1. Challenges of Chronic Hepatitis B Treatment
Chronic hepatitis B (CHB) infection, as highlighted by the World Health Organization (WHO), plays a pivotal role in the pathogenesis of hepatocellular carcinoma (HCC) and liver cirrhosis (LC), resulting in significant morbidity and mortality. Despite recent success in curing chronic Hepatitis C infection, a safe and effective cure for CHB remains elusive. Current therapeutic limitations pose substantial obstacles to achieving the WHO’s ambitious goal for 2030 of reducing viral hepatitis-related mortality by 65% [
1,
2].
Considerable efforts have been directed toward developing CHB cures. Therapies aimed at achieving a “functional cure” attempt to achieve sustained undetectable levels of HBsAg (hepatitis B surface antigen) and HBV DNA have mainly proved unachievable. Thus, the concept of a “partial HBV cure” remains relevant. A partial HBV cure would involve still detectable HBsAg levels but persistently low or undetectable HBV DNA in serum after completing treatment. Achieving a partial cure where normal ALT/AST levels, inactive liver disease, and favorable clinical outcomes contribute to disease remission could represent a step toward a complete HBV cure [
2,
3].
Current CHB therapies have failed to induce sustained viral suppression of treatment and have not succeeded in inducing HBsAg loss and anti-HBsAg seroconversion. Additionally, their prolonged use can cause kidney and bone complications. Real-life irregular use of nucleotide (s)ide analogues (NUCs) [
4] and the residual risk of HCC in patients under NUC treatment are also matters of concern [
5].
Therapeutic CHB vaccines have been under development for decades. They aim to leverage the immune system to control CHB infection. Their low cost would make them suitable as potential first-line treatments or add-ons, allowing discontinuation of NUCs. Such an approach could expand the number of patients achieving a CHB functional cure or partial cure.
This review focuses on the limitations of current CHB treatments and emphasizes the need to continue to look for an effective vaccine against CHB [
6]. The goal is to understand the potential and limitations of therapeutic vaccines and to discuss the future of therapeutic vaccination.
The importance of controlling HBV extends beyond just liver cancer, as shown in a prospective study that followed about 4 million individuals in Korea for 8 years [
7]. In this study CHB infection was found to not only increase the risk of liver cancer, but it was also associated with a heightened risk of multiple extrahepatic cancers (including hematologic, gallbladder, pancreas, stomach, lung, colorectal, and thyroid). These further underscores the broad impact of this chronic infection.
2. Current Treatments for CHB: A Systematic Comparison
2.1. Safety and Tolerability
IFN-mediated treatments are not recommended for patients with low ALT levels [
8]. This recommendation arises from the lower efficacy of IFN products in patients with lower ALT levels [reviewed in [
9]] and the extensive list of adverse reactions associated with interferon and PegIFN therapies. Many of these reactions have the potential for severity, necessitating special monitoring by healthcare personnel. In this context, the risk-to-benefit balance does not favor enrolling patients with low ALT levels unless other variables, such as family history or increased liver inflammation or fibrosis, are detected [
8].
On the other hand, NUCs are not recommended for CHB patients with low viral loads (under 104 copies/mL), even when they are well-tolerated oral pills. Their chemically mediated HBV suppressor effect does not offer additional benefits in this setting. Physicians typically recommend using NUCs in more advanced stages of the disease, considering that lifelong intake may lead to bone and kidney problems and other conditions that may develop after several years.
HeberNasvac has been developed as an alternative for first-line CHB treatment. Its attractive safety profile compared to PegIFN was demonstrated in the phase II/III trial [
6]. It follows a finite, dedicated, relatively short treatment schedule of 10 immunizations over 20 weeks. The regimen includes a first cycle of five intranasal (IN) administrations every 2 weeks, followed by a second cycle of five IN/subcutaneous (SC) immunizations one month later with a dose of 100 μg per antigen (HBcAg and HBsAg).
PegIFN is a finite treatment administered via the SC route for 48 weeks but with considerably higher reactogenicity. During the Phase II/III study, 2.5% of the patients were excluded due to early signs of decompensation. A similar proportion abandoned treatment because of adverse reactions. None of the patients in the HeberNasvac-treated group required discontinuation [
6]. In addition, there is still room to optimize HeberNasvac into an exclusively IN product. Novel formulations are currently under preclinical and clinical development and will be introduced subsequently.
2.2. Antiviral Efficacy and Liver-Protecting Effect
The ability to inhibit the replication of HBV and reduce viral loads to low or undetectable levels is a significant variable. It is associated with the normalization of transaminases (ALT) and reduced disease progression. PegIFN has shown significant levels of antiviral effect during treatment, but NUCs are the products with more potent antiviral activity. However, the antiviral effect is often reduced after treatment cessation in both cases. Virus-induced biochemical exacerbations are frequently observed after stopping NUCs or PegIFN. Despite this, in real-life settings, the discontinuation of both therapies is a common occurrence. Considering the long-lasting duration of NUC treatment and the reactogenicity of PegIFN, a high level of patient commitment to managing the disease is required. Financial reasons also play a role in the decision to stop CHB treatment.
HeberNasvac demonstrated a similar antiviral effect at the end of treatment (EOT) compared to the end of PegIFN treatment; however, 6 months later, a sustained antiviral effect was detected in the HeberNasvac-treated group [
6]. Subsequently, a generalized trend of HBV reduction over time was detected [
10,
11], motivating an in-depth study of action mechanisms.
During COVID-19 pandemic, HeberNasvac was used as an innate immune stimulator due to the capacity of its antigens to stimulate multiple Toll-like receptors in vitro and in vivo at the oropharynx of aged subjects, suggesting a role in post-exposure prophylaxis. An increase in antigen presentation by monocytes and lymphocytes was detected in aged (>60 years old) volunteers who were household contacts of patients with respiratory and febrile infections [
12,
13].
After local administration of HeberNasvac, several interferon-stimulated genes (ISGs) increased their expression with a similar intensity as induced by the nasal administration of interferon alpha 2b (Nasalferon
®) [
12]. Specifically, an increase in the relative mRNA expression of OAS1, ISG15, ISG20, STAT1, STAT3, and DRB1-HLA II genes was detected. In summary, the mode of action mechanism of HeberNasvac involves a dual effect: it acts on innate immunity as an interferon inducer (with immunomodulatory effects) and stimulates antigen-specific adaptive immunity (adaptive immunity induction). [Reviewed in [
14]] This duality explains the long-lasting antiviral effect observed in vaccinated patients compared to PegIFN-treated CHB patients [
6].
The long-term follow-up of HeberNasvac patients was also related to the absence of relevant abnormalities in the markers of liver function and damage, as well as its capacity to prevent LC. In a preliminary report of the 10 years follow-up, 11 out of 80 patients who started the study developed LC in the group treated with PegIFN, whereas none of the patients developed LC in the group treated with HeberNasvac [
15], even when genotypes C and D were prevalent under the studied conditions.
2.3. ‘Functional’ vs ‘Partial’ HBV Cure
PegIFN and NUCs are effective in reducing HBV DNA during patient treatment. However, the effect on HBsAg levels during treatment is limited. The IFN treatment results in a comparatively higher proportion of CHB patients with HBsAg loss. They are in the range of 3-12% after long-term follow-up, with lower proportions in Asian patients and higher in those with very low HBsAg levels in blood. Therefore, current treatments prioritize virus control and prevention of complications. Thus, the concept of partial HBV cure is now considered as a realistic and attainable goal.
Regarding serological responses, preliminary HeberNasvac phase IV clinical data demonstrate that a relevant proportion of patients (10%) achieve HBsAg to anti-HBsAb seroconversion after 3 to 4 years of follow-up [
16]. The loss of HBsAg appears in a general background of patients progressing in HBsAg reduction trend, normal liver enzymes and stable disease by fibroscan; see
Figure 1 [
15,
16,
17,
18,
19,
20]. In addition, the clinical use of HeberNasvac in combination with the mucoadhesive excipient CVP (carboxyl-vinyl-polymer) from Toko Yakuhin (Kogyo, Japan) was evaluated in clinical trials at Ehime University (Matsuyama). The obtained results showed serological responses, including HBsAg loss and anti-HBsAg seroconversion, which were in turn associated to the generalized reduction of core-related antigens (HBcrAg) in most patients [
19,
20].
Two groups of patients not recommended for treatment—namely, a) asymptomatic carriers and b) patients already under NUCs—were vaccinated using HeberNasvac mixed with the mucoadhesive at the time of use. The study’s objective in terms of efficacy was to induce functional cure. Per protocol analysis among vaccinated CHB patients treated with NUCs (n = 27) and HBV carriers (n = 36), 74.1% and 75.0%, respectively, exhibited reductions in their baseline HBsAg levels. These mean reductions were statistically significant after 18 and 48 months [
19,
20]. Anti-HBsAg antibody responses were detected in 40.7% and 58.3% of patients treated with and without NUCs, respectively. Notably, six out of 63 (9.5%) patients were functionally cured after an 18-month follow-up. A 48-month follow-up detected an increase up to 12.7% (8 out of 63 patients), suggesting that a booster dose implemented at 18 months in a proportion of patients may be a suitable continuation for some of the patients boosting the anti-HBsAg response [
20]. The positive trend of HBsAg and anti-HBsAg markers after 18 and 48 months is highly encouraging in genotype C Japanese patients. In fact, the HBsAg loss after PegIFN treatment has been reported to be higher in patients infected with HBV genotype A than C [
29].
In a 2024 review assessing the situation of current treatments for CHB toward 2030 WHO goals [
29], Dr Anna Lok summarized the effect of current treatments. After a 48-week course of PegIFN-α treatment 20-25% of patients achieve a sustained decrease in HBV DNA levels, with HBsAg loss increasing from 2-3% at the end of treatment to 8-14% after 3-5 years post treatment follow-up [
30,
31,
32]. Interestingly, the loss of HBsAg depended on the HBV genotype, with 3% for genotype C and 14% for genotype A [
33]. In the case of NUCs, although more effective than PegIFN-α in inhibiting HBV DNA replication, only 2-5% of patients experience HBsAg loss after 10 years of continuous treatment [
32]. Taking into account these results obtained with PegIFN-α and NUCs, it is remarkable that about 10% HBsAg loss and anti-HBsAg seroconversion were achieved at 18 months, and 12.7% after 48 months (8 out of 63 patients) of HeberNasvac-CVP treatment, as reported at the 2023 edition of the AASLD meeting [
19,
20]. It is important to highlight that these results were observed in genotype C Japanese patients, who were selected from groups that typically have low ALT levels and are consequently low responders to immunotherapy with PegIFN—the drug category that aligns with HeberNasvac.
2.4. Other Serological Markers of Disease Progression
The HBcrAg is a novel marker that correlates with serum HBV DNA, intrahepatic total HBV DNA, and hepatitis B covalently closed circular DNA (cccDNA) [
21,
22,
23,
24,
25,
26,
27,
28]. Since most patients on long-term Entecavir (ETV) have undetectable HBV DNA, serum HBcrAg levels may serve as a potential marker of intrahepatic viral activity in virally suppressed patients. Additionally, HBcrAg levels predict hepatocellular carcinoma (HCC) development in patients with undetectable HBV DNA under NUC therapy [
26]. High HBcrAg levels have also been associated with reactivation of hepatitis after cessation of lamivudine therapy [
27,
28].
Quantitative HBeAg reduction was observed in all HBeAg-positive Japanese patients, consistent with a significant proportion of HBeAg loss and anti-HBeAg seroconversion. A significant reduction in hepatitis B core-related antigen (HBcrAg) levels was detected in 19 out of 21 patients treated with the HeberNasvac-CVP formulation (2 remained with stable levels) [
19,
20]. The results obtained in Japan indicate a delayed effect, in addition to the effect induced after treatment, suggesting that therapeutic vaccination may exhibit late responses similar to those seen with IFN-based treatments. Considering the encouraging serological responses achieved in Japanese clinical trials, a double-blind placebo-controlled clinical trial has commenced in Japan to confirm the effect of HeberNasvac in the targeted populations.
2.5. Treatment Complications after Long-Term Use
The currently approved antivirals are administered in a once-daily pill, improving the treatment adherence compared to PegIFN treatment, even compared to injectable therapeutic vaccines. However, the indefinite often-lifelong oral therapies complicate the adherence to these treatments for some patients, in particular considering the increase of kidney and bone problems. This long-term adverse reactions have been detected since the first nucleotide analogue-related nephrotoxicity has been reported in subsequent cohort studies, with a prevalence of 3.0–9.9 % for Adefovir (ADV) monotherapy or in case of their combination with Lamivudine (LAM) combination therapy [
34,
35].
It is recognized that the long-term use of NUCs increased the risk of nephrotoxicity in patients with CHB or human immunodeficiency virus (HIV). Thus, the regular assessment of renal function is recommended for all patients with CHB, particularly those treated with a nucleotide analogue, complicating the management of the treatment [
36]. In a recently published 7-year safety and efficacy study of TDF in CHB, 3.6% of patients had developed nephrotoxicity [
37]. The nephrotoxicity of TDF was already observed for the treatment of HIV, but in HIV, other antiviral drugs might also contribute to renal dysfunction. One recent retrospective study found that 5.6% of patients with CHB had evidence of nephrotoxicity after 2 years of treatment with TDF [
38].
Prolonged exposure to TDF has also been associated with a decrease in bone mineral density (BMD) and increased fracture risk. However, limited discussion exists on its effects on various aspects of bone quality. A comprehensive overview of the impact of TDF on bone quality appeared in 2024 [
40]. Findings indicate that prolonged exposure to TDF adversely affects bone microarchitecture and strength, impeding fracture healing and skeletal microdamage repair. Complex interplay involving bone cell signaling, cytokines and bone remodeling processes are potential mechanisms underlying TDF’s impact on bone quality.
2.6. The Route and Schedule of Immunization
HeberNasvac may potentially become an exclusively intranasal (IN) product, as demonstrated in Japanese clinical trials where the vaccine is administered by IN route alone. The relevance of the route and schedule of administration has been studied in HBV-carrier mouse models and patients, revealing that the anti-HBsAg response was preferentially induced by the IN route in a murine model of CHB infection [
41].
The IN route was also the most efficacious at inducing cellular immune responses, in particular CD4+ T cells. In HBV-carrier mice, high frequencies of HBs-specific CD4+ T cells secreting interferon-γ, interleukin (IL)-2 and tumor necrosis factor (TNF)-α were found in liver only after IN immunization and not by SC immunizations. This demonstrates that the route of therapeutic immunization may be relevant to the final effect of the product. Increased frequencies of CD4+ T cells expressing the integrin CD49a in liver suggest a role of the nasal cavities as inductive site in promoting a hepatotropic cellular homing process. Multiple dose schedules were also a prerequisite for HeberNasvac to overcome immunotolerance [
41].
To compare different schedules and routes of immunizations, a group of patients received four different vaccination regimens in a placebo-controlled factorial study. Patients were followed for a minimum of 48 weeks. Samples collected at the end of the follow-up were compared with initial samples. Groups I and II received the product by IN/SC routes, every 14 and 7 days, respectively. Groups III and IV were treated by the SC route alone following a 14 and 7-day schedule. After more than 48 weeks of treatment-free follow-up, HeberNasvac-treated patients demonstrated superior responses compared with the placebo group in terms of antiviral and serological responses. The factorial analysis evidenced that the schedule combining the IN/SC routes of immunization and the frequency of 14 days resulted in stronger antiviral and serological responses. The present results support the study of the IN route as well as the development of IN-only immunization schedules [
42].
In summary, the composition of HeberNasvac, the IN administration route, and the procedure of multiple immunizations are relevant variables affecting the final efficacy of the product. Additionally, the product can be further optimized by considering a vaccination schedule every two weeks. However, we do not consider this chapter closed; on the contrary, several variables remain to be explored, for example a potential role of trained innate immunity in the mode of action mechanism of HeberNasvac.
2.7. Accessibility to Patients
Iterative HBV DNA quantifications using qPCR technology and ALT determinations, which are required to assess guideline cut-offs, also represent an additional cost for patients and healthcare systems. Reducing the costs associated with the treatment decision process would increase the accessibility of the product. In parallel, governments and health authorities should work on reducing the expenses related to HBV DNA qPCR technologies. In this context, the development of HeberNasvac has been associated with the creation of an in-house system, validated against multiple international kits. The aim is to administer the product within the scope of a sanitary intervention. This qPCR system is also valuable for assessing patient outcomes after immunomodulatory vaccine treatment [
43,
44,
45] and as a cost-effective method to quantify HBsAg levels over time, as previously used and reported [
42,
46]. Further cost reduction may be achieved by using point-of-care serological assays for timely patient follow-up.
Treatment accessibility can be interpreted considering the range of patients who may benefit from it. While current treatments are recommended only for patients meeting guideline-related cut-offs, HeberNasvac has also been used in HBeAg-negative patients with low HBV DNA and intermittently abnormal ALT levels. In countries like Bangladesh, where the patients with this profile are also progressing to cirrhosis [
47,
48,
49], HeberNasvac offers an alternative treatment option for a broader patient population.
The availability and affordability of therapeutic vaccines, including those in countries with limited resources, may be a key advantage compared to currently established products. Although HeberNasvac does not follow a traditional two- or three-dose schedule, it benefits from the well-established understanding of vaccination as one of the most cost-effective public health interventions. If mass-scale production can be achieved, there is ample opportunity to treat patients in low-income countries. Additionally, new developments, such as obtaining an IN-only product and using the vaccine to discontinue NUC treatments will open new windows of opportunities.
3. Residual Risk of HCC: An Opportunity for Immunotherapies?
It is generally accepted that IFN-α-based therapies are the best choice for patients with CHB to achieve a higher percentage of functional cure and a lower risk of HCC. In contrast, HBsAg loss is rare with NUC monotherapy, and the risk of HCC persists steadily despite long-term ETV or TDF treatment. The 5-year cumulative HCC incidence rate exceeds 9% with ETV/TDF, whereas it remains below 1% with IFN-α therapy. In summary, both PegIFN and NUCs are recommended as first-line therapies for CHB infection by the EASL, AASLD, and APASL guidelines, however, real-life studies show that long-term follow-up benefits CHB patients treated with IFN-based regimens, particularly in preventing liver cirrhosis (LC) and HCC [
50,
51,
52,
53].
Even though lifelong and optimal suppression of HBV DNA is believed to be crucial for preventing HCC, PegIFN-experienced patients exhibit a lower HCC incidence than NUC-treated patients. Notably, viral loads in ETV-treated patients remain consistently suppressed below those of PegIFN-treated patients, however the occurrence of oncogenic surface antigen truncation mutations associated with HCC is less frequent in the PegIFN group, thereby highlighting the beneficial effects of immune modulation [
52].
The impact of undetectable versus low-level HBV viremia (<2,000 IU/mL) on HCC development has been recognized in a retrospective cohort study of 875 treatment-naive chronic HBV monoinfected patients who received ETV monotherapy. Over a median follow-up of 4.5 years (range 1.0-8.7 years), HCC was diagnosed in 85 patients (9.7%). HCC occurred more frequently in patients experiencing low levels or intermittent increases below 2,000 IU/mL (14.3% versus 7.5% at 5 years, P = 0.015). Among patients with LC, the risk remained higher for those with low-level viremia (23.4% versus 10.3% at 5 years) compared to those with undetectable viremia [
54]. In summary, the association of HBV DNA and HCC risk is clear still at low-level viremia; however, the residual HCC risk is still high even in those with HBV DNA undetectable. An unexplored effect of NUCs, opposed to their antiviral effect, may explain the residual risk of HCC compared to PegIFN.
Recent results suggest that IFN add-on therapy with NUCs may offer dual benefits by reducing HCC development and facilitating HBsAg loss among NUC-treated CHB patients with intermediate to high HCC risk. The NUC-treated group maintained monotherapy, whereas patients in the IFN + NUCs group received IFN add-on therapy for 48 weeks before switching to NUCs monotherapy. A total of 196 patients were included in the interim analysis (NUCs group: 68; IFN + NUCs group: 128). The 96-week cumulative HCC incidence was lower in the IFN + NUCs group than in the NUCs monotherapy group (0% vs. 4.5%, p < 0.05). Additionally, the IFN + NUCs group exhibited significantly higher rates of HBsAg loss at weeks 48 and 96 (22.7% vs. 0%; 16.7% vs. 0%, both p < 0.05) [
55].
The experience of PegIFN reducing this risk is relevant to the development of immune therapies with mechanisms involving innate immune stimulation. Similar mechanisms have been described for HeberNasvac, considered an interferon-inducer with effects comparable to IFN alfa-2b in stimulating interferon-stimulated genes (ISGs) in blood samples when both products were administered locally (intranasal and sublingual). The physicochemical nature of the HBcAg, comprising multiple innate immunity ligands, justifies this property [
12,
13].
The detection of oncogenic surface antigen truncation mutations in NUC-treated patients who developed HCC, but not in those treated with PegIFN, suggests a potential link with HCC development [
53]. These mutations are associated with an increased HCC risk because they can lead to the production of altered forms of HBsAg that may promote oncogenesis. It is possible to hypothesize that the effects observed on the HBsAg sequence could also manifest in other proteins. NUCs are known to be incorporated into growing DNA strands, acting as chain terminators and stopping viral DNA polymerase. If similar truncation mutations were to occur in other viral or cellular proteins, they could potentially disrupt normal cellular functions and contribute to carcinogenesis.
Whether the residual risk of HCC results from two opposing vectors deserves further study. Product monographs serve as important sources of information to better understand the process of NUC carcinogenicity. Some reports warrant closer examination [
56,
57,
58], balancing their effects with the well-recognized oncogenic impact of HBV, which has been associated not only with HCC but also with other types of cancer [
7].
3.1. Carcinogenic Effect of NUCs According to Product Monographs
While interferon products are well-known because of their effect against cancer, animal study results provide evidence of ETV’s carcinogenicity in mice and rats, as reported in the Product Monographs of Baraclude and Viread [
57,
58]. This effect is more pronounced in the case of Baraclude.
Long-term oral carcinogenicity studies of ETV showed positive findings related to carcinogenicity in both mouse and rat models. Lung adenomas were increased in male and female mice at exposures 3 and 40 times those in humans. Lung carcinomas also increased at exposures 40 times those in humans. Tumor development -preceded by pneumocyte proliferation in the lung-was not observed in rats, dogs, or monkeys administered ETV. It was suggested that lung tumors in mice might be a species-specific event [
57].
Hepatocellular carcinomas were increased in males, and combined liver adenomas and carcinomas were increased at exposures 42 times those in humans. Vascular tumors in female mice (haemangiomas of ovaries and uterus and hemangiosarcomas of spleen) were increased at exposures 40 times those in humans. In rats, hepatocellular adenomas were increased in females at exposures 24 times those in humans. Brain gliomas were induced in males and females at exposures 35 and 24 times those in humans. Skin fibromas were induced in females at exposures 4 times those in humans. As discussed in the genotoxicity chapter, a general tumorigenic mechanism may involve a cumulative mutational effect of prolonged nucleotide pool imbalances [
57].
Long-term oral carcinogenicity studies of TDF in mice and rats were carried out at exposures up to approximately 16 times (mice) and 5 times (rats) those observed in humans at the therapeutic dose. At the high dose in female mice, liver adenomas were increased at exposures 16 times that in humans. TDF was mutagenic in the in vitro mouse lymphoma assay. The other tests were reported as unfavorable [
58].
It has been reported that ETV can be incorporated and embedded into the human genome via primer extension or subsequent ligation, which may contribute to a putative mechanism of carcinogenicity [
59]. A second study found that ETV induced DNA damage at a nanomolar concentration in DT40 cells in the more sensitive DNA repair-deficient cells. Considering that, ETV inhibited HBV DNA synthesis in the same nanomolar range; the authors suggested restricting ETV treatment period [
60]. However, interpreting and extrapolating animal data to humans is not straightforward. In this sense, it is of value to consider the information from large clinical trials assessing the carcinogenic effect of NUCs in humans.
3.2. Effect of NUCs in Human Carcinogenesis
The preventive effect of NUCs on HCC development in patients with CHB is controversial due to the difficulty of conducting randomized controlled trials. While some studies suggest a reduced risk of HCC with nucleotide analogue therapy, others indicate that the benefit may be limited to certain patient subgroups, such as those with LC [
61]. On the other hand, since NUCs are intended to be used over a long period, the potential carcinogenic effects of ETV detected in animal studies [
57] have been examined in a large-scale, population-based study designed to explore the potential causal effects of NUCs on the risk of malignancies in humans [
62].
The retrospective cohort study by Wong and colleagues examined more than four thousand CHB patients in Hong Kong and found that ETV did not appear to increase the risk of common cancers in patients with CHB—although a statistically significant increase in cervical and colorectal cancers was found, prompting the authors to advise further exploration of this finding. A surprising result from this study was that the authors failed to show a statistically significant association between antiviral treatment and a lower risk of HCC [
62], suggesting that this may be due to the lower prevalence of cirrhosis among the studied patients. This comment implies that the author follows the hypothesis that the benefit may be limited to patient with cirrhosis.
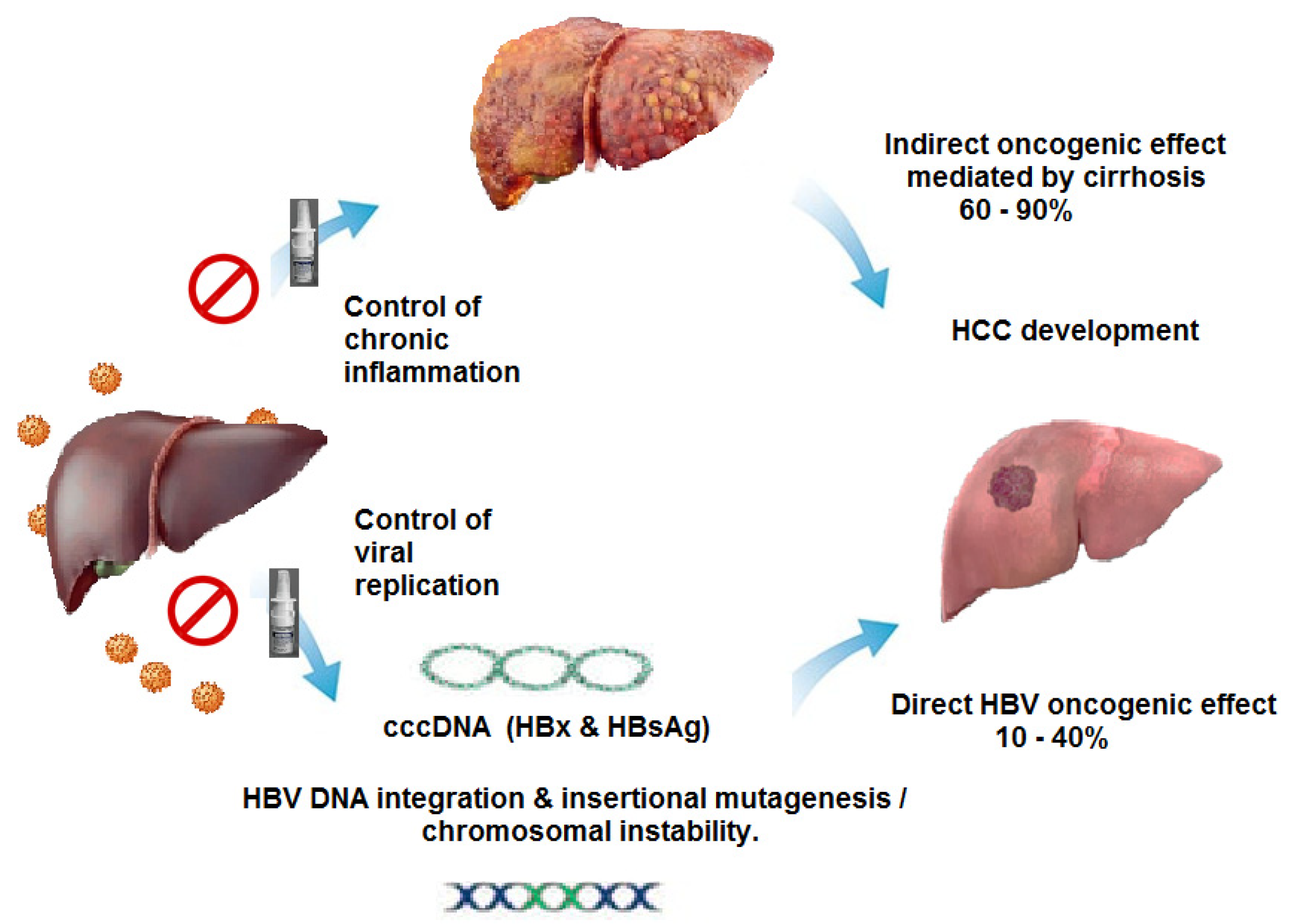
The oncogenic potential of HBV is attributed to several factors: integration of HBV DNA into the host genome, insertional mutagenesis and activation of oncogenes, chronic inflammation caused by long-term HBV infection leading to cellular damage and increased cell turnover, which may contribute to carcinogenesis and also to viral proteins such as HBxAg that can interfere with host cell regulatory pathways, promoting cell growth and survival. While the strongest association is with liver cancer, the mechanisms by which HBV contributes to other cancers are still being investigated. It is clear that the virus can have far-reaching effects beyond the liver. Thus, it is reasonable to expect that treatment with NUCs, which strongly reduce viral replication, would reduce the oncogenic potential of the virus not only in the liver but also in other tissues, reducing the incidence of all types of cancers.
In contrast to the rational expectation, NUC treatment did not lead to a reduction in the risk of HCC and other common cancers in CHB patients subsequent to the strong reduction in HBV levels [
62]. These results should be considered very carefully when compounded with the observed statistical significance in the case of colorectal and cervical cancer (see above).
In fact, a closer look into the reported data in NUC treated patients evidences a generalized trend to the increase in the incidence rate of almost all types of cancer (presented in per 10
5 person years) independently of their statistical signification. This was detected for the category of ‘all malignancies’ in the NUC treated vs untreated groups (1672.0 vs 473.5), and for the specific cancers: HCC (1069.7 vs 226.8); colorectal cancer (87.9 vs 23.1); Lymphoma (58.6 vs 14.6) and cervical cancer (45.7 vs 22.2), respectively [
62]. This analysis involved 44′494 patients in total. The differences in the incidences were even more dramatic when the patients were selected considering those highly exposed to the drug or never exposed to any antiviral treatment in the untreated group: ‘all malignancies’ (1884.4 vs 417.9); HCC (1279.4 vs 183.0); colorectal cancer (98.0 vs 22.5); Cervical cancer (73.7 vs 22.7) and lymphoma (53.5 vs 11.8). This second analysis involved 38,181 patients [
62].
Interestingly, this clear increasing trend of incidence does not match a hypothetical protective activity of NUCs against HCC or any other common cancer after long years of treatment, but rather the opposite. These findings appear to support the suggestion of restricting the ETV treatment period [
60], and render necessary further exhaustive studies to rule out any contribution of long-term NUC therapies to a pro-oncogenic effect.
Considering the current results observed during implementation of HeberNasvac, which show a lower proportion of patients with progression to LC [[
15],
Figure 1], one would expect a reduced number of HCC cases after therapeutic vaccination. HeberNasvac was also competitive during the phase II/III study. Out of 80 enrolled patients treated with PegIFN, 11 progressed to LC according to fibroscan measures after several years of treatment-free follow-up. In contrast, none of the 78 vaccinated volunteers progressed to LC [
15]. Underlying mechanisms for the conferred protection are depicted in
Figure 2.
On the other hand, a considerable body of clinical evidence support the fact that immunomodulatory therapy with PegIFN reduces the risk of residual HCC, as compared to treatment with NUCs. Further research should focus on the long-term protective effects of HeberNasvac and other therapeutic vaccines with regard to HCC, LC, and mortality. It is expected that new finite therapies based on the combination of immune modulatory strategies and PegIFN would allow tackling the high levels of mortality associated with CHB.
A therapeutic vaccination approach benefits from the induction of both adaptive and innate immunity. There is an increased expression of IFN-stimulated genes in the systemic compartment after local administration of HeberNasvac. This increment is similar to what observed after local administration of IFN-α 2b (Nasalferon
®). This in turn opens a new window for combined therapies based on HeberNasvac and Nasalferon, which have a low reactogenicity profile, as an alternative treatment for patients treated with NUCs [
12].
4. Irregular Medication, ACLF and the Need of a Safe Discontinuation
Long-term treatment with NUCs has been linked to their irregular use, known as “irregular medication of nucleot(s)ide analogues” (IMNA). An in-depth analysis revealed that the most common reasons for IMNA were economic constraints, followed by suboptimal response or unaltered course of disease upon long-term antiviral treatment, patients concerns about the side effects, irregular daily routines leading to irregular medication, and patients switch to cheaper traditional medicines over conventional antiviral treatment [
4]. Although these problems have been detected in China, undoubtedly the same scenario can be replicated in most developing countries where CHB has higher prevalence.
Irregular medication has been suggested as the cause of disease exacerbation and HBV-associated liver failure resulting from the “acute-on-chronic” insult (ACLF) [
4]. ACLF is a serious and life-threatening complication of chronic liver disease, characterized by acute decompensation of liver function in the context of chronic liver disease (50% mortality). A recent study assessed the prevalence and severity of HBV-associated ACLF resulting from IMNA. The percentage of ACLF caused by IMNA was 9.01% in CHB non-cirrhotic patients of nine tertiary-level “A” hospitals in the Chinese province of Heilongjiang over a ten-year period. After studying more than 1000 cases of ACLF, the proportion was nearly double in the case of cirrhotic patients [
4]. In short, while NUCs prevent deterioration in some patients, their misuse, conditioned by patients’ economic situations and other factors, may lead to complications and death in a significant proportion of these patients.
It is important to highlight that CHB patients without LC are not expected to die at this stage of their disease. However, the irregular use of antiviral medication, with some cessation-restart cycles of medication, leads to exacerbated damage in the liver caused by the emergence of HBV replication and the subsequent uncontrolled reactivation of the antiviral response. As a result, the liver is damaged in a process resulting from the irregular treatment. Remarkably, the worst ACLF from all studied causes resulted from IMNA, leading to a higher proportion of fatal cases [
4]. Thus, the suitability of treatment with NUCs should be carefully evaluated, considering the potential misuse of the NUCs, according to the characteristic of the health care system. This may help to reduce morbidity and mortality in HBV-associated ACLF by enhancing patients’ compliance.
In order to prevent the negative consequences of IMNA during long-term treatments, it will be important to develop a therapeutic vaccine approach capable of averting or delaying HBV rebound and ALT exacerbations. In this setting it would be expected a subsequent reduction of HBsAg levels, if vaccine induced immunity can be linked to the natural immune reactivation post NUC cessation.
5. Towards a “Smart” Approach for the Safe Discontinuation of NUCs
Therapeutic vaccination may be a valid alternative in the context of NUC treatment discontinuation. However, current results of treatment cessation after therapeutic vaccination have led to disappointing outcomes and the resumption of antiviral treatment [
63,
64,
65]. The evaluation of HeberNasvac, used in combination with NUCs in the setting of treatment discontinuation, did not achieve the study’s goal of generating a sustained antiviral response post-cessation, as compared to the control group [
63]. This phase IIb trial, conducted in several Asian countries (NCT02249988), assessed the vaccination under viral suppression, with the discontinuation of NUCs occurring one month after the end of the vaccination.
HeberNasvac vaccination in HBeAg(-) CHB patients, who had been under extensive and effective antiviral treatment for several years, evaluated the vaccine’s capacity to prevent viral relapse after NUC discontinuation. At Week 24, one month after the completion of vaccination, antiviral therapy was halted in all patients. The vaccination was safe and well-tolerated, with only 2.2% severe adverse events in both treatment arms (not drug-related). However, it did not prevent viral relapse after the cessation of NUCs and patients were retreated after detecting the viral relapse [
63].
A systematic review and meta-analysis on NUC discontinuation concluded that the prompt retreatment following viral DNA rebound—within the scope of NUC discontinuation—was an overly cautious or conservative approach [
66]. It might hinder the potential immune reactivation that could effectively control the viral peak. In line with this, some NUC stopping-restarting guidelines were established, detailed at the EASL 2018 Postgraduate course, after considering the experience from several NUC discontinuation clinical trials [
67,
68,
69].
In our view, the design of clinical trials using therapeutic vaccines in the context of NUC discontinuation [
63,
64,
65] would differ if drafted today. The swift termination of studies combining vaccines and NUC cessation precluded a comprehensive understanding of the virological and serological responses after HBV rebound and immune reactivation. In light of the recent results by Jiang and colleagues [
55], which demonstrate the add-on effect of PegIFN during NUC treatment, it would be useful to explore the long-term effect of therapeutic vaccination on the residual risk of HCC with a less reactogenic immune modulator. Thus, smarter designs are required to capitalize the temporal association of the immune response induced by the vaccine and the host-induced response post-discontinuation. A clinical trial exploring this scenario is currently ongoing.
HeberNasvac’s safety and efficacy, demonstrated in treatment-naïve CHB patients [
6,
15,
16,
17,
18,
42,
46], and its capacity as an innate immunity stimulator [
70,
71], make this product a suitable choice for this indication. The local administration of HeberNasvac (comprising a nucleoprotein with multiple ligands for TLR2, 3, 7, 8, and 9) leads to the strong expression of IFN-induced genes in the blood, resulting from the stimulation of innate immunity in the oropharyngeal mucosa [
12,
13,
14].
Whether the combination of NUCs and therapeutic vaccines may lead to sustained viral suppression is not obvious until clinically proven. Alternative approaches using therapeutic vaccination in patients who do not stop NUC treatment (add-on) [
19,
20] or in those who stop NUC treatment to start vaccination (switch-on) are ongoing and will shed light on this potential use.
A smart and immunologically rational schedule of immunization, combining therapeutic vaccination and NUC discontinuation, would ensure a soft viral take-off post-discontinuation. Optimistically, it could lead to HBV suppression for several months or even years. This approach may represent a way to mitigate the consequences of irregular NUC use, reduce the risk of HCC, and recover kidney and bone structure and functionality by preventing virus-induced flares [
72,
73].
6. Surfing the New Wave of Treatments
The achievement of a “cure” seems far from today’s perspective. One-year PegIFN treatment or long-term NUCs are not suitable treatments to achieve an HBV cure, considering the 300 million CHB patients. Stopping NUC therapy may lead to a functional cure but mostly in a limited percentage of Caucasian patients with low HBsAg levels at baseline. However, a wave of novel products has inspired faith in recent years.
The task of developing a novel product addressing a global health challenge like CHB requires mingling vaccine innovation with industrial and clinical development, emphasizing safety, efficacy, and accessibility. Viral entry inhibitors, inhibitors of translation and secretion of HBsAg, capsid assembly modulators, and products targeting cccDNA transcription/degradation have shown encouraging results in clinical trials. Immunomodulatory approaches include checkpoint inhibitors, metabolic modulation of T cells, therapeutic vaccines, adoptive transfer of genetically engineered T cells, and stimulation of innate and B-cell immune responses. Recent reviews offer a systematic description of the novel wave of products [
74,
75].
The suppression of HBsAg production from cccDNA and integrated HBV DNA, as well as the restoration of the HBV-specific immune response, are required to achieve a functional cure or sustained undetectable HBsAg / HBV DNA at week 24 post-treatment cessation. New antivirals in clinical trials, notably siRNA and antisense oligonucleotide, result in a 2–3 log10 decrease in HBsAg levels after 24–48 weeks of treatment, but sustained HBsAg seroclearance is still uncommon. Adding PegIFN or immune-modulatory therapy after HBV DNA suppression and HBsAg reduction may enhance the likelihood of a functional cure [
75].
The combination of novel and current treatment in personalized strategies to maximize the chance of an HBV cure has been considered. However, a “gourmet” product may not be affordable for low- and middle-income countries in the short to medium term. These approaches would also carry over some of the current limitations of established treatments, i.e., HBV and ALT cut-offs for treatment decisions and some of the safety concerns related to existing products. A complex monitoring is required in the case of PegIFN and this may result in a dose reduction, temporary dose cessation, or discontinuation of therapy complicating the future of combined treatments (
Table 1 summarizes the adverse reactions described in the product monograph of Pegasys).
The use of novel adjuvants based on innate immunity stimulators—agonists of innate immunity discovered in the last two decades; a broader antigenic composition mainly including pre-S and core antigens; and the use of prime-boosting approaches, are fueling a new wave of development in the field of therapeutic vaccination. In addition, the understanding the immunology behind the combination of therapeutic vaccines and NUCs is still a matter of debate and a potential driving force for novel strategies [
73].
6.1. The Rationale of Combining Therapeutic Vaccination and Antiviral Drugs
Major associations for the study of liver diseases do not recommend the routine use of interferon in combination with NUCs for the treatment of CHB [
8]. The benefits of combination therapy have not been consistently demonstrated across studies. Therapeutic vaccines are also immunomodulatory treatments that stimulate both innate and adaptive immunity. Should we expect something different? It is not an easy answer.
We know that HBV-specific immune responses can be induced [
64], and several attempts of vaccination under NUCs have been unsatisfactory [
64,
65,
76]. As in the case of combinations with PegIFN, the use of novel therapies with the NUCs may add safety concerns. Although considered safe when used for several years, some risks are inserted as warning notes of Baraclude and Viread product monographs (see summary in
Table 2), and they should be considered in the design of NUC cessation approaches -and in general for their combination of NUCs and therapeutic vaccines.
HeberNasvac induced a significant HBsAg neutralizing response both under NUCs and in asymptomatic carriers when administered with the mucoadhesive CVP [
19]. In line with the early results of several vaccine candidates, stronger HBsAg reductions appear in patients with HBsAg levels below 100 IU/mL immunized with HeberNasvac. However, the proportion of patients with HBsAb titres and HBsAg loss is higher in the setting of patients without NUCs. The assessment of the response after long-term follow-up (late effect), the use of booster doses, and the improvement of the formulation represent alternatives to induce superior responses, considering the trend of HBsAg reduction over time.
Some theoretical disadvantages related to inducing adaptive immunity in patients under NUCs arise. Vaccine-induced T cells should exert their function in the liver. However, the inflammatory liver environment is reduced soon after the start of NUC treatment. This is reflected by the reduction in ALT levels in most patients a few weeks after the start of antiviral treatment. In line with this, it has been demonstrated that hepatocytes do not express HLA class II, except under inflammatory conditions [
77,
78,
79], a logical and natural adaptation to the tolerogenic role of the liver.
The suppression of viral replication in patients under NUC therapy leads to a reduction in the number of hepatocytes presenting viral antigens, such as cytoplasmic HBcAg. It has been demonstrated that control of replication can be predicted by the low intracellular expression of HBcAg [
80]. Taken together, in virally suppressed patients, there is a reduced presentation of HBV antigens and consequently a reduction in the presentation of viral peptides to vaccine-induced T cells by both HLA class I and II.
Viral replication reactivates the immune system’s detection mechanisms, restarting the immune response. Indeed, discontinuing NUC treatment may be beneficial for enhancing immunotherapeutic efficacy. However, the design of the discontinuation and restart treatment protocol requires a smart approach. Discontinuation protocols should adhere to the established rules for restarting therapy. Transient and moderate increases in ALT should be considered normal in this context, and patients nearing cirrhosis should not be included in the initial trials. A new horizon of opportunities can be created for therapeutic vaccination approaches if the immunological scenario is considered.
On the other hand, using NUCs for an extended period after therapeutic vaccination may take longer to induce an effective response at the HBsAg level. However, this approach may be suitable for patients with more advanced degrees of fibrosis. The initial results of HeberNasvac administered under NUCs indicate a trend of reduction in HBsAg and HBcrAg levels, as well as noticeable progress in HBeAg serology. HeberNasvac add-on explores HBsAg seroconversion without forfeiting the virological, serological, and biochemical benefits of NUCs. In brief, the immune system may require more time to function under viral suppression [
19,
20].
HeberNasvac also activates a wide array of TLRs from both TRIF and MyD88 pathways, leading to the stimulation of type I and type II interferons and multiple ISGs [
12] after local administration in aged >60 year-old volunteers. Therefore, there is still potential for a significant response in the combination with NUCs. In addition, multiple factors need consideration, including the potential to reduce HCC risk.
7. Therapeutic Vaccination: From Bench to Large-Scale Intervention
HeberNasvac has been developed as a ‘South-North-South’ cooperation in the practical sense. This experience deserves a closer look as an example of collaboration between academic institutions, the pharmaceutical industry, hospitals, and regulators.
The pharmacological studies of HeberNasvac brought together collaborators from the Center for Genetic Engineering and Biotechnology (Cuba), Vaxine Pty (Australia), Ehime University (Japan), Institut Pasteur and the Center for Atomic Energy (France) and the Helmholtz Centre for Infection Research (Germany). Multiple studies demonstrated the strength and immunomodulatory capacity of the induced antibody and T cell response in different lines of mice, as well as the serological responses in HBsAg-tg and HBV-transfected animal models. The nasal route of immunization has shown to be far beyond an attractive and safe administration route. This route ensure the antigen uptake and induction of antigen-specific T cells preferentially recirculating to the liver. In comparison, the parenteral administration of the same product induced a significantly lower response in frequency and intensity. This is part of product rationale and action mechanism [
10,
11,
70,
71].
After industrial development and confirmation of preclinical stability, HeberNasvac completed acute toxicity, local tolerance, and repeated-dose toxicology studies for IN as well as for IN and SC combined administration. The Mucosal Irritability study also confirmed that HeberNasvac was not irritating to the mucosa and there was no damage to the brain due to nose-to-brain mechanisms.
Clinical safety and evidences of immunogenicity and antiviral effect was demonstrated in two phase I clinical trials, one in healthy volunteers [
81] and the other in IFN refractory patients. The second study, even reduced in sample size, was the first evidence of the late response associated to immune stimulation. Two out of 6 patients seroconverted to HBsAg and the three HBeAg positive loss the antigen, in two cases with seroconversion. DNA titres were undetectable in all after five years [
46].
The product was also studied in a phase I/II trial enrolling CHB patients in Bangladesh [
82] and in a phase IIa trial in Cuba [
42]. These studies further supported the safety and immunogenicity of the product. In addition, they showed that administration every 2 weeks in the 10-dose standard schedule was superior compared to the reinforced schedule of weekly administration of 20 doses.
A phase II/III study of HeberNasvac was conducted to assess the efficacy of the product. Antiviral response was superior in the group treated with HeberNasvac compared to PegIFN. Although the proportion of patients with HBV DNA suppression below 250 copies/mL was similar at the end of treatment for both groups, it was >20% higher in vaccinated patients 6 months after the end of each treatment, confirming the hypothesis of the study. The superiority of the product was also confirmed in other clinically relevant variables (proportion of patients with HBV DNA below (10
4) copies/mL, as well as in terms of HBeAg loss and seroconversion) [
6].
The long-term follow-up of the vaccinated patients 1, 2, 3, and 5 years after the EOT [
16,
17,
18] detected a general trend of reduction in mean values and proportions. More than 75% of patients had HBV DNA levels below the limit of detection after 5 years of treatment-free follow-up. These results were associated with a lower proportion of patients with cirrhosis according to the fibroscan test compared to PegIFN-treated patients [
15], as previously discussed.
The assessment of the antiviral response across genotypes in patients from the phase II/III clinical trial has shown that HeberNasvac developed a uniformly superior antiviral response compared to PegIFN for all genotypes involved in the study (A, C, and D). It was remarkable to find that genotype D patients, known to be less responsive to PegIFN treatment compared to genotype A, exhibited the highest relative antiviral response, while maintaining lower relative responsiveness as expected in the PegIFN-treated patients. An early peak of ALT values (week 8) was detected in genotype D compared to genotype A patients, who peaked at week 12 [
83]. In all cases, there was a subsequent and generalized normalization of transaminases over time.
HeberNasvac, now registered as a “monotherapy” for CHB treatment, is currently accessible to HBsAg-positive non-cirrhotic patients in Cuba [
84]. A phase IV trial is ongoing in more than 300 patients across the island [
85]. The preliminary results of phase IV were encouraging in terms of HBsAg loss and seroconversion [
16], and the five-year follow-up results are expected by the end of 2024. HeberNasvac has been proposed for a sanitary intervention in Havana to cover all HBsAg(+) patients in the future.
HeberNasvac clinical development has been associated with the development of standardized and validated cost-effective virologic and serologic assays targeting future expansion in the number of patients. The CIGB validated a cost-effective and implemented a low-priced in-house HBV DNA determination and HBsAg quantitative systems to ensure the control of a large number of patients enrolled as part of the future sanitary intervention [
86,
87,
88].
8. Enhancing Innate Immunity: A Driving Approach for Vaccine Optimization
HeberNasvac is being developed into an exclusively nasal product that is affordable and accessible to all CHB patients without requiring injections. The mixture of two recombinant virus-like particles in a liquid presentation is the most straightforward formulation for expanding their use in low-income countries. The use of the CVP mucoadhesive will obviate the need for parenteral administrations during the second cycle of the standard protocol of immunizations [
19,
20]. A secondary benefit will be the induction of solid immunogenicity by the IN route alone. Results in Japanese vaccine non-responders have evidenced the strong immunity conferred by the IN formulation [
89].
Another interesting approach for developing HeberNasvac, whether linked to mucoadhesives or not, maybe using c-di-AMP, an active excipient used to induce strong innate immunity stimulation. This excipient has a recognized adjuvant effect in many vaccine settings [
90,
91,
92]. The mucosal adjuvanticity of c-di-AMP has been demonstrated in multiple models, even in formulations that can be lyophilized to improve thermal stability or administered in powder form through the respiratory mucosa [
93].
The compound c-di-AMP is produced by enzymatic synthesis as a highly pure GMP product. The c-di AMP is recognized by host cells inducing a type I interferon (IFN) response via STING (Stimulator of Interferon Genes), activating the nuclear factor kappa B (NF-κB) pathway, the inflammasome, and also the host autophagy system, promoting the production and secretion of cytokines [Reviewed in [
92]]. The c-di-AMP has demonstrated the capacity to trigger a host mucosal immune response as a mucosal adjuvant in formulation with HeberNasvac [
94]. As a pathogen-associated molecular pattern, c-di AMP will reinforce host immunity and should be tested in patients following satisfactory results in the non-human primate model. HeberNasvac, with an exclusively IN administration procedure, will be an attractive product for the massive introduction in challenging sanitary settings where CHB is more prevalent.
CHB patients exhibit a dysfunctional immune response, and inhibitory receptors such as programmed death receptor 1 (PD-1) are overexpressed on T cells, leading to an ineffective immune response. Consequently, some companies plan to combine therapeutic vaccines with immune checkpoint inhibitors (ICI). The PD-1 inhibitor, Nivolumab, was safe and effective for treating virally suppressed patients with CHB, with or without administering the therapeutic vaccine GS-4774. However, the effect of the vaccine was negligible under the studied conditions, and the only patient eliminating HBsAg during the study was from the non-vaccinated group. The study was conducted with patients under antiviral treatment with NUCs [
95].
It is essential to highlight that Nivolumab can suppress the host’s immune control over HBV replication in certain patients. HBV reactivation has also been increasingly recognized in oncologic patients with concomitant HBV infection treated with Nivolumab. Recently, hyper-progressive disease has been described in a subset of patients with HCC treated with a PD-1 inhibitor, and it was associated with worse progression-free and overall survival [
96]. In summary, additional studies are needed to elucidate the impact of checkpoint inhibitors on the risk of HBV reactivation to exploit the benefits of this approach, albeit reducing any potential risk [
97].
The rationale and status of the 10 most advanced approaches to therapeutic vaccination as of 2024 are summarized in
Table 3. This selection largely similar to the list of the EASL in June 2024. Some conclusions can be draw from the group of front-runner approaches: In addition to HeberNasvac, where the HBcAg represents a nucleoprotein with a multi-TLR agonist effect, most vaccine candidates are enhancing the immunogenicity of their antigens by stimulating innate immunity using PegIFN or agonists of the innate immunity receptors (7 candidates). In addition, new antigens are being used, including vaccines with PreS regions (5 candidates), HBcAg (4 candidates), antigens in vectors (2 candidates), peptides from conserved regions (1 candidate), or selecting HBsAg with neutralizing epitopes associated with Hep B cure (1 candidate). In addition, some studies declare their intention to recruit patients with low baseline HBsAg levels, those with a higher probability to benefit from the functional cure, even when they are not within the scope of current therapies.
There is a call for a simplified recommendation to reduce the disease burden. However, recent reviews acknowledge there is insufficient evidence to support treatment expansion to all HBsAg seropositive patients using current antiviral agents [
105]. The use of therapeutic vaccines in well-designed studies, preferably prospective cohorts, or randomized controlled trials, with appropriate monitoring planning, is required to establish them as treatment for individuals who do not currently meet the eligibility criteria for antiviral treatment by current guidelines’ recommendation. The recent interest of different companies in treating CHB patients with low levels of HBsAg concentration in blood confirms the validity of the HeberNasvac-based approach of studying all HBsAg positive patients. However, the current data in the case of HBeAg positive patients remains insufficient and in this sense additional studies and the analysis of the phase IV clinical trial results would offer a clear understanding of the potential usefulness of this product in the immune-tolerant subjects as well as in the rest of CHB patients.
9. Concluding Remarks and Five-Year Prospects
Despite vaccination efforts, CHB remains one of the significant health problems worldwide. Current CHB treatments face safety, efficacy, and effectiveness limitations, restricting their use to a small segment of patients. After a finite treatment, novel therapies aim for a “functional cure” or sustained undetectable HBsAg and HBV DNA. However, so far, product efficacy has fallen below expectations.
NUCs can be considered a safe treatment; however, the long-lasting treatment has been associated with an increased frequency of kidney and bone problems and the emergence of oncogenic mutants in viral proteins, potentially linked to the “residual” risk of HCC after long-term treatments. The damage caused by waves of viral suppression and relapse during irregular medication with NUCs affects the liver and is responsible for about 10% of the most severe form of ACLF in CHB patients. Taken together, the long-term limitations of NUC treatments should be considered in the risk-to-benefit balance, taking into account the economic and educational level of patients, reassessing the “sacrifice” of using a more reactogenic interferon treatment, averting long-term complications, and reducing the risk of HCC.
Therapeutic vaccination is a promising tool for managing CHB in many HBsAg(+) individuals. HeberNasvac R&D’s positive and negative experiences may help understand the present and shape the future of these products. The HBV DNA reduction trend detected several years post-treatment completion in all clinical trials is associated with a constant improvement of serological variables and generalized biochemical normalization and a reduced proportion of LC compared to PegIFN treatment. These results suggest that the variable “time” is a significant player in reverting the deep-rooted tolerance generated during CHB infection.
The combination of therapeutic vaccination and NUCs in add-on or switch-on protocols requires new smart designs that consider the immunological background of the target patient to allow optimal T-cell functionality under/after viral suppression. After initial drawbacks, several therapeutic vaccines are being developed enthusiastically and are expected to bring exciting results, leading to innovative health interventions in the next five years.
New candidates are characterized by a broader representation of viral antigens, where the Pre-S and core are frequently used, both as recombinant proteins and in viral vectors, used alone or in rationally designed prime-boosting approaches. The generalized use of agonists of innate immunity as part of the new formulations is a common approach in novel vaccine formulations. The “adjuvant” concept has evolved into a broad range of compounds with defined molecular targets and subsequent modes of action mechanisms. The use of TLR ligands, c-di-AMP, delta inulin, and mucoadhesives will ensure biodistribution and higher efficacy, reduce the use of syringes and the amount of antigen, and promote product user-friendliness. Special attention will be given to the stimulation of innate immunity and training to promote an optimal immune response.
Global WHO 2030 goals for mortality reduction may not be accomplished. However, a therapeutic vaccine with moderate efficacy would significantly impact CHB epidemiology. The initial use of HeberNasvac in the setting of sanitary interventions in Cuba has also involved validating PCR systems and quantitative HBsAg serology. Point-of-care serology, affordable biochemistry, and ultrasound tools will be implemented to control patient retention, treatment, and follow-up. In addition, information technologies will be required to organize and inform vaccination centers, labs, clinicians, family doctors, social workers, and patients to ensure the quality of the overall process. Although multiple limitations are on the horizon, the first experience of therapeutic vaccination on a large scale would result in a valuable experience for countries with economic limitations, where the CHB has a higher prevalence.
Author Contributions
J.C.A. and SMFA wrote the manuscript and were equal contributors. All authors participated in discussions, analysis, revision, and editions of the text. All authors have read and agreed to the published version of the manuscript.
Funding
This manuscript received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare that this review was conducted without any commercial or financial relationships that could be construed as potential conflicts of interest.
References
- World Health Organization. Global hepatitis report 2017. Geneva: WHO; 2017. Available online: https://www.who.int/publications/i/item/9789241565455 (Accessed on 30.06.2024).
- Wong, G.L.H.; Gane, E.; Lok, A.S.F. How to achieve functional cure of HBV: Stopping NUCs, adding interferon or new drug development? J Hepatol. 2022, 76(6), 1249-1262. doi: 10.1016/j.jhep.2021.11.024. [CrossRef]
- Cornberg, M.; Lok, A.S.; Terrault, N.A.; Zoulim, F.; 2019 EASL-AASLD HBV Treatment Endpoints Conference Faculty. Guidance for design and endpoints of clinical trials in chronic hepatitis B—Report from the 2019 EASL-AASLD HBV Treatment Endpoints Conference. J Hepatol. 2020, 72(3), 539-557. doi: 10.1016/j.jhep.2019.11.003. [CrossRef]
- Zheng, Y.; Chen, S.; Huang, Y.; Jiang, L.; Li, Y.; Lan, Y.; Li, S.; Xu, Y.; Li, X.; Zhao, H.; Wang, Y.; Shen, Y.; Wei, C.; Zhou, H.; Fan, R.; Zeng, X.; Jiang, M.; Song, S.; Xu, M. Prevalence and severity of HBV-associated acute-on-chronic liver failure due to irregular medication of nucleos(t)ide analogs. Infect. Microbes Dis. 2021, 3(4), 205-209. doi: 10.1097/IM9.0000000000000076. [CrossRef]
- Wong, G.L.; Lampertico, P. Residual risk of HCC during long-term oral nucleos(t)ide analogues (NUCs) in patients with CHB—Is one NUC better than the other? J Hepatol. 2019, 71(3), 453-455. doi: 10.1016/j.jhep.2019.05.017. [CrossRef]
- Al Mahtab, M.; Akbar, S.M.F.; Aguilar, J.C.; Guillen, G.; Penton, E.; Tuero, A.; Yoshida, O.; Hiasa, Y.; Onji, M. Treatment of chronic hepatitis B naïve patients with a therapeutic vaccine containing HBs and HBc antigens (a randomized, open and treatment controlled phase III clinical trial). PLoS One. 2018, 13(8): e0201236. doi: 10.1371/journal.pone.0201236. [CrossRef]
- Hong, C. Y.; Sinn, D. H.; Kang, D.; Paik, S. W.; Guallar, E.; Cho, J.; Gwak, G. Y. Incidence of extrahepatic cancers among individuals with chronic hepatitis B or C virus infection: A nationwide cohort study. J. Viral Hepat. 2019, 27(9), 896-903. doi: 10.1111/jvh.13304. [CrossRef]
- European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the Management of Hepatitis B Virus Infection. J Hepatol. 2017, 67, 370–398. doi: 10.1016/j.jhep.2017.03.021. [CrossRef]
- Ye J.; Chen J. Interferon and Hepatitis B: Current and future perspectives. Front. Immunol. 2021, 12, 733364. doi: 10.3389/fimmu.2021.733364. [CrossRef]
- Aguilar J.C.; Lobaina Y.; Muzio V.; Garcia D.; Penton E.; Iglesias E.; Pichardo D.; Urquiza D.; Rodriguez D.; Silva D.; Petrovsky N.; Guillen G. Development of a nasal vaccine for chronic hepatitis B infection that uses the ability of hepatitis B core antigen to stimulate a strong Th1 response against hepatitis B surface antigen. Immunol. Cell Biol. 2004, 82(5), 539-546. doi: 10.1111/j.0818-9641.2004.01278.x. [CrossRef]
- Mancini-Bourgine M.; Guillen G.; Michel M.L.; Aguilar J.C. Impact of the immunogen nature on the immune response against the major HBV antigens in an HBsAg and HLA-humanized transgenic mouse model. Euroasian J Hepatogastroenterol. 2014, 4(1), 36-44. doi: 10.5005/jp-journals-10018-1094. Erratum in: Euroasian J Hepatogastroenterol. 2014, 4(2), 112. PMID: 29264317. [CrossRef]
- Aguiar, J.; Marrero, M.; Figueroa, D.; Aguiar, J.; Idavoy, A.; Martinez, S.; Morán, I.; Rodríguez, M.; Canales López, E.; Hernández Esteves, I.; Silva Girado, J.A.; Estrada Vázquez, R.C.; Gell Cuesta, O.; Mendoza-Marí, Y.; Valdés Prado, I.; Rodríguez Ibarra, C.; Palenzuela Gardon, D.O.; Pentón Arias, E.; Guillén Nieto, G.; Aguilar Rubido, J.C. Preparing for the next pandemic: Increased expression of interferon-stimulated genes after local administration of Nasalferon or HeberNasvac. DNA Cell Biol. 2024, 43(2), 95-102. doi: 10.1089/dna.2023.0283. [CrossRef]
- Fleites, Y.A.; Aguiar, J.; Cinza, Z.; Bequet, M.; Marrero, E.; Vizcaíno, M.; Esquivel, I.; Diaz, M.; Sin-Mayor, A.; Garcia, M.; Martinez, S.M.; Beato, A.; Galarraga, A.G.; Mendoza-Marí, Y.; Valdés, I.; García, G.; Lemos, G.; González, I.; Canaán-Haden, C.; Figueroa, N.; Oquendo, R.; Akbar, S.M.F.; Mahtab, M.A.; Uddin, M.H.; Guillén, G.E.; Muzio, V.L.; Pentón, E.; Aguilar, J.C. HeberNasvac, a therapeutic vaccine for chronic hepatitis B, stimulates local and systemic markers of innate immunity: Potential use in SARS-CoV-2 postexposure prophylaxis. Eur. Asian J. Hepatogastroenterol. 2021, 11(2), 59-70. doi: 10.5005/jp-journals-10018-1344. [CrossRef]
- Aguilar, J.C.; Aguiar, J.; Akbar, S.M.F. Action mechanisms and scientific rationale of using nasal vaccine (HeberNasvac) for the treatment of chronic hepatitis B. Vaccines 2022, 10: 2087. doi: 10.3390/vaccines10122087. [CrossRef]
- Akbar, S.M.F.; Al Mahtab, M.; Yoshida, M.; Aguilar, J.C.; Guillen, G.; Hiasa, Y. Anti-viral and Anti-fibrotic Effect of Nasvac for Treating CHB Patients Guiding to Discontinuation of NUCs: A step of unlimited prospect and challenge for elimination of hepatitis by 2030 in developing countries. In proceedings of: BioHabana 2024, Oral Presentation. Varadero, Cuba (01-05 Apr 2024).
- Santos, I.L.; Rodriguez, C.; Cinza, Z.; Penton, E.; Freyre, F.; Guillen, G.; Aguilar, J.C. Long term evaluation of HBsAg levels in the serum of samples from chronic hepatitis b patients treated with HeberNasvac in the Phase IV Clinical Trial. In proceedings of: BioHabana 2024, Oral Presentation. Varadero, Cuba (01-05 Apr 2024).
- Akbar, S.M.F.; Al Mahtab, M.; Aguilar, J.C.; Yoshida, O.; Khan, S.; Penton, E.; Hiasa, Y. The safety and efficacy of a therapeutic vaccine for chronic hepatitis b: A follow-up study of phase III clinical trial. Vaccines 2021, 10(1), 45. doi: 10.3390/vaccines10010045. [CrossRef]
- Al Mahtab, M.; Akbar, S.M.F.; Aguilar, J.C.; Yoshida, O.; Khan, S.; Gerardo, G.N.; Hiasa, Y. Safety profile, antiviral capacity, and liver protection of a nasal therapeutic vaccine in patients with chronic hepatitis B: Five-year-follow-up outcomes after the end of treatment. Front. Med. (Lausanne) 2023, 10: 1032531. doi: 10.3389/fmed.2023.1032531. [CrossRef]
- Yoshida, O.; Akbar, S.M.F.; Imai, Y.; Sanada, T.; Tsukiyama-Kohara, K.; Miyazaki, T.; Kamishita, T.; Miyake, T.; Tokumoto, Y.; Hikita, H.; Tsuge, M.; Shimizu, M.; Al Mahtab, M.; Aguilar, J.C.; Guillen, G.; Kohara, M.; Hiasa, Y. Intranasal therapeutic vaccine containing HBsAg and HBcAg for patients with chronic hepatitis B; 18 months follow-up results of phase IIa clinical study. Hepatol. Res. 2023, 53(3), 196-207. doi: 10.1111/hepr.13851. [CrossRef]
- Yoshida, O.; Akbar, S.M.F., Imai, Y.; Sanada, T.; Tsukiyama-Kohara, K.; Miyazaki, T.; Kamishita, T.; Miyake, T.; Tokumoto, Y.; Hikita, H.; Tsuge, M.; Shimizu, M.; Al Mahtab, M.; Aguilar, J.C.; Guillen, G.; Kohara, M.; Hiasa, Y. Long term HBsAg reduction by a nasal administrative therapeutic vaccine containing HBsAg and HBcAg mixed with mucoadhesive CVP (CVP-NASVAC) in patients with chronic HBV infection: The results of 48 months follow up. In proceedings of: AASLD: The Liver Meeting. U.S.A. (10- 14, Nov, 2023).
- Kimura, T.; Rokuhara, A.; Sakamoto, Y.; Yagi, S.; Tanaka, E.; Kiyosawa, K.; Maki, N. Sensitive enzyme immunoassay for hepatitis B virus core-related antigens and their correlation to virus load. J. Clin. Microbiol. 2002, 40, 439–445. doi: 10.1128/JCM.40.2.439-445.2002. [CrossRef]
- Wong, D.K.; Tanaka, Y.; Lai, C.L.; Mizokami, M.; Fung, J.; Yuen, M.F. Hepatitis B virus core-related antigens as markers for monitoring chronic hepatitis B infection. J. Clin. Microbiol. 2007, 45, 3942–3947. doi: 10.1128/JCM.00366-07. [CrossRef]
- Suzuki, F.; Miyakoshi, H.; Kobayashi, M.; Kumada, H. Correlation between serum hepatitis B virus core-related antigen and intrahepatic covalently closed circular DNA in chronic hepatitis B patients. J. Med. Virol. 2009, 81, 27–33. doi: 10.1002/jmv.21339. [CrossRef]
- Rokuhara, A.; Tanaka, E.; Matsumoto, A.; Umemura, T.; Yoshizawa, K.; Kimura, T.; Sata, M.; Uchida, T.; Gotoh, K.; Masaki, N.; Tanaka, K.; Shinkai, N.; Nojiri, S.; Joh, T. Clinical evaluation of a new enzyme immunoassay for hepatitis B virus core-related antigen; a marker distinct from viral DNA for monitoring lamivudine treatment. J. Viral Hepat. 2003, 10, 324–330. doi: 10.1046/j.1365-2893.2003.00437.x. [CrossRef]
- Tanaka, E.; Matsumoto, A.; Yoshizawa, K.; Tsuji, K.; Yatsuhashi, H.; Okanoue, T.; Iino, S.; Tanaka, Y.; Kumada, H. Hepatitis B core-related antigen assay is useful for monitoring the antiviral effects of nucleoside analogue therapy. Intervirology 2008, 51 (Suppl 1), 3–6. doi: 10.1159/000122592. [CrossRef]
- Cheung, K.S.; Seto, W.K.; Wong, D.K.; Lai, C.L.; Yuen, M.F. Relationship between hepatocellular carcinoma development and serum viral markers in chronic hepatitis B patients who achieved undetectable serum HBV DNA while on long-term nucleoside analogue therapy. Hepatology 2015, 62 (Suppl 1), 273A. AASLD abstract.
- Matsumoto, A.; Tanaka, E.; Minami, M.; Okanoue, T.; Yatsuhashi, H.; Komatsu, T.; Suzuki, F.; Itoh, Y.; Maki, N.; Akahane, Y.; Kiyosawa, K.; Tanaka, Y. Low serum level of hepatitis B core-related antigen indicates unlikely reactivation of hepatitis after cessation of lamivudine therapy. Hepatol. Res. 2007, 37, 661–666. doi: 10.1111/j.1872-034X.2007.00094.x. [CrossRef]
- Shinkai, N.; Tanaka, Y.; Orito, E.; Ito, K.; Ohno, T.; Hirashima, N.; Hasegawa, I.; Sugauchi, F.; Ueda, R.; Mizokami, M. Measurement of hepatitis B virus core-related antigen as predicting factor for relapse after cessation of lamivudine therapy for chronic hepatitis B virus infection. Hepatol. Res. 2006, 36, 272–276. doi: 10.1016/j.hepres.2006.08.005. [CrossRef]
- Lok A.S.F. Toward a functional cure for hepatitis B. Gut Liver. Published online March 27, 2024. doi:10.5009/gnl240023. [CrossRef]
- Buster, E.H.C.J.; Flink, H.J.; Cakaloglu, Y.; Simon, K.; Trojan, J.; Tabak, F.; So, T.M.K.; Feinman, S.V.; Mach, T.; Akarca, U.S.; Schutten, M.; Tielemans, W.; van Vuuren, A.J.; Hansen, B.E.; Janssen, H.L.A. Sustained HBeAg and HBsAg loss after long-term follow-up of HBeAg-positive patients treated with peginterferon alpha-2b. Gastroenterology 2008, 135, 459-467. doi:10.1053/j.gastro.2008.05.031. [CrossRef]
- Marcellin, P.; Bonino, F.; Yurdaydin, C.; Hadziyannis, S.; Moucari, R.; Kapprell, H.P.; Rothe, V.; Popescu, M.; Brunetto, M.R. Hepatitis B surface antigen levels: association with 5-year response to peginterferon alfa-2a in hepatitis B e-antigen-negative patients. Hepatol. Int. 2013, 7, 88-97. doi:10.1007/s12072-012-9343-x. [CrossRef]
- Jeng, W.J.; Lok, A.S. Should treatment indications for chronic hepatitis B be expanded?. Clin. Gastroenterol. Hepatol. 2021, 19, 2006-2014. doi:10.1016/j.cgh.2020.04.091. [CrossRef]
- Flink, H.J.; van Zonneveld, M.; Hansen, B.E.; de Man, R.A.; Schalm, S.W.; Janssen, H.L.A. Treatment with Peg-interferon alpha-2b for HBeAg-positive chronic hepatitis B: HBsAg loss is associated with HBV genotype. Am. J. Gastroenterol. 2006, 101, 297-303. doi:10.1111/j.1572-0241.2006.00418.x. [CrossRef]
- Tamori, A.; Enomoto, M.; Kobayashi, S.; Iwai, S.; Morikawa, H.; Sakaguchi, H.; Kawada, N. Add-on combination therapy with adefovir dipivoxil induces renal impairment in patients with lamivudine-refractory hepatitis B virus. J Viral Hepat 2010, 17(2), 123–129. doi:10.1111/j.1365-2893.2009.01160.x. [CrossRef]
- Hartono, J.L.; Aung, M.O.; Dan, Y.Y.; Gowans, M.; Lim, K.; Lee, Y.M.; Chow, W.C.; Lim, S.G. Resolution of adefovir-related nephrotoxicity by adefovir dose-reduction in patients with chronic hepatitis B. Aliment. Pharmacol. Ther. 2013, 37(7), 710-719. doi:10.1111/apt.12251. [CrossRef]
- Thu, A.M.; Poovorawan, K.; Kittitrakul, C.; Sukeepaisarnjaroen, W.; Tangkijvanich, P.; Tanwandee, T.; Sombat, S.; Treeprasertsuk, S.; Tanaka, Y.; Mizokami, M. Nephrotoxicity caused by oral antiviral agents in patients with chronic hepatitis B treated in a hospital for tropical diseases in Thailand. BMC Pharmacol. Toxicol. 2015, 16, 38. doi:10.1186/s40360-015-0037-6. [CrossRef]
- Buti, M.; Tsai, N.; Petersen, J.; Flisiak, R.; Gurel, S.; Krastev, Z.; Shiffman, M.L.; Marcellin, P.; Janssen, H.L.A.; Craxi, A.; Diago, M.; Moreno, C.; Schiff, E.; Berg, T.; Mo, S.; Hadziyannis, S.; Wolf, E.; McCloud, P.; Baumann, U.; Gallegos-Orozco, J.F.; Fung, S.; Chang, T.T.; Xie, Q.; Colombo, M. Seven-year efficacy and safety of treatment with tenofovir disoproxil fumarate for chronic hepatitis B virus infection. Dig. Dis. Sci. 2015, 60(5), 1457–1464. doi:10.1007/s10620-014-3486-7. [CrossRef]
- Baran, B.; Soyer, O.M.; Ormeci, A.C.; Gokturk, S.; Evirgen, S.; Bozbey, H.U.; Idilman, R.; Yurdaydin, C.; Karasu, Z.; Akarca, U.S.; Tozun, N.; Kaymakoglu, S.; Badur, S.; Ustun, Y.; Demir, K.; Besisik, F.; Cakaloglu, Y.; Kayacetin, E.; Sengul, C.; Yalcin, K.; Tuncer, I.; Simsek, H.; Balik, I.; Erden, E.; Heper, A.O.; Ozdil, S.; Gunsar, F.; Mungan, Z.; Yilmaz, S.; Gurel, S.; Senturk, H.; Purnak, T.; Koruk, M.; Tukek, T.; Tabak, F.; Mert, A.; Ozbay, G.; Mert, D.; Dolar, E.; Kiyici, M.; Gulten, M.; Dizdar, O.S.; Keskin, O.; Arslan, S.; Kose, S.; Tuncbilek, S.; Aygen, B.; Sener, A.; Ural, O.; Gulluoglu, M.; Akdogan, M.; Sokmensuer, C.; Tokat, Y.; Yuzer, Y.; Kalayci, C.; Karasu, Z.; Ozdogan, O.; Bozdayi, A.M.; Degertekin, H.; Tozun, N. Efficacy of tenofovir in patients with Lamivudine failure is not different from that in nucleoside/nucleotide analogue-naive patients with chronic hepatitis B. Antimicrob. Agents Chemother. 2013, 57(4), 1790-1796. doi:10.1128/AAC.02600-12. [CrossRef]
- Baxi, S.M.; Scherzer, R.; Greenblatt, R.M.; Minkoff, H.; Sharma, A.; Young, M.; Cohen, M.; Szczech, L.A.; Feldman, J.G.; Lin, F.; Delapenha, R.; Terrell, F.; Gange, S.J.; Norris, P.J.; Weiser, S.D.; Tien, P.C. Higher tenofovir exposure is associated with longitudinal declines in kidney function in women living with HIV. AIDS 2016, 30, 609–618. doi:10.1097/QAD.0000000000000958. [CrossRef]
- Hashwin Singh, T.S.; Jashwin Singh, T.S.; Chin, K.-Y. Effects of tenofovir disoproxil fumarate on bone quality beyond bone density—a scoping review of the literature. Pharmaceuticals 2024, 17, 146. doi:10.3390/ph17020146. [CrossRef]
- Bourgine, M.; Crabe, S.; Lobaina, Y.; Guillen, G.; Aguilar, J.C.; Michel, M.L. Nasal route favors the induction of CD4+ T cell responses in the liver of HBV-carrier mice immunized with a recombinant hepatitis B surface-and core-based therapeutic vaccine. Antiviral Res 2018, 153, 23-32. doi:10.1016/j.antiviral.2018.02.019. [CrossRef]
- Freyre, F.M.; Aguiar, J.A.; Cinza, Z.; Figueroa, N.; Diaz, P.A.; Muzio, V.L.; Lemos, G.; Freyre, G.; Coizeau, E.; Rodríguez, C.; Pentón, E.; Campos, M.; Santos, I.L.; Mahtab, M.A.; Akbar, S.M.F.; Guillen, G.E.; Aguilar, J.C. Impact of the route and schedule of immunization on the serological and virological response of chronic hepatitis B patients treated with Heber-Nasvac. Euroasian J Hepato-Gastroenterol 2023, 13(2), 73-78. doi:10.5005/jp-journals-10018-1402. [CrossRef]
- Aguiar J.; Rodriguez L.; León Y.; Freyre F.; Silva J.A.; Montalvo M.C.; Gell O.; Estrada R.; Canales E.; Muzio V.; Guillén G.; Pentón E.; Aguilar J.C. Hepatitis B virus DNA quantification using a cost effective and simple real time PCR in Cuban carriers. J Infect Dis Ther. 2014; 2(1):49-58. No DOI available.
- Aguiar, J.; García, G.; León, Y.; Canales, E.; Silva, J.A.; Gell, O.; Estrada, R.; Morán, I.; Muzio, V.; Guillén, G.; Pentón, E.; Aguilar, J.C. High functional stability of a low-cost HBV DNA qPCR primer pair and plasmid standard. Eur. J. Hepato-Gastroenterol. 2016, 6(1), 19-24. doi:10.5005/jp-journals-10018-1160. [CrossRef]
- Aguiar, J.; Silva, J.A.; García, G.; Guillén, G.; Aguilar, J.C. Cross-validation studies of a novel low-cost hepatitis B virus quantitative polymerase chain reaction system. Eur. J. Hepato-Gastroenterol. 2018, 8(1), 38. doi:10.5005/jp-journals-10018-1255. [CrossRef]
- Fernández, G.; Sanchez, A.L.; Jerez, E.; Anillo, L.E.; Freyre, F.; Aguiar, J.A.; Leon, Y.; Cinza, Z.; Diaz, P.A.; Figueroa, N.; Muzio, V.; Guillen, G.; Lobaina, Y.; Aguilar, A.; Penton, E.; Aguilar, J.C. Five-year follow-up of chronic hepatitis B patients immunized by nasal route with the therapeutic vaccine HeberNasvac. Eur. J. Hepato-Gastroenterol. 2018, 8(2), 133-139. doi:10.5005/jp-journals-10018-1279. [CrossRef]
- Al-Mahtab, M.; Rahman, S.; Khan, M.; Al Mamun, A. HBeAg negative chronic hepatitis B with persistently normal serum transaminase and low HBV DNA can cause significant liver disease. Indian J. Gastroenterol. 2007, 26(6), 297. No doi available.
- Rahman, S.; Khan, M.; Mahtab, M. Precore/core promoter mutant hepatitis b virus produces more severe histologic liver disease than wild type hepatitis b virus. Hung. Med. J. 2007, 1(1), 41-46. No doi available. [CrossRef]
- Chu, C.; Liaw, Y. Spontaneous relapse of hepatitis in inactive HBsAg carriers. Hepatol. Int. 2007, 1(2), 311-315. doi: 10.1007/s12072-007-9002-9. [CrossRef]
- Lee, H.W.; Lee, J.I.; Kim, S.; Chang, H.Y.; Lee, K.S. Cumulative incidence of hepatocellular carcinoma and hepatitis B surface antigen seroclearance after nucleos(t)ide analogue-induced hepatitis B e antigen seroclearance. BMC Gastroenterol. 2020, 20, 113. doi:10.1186/s12876-020-01236-9. [CrossRef]
- Sou, F.M.; Hu, T.H.; Hung, C.H.; Lai, H.C.; Wang, J.H.; Lu, S.N., et al. Incidence and predictors of hepatocellular carcinoma beyond year 5 of ETV therapy in chronic hepatitis B patients. Hepatol Int. 2020, 14, 513-520. doi: 10.1007/s12072-020-10031-3. [CrossRef]
- Liang, K.H.; Hsu, C.W.; Chang, M.L.; Chen, Y.C.; Lai, M.W.; Yeh, C.T. Peginterferon is superior to nucleos(t)ide analogues for prevention of hepatocellular carcinoma in chronic hepatitis B. J. Infect. Dis. 2016, 213, 966-974. doi: 10.1093/infdis/jiv547. [CrossRef]
- Ren, P.; Cao, Z.; Mo, R.; Liu, Y.; Chen, L.; Li, Z., et al. Interferon-based treatment is superior to nucleos(t)ide analog in reducing HBV-related hepatocellular carcinoma for chronic hepatitis B patients at high risk. Expert Opin. Biol. Ther. 2018, 18, 1085-1094. doi: 10.1080/14712598.2018.1518423. [CrossRef]
- Kim, J.H.; Sinn, D.H.; Kang, W.; Gwak, G.Y.; Paik, Y.H.; Choi, M.S.; Lee, J.H.; Koh, K.C.; Paik, S.W. Low-level viremia and the increased risk of hepatocellular carcinoma in patients receiving ETV treatment. Hepatology. 2017, 66(2), 335-343. doi: 10.1002/hep.28916. [CrossRef]
- Jiang, S.; Guo, S.; Huang, Y.; Xu, J.; Li, Y.; Zeng, Y.; Xie, Q. Interim analysis of the PARADISE study: benefits of add-on peginterferon-α in NA-treated patients with CHB. Antiviral Res. 2024, 226: 105892. doi: 10.1016/j.antiviral.2024.105892. [CrossRef]
- Pegasys (Pegylated Interferon alfa 2a), Injection for subcutaneous use. Hoffmann-La Roche Inc.; September 2011. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/103964s5204lbl.pdf. (Accessed on 30.06.2024).
- Baraclude (Entecavir), Tablets 0.5 mg, Antiviral. Bristol-Myers Squibb Canada; September 23, 2020. Available online: https://www.bms.com/assets/bms/ca/documents/productmonograph/BARACLUDE_EN_PM.pdf. (Accessed on 30.06.2024).
- Viread (Tenofovir disoproxil fumarate) Tablets: 150, 200, 250 and 300 mg; Oral powder: 40 mg per 1 g of oral powder, Antiviral. Gilead Sciences Inc.; January 2012. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022577lbl.pdf. (Accessed on 30.06.2024).
- Brown, J.A.; Pack, L.R.; Fowler, J.D.; Suo, Z. Presteady state kinetic investigation of the incorporation of anti-hepatitis B nucleotide analogues catalyzed by noncanonical human DNA polymerases. Chem. Res. Toxicol. 2012, 25(1), 225–233. doi: 10.1021/tx200458s. [CrossRef]
- Jiang, L.; Wu, X.; He, F.; Liu, Y.; Hu, X.; Takeda, S.; Qing, Y. Genetic evidence for genotoxic effect of entecavir, an anti-hepatitis B virus nucleotide analog. PLoS ONE 2016, 11(1), e0147440. doi: 10.1371/journal.pone.0147440. [CrossRef]
- Moriyama, M.; Tateishi, R.; Kinoshita, M.N.; Fukumoto, T.; Yamada, T.; Wake, T.; Nakagawa, H.; Fujinaga, H.; Asaoka, Y.; Tanaka, Y.; Otsuka, M.; Koike, K. Impact of nucleos(t)ide analogues on the risk of hepatocellular carcinoma in chronic hepatitis B patients: A time-dependent Cox regression analysis. medRxiv 2023-09. doi: https://doi.org/10.1101/2023.09.27.23296247. [CrossRef]
- Wong, G.H.; Tse, Y.K.; Yip, T.F.; Chan, H.Y.; Tsoi, K.F.; Wong, V.S. Long-term use of oral nucleos(t)ide analogues for chronic hepatitis B does not increase cancer risk–a cohort study of 44,494 subjects. Aliment. Pharmacol. Ther. 2017, 45(9), 1213-1224. doi: 10.1111/apt.14015. [CrossRef]
- Höner zu Siederdissen, C.; Hui, A.J.; Sukeepaisarnjaroen, W.; Tangkijvanich, P.; Su, W.W.; Nieto, G.E.G.; Crabé, S.; Stepien, S.; Manns, M.P.; Wedemeyer, H.; Cornberg, M. Contrasting timing of virological relapse after discontinuation of tenofovir or entecavir in hepatitis B e antigen–negative patients. J. Infect. Dis. 2018, 218(9), 1480-1484. doi: 10.1093/infdis/jiy350. [CrossRef]
- Fontaine, H.; Kahi, S.; Chazallon, C.; Bourgine, M.; Varaut, A.; Buffet, C.; Godon, O.; Meritet, J.F.; Saïdi, Y.; Michel, M.L.; Scott-Algara, D.; Aboulker, J.P.; Pol, S. Anti-HBV DNA vaccination does not prevent relapse after discontinuation of analogues in the treatment of chronic hepatitis B: A randomised trial–ANRS HB02 VAC-ADN. Gut 2015, 64, 139–147. doi: 10.1136/gutjnl-2013-305707. [CrossRef]
- Vandepapeliere, P.; Lau, G.K.; Leroux-Roels, G.; Horsmans, Y.; Gane, E.; Tawandeee, T.; bin Merican, M.I.; Wing, K.M.; Trepo, C.; Cooksley, G. Therapeutic vaccination of chronic hepatitis B patients with virus suppression by antiviral therapy: A randomized, controlled study of co-administration of HBsAg/AS02 candidate vaccine and lamivudine. Vaccine 2007, 25, 8585–8597. doi: 10.1016/j.vaccine.2007.09.072. [CrossRef]
- Papatheodoridis, G.; Vlachogiannakos, I.; Cholongitas, E.; Wursthorn, K.; Thomadakis, C.; Touloumi, G.; Petersen, J. Discontinuation of oral antivirals in chronic hepatitis B: A systematic review. Hepatology 2016, 63, 1481–1492. doi: 10.1002/hep.28438. [CrossRef]
- Hadziyannis, S.J.; Sevastianos, V.; Rapti, I.; Vassilopoulos, D.; Hadziyannis, E. Sustained responses and loss of HBsAg in HBeAg-negative patients with chronic hepatitis B who stop long-term treatment with adefovir. Gastroenterology 2012, 143, 629–636. doi: 10.1053/j.gastro.2012.05.039. [CrossRef]
- van Bömmel, F.; Stein, K.; Heyne, R.; Petersen, J.; Buggisch, P.; Berg, C.; Berg, T. A multicenter randomized-controlled trial of nucleos(t)ide analogue cessation in HBeAg-negative chronic hepatitis B. J. Hepatol. 2023, 78(5), 926-936. doi: 10.1016/j.jhep.2022.12.018. [CrossRef]
- Höner Zu Siederdissen, C.; Rinker, F.; Maasoumy, B.; Wiegand, S.B.; Filmann, N.; Falk, C.S.; Deterding, K.; Port, K.; Mix, C.; Manns, M.P. Viral and host responses after stopping long-term nucleos(t)ide analogue therapy in HBeAg-negative chronic hepatitis B. J. Infect. Dis. 2016, 214, 1492–1497. doi: 10.1093/infdis/jiw412. [CrossRef]
- Akbar, S.M.F.; Yoshida, O.; Chen, S.; Cesar, A.J.; Abe, M.; Matsuura, B.; Hiasa, Y.; Onji, M. Immune modulator and antiviral potential of dendritic cells pulsed with both hepatitis B surface Ag and core antigen for treating chronic HBV infection. Antivir. Ther. 2010, 15, 887–895. doi: 10.3851/IMP1637. [CrossRef]
- Lobaina, Y.; Hardtke, S.; Wedemeyer, H.; Aguilar, J.C.; Schlaphoff, V. In vitro stimulation with HBV therapeutic vaccine candidate Nasvac activates B and T cells from chronic hepatitis B patients and healthy donors. Mol. Immunol. 2015, 63, 320–327. doi: 10.1016/j.molimm.2014.08.003. [CrossRef]
- Flink, H.J.; Sprengers, D.; Hansen, B.E.; van Zonneveld, M.; De Man, R.A.; Schalm, S.W.; Janssen, H.L.A. Flares in chronic hepatitis B patients induced by the host or the virus? Relation to treatment response during Peg-interferon α-2b therapy. Gut 2005, 54(11), 1604-1609. doi: 10.1136/gut.2004.062208. [CrossRef]
- Aguilar, J.C. On the future of therapeutic vaccination in chronic hepatitis B. Future Sci. OA 2019, 5(10), FSO426. doi: 10.2144/fsoa-2019-0104. [CrossRef]
- Lim, S.G.; Baumert, T.F.; Boni, C.; Gane, E.; Levrero, M.; Lok, A.S.; Zoulim, F. The scientific basis of combination therapy for chronic hepatitis B functional cure. Nat. Rev. Gastroenterol. Hepatol. 2023, 20(4), 238-253. doi: 10.1038/s41575-022-00724-5. [CrossRef]
- Jeng, W.J.; Lok, A.S. How to achieve a functional cure for chronic hepatitis B infection. Clin. Liver Dis. 2024, 23(1), e0134. doi: 10.1097/CLD.0000000000000134. [CrossRef]
- Boni, C.; Janssen, H.L.; Rossi, M.; Yoon, S.K.; Vecchi, A.; Barili, V.; Ferrari, C. Combined GS-4774 and tenofovir therapy can improve HBV-specific T-cell responses in patients with chronic hepatitis. Gastroenterology 2019, 157(1), 227-241. doi: 10.1053/j.gastro.2019.03.044. [CrossRef]
- Knolle, P.A. Staying local-antigen presentation in the liver. Curr. Opin. Immunol. 2016, 40, 36–42. doi: 10.1016/j.coi.2016.02.009. [CrossRef]
- Krawitt, E.L.; Zannier, A.; Chossegros, P.; Desmet, V.J. Expression of HLA antigens and T-cell infiltrates in chronic viral hepatitis. A comparison of biopsy and fine-needle aspiration findings. J. Hepatol. 1991, 12(2), 190–194. doi: 10.1016/0168-8278(91)90937-7. [CrossRef]
- Van den Oord, J.J.; de Vos, R.; Desmet, V.J. In situ distribution of major histocompatibility complex products and viral antigens in chronic hepatitis B virus infection: evidence that HBc-containing hepatocytes may express HLA-DR antigens. Hepatology 1986, 6(5), 981–989. doi: 10.1002/hep.1840060529. [CrossRef]
- Uzun, Y.; Bozkaya, H.; Erden, E. Hepatitis B core antigen expression pattern reflects the response to anti-viral treatment. J. Gastroenterol. Hepatol. 2006, 21(6), 977–981. doi: 10.1111/j.1440-1746.2006.04263.x. [CrossRef]
- Betancourt, A.A.; Delgado, C.G.; Estévez, Z.C.; Martínez, J.C.; Ríos, G.V.; Aureoles-Roselló S.M.; Rubido J.A. Phase I clinical trial in healthy adults of a nasal vaccine candidate containing recombinant hepatitis B surface and core antigens. Int. J. Infect. Dis. 2007, 11(5), 394-401. doi: 10.1016/j.ijid.2006.09.010. [CrossRef]
- Al-Mahtab, M.; Akbar, S.M.F.; Aguilar, J.C.; Uddin, M.H.; Khan, M.S.; Rahman, S. Therapeutic potential of a combined hepatitis B virus surface and core antigen vaccine in patients with chronic hepatitis B. Hepatol. Int. 2013, 7(4), 981–989. doi: 10.1007/s12072-013-9486-4. [CrossRef]
- Al-Mahtab, M.; Akbar, S.M.F.; Yoshida, O.; Aguilar, J.C.; Guillen, G.; Hiasa, Y. Antiviral response across genotypes after treatment of chronic hepatitis B patients with the therapeutic vaccine Nasvac or Pegylated Interferon. Vaccines 2023, 11(5), 962. doi: 10.3390/vaccines11050962. [CrossRef]
- HeberNasvac: Resumen de las Caracteristicas del Producto (RCP) CECMED. Available online: https://www.cecmed.cu/sites/default/files/adjuntos/rcp/biologicos/rcp_hebernasvac_0.pdf. (Accessed on 30.06.2024).
- HeberNasvac Phase IV in chronic hepatitis B. Cuban public registry of clinical trials. RPCEC. Available online: https://rpcec.sld.cu/en/trials/RPCEC00000283-En (Accessed on 30.06.2024).
- Aguiar, J.; Rodriguez, L.; León, Y.; Freyre, F.; Silva, J.A.; Montalvo, M.C.; Omar, Gell; Estrada, R.; Canales, E.; Muzio, V.; Guillén, G.; Pentón, E.; Aguilar, J.C. Hepatitis B virus DNA quantification using a cost effective and simple real time PCR in Cuban carriers. J. Infect. Dis. Ther. 2014, 2(1), 49-58. No DOI available. E-ISSN: 2310-9386/14.
- Aguiar, J.; García, G.; León, Y.; Canales, E.; Silva, J.A.; Gell, O.; Estrada, R.; Muzio, V.; Guillén, G.; Pentón, E.; Aguilar, J.C. High functional stability of a low-cost HBV DNA qPCR primer pair and plasmid standard. Euroasian J. Hepato-Gastroenterol. 2016, 6(1), 19. doi: 10.5005/jp-journals-10018-1160. [CrossRef]
- Aguilar, J.C.; León, Y.; Lobaina, Y.; Freyre, F.; Fernández, G.; Sanchez, A.L.; Penton, E. Five-year follow-up of chronic Hepatitis B patients immunized by nasal route with the therapeutic vaccine HeberNasvac. Euroasian J. Hepato-Gastroenterol. 2019, 8(2), 133-139. doi: 10.5005/jp-journals-10018-1279. [CrossRef]
- Shiraishi, K.; Yoshida, O.; Imai, Y.; Akbar, S.M.F.; Sanada, T.; Kohara, M.; Miyazaki, T.; Kamishita, T.; Miyake, T.; Hirooka, M.; Tokumoto, Y.; Abe, M.; Rubido, J.C.A.; Nieto, G.G.; Hiasa, Y. Intranasal HBsAg/HBcAg-containing vaccine induces neutralizing Anti-HBs production in hepatitis B vaccine non-responders. Vaccines (Basel) 2023, 11(9), 1479. doi: 10.3390/vaccines11091479. [CrossRef]
- Lirussi, D.; Weissmann, S.F.; Ebensen, T.; Nitsche-Gloy, U.; Franz, H.B.; Guzmán, C.A. Cyclic di-adenosine monophosphate: a promising adjuvant candidate for the development of neonatal vaccines. Pharmaceutics 2021, 13(2), 188. doi: 10.3390/pharmaceutics13020188. [CrossRef]
- Sanchez-Alberti, A.; Bivona, A.E.; Matos, M.N.; Cerny, N.; Schulze, K.; Weißmann, S.; Ebensen, T, González, G, Morales, C, Cardoso, A.C.; Cazorla, S.I.; Guzmán, C.A.; Malchiodi, E.L. Mucosal heterologous prime/boost vaccination induces polyfunctional systemic immunity, improving protection against Trypanosoma cruzi. Front. Immunol. 2020, 11, 128. doi: 10.3389/fimmu.2020.00128. [CrossRef]
- Cheng, X.; Ning, J.; Xu, X.; Zhou, X. The role of bacterial cyclic di-adenosine monophosphate in the host immune response. Front. Microbiol. 2022, 13, 958133. doi: 10.3389/fmicb.2022.958133. [CrossRef]
- Ebensen, T.; Arntz, A.; Schulze, K.; Hanefeld, A.; Guzmán, C.A.; Scherließ, R. Pulmonary application of novel antigen-loaded chitosan nanoparticles co-administered with the mucosal adjuvant c-di-AMP resulted in enhanced immune stimulation and dose sparing capacity. Pharmaceutics. 2023, 15, 1238. doi: 10.3390/pharmaceutics15041238. [CrossRef]
- Mauras, A. Optimization of the HeberNasvac’s immune response in a therapeutic vaccination scheme. In proceedings of: Biohabana 2024. Varadero, Cuba (01-05 Apr 2024).
- Gane, E.; Verdon, D.J.; Brooks, A.E.; Gaggar, A.; Nguyen, A.H.; Subramanian, G.M.; et al. Anti-PD-1 blockade with nivolumab with and without therapeutic vaccination for virally suppressed chronic hepatitis B: a pilot study. J. Hepatol. 2019, 71(5), 900–907. doi: 10.1016/j.jhep.2019.06.028. [CrossRef]
- Kim, C.G.; Kim, C.; Yoon, S.E.; Kim, K.H.; Choi, S.J.; Kang, B.; Kim, H.R.; Park, S.H.; Shin, E.C.; Kim, Y.Y.; Kim, D.J.; Chung, H.C.; Chon, H.J.; Choi, H.J.; Lim, H.Y. Hyperprogressive disease during PD-1 blockade in patients with advanced hepatocellular carcinoma. J. Hepatol. 2021, 74(2), 350–359. doi: 10.1016/j.jhep.2020.08.010. [CrossRef]
- Lau, G.; Yu, M.L.; Wong, G.; Thompson, A. Are immune-checkpoint inhibitors immunosuppressive to hepatitis B virus? Hepatol Int. 2022, 16, 482–483. doi: 10.1007/s12072-022-10318-7. [CrossRef]
- Tait, D. A phase 2b, open-label study to evaluate the efficacy, safety, tolerability, immunogenicity and treatment regimens of VTP-300 combined with low-dose Nivolumab in chronic hepatitis b infection. In proceedings of: The Liver Meeting. Boston, MA. U.S.A. (10–14, Nov. 2023).
- VBI vaccines partner Brii Biosciences announces topline interim results of phase 2 study evaluating BRII-179 (VBI-2601) in combination with PEG-IFNα for the Treatment of Chronic Hepatitis B. (Press release). Available online: https://www.vbivaccines.com/press-releases/vbi-vaccines-partner-brii-biosciences-announces-topline-interim-results-of-phase-2-study-evaluating-brii-179-vbi-2601-in-combination-with-peg-ifn-for-the-treatment-of-chronic-hepatitis-b/ (Accessed on 30.06.2024).
- Cooper P.D.; Petrovsky N. Delta inulin: a novel, immunologically active, stable packing structure comprising β-D-[2 → 1] poly(fructo-furanosyl) α-D-glucose polymers. Glycobiology. 2011, 21(5), 595–606. https://doi.org/10.1093/glycob/cwq201. [CrossRef]
- Stewart, E.L.; Counoupas, C.; Steain, M.; Ashley, C.; Alca, S.; Hartley-Tassell, L.; von Itzstein, M.; Britton, W.J.; Petrovsky, N.; Triccas, J.A. Dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN) is a cellular receptor for delta inulin adjuvant. Immunol Cell Biol. 2024 May 17. Epub ahead of print. doi: 10.1111/imcb.12774. [CrossRef]
- Su, J.; Brunner, L.; Ates, Oz, E.; Sacherl, J.; Frank, G.; Kerth, H.A.; Thiele, F.; Wiegand, M.; Mogler, C.; Aguilar, J.C.; Knolle, P.A.; Collin, N.; Kosinska, A.D.; Protzer, U. Activation of CD4 T cells during prime immunization determines the success of a therapeutic hepatitis B vaccine in HBV-carrier mouse models. J. Hepatol. 2023, 78(4), 717-730. doi: 10.1016/j.jhep.2022.12.013. [CrossRef]
- Kosinska, A.D.; Festag, J.; Mück-Häusl, M.; Festag, M.M.; Asen, T.; Protzer, U. Immunogenicity and antiviral response of therapeutic hepatitis B vaccination in a mouse model of HBeAg-negative, persistent HBV infection. Vaccines (Basel). 2021, 9(8), 841. doi: 10.3390/vaccines9080841. [CrossRef]
- Sacherl, J.; Kosinska, A.D.; Kemter, K.; Kächele, M.; Laumen, S.C.; Kerth, H.A.; Öz, E.A.; Wolff, L.S.; Su, J.; Essbauer, S.; Sutter, G.; Scholz, M.; Singethan, K.; Altrichter, J.; Protzer, U. Efficient stabilization of therapeutic hepatitis B vaccine components by amino-acid formulation maintains its potential to break immune tolerance. JHEP Rep. 2022, 5(2), 100603. doi: 10.1016/j.jhepr.2022.100603. [CrossRef]
- Liu, Y.C.; Jeng, W.J. Should Indications for Antiviral Therapy for Hepatitis B Be Broadened to Include Immune-Tolerant Patients, Inactive Carriers, or Patients in the “Gray Zone”?. Curr. Hepatology Rep. 2024, 23, 11–21. doi: https://doi.org/10.1007/s11901-024-00635-w. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).