's ability to mount an effective immune response against potential pathogens. The emergence of
Staphylococcus xylosus in this case further underscores the intriguing connection between gabapentin-induced hypocortisolemia and the development of infections [
30]. The patient's altered adrenal response due to gabapentin likely contributed to a weakened immune defense, increasing susceptibility to infections, including unusual pathogens like
Staphylococcus xylosus [
30,
31]. Complications included infections with
Staphylococcus hominis and
Staphylococcus xylosus, pathogens typically considered low-virulence but capable of causing significant infections in immunocompromised hosts [
32,
33]. The presence of
Staphylococcus xylosus is particularly noteworthy, as it is a coagulase-negative staphylococcus (CoNS) and is generally considered a contaminant [
32,
33]. However, in this immunosuppressed state induced by GBP,
Staphylococcus xylosus emerged as a primary pathogen, underscoring the importance of considering the patient's immunological status when interpreting microbiological findings [
30,
31,
32,
33].
This scenario aligns with findings from the study by Brand and Rufer (2021) [
30], where
Staphylococcus xylosus was identified as the causative agent in a late prosthetic joint infection (PJI). In this study, a 70-year-old male patient developed a knee joint infection 18 years after total knee arthroplasty (TKA), which was attributed to
Staphylococcus xylosus [
30]. The patient’s history included recent trauma and possible sources of hematogenous seeding, which likely contributed to the infection [
30]. The study emphasized that CoNS, including
Staphylococcus xylosus, should not be routinely dismissed as contaminants, particularly in immunocompromised or stressed patients, where these organisms can act as opportunistic pathogen [
30].
Similarly, the study by Scorzolini et al. (2014) [
31] demonstrated the increased sensitivity of microbiological detection methods such as sonication in identifying pathogens like CoNS in prosthetic joint infections (PJIs) [
31]. This study highlighted the importance of accurately diagnosing and treating PJIs, particularly in patients with compromised immune systems, where pathogens like
Staphylococcus xylosus can cause significant infections despite their typically low virulence [
30,
31,
32,
33]. The study's findings further support the need for vigilance in interpreting culture results in the context of GBP-induced immunosuppression.
When comparing the patients in the two previous reported studies to the patient in our case report, several similarities emerge. All patients were immunocompromised, either due to their underlying conditions, surgical stress, or GBP-induced hypocortisolemia, which facilitated the emergence of
Staphylococcus xylosus as a significant pathogen. In both the Brand and Rufer case and our case report,
Staphylococcus xylosus caused serious infections that required intensive treatment [
30]. These cases emphasize the clinical significance of GBP-induced cortisol suppression, which can impair the body’s ability to fend off infections, especially from opportunistic pathogens like CoNS. The presence of
Staphylococcus xylosus as the main pathogen in these cases underscores the need for careful consideration of the potential for GBP to exacerbate infection risks in vulnerable patients [
30,
31,
32].
In conclusion, the risk of infection in patients receiving GBP, particularly those undergoing surgery, is heightened due to the drug's impact on cortisol levels and immune function. The emergence of Staphylococcus xylosus as a significant pathogen in these cases serves as a reminder that even typically low-virulence organisms can cause severe infections in the context of GBP-induced immunosuppression. Clinicians should be aware of these risks and consider them when managing patients on GBP, especially in perioperative settings or in those with pre-existing conditions that may compromise their immune responses.
4. Conclusions
This review and case report illustrate the complex interplay between GBP use, adrenal insufficiency, and infection risk, particularly in patients undergoing major surgical procedures. The findings emphasize the importance of understanding GBP's multifaceted effects on the HPA axis and cortisol regulation, as well as the need for vigilant monitoring of patients receiving GBP, especially in perioperative settings. Further research is essential to elucidate the underlying mechanisms of GBP's impact on the HPA axis and to develop evidence-based guidelines for managing such cases.
Author Contributions
Conceptualization, E.P and A.C.; investigation, MA.Z. A.C.; data curation, O.C., N.P.; writing—original draft preparation, E.P., A.C. and MA.Z..; writing—review and editing, N.S., A.C., P.Z. and R.M.; visualization, E.P. and S.K.; supervision, R.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This case presentation follows the Declaration of Helsinki and internal policies of the General Children’s Hospital of Athens. Due to the fact that this is a case report (not a prospective nor a clinical study) and a review, ethical review and approval before the event are not applicable.
Informed Consent Statement
Informed consent was obtained from the parents of the patient involved in this study. Written informed consent has been obtained from the patients’ parents to publish this paper.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors are sincerely thankful to the big medical team of the 1st Department of Orthopaedics of “P&A Kyriakou” Children’s Hospital and everyone that participated in the treatment of the adolescent (Endocrinology Department, Infectious Diseases Department, Radiology Department, Anesthesiology Department etc) and embraced his fears and hopes.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hayes, W.J.; Ferdinand, A.; Neabore, S.; Kappes, J.A.; Hayes, K.M.; Berendse, J. Patient Case Report: Gabapentin-Induced Hypoglycemia. J Pharm Pract 2022, 35, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Product Information. Neurontin(R) Oral Capsules, Tablets, Solution, Gabapentin Oral Capsules, Tablets, Solution. ; 2010.
- Taylor, C.P. Mechanisms of action of gabapentin. Rev Neurol (Paris) 1997, 153 Suppl 1, S39–45. [Google Scholar]
- Taylor, C.P.; Gee, N.S.; Su, T.Z.; Kocsis, J.D.; Welty, D.F.; Brown, J.P.; Dooley, D.J.; Boden, P.; Singh, L. A summary of mechanistic hypotheses of gabapentin pharmacology. Epilepsy Res 1998, 29, 233–249. [Google Scholar] [CrossRef]
- Cullinan, W.E.; Ziegler, D.R.; Herman, J.P. Functional role of local GABAergic influences on the HPA axis. Brain Struct Funct 2008, 213, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Maguire, J. The relationship between GABA and stress: 'it's complicated'. J Physiol 2018, 596, 1781–1782. [Google Scholar] [CrossRef] [PubMed]
- Maguire, J.; Mody, I. GABA(A)R plasticity during pregnancy: relevance to postpartum depression. Neuron 2008, 59, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Camille Melon, L.; Maguire, J. GABAergic regulation of the HPA and HPG axes and the impact of stress on reproductive function. J Steroid Biochem Mol Biol 2016, 160, 196–203. [Google Scholar] [CrossRef]
- Alam, W.R., Srinivasan. Gabapentin induced low cortisol. Presented at Society for Endocrinology BES 2017, Harrogate, UK. Endocrine Abstracts 50 PL1 2022.
- Karbic, V.O.; Skoda, M.; Antoncic, D.; Kristofic, I.; Komar, D.; Trobonjaca, Z. Gabapentin-induced changes of plasma cortisol level and immune status in hysterectomized women. Int Immunopharmacol 2014, 23, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Kong, V.K.; Irwin, M.G. Gabapentin: a multimodal perioperative drug? Br J Anaesth 2007, 99, 775–786. [Google Scholar] [CrossRef]
- Smeland, S.; Bielack, S.S.; Whelan, J.; Bernstein, M.; Hogendoorn, P.; Krailo, M.D.; Gorlick, R.; Janeway, K.A.; Ingleby, F.C.; Anninga, J.; et al. Survival and prognosis with osteosarcoma: outcomes in more than 2000 patients in the EURAMOS-1 (European and American Osteosarcoma Study) cohort. Eur J Cancer 2019, 109, 36–50. [Google Scholar] [CrossRef]
- Takeuchi, A.; Yamamoto, N.; Hayashi, K.; Matsubara, H.; Miwa, S.; Igarashi, K.; Tsuchiya, H. Joint-preservation surgery for pediatric osteosarcoma of the knee joint. Cancer Metastasis Rev 2019, 38, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Bockbrader, H.N.; Wesche, D.; Miller, R.; Chapel, S.; Janiczek, N.; Burger, P. A comparison of the pharmacokinetics and pharmacodynamics of pregabalin and gabapentin. Clin Pharmacokinet 2010, 49, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Lizhao, C.M. , XU; Jihong, ZHOU.. Gabapentin regulates expression of corticotropin-releasing hormone in the hypothalamus through the μ-opioid receptor and ERK signaling pathway. Acta Biochim Biophys Sin 2018, 50, 1257–1263. [Google Scholar]
- Taylor, C.P. Mechanisms of analgesia by gabapentin and pregabalin--calcium channel alpha2-delta [Cavalpha2-delta] ligands. Pain 2009, 142, 13–16. [Google Scholar] [CrossRef]
- Baram, T.Z.; Hatalski, C.G. Neuropeptide-mediated excitability: a key triggering mechanism for seizure generation in the developing brain. Trends Neurosci 1998, 21, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Johannessen Landmark, C.; Henning, O.; Johannessen, S.I. Proconvulsant effects of antidepressants - What is the current evidence? Epilepsy Behav 2016, 61, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr Physiol 2016, 6, 603–621. [Google Scholar] [CrossRef] [PubMed]
- Van Bockstaele, E.J.; Valentino, R.J. Neuropeptide regulation of the locus coeruleus and opiate-induced plasticity of stress responses. Adv Pharmacol 2013, 68, 405–420. [Google Scholar] [CrossRef] [PubMed]
- Theophilus, S.C. , NR; Abrams, R; Silverman, DG; Silverman, DG.. Perioperative gabapentin and postoperative pain: a systematic review. Anesth Analg. 2009, 108, 682–687. [Google Scholar]
- Ho, K.Y.; Gan, T.J.; Habib, A.S. Gabapentin and postoperative pain--a systematic review of randomized controlled trials. Pain 2006, 126, 91–101. [Google Scholar] [CrossRef]
- Finnerty, C.C.; Mabvuure, N.T.; Ali, A.; Kozar, R.A.; Herndon, D.N. The surgically induced stress response. JPEN J Parenter Enteral Nutr 2013, 37, 21S–29S. [Google Scholar] [CrossRef] [PubMed]
- Prete, A.; Yan, Q.; Al-Tarrah, K.; Akturk, H.K.; Prokop, L.J.; Alahdab, F.; Foster, M.A.; Lord, J.M.; Karavitaki, N.; Wass, J.A.; et al. The cortisol stress response induced by surgery: A systematic review and meta-analysis. Clin Endocrinol (Oxf) 2018, 89, 554–567. [Google Scholar] [CrossRef]
- Hudec, C.P.; Griffin, C.E. Changes in the stress markers cortisol and glucose before and during intradermal testing in cats after single administration of pre-appointment gabapentin. J Feline Med Surg 2020, 22, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Gilron, I.; Bailey, J.M.; Tu, D.; Holden, R.R.; Jackson, A.C.; Houlden, R.L. Nortriptyline and gabapentin, alone and in combination for neuropathic pain: a double-blind, randomised controlled crossover trial. Lancet 2009, 374, 1252–1261. [Google Scholar] [CrossRef]
- Tiippana, E.M.; Hamunen, K.; Kontinen, V.K.; Kalso, E. Do surgical patients benefit from perioperative gabapentin/pregabalin? A systematic review of efficacy and safety. Anesth Analg 2007, 104, 1545–1556, table of contents. [Google Scholar] [CrossRef]
- Fareau, G.G.; Vassilopoulou-Sellin, R. Hypercortisolemia and infection. Infect Dis Clin North Am 2007, 21, 639–657, viii. [Google Scholar] [CrossRef]
- Cain, D.W.; Cidlowski, J.A. Immune regulation by glucocorticoids. Nat Rev Immunol 2017, 17, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Brand, Y.E.; Rufer, B. Late prosthetic knee joint infection with Staphylococcus xylosus. IDCases 2021, 24, e01160. [Google Scholar] [CrossRef]
- Scorzolini, L.; Lichtner, M.; Iannetta, M.; Mengoni, F.; Russo, G.; Panni, A.S.; Vasso, M.; Bove, M.; Villani, C.; Mastroianni, C.M.; et al. Sonication technique improves microbiological diagnosis in patients treated with antibiotics before surgery for prosthetic joint infections. New Microbiol 2014, 37, 321–328. [Google Scholar]
- Dordet-Frisoni, E.; Dorchies, G.; De Araujo, C.; Talon, R.; Leroy, S. Genomic diversity in Staphylococcus xylosus. Appl Environ Microbiol 2007, 73, 7199–7209. [Google Scholar] [CrossRef]
- Leroy, S.; Lebert, I.; Andant, C.; Talon, R. Interaction in dual species biofilms between Staphylococcus xylosus and Staphylococcus aureus. Int J Food Microbiol 2020, 326, 108653. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).