1. Introduction
Currently, the increasing prevalence of chronic metabolic disorders, with overweight, obesity and diabetes as primary factors, has become a significant public health concern in both developed and developing countries [
1,
2,
3]. Obesity, recognized for its complexity and myriad associated complications, is regarded as a major public health issue. Various factors contribute to the early onset of these pathologies, including genetic predispositions, environmental variables such as pollution, and sedentary lifestyles. However, the most prevalent cause is the imbalance between excessive caloric intake and insufficient physical activity [
4,
5]. According to The Lancet, more than 1 billion people globally are expected to face obesity by the year 2022 [
6]. Additionally, there has been a doubling in the number of overweight cases worldwide since 1990 and a fourfold increase in the number of children aged 5 to 19 affected by this condition [
6]. In 2019, European countries reported that between 40-65% of their populations were diagnosed with overweight [
7].
In addition to the global statistics, it has been observed that ethnic and racial minority groups present an additional risk of overweight and type 2 diabetes, thereby contributing to an increased risk of cardiovascular diseases [
8,
9,
10]. The diagnosis of obesity is more frequent among women, with the highest increases reported among black populations [
11]. Although all populations show an increase in the prevalence of obesity, the development of type 2 diabetes as a complication of obesity at a lower BMI (body mass index) value has been observed in Non-Hispanic Blacks (NHB) compared to Non-Hispanic Whites (NHW) [
10,
11,
12,
13]. This disparity is mainly attributed to significant differences in diet quality and eating habits [
14,
15,
16].
Lately, there have been significant advances in understanding the pathophysiological mechanisms underlying the development of excess weight. Despite these substantial research efforts, these findings have yet to be widely applied in clinical practice, where obesity continues to be diagnosed and treated in a general manner [
17]. The factors leading to obesity are multiple and interconnected, encompassing both genetic and environmental influences, with many being modifiable: food consumption, physical activity, social and individual psychology [
18,
19,
20]. Moreover, it has been observed that urbanization, wide access to high-calorie and refined foods, and a sedentary lifestyle are the main contributors to excess weight [
1,
20]. From a nutritional standpoint, excess weight has been correlated with various eating patterns, such as meal frequency, meal volume or quantity, types of snacks, the removal of breakfast as the main meal, and overall diet quality [
21,
22]. Among these factors, the most recognized and significant contributor to the current obesity epidemic is quantity of food intake, which disrupts the energy balance [
23]. A diversity of foods and nutrients have been identified and associated with both the risk of obesity and the management of this pathology [
24,
25,
26,
27].
The daily and conscious eating habits of an individual, influenced by cultural and social factors, include the amount, type, and frequency of food consumed and are collectively defined as “eating patterns” [
28,
29,
30,
31]. Given that unhealthy food choices and, consequently, poor diet quality predominantly contribute to excess weight, identifying and analyzing food patterns is currently the most effective method for evaluating the influence of general eating routines among people with obesity [
20]. Additionally, this approach allows for the association of dietary patterns with nutritional status variables, such as anthropometric measurements and cardiometabolic markers [
32,
33]. Increased portion sizes and the frequency of hypercaloric foods are primary precursors of energy imbalance, and thus, obesity [
29]. Accurately quantifying food patterns can be challenging due to differences in perception, including gender differences [
34]. Moreover, assessing a person’s dietary pattern provides greater insights compared to evaluating daily individual macronutrient intake [
35].
Although the report justifying the value of the most widely used anthropometric indicator, BMI, was proposed approximately 200 years ago, it was only 50 years ago that a concrete definition was implemented for this concept [
36,
37,
38]. This delay resulted from prolonged debates in the field of research to confirm that BMI is a useful and accurate tool for diagnosing overweight and obesity [
36,
39]. Therefore, to evaluate the nutritional status of an adult, BMI is the most commonly used parameter, as it defines the excess of adipose tissue with generalized distribution [
40]. Despite its widespread use, uncertainties persist regarding the relationship between BMI and mortality risk [
39]. Nonetheless, studies have identified associations between BMI and both general and cardiovascular mortality risks, with stronger correlations observed among young adults and more significant associations in men compared to women [
42,
43]. A significant limitation of BMI is its inability to provide detailed information about the distribution and percentage of adipose tissue in the body [
43,
44,
45]. Moreover, this parameter can provide misleading information in various situations, such as during different stages of life like childhood and adolescence, for performance athletes, or during the dynamic monitoring of a weight loss process [
46]. Additionally, the percentage of adipose tissue varies by sex and age, regardless of BMI [
44]. However, a universal definition of adult obesity based strictly on the percentage of adipose tissue has not been established [
47,
48]. The fat mass index (FMI) is a more precise indicator for evaluating body fat, but its interpretation is also limited [
47,
48,
49,
50]. Specific thresholds for FMI values have not been identified, unlike the percentage of adipose tissue used for diagnosing obesity [
51,
52]. Conversely, the percentage of adipose tissue determined by electrical bioimpedance techniques can fall within optimal limits based on age, sex, and ethnicity, as indicated by the device-specific reference charts for both adults and children [
53,
54,
55].
Obesity is known to negatively impact quality of life and life expectancy. However, the risk of developing complications varies significantly among individuals, a variation that cannot be fully explained by BMI or the degree of adipose tissue alone [
56]. Metabolically healthy obesity (MHO) is a well-recognized condition, with a prevalence between 10-30%, and is influenced by factors such as gender and age [
56,
57]. While there is no standard definition of MHO, it is characterized by a lower cardiometabolic risk compared to metabolically unhealthy obesity (MHU) [
58,
59,
60]. Despite the significant role of adipose tissue distribution in defining obesity subtypes, MHO remains a transient phenotype. Therefore, nutritional intervention aimed at weight loss is crucial for MHO individuals, as the presence of excess adipose tissue is more critical than its distribution when compared to normal-weight individuals [
56].
Adipose tissue, due to its complexity and various functions—such as mechanical protection, lipid storage, thermogenesis, and regulation of systemic energy and nutrient homeostasis—is considered a metabolically active endocrine tissue with a remarkable ability to change size in response to different factors [
44,
61,
62,
63]. This dynamic nature of adipose tissue underscores the importance of comprehensive methods for evaluating body composition and health risks beyond BMI alone. This component belongs to the endocrine system, being essential in regulating homeostasis [
64,
65]. Consequently, its distribution, especially at the abdominal level, is a critical factor for the development of metabolic syndrome, type 2 diabetes and, implicitly, for the increase in cardiovascular risk [
66].
With the advent of advanced technology, it is now possible to accurately determine not only the total amount but also the distribution of adipose tissue, both in percentage and in kilograms. Various devices have been developed and validated to assess the percentage of adipose tissue, including plethysmography, dual-energy X-ray absorptiometry (DXA), computed tomography (CT), and magnetic resonance imaging (MRI) [
67,
68]. However, the most commonly used technique in recent years is BIA [
69]. Compared to other methods of quantifying body composition, BIA offers several advantages: it is easy to use, relatively low-cost, non-invasive and can be applied to most individuals regardless of age [
70]. These advantages make BIA a practical and accessible tool for widespread use in clinical and research settings.
Therefore, the aim of this study is to identify and analyze the impact of a wide range of eating habits and various medical conditions on body weight, as well as the potential risk factors contributing to overweight. This will be achieved using two different classifications of nutritional status: the BMI value and the percentage of adipose tissue, assessed through electrical bioimpedance. These models were constructed to identify and quantify the impact of various predictors on the probability of obesity. By contrasting the outcomes derived from the BMI-based and BFP-based models, this study seeks to highlight the similarities and differences in obesity risk factors as determined by these two classification systems. This comparative analysis offers a more detailed understanding of how different measures of body composition may influence the identification of obesity-related risk factors.
2. Materials and Methods
The prospective observational study was conducted over approximately four years, from July 2020 to June 2024, in our endocrinology unit. The total cohort comprised 1255 adults, 471 males (34%) and 927 females (66%), with a mean age of 36 ± 11.90, who were willing to assess their eating habits and undergo a comprehensive evaluation of their nutritional status with the aim of lifestyle modification. All participants provided informed consent. The study adhered to the ethical standards of the Helsinki Declaration and was approved by the Scientific Research Ethics Committee (CECS) of the “Victor Babeș” University of Medicine and Pharmacy Timișoara (No. 69/03.10.2022). To allow a complex comparative analysis of the final set of evaluated data, the entire group was divided as follows:
Based on the
nutritional status evaluation, the cohort was divided into three subgroups according to BMI values [
71,
72]:
The control group (normal weight, BMI 18-24.9 kg/m²), which consisted of individuals with no family or personal medical history of metabolic and cardiovascular diseases.
The overweight group: BMI 25-29.9 kg/m²
The obese group: BMI ≥ 30 kg/m².
Based
on age and sex, independent of BMI value, according to the severity of excess adipose tissue [
53,
54], as follows:
- ▪
-
Normal weight women with distribution of adipose tissue according to age:
20-39 years (21-32.9% adipose tissue)
40-59 years (23-33.9% adipose tissue)
60-79 years (24-35.9% adipose tissue)
- ▪
-
Normal weight men with distribution of adipose tissue according to age:
20-39 years (8-20% adipose tissue)
40-59 years (11-22% adipose tissue)
60-79 years (13-25% adipose tissue)
- ▪
-
Overweight women with distribution of adipose tissue according to age:
20-39 years (33-38.9% adipose tissue)
40-59 years (34-39.9% adipose tissue)
60-79 years (36-42% adipose tissue)
- ▪
-
Overweight men with distribution of adipose tissue according to age:
20-39 years (adipose tissue)
40-59 years (adipose tissue)
60-79 years (adipose tissue)
- 3.
Obesity Group (excess adipose tissue):
- ▪
-
Women with excess distribution of adipose tissue according to age:
20-39 years (adipose tissue)
40-59 years (adipose tissue)
60-79 years (adipose tissue)
- ▪
-
Men with excess distribution of adipose tissue according to age:
20-39 years (adipose tissue)
40-59 years (adipose tissue)
60-79 years (adipose tissue)
2.1. Patient Inclusion and Exclusion Criteria
Inclusion Criteria: Participants were required to be adults, both men and women, with overweight or obesity, who have maintained the same residence for at least five years. They must have accurately completed a comprehensive questionnaire regarding eating habits, lifestyle habits, and personal and family history of cardiometabolic diseases. Additionally, a control group of normal-weight individuals without personal or family medical history of metabolic or cardiovascular conditions was included. Only those who consented to a thorough anamnestic and clinical evaluation by signing the informed consent form were included in the final assessment.
Exclusion Criteria: Patients with secondary obesity, regardless of its etiology (e.g., endocrinological conditions such as hypothyroidism or Cushing’s syndrome; genetic disorders like Prader-Willi syndrome; or iatrogenic causes such as glucocorticoid or insulin therapy within tha past 12 weeks) were excluded from the study [
73,
74,
75]. Additionally, subjects who had followed a hypocaloric diet or received anti-obesity treatments (e.g., liraglutide, semaglutide, Orlistat, Bupropion/Naltrexone) within the past 16 weeks were excluded [
73]. Individuals with documented psychiatric conditions and children were also not included in the research.
2.2. Patient Complete Evaluation:
Each participant was thoroughly informed about the stages of the study, including anamnesis, clinical and paraclinical evaluation, and provided with informed consent documentation. A detailed anamnesis was conducted for each patient, which encompassed the food survey, personal medical history of documented cardiometabolic pathologies, and family history, with particular attention to the presence of these conditions among first-degree relatives. Body analysis by bioimpedance was employed as the primary non-invasive method to estimate segmental body composition. Consequently, the following parameters were included in the initial evaluation of the participants:
Body Weight Measurement: Body weight was assessed using a mechanical scale with metrological certification, capable of measuring up to 200 kg. Participants were instructed to stand in a vertical posture on the scale while wearing minimal clothing.
Height Measurement: Height was measured using a calibrated wall-mounted stadiometer. Participants were instructed to stand in a vertical posture on the platform without wearing shoes.
Nutritional Status: The nutritional status of each participant in our study was assessed using BMI, a widely utilized, cost-effective parameter. The BMI was calculated using the formula: BMI = weight (in kg) / height² (in m²) [
71,
72], as previously mentioned.
Family medical history and conditions: A detailed family medical history focusing on cardiometabolic pathologies was obtained from each participant. The pathologies of interest, particularly concerning first-degree relatives, included obesity, overweight, type 2 diabetes, stroke, essential hypertension, and acute myocardial infarction.
Personal medical history and conditions: Similar to those mentioned previously, a pre-announced evaluation through directed questioning of the personal medical history was conducted individually for each participant. The primary pathologies emphasized were within the cardiovascular and metabolic categories, including diagnosed essential hypertension or use of antihypertensive treatment, prediabetes, type 2 diabetes, metabolic syndrome, lipid profile alterations, and asymptomatic hyperuricemia. Additionally, for patients who were undergoing insulin treatment in the past (more than 6 months), potential weight gain following its initiation was assessed. Among women, other parameters of interest included in the anamnesis were weight gain postpartum, diagnosis of polycystic ovary syndrome (regardless of subtype), daily use of oral contraceptives, onset of menopause, preclimax, and increased appetite during the premenstrual period. Regardless of gender and medical history, other significant factors addressed were previous weight loss, food intolerances, and weight gain after smoking cessation.
Demographic and lifestyle factors: In this category, parameters associated with the daily routine were also included due to the complexity of the anamnesis:
- ◦
Cigarette smoking status: this was defined as smoking at least one cigarette every day for more than a year.
- ◦
Alcohol consumption: To quantify alcohol consumption, participants reported the number of units of alcohol consumed (equivalent to 10 ml of pure ethanol) via self-reporting. The units were defined as follows: two units equated to a pint or can of beer, one unit to a 25 ml shot of hard liquor, and one unit to a standard 175 ml glass of white or red wine. Participants consuming more than two units of alcohol daily were categorized as “alcoholic,” while those who had never consumed alcohol were classified as “non-alcoholic” [
76].
- ◦
Physical activity level: To be excluded from the sedentary category, it was necessary to confirm a sustained physical effort of at least 30 minutes per day or 150 minutes per week (activity level > active plus basal).
- ◦
Sleep schedule: The duration of sleep for each subject was assessed, with a nightly duration of less than 7 hours being classified as sleep deprivation or an insufficient sleep schedule [
77].
Eating habits and preferences: The dietary habits of participants were meticulously documented from multiple perspectives. Key criteria included: daily breakfast consumption, adherence to the three main meals of the day, and the inclusion of two main courses at lunch. Additionally, the focus was placed on portion sizes relative to individual energy requirements, snacking between meals, the need for additional servings due to reduced satiety, and daily intake of fruits and whole foods. Eating habits were further categorized by quality, such as the consumption of home-cooked meals, dining at restaurants, fast-food intake, and dessert consumption. The study also examined the quantity of non-caloric clear liquids consumed, specifically plain water, as well as coffee and dairy consumption.
Psychological and emotional factors: Finally, psychological and emotional factors were assessed through targeted questions during the anamnesis, similar to the previous sections. The focus was on the presence of overeating or excessive eating triggered by negative and positive emotions, including eating for pleasure, as a reward, and secondary to loneliness, psychological stress, fatigue, or boredom.
2.3. Bioimpedance Body Analyzed Variables
All subjects included in the study underwent an initial examination of their nutritional status using body bioimpedance analysis with the Tanita Body Composition Analyzer BC-418 MA III device (T5896, Tokyo, Japan). This analysis focused particularly on the percentage and distribution of adipose tissue. This involved a detailed analysis of the complete body composition utilizing a constant high-frequency current source (50 kHz, 500 μA) and employing a tetra-polar eight-point tactile electrode system. Participants were instructed to maintain an upright posture and grasp the analyzer’s handles to ensure contact with a total of eight electrodes, two for each foot and hand [
78]. During the bioelectrical impedance analysis, a low-level electrical current passed through each participant’s body, and impedance (resistance to the current flow) was measured [
79]. The entire procedure lasted approximately three minutes, and the results were thoroughly explained and recorded for each patient. Based on the results, the analyzed parameters were categorized into:
Current weight or weight at the time of examination (kg)
Metabolic basal rate (BMR) (kcal)
Percentage of adipose tissue (%)
Percentage of lean mass or muscle tissue (%)
Percentage of total body water or hydration status (%)
The following personal information was collected and entered into the operating system of the instrument model used: identification data, gender, birth date and height (cm). Upon entering the personal data for each patient, the instrument’s operating system automatically generated the BMR values.
2.4. Statistical Analysis
Numerical variables, based on their distribution type, were presented as median and interquartile range, while categorical variables were presented as frequency and proportions. The normality of distributions was assessed using the Shapiro-Wilk test, with a p-value < 0.05 indicating a non-Gaussian distribution. The test indicated non-normality for all numerical variables; hence, non-parametric methods were employed. To investigate differences between numerical variables, the Mann-Whitney U test was used. For exploring statistically significant differences between categorical variables, the Pearson Chi-square test was employed. To identify risk factors for obesity, multivariate logistic regression was used. The Nagelkerke R² was used to assess model quality. The ROC curve parameters, including specificity, sensitivity, accuracy, and AUC, were utilized to compare the two models. The results were presented in both tabular and graphical formats. The statistical analysis was performed using R (R Core Team, 2024), a language and environment for statistical computing provided by the R Foundation for Statistical Computing, Vienna, Austria. A p-value < 0.05 was considered statistically significant, with a 95% confidence interval.
4. Discussion
The diversity of eating habits, lifestyle factors, psychological and emotional influences, and genetic components, all contribute significantly to an individual’s nutritional status, impacting the distribution of adipose and lean tissue masses. Obesity remains a contentious topic, often criticized for the limitations in its identification and classification. The importance of understanding these factors is critical for developing effective obesity management strategies. Although BMI has long been used as a standard measure to classify individuals based on their weight relative to height, it fails to account for the distribution and composition of body fat, which are crucial for understanding the health risks associated with obesity [
80]. Contrary to expectations, the present study underscored the importance of BMI determination in clinical practice among overweight and obese individuals. While recognizing BMI’s limitations, our findings highlight its utility in providing a quick, accessible, and general indication of obesity, which can be essential for initial screenings and epidemiological studies. The implications of these findings are particularly relevant for nutrition clinicians, offering valuable insights for enhancing nutritional counseling and long-term monitoring of the nutritional status of overweight individuals. This approach can aid in developing personalized intervention strategies that address both dietary and lifestyle factors contributing to obesity. Furthermore, the study emphasizes the necessity of a comprehensive dietary assessment to identify the underlying factors leading to excess weight. This assessment should be part of a multidisciplinary team approach, allowing for the treatment of potential causes of obesity from various perspectives. Additionally, our research highlights the impact of different obesity classification models on the nutritional status of adults, regardless of their body weight, demonstrating that both BMI and adipose tissue measurements should be considered for a more accurate evaluation and management of obesity.
Furthermore, alongside modifiable risk factors for excess weight, such as dietary habits, several other parameters contribute to weight gain in individuals. Age is a prominent, yet immutable risk factor for overweight, primarily due to the progressive decline in basal metabolic rate with advancing age and the concomitant reduction in physical activity levels [
81,
82]. Additionally, aging is associated with a redistribution of adipose tissue, favoring visceral fat accumulation over subcutaneous fat [
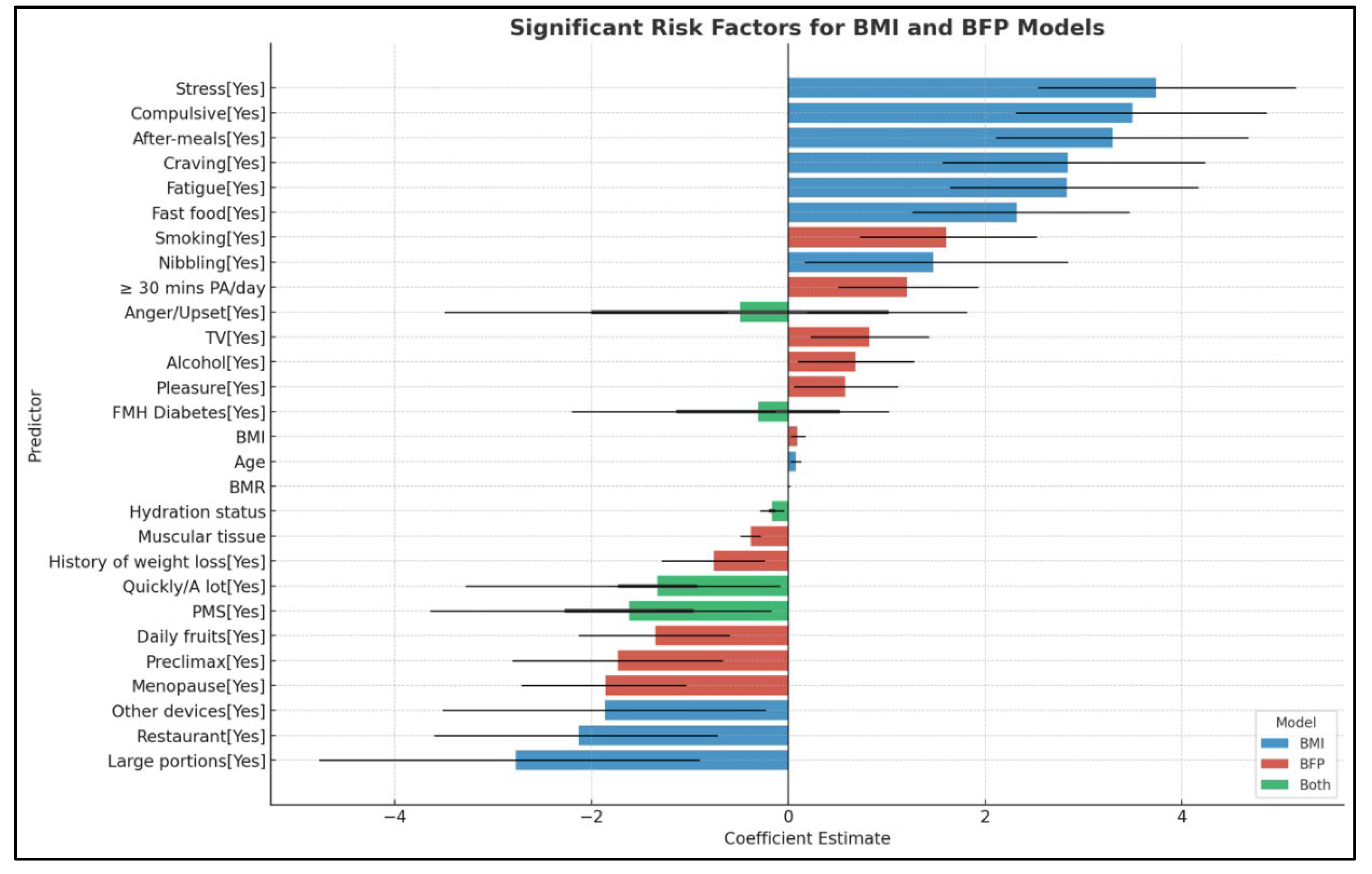
83]. In our study, statistically significant differences were observed between the study and control groups in both the BMI-based and BFP-based models (p<0.001). Notably, in the BFP-based model, an increase in age was directly proportional to the degree of adiposity, contrasting with the BMI-based model, where younger ages were predominantly in the overweight category. However, age emerged as a predictor for obesity onset exclusively in the BMI-based model.
Measurements were conducted under comparable levels of hydration to avoid misinterpretation, as BIA is particularly sensitive to total body water. This method was utilized to estimate total body water, fat mass percentage, muscle mass and total body water [
84,
85]. In the context of its application in sports and medicine, the raw BIA variable of phase angle, representing the ratio of resistance to reactance, has gained prominence and is provided by certain BIA devices [
85]. Numerous studies have demonstrated the reliability of both single-frequency and multi-frequency BIA instruments, concluding that BIA can serve as a substitute for DXA in the analysis of whole-body and segmental body composition in large populations [
86,
87]. Recently, the association BMR and muscle tissue has been confirmed, both identified through body composition analysis [
3,
88]. Sarcopenia, the decline in muscle mass, frequently coexists with excess weight, especially among the elderly, representing an age-related abnormality [
3,
88,
89]. The reduction in BMR is linked to excess weight, while variations in hydration status or total body water have been observed in both children and individuals with obesity [
90,
91,
92]. Additionally, eating habits have been shown to negatively impact the percentage of adipose tissue, BMR, and hydration status [
93,
94]. In the current study, significant differences were observed in the parameters evaluated by BIA among the normal weight, overweight, and obese groups in both the BMI-based and BFP-based models. The highest percentage of adipose tissue was found in the obesity group, while the highest percentage of muscle tissue was attributed to the control group. BMR was comparable between the obese and normal weight groups but significantly lower in the overweight group. However, predictors for the development of obesity were identified only in the BFP-model. Specifically, an increased percentage of muscle tissue was found to be protective, reducing the chances of obesity by 32%. Additionally, a higher percentage of total body water also proved to be protective. BMR showed a small but significant increase in the odds ratio per unit (OR: 1.01, CI: 1.00-1.01, p<0.001).
Various factors, some more extensively researched than others contribute to the development of obesity. Physical activity, defined as “any type of body movement performed by skeletal muscles that results in energy expenditure”, plays a critical role [
95]. Insufficient physical activity is linked to the onset of obesity, reduced cardiovascular fitness from childhood, and the development of various chronic cardiometabolic conditions in adulthood [
96,
97]. Consequently, a sedentary lifestyle is recognized as a significant contributor to obesity [
96,
98]. Additionally, regular physical activity has been associated with improvements in body composition and reductions in insulin resistance among adults [
99,
100]. Similar findings were observed in our study. In both evaluated models, the recommended physical activity of at least 30 minutes per day decreased proportionally with increases in BMI and adipose tissue percentage, being lowest in the group with obesity. The differences between the groups were significant (p<0.001). Furthermore, a decrease in physical activity duration to less than 30 minutes per day was identified as a predictive factor for obesity development only in the BFP-based model.
There are discernible differences in dietary patterns and eating behaviors concerning the quality and quantity of food by gender [
101]. Gender-specific differences are also evident in the complications associated with obesity. A higher prevalence of obesity is typically observed among women, whereas men are more likely to develop metabolic complications secondary to excess weight [
102,
103]. Our study aligns with previous observations indicating that the prevalence of obesity is higher among women compared to men, with these gender differences being evident in most regions worldwide [
104,
105]. The type of adipose tissue expansion also varies by gender [
104]. Although the overall obesity rate is 10% higher in women than in men, women tend to have a higher percentage of visceral fat [
106,
107,
108]. In the current study, a significantly higher percentage of women was observed in the overweight (79%) and obesity (68%) groups within the BMI-based model. Similarly, in the adiposity-based model, 76% of the overweight group and 65% of the obese group were women. These findings indicate a higher prevalence of excess weight among females, with statistically significant differences (p<0.001). However, gender was not identified as a risk factor for the development of obesity in the logistic regression analysis.
An association has been observed between excess weight and functional as well as structural changes in the brain’s reward system [
109,
110]. The consumption of hypercaloric and palatable foods in excess can trigger reward phenomena [
104]. Eating behaviors and preferences vary according to gender and age [
104,
111]. Cross-sectional studies have shown that women consume more fruits compared to men [
111,
112]. Additionally, women tend to prefer sweet and easily accessible snacks such as candies, whereas men are more likely to opt for fast food items like pizza [
113]. It has also been observed that men tend to eat more quickly, while women may eat uncontrollably, even in the absence of hunger [
114]. These differences highlight the need for gender-specific approaches in dietary interventions and obesity management. In the current study, various dietary behavioral factors were identified as predictors of weight gain and obesity, with both similarities and differences observed between obesity classification based on BMI and that based on the percentage of adipose tissue. This comparative approach provided a more comprehensive understanding of how different body composition measures can influence the assessment and identification of obesity risk factors. Specifically, daily consumption of fast food, snacking immediately after the main meal, nibbling, and experiencing cravings significantly increased the risk of obesity in the BMI-based model. Conversely, behaviors such as quickly eating, dining in social settings like restaurants, and consuming larger portions were identified as compensatory behaviors and were not associated with an increased risk of obesity in the same model. All these eating behaviors showed statistically significant differences between the evaluated groups (p<0.001). In contrast, the adiposity-based model identified different predictive factors for excess of adipose tissue. For instance, watching TV during meals increased the risk of obesity, while daily fruit consumption was a protective factor. Additionally, eating quickly, on the run, did not predict the development of obesity in either model.
Both diet and psycho-affective factors play crucial roles in maintaining quality of life and preventing chronic diseases. Emotional eating, a problematic eating pattern, often negatively impacts eating decisions and is associated with various degrees of obesity and the emotional factors underlying its etiology [
115]. Adult women are most frequently affected by emotional eating [
116,
117], which is linked to both psychological state and nutritional status [
118]. Emotional eating results from an accumulation of emotions and behaviors associated with eating, rather than being considered a separate eating disorder [
118,
119]. Amplification of negative emotions plays a significant role in the onset and progression of obesity, demonstrating a bidirectional correlation between these entities [
120,
121]. Through a detailed anamnesis, our study identified psycho-emotional predictive factors for the development of obesity. Significant differences were observed between the normal weight, overweight, and obesity groups in terms of stress, fatigue, compulsive eating, reward, and pleasure. Notably, most of these factors were risk factors for obesity only in the BMI-based model. Eating for pleasure was the only predictive factor within the adiposity-based model, while upset/anger eating was protective in the BMI model but a risk factor for obesity in the adiposity-based model. These findings highlight the complex interactions between different models of body composition and their influence on the risk of developing obesity from distinct perspectives.
The current research also focused on identifying and analyzing parameters specific to females. In the BMI-based model, significant differences were observed among the control, overweight, and obese groups concerning menopause (p<0.001). Conversely, in the BFP-based model, these differences were not significant (p=0.15). Thus, menopause was identified as a risk factor for increased BMI but not for excess adipose tissue. However, literature indicates that menopause is associated with changes in body composition, particularly an increase in the percentage of visceral adipose tissue, which amplifies cardiometabolic risk and deteriorates the quality of life among women [
122]. This is primarily due to the absence of estrogen hormones during menopause, which significantly contributes to weight gain [
123]. The reduction in circulating estrogens is also associated with a redistribution of adipose tissue, decreasing subcutaneous fat and increasing abdominal fat [
124]. Additionally, it has been observed that the rate of developing obesity during menopause is three times higher compared to the pre-menopausal phase [
125]. Therefore, in the present study, statistical differences were reported between the evaluated groups concerning pre-menopause diagnosis. Thus, pre-menopause was not noted as a risk factor for increased adiposity or BMI. Further research is necessary to clarify how hormonal fluctuations during menopause influence fat distribution, metabolic rate, and overall weight gain. Additionally, the synergistic effects of aging and menopause on these processes warrant more extensive investigation to inform the development of targeted interventions aimed at mitigating the associated health risks. Excess weight and the use of COCs are independent cardiovascular risk factors. Their concurrent use among women significantly increases the risk of pulmonary thromboembolism, with risk estimates ranging from 12 to 24 times higher [
126]. In the current study, the use of COCs was not identified as a risk factor for the development of obesity. However, differences were observed between the groups; the overweight group had the highest percentage of COC use (7%) in both evaluated models.
The duration of sleep plays a crucial role in predicting cardiometabolic risk. Adequate sleep is crucial for maintaining cardiovascular health and metabolic function, as insufficient sleep has been associated with an increased risk of hypertension, obesity, diabetes mellitus, and cardiovascular disease (CVD) [
127]. Short sleep duration (less than 7 hours per night) has been linked to an elevated risk of cardiometabolic diseases [
128]. In particular, short sleep duration can lead to alterations in glucose metabolism, increased appetite, and reduced insulin sensitivity [
129]. In our study, the normal weight group exhibited a significantly longer sleep duration compared to the overweight and obese groups in both evaluated models, with statistically significant differences (p<0.001). However, a sleep duration of less than 7 hours per night was not identified as a predictor for obesity in this analysis.
Both overweight and smoking status are significant factors for cardiovascular risk [
130]. Quitting smoking reduces cardiovascular risk, but it is often accompanied by increased appetite and subsequent weight gain [
131]. While weight gain is a common consequence of smoking cessation, the health benefits of quitting smoking far outweigh the risks associated with moderate weight gain. Nicotine, the primary addictive substance in cigarettes, increases metabolic rate. When smoking is discontinued, the metabolic rate decreases, leading to fewer calories being burned at rest [
132]. Research indicates that quitting smoking results in an average weight gain of 4-5 kilograms within the first year, though this can vary widely among individuals [
133]. Interestingly, despite the weight gain following smoking cessation, cardiovascular risk was still reduced compared to those whose weight remained constant [
134]. In the present study, smoking was more prevalent among individuals with normal weight, confirming the relationship between lower weight, higher metabolic rate, and smoking status. Additionally, both the BMI-based and BFP-based models showed a significantly reduced percentage of weight gain after quitting smoking. However, smoking status, specifically smoking at least one cigarette daily for more than a year, was identified as a predictive factor for obesity when classified according to the percentage of adipose tissue.
Similar to smoking, alcohol consumption is another harmful factor within the category of lifestyle choices. The impact of alcohol consumption on nutritional status is influenced by several factors, leading to significant inter-individual variations [
135]. Cross-sectional studies have found that alcohol consumption is not consistently associated with BMI, regardless of gender [
135,
136]. However, gender differences have been observed, with a stronger association between alcohol consumption and BMI among men. This disparity is primarily attributed to the quantity and type of alcohol consumed [
137]. The current results identified significant differences in alcohol consumption between the control group and the overweight and obese groups (p<0.001), with consumption being higher among obese individuals in both evaluated models. Furthermore, alcohol consumption was identified as a risk factor for excess adipose tissue in the BFP-model.
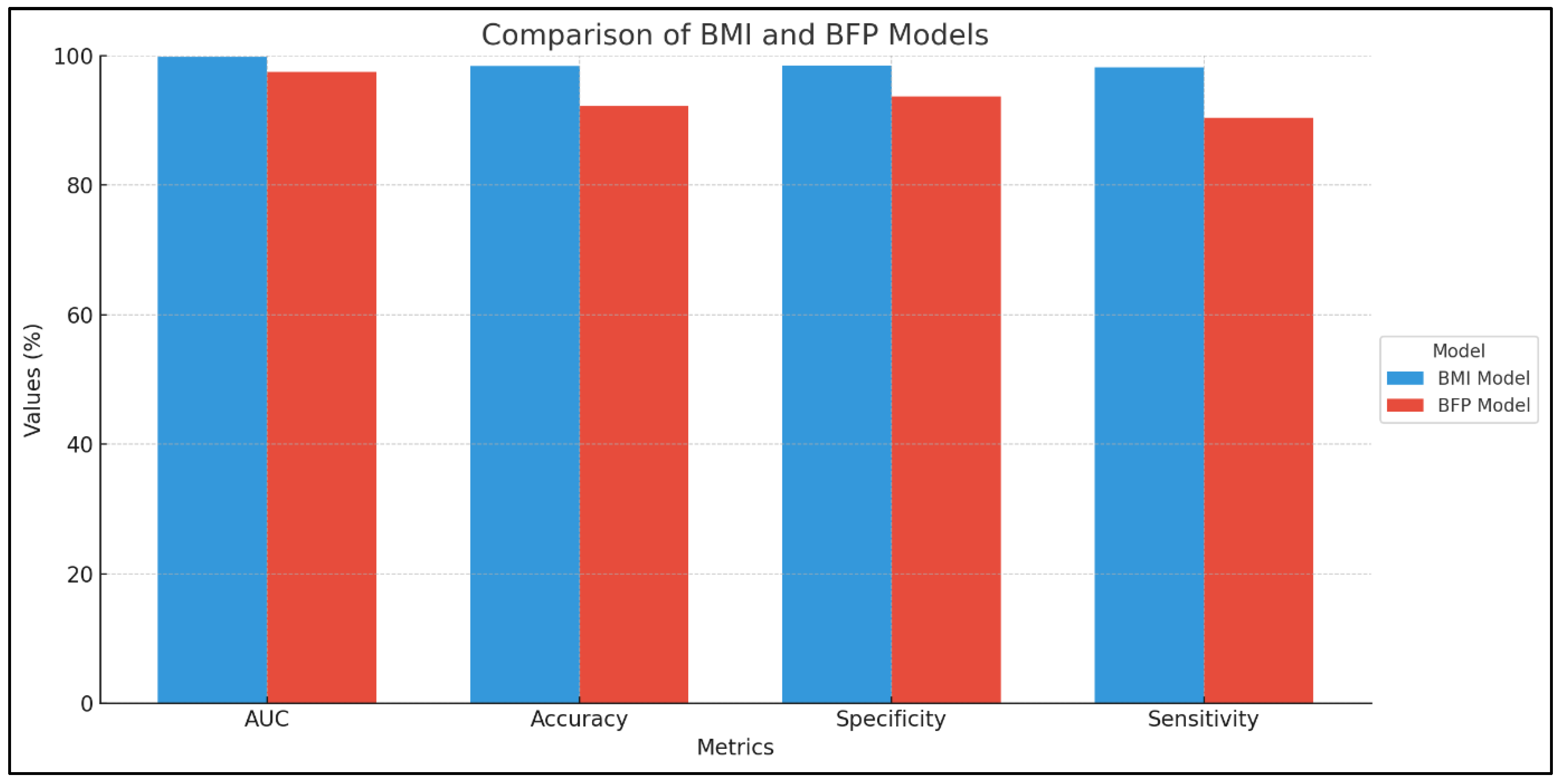
According to the results, several risk factors were identified in both models—namely, BMI value and percentage of adipose tissue. However, certain predictors were associated only within a single model. This indicates that both models complement each other in the comprehensive assessment of an adult’s nutritional status. Furthermore, the comparative analysis of these models highlighted important differences in their predictive performance regarding various behaviors and eating patterns. The BMI-based model demonstrated an almost perfect discrimination between individuals with and without obesity, with an accuracy of 0.983, a specificity of 0.985, and a sensitivity of 0.982. These findings suggest that while each model has its strengths, their combined use provides a more holistic understanding of obesity-related risk factors.
Despite the promising results, there are several limitations associated with this study. While no other study has approached this subject with such precision, these findings must be interpreted within the context of these limitations. The study does not account for ethnic, cultural, and regional differences in the evaluated parameters, which negatively impacts the generalizability of the findings to populations with different eating habits compared to the evaluated groups. Another limitation is the use of the bioimpedance body analyzer, a device that may not be accessible to all clinicians for evaluating nutritional status and body composition, unlike the more readily available measurement of BMI. Additionally, the study did not aim to correlate the scores obtained from validated food and eating behavior questionnaires but rather to identify possible predictive factors for obesity from the perspective of two different classifications in a significant group of subjects. Furthermore, eating habits and their impact on nutritional status can change over time, an aspect that was not dynamically monitored in this study. Therefore, this research represents a preliminary step toward a more comprehensive approach in the field of nutrition and obesity research.