Submitted:
16 August 2024
Posted:
20 August 2024
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. Materials and Methods
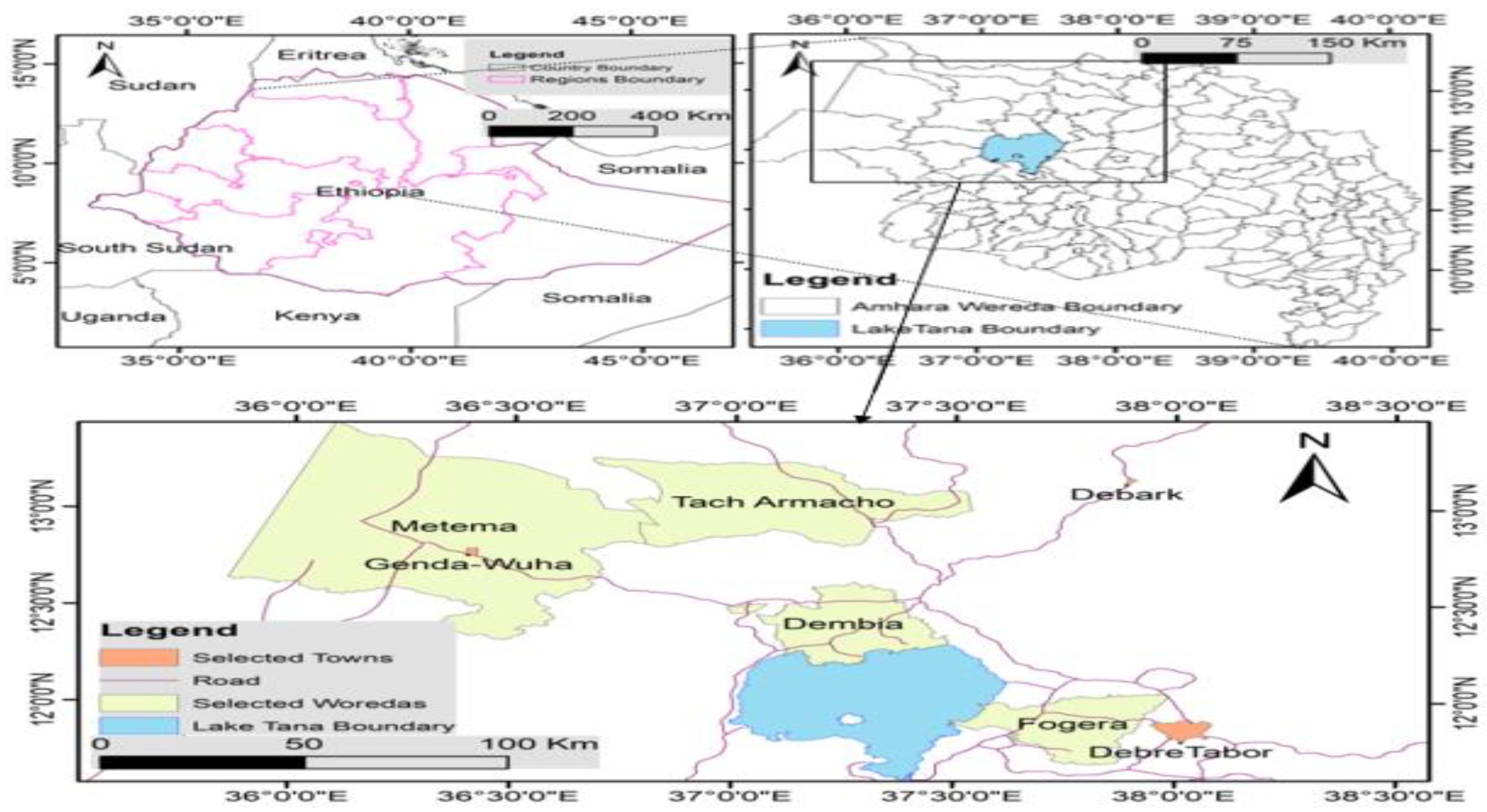
2.1. Description of the Study Area
2.2. Study Population
2.3. Sampling Methods
2.4. Design of the Questionnaire and Data Collection
| Variable | Levels |
|---|---|
| Do you know rabies existence? | No/Yes |
| Do you know rabies affects human? | No/Yes |
| Do you know way of transmission of rabies? | No/Yes |
| Do you have a dog(s)? | No/Yes |
| Do you know dog as a predisposing risk factor of rabies? | No/Yes |
| Do you believe traditional medicine can treat rabies? | No/Yes |
| Do you vaccinate your dog against rabies? | No/Yes |
| Do you perceive rabies as severe/fatal? | No/Yes |
| Do you or member of family ever bitten by rabid animal | No/Yes |
| What was the species of the animal that bite you or your family? | Dog/cat/Equines/wild carnivore. |
| Do you wash the bite wound | No/Yes |
| Do you know presence of post exposure prophylaxis (PEP)? | No/Yes |
| Do you or your family ever take PEP | No/Yes |
| Do your dog contact with other dogs frequently | No/Yes |
| Do you castrate your dog (s) | No/Yes |
| Is there any wild carnivore nearby your area? | No/Yes |
2.5. Sample Size Determination
2.6. Data Analysis
3. Results
3.1. Respondents’ Awareness and Management Of Rabies
3.3. Sources of Rabies Exposure
3.4. Risk Factors Associated with Rabies Exposure
3.5. Multivariate Logistic Regression Model for Risk Association of Rabies Exposure
4. Discussion
5. Conclusion and Recommendations
Author Contributions
Funding
Ethical Approval and Consent to Participate
Informed Consent
Data Sharing Statement
Acknowledgments
Conflicts of Interest
References
- Hayman, DTS., Johnson, N., Horton, DL., Hedge, J., Wakeley, PR., Banyard, AC., Zhang, S., Alhassan, A., Fooks, AR. (2011) Evolutionary History of Rabies in Ghana. PLOS Neglected Tropical Diseases 5(4). [CrossRef]
- Coetzer, A., Gwenhure, L., Makaya, P., Markotter, W. & Nel, L. (2019). Epidemiological aspects of the persistent transmission of rabies during an outbreak (2010 - 2017) in Harare, Zimbabwe. PLoS One, 14, e0210018.
- Hampson, K., Coudeville, L., Lembo, T., Sambo, M., Kieffer, A., Attlan M, Barrat J, Blanton JD, Briggs DJ, Cleaveland S, Costa P, Freuling CM, Hiby E, Knopf, L., Leanes F., Meslin, FX., Metlin, A., Miranda, ME., Müller, T., Nel, LH., Recuenco, S., Rupprecht, CE., Schumacher, C., Taylor, L., Vigilato, MA., Zinsstag, J., Dushoff, J. (2015) Estimating the Global Burden of Endemic Canine Rabies. PLOS Neglected Tropical Diseases 9(4): e0003709. [CrossRef]
- Al-Mustapha AI, Tijani AA, Bamidele FO, Muftau O, Ibrahim A, Abdulrahim I, et al. (2021). Awareness and knowledge of canine rabies: A state-wide cross-sectional study in Nigeria. PLoS ONE 16(3): e0247523. [CrossRef]
- Jackson, A.C. (2007). Human disease and pathogenesis. In: Jackson AC, Wunner WH, (eds). Rabies. Second edition. Academic Press, London, UK. pp. 309–333 and 341-381.
- Ugolini G. (2008). Use of rabies virus as a trans-neuronal tracer of neuronal connections: implications for the understanding of rabies pathogenesis. Dev Biol (Basel).131:493-506. [PubMed]
- Mitrabhakdi, E., Shuangshoti, S., Wannakrairot, P., Lewis, RA., Susuki, K., Laothamatas, J. Hemachudha, T. (2005). Difference in neuro-pathogenetic mechanisms in human furious and paralytic rabies. J Neurol Sci. Nov 15;238(1-2):3-10.
- Shite, A., Guadu, T. and Admassu, B., (2015). Challenges of rabies. International Journal of Basic and Applied Virology, 4(2), pp.41-52.
- Ichhpujani, RL, Mala, C, Veena M, Singh J, Bhardwaj M, Bhattacharya D, Pattanaik SK, Balakrishnan N, Reddy AK, Samnpath G, Gandhi N, Nagar SS, Shiv L.(2008) Epidemiology of animal bites and rabies cases in India. A multicentric study. J Commun Dis. Mar;40(1):27-36. PMID: 19127666. [PubMed]
- 1Salomão, C., Nacima, A., Cuamba, L., Gujral, L., Amie,l O., Baltazar, C., Cliff, J., Gudo, ES. (2017) Epidemiology, clinical features and risk factors for human rabies and animal bites during an outbreak of rabies in Maputo and Matola cities, Mozambique, 2014: Implications for public health interventions for rabies control. PLoSNegl Trop Dis 11(7): e0005787. [CrossRef]
- Lembo, T., Hampson, K., Kaare, MT., Ernest, E., Knobel, D., Kazwala, RR., Haydon, DT., Cleaveland, S. (2010) The feasibility of canine rabies elimination in Africa: dispelling doubts with data. PLoS Negl Trop Dis. Feb 23;4(2): e626. [CrossRef] [PubMed]
- Wilde, H., Khawplod, P., Khamoltham, T., Hemachudha, T., Tepsumethanon, V., Lumlerdacha, B., Mitmoonpitak, C., Sitprija, V. (2005). Rabies control in South and Southeast Asia. Vaccine. Mar 18;23(17-18):2284-9. [CrossRef] [PubMed]
- Yousaf, MZ., Qasim, M., Zia, S., Khan, Mu., Ashfaq, UA., Khan, S. (2012). Rabies molecular virology, diagnosis, prevention and treatment. Virol J. Feb 21; 9:50. [CrossRef] [PubMed]
- Jibat, T., Mourits, M.C. and Hogeveen, H., (2016). Incidence and economic impact of rabies in the cattle population of Ethiopia. Preventive Veterinary Medicine, 130, pp.67-76.
- 1Yibrah, M., and Damtie, D.(2015). Incidence of human rabies exposure and associated factors at the Gondar Health Center, Ethiopia: a three-year retrospective study. Infect Dis Poverty 4, (3). [CrossRef]
- Beyene, TJ, Mourits, MCM., Kidane, AH. and Hogeveen, H. (2018). Estimating the burden of rabies in Ethiopia by tracing dog bite victims. PLoS ONE 13(2): e0192313. https://doi.org/10.1371/journal.pone.0192313. [CrossRef]
- Pankhurst, R., (1970). The history and traditional treatment of rabies in Ethiopia. Medical history, 14(4), pp.378-389.
- Nomoto H, Yamamoto K, Kutsuna S, Asai Y, Kasamatsu Y, Shirano M, et al. (2023) Evaluation of potential rabies exposure among Japanese international travelers: A retrospective descriptive study. PLoS ONE 18(8): e0287838.
- Central Statistics Agency (CSA) (2007). Federal Democratic Republic of Ethiopia Central Statistical Agency.
- Waktola, A., (1999). Exploratory Study of two regions in Ethiopia to Identify target areas and partners for intervention. DCG.
- Yalew, S., Teferi, E., Van Griensven, A., Uhlenbrook, S., Mul, M., Van Der Kwast, J. and Van der Zaag, P., (2012). Land use change and suitability assessment in the Upper Blue Nile basin under water resources and socio-economic constraints: a drive towards a decision support system.
- CSA (2017) LSMS—Integrated Surveys on Agriculture, Ethiopia Socioeconomic Survey (ESS) 2015/16, situation analysis of children and women: Amhara Region: CSA, 2017 p. 8.
- Krishna, A.K., Satyanarayanan, M. and Govil, P.K., (2009). Assessment of heavy metal pollution in water using multivariate statistical techniques in an industrial area: a case study from Patancheru, Medak District, Andhra Pradesh, India. Journal of hazardous materials, 167(1-3), pp.366-373.
- Otranto, D., Dantas-Torres, F., Giannelli, A., Latrofa, M.S., Cascio, A., Cazzin, S., Ravagnan, S., Montarsi, F., Zanzani, S.A., Manfredi, M.T. and Capelli, G., (2014). Ticks infesting humans in Italy and associated pathogens. Parasites & vectors, 7, pp.1-9.
- Thrusfield, M., (2018). Veterinary epidemiology. John Wiley & Sons.
- Bennett, M.D. and Smith, J.B., (1991). Nuclear DNA amounts in angiosperms. Philosophical Transactions: Biological Sciences, pp.309-345.
- Ntampaka, P., Nyaga, P.N., Niragire, F., Gathumbi, J.K. and Tukei, M., (2019). Knowledge, attitudes and practices regarding rabies and its control among dog owners in Kigali city, Rwanda. PloS one, 14(8), p.e0210044.
- Bahiru, A., Molla, W., Yizengaw, L., Mekonnen, S.A. and Jemberu, W.T., (2022). Knowledge, attitude and practice related to rabies among residents of Amhara region, Ethiopia. Heliyon, 8(11).
- Hampson, K., Dobson, A., Kaare, M., Dushoff, J., Magoto, M., Sindoya, E. and Cleaveland, S., (2008). Rabies exposures, post-exposure prophylaxis and deaths in a region of endemic canine rabies. PLoS neglected tropical diseases, 2(11), p.e339.
- Deressa, A., Ali, A., Bayene, M., Selassie, BN., Yimer, E., and Hussen, K. (2010). The status of rabies in Ethiopia: A retrospective record review. Ethiopian Journal of Health Development, 24(2).
- Tenzin, Wangdi, K., Ward, M.P., (2012). Human and animal rabies prevention and control cost in Bhutan, 2001-2008: The cost-benefit of dog rabies elimination. Vaccine 31, 260 270. https://doi.org/10.1016/j.vaccine.2012.05.023. [CrossRef]
- Acosta-Jamett, G., Cleaveland, S., Cunningham, A.A. and Bronsvoort, B.D., (2010). Demography of domestic dogs in rural and urban areas of the Coquimbo region of Chile and implications for disease transmission. Preventive veterinary medicine, 94(3-4), pp.272-281.
- Mustiana, A., (2013). Assessment of the risk for rabies introduction and establishment in Lombok, Indonesia (Master's thesis, University of Sydney).
- World Health Organization WHO (1987). Guidelines for dog rabies control. VPH/83.43 Rev.1.World Health Organization, Geneva.
- Seligsohn, D., (2014). Dog bite incidence and associated risk factors—a cross-sectional study on school children in Tamil Nadu. Degree Project, Faculty of Veterinary Medicine and Animal Science, Department of Biomedical Sciences and Veterinary Public Health, Swedish University of Agricultural Sciences, http://stud. epsilon. slu. se/6622.
- Jemberu, W.T., Molla, W., Almaw, G. and Alemu, S., (2013). Incidence of rabies in humans and domestic animals and people's awareness in North Gondar Zone, Ethiopia. PLoS neglected tropical diseases, 7(5), p.e2216.
- Belete S, Meseret M, Dejene H, Assefa A. (2021). Prevalence of dog-mediated rabies in Ethiopia: a systematic review and Meta-analysis from 2010 to 2020. One Health Outlook 3, 16 (2021). [CrossRef]
- Mediouni, S., Brisson, M., Ravel, A., (2020). Epidemiology of human exposure to rabies in Nunavik: incidence, the role of dog bites and their context, and victim profiles. BMC Public Health 29;20(1):584. [CrossRef]
- Tenzin., Dhand, N.K., Dorjee, J. and Ward, M.P. (2010). Re-emergence of rabies in dogs and other domestic animal in eastern Bhutan, 2005-2007. Epidemiology and Infection.
- Evangelio, SA., Satur, DA., Lachica, ZPT., Mata, MAE. and Alviola, PA., (2020). Risk Factor Analysis for Dog Bite Victims in Davao City, Southern Philippines. Philippine Journal of Science, 149(2).
- Arias, CM., Xavier, D, Arias, CC, Andrade, E, and Abel, I. (2019) Epidemiological scenarios for human rabies exposure notified in Colombia during ten years: A challenge to implement surveillance actions with a differential approach on vulnerable populations. PLOS ONE 14(12): e0213120.
- Buso, D.S., da Somenzari, M.A., Silva, J.E., Oliveira, T.C.B. and Queiroz, L.H.Q., (2016). Risk Factors for Dog Bites to People in São Paulo, Brazil. JSM Trop. Med. Res, 1, p.1006.
- World Health Organization (WHO) (2018) Expert consultation on rabies. First report; technical report series 918. Switzerland: Geneva; p. 1–130.
- Voupawoe, G., Varkpeh, R., Kamara, V., Sieh, S., Traoré, A., Battisti, C., Angot, A., Loureiro, L., Soumare, B., Dauphin, G., Abebe, W., and Coetzer, A., Scott, T., Nel, L., Blanton, J., Dacheux, L., Bonas, S.,Bourhy, H.,Gourlaouen, M., Mauti, S., (2020). Rabies control in Liberia: Joint efforts towards Zero by 30. Acta Tropica. 216. 105787.
- Shwiff, S., Hampson, K. and Anderson, A., (2013). Potential economic benefits of eliminating canine rabies. Antiviral research, 98(2), pp.352-356.
- Ditsele, B., (2016). The epidemiology of Rabies in domestic ruminants in Botswana (Doctoral dissertation, Murdoch University).
- Tang, X., Luo, M., Zhang, S., Fooks, A.R., Hu, R. and Tu, C., (2005). Pivotal role of dogs in rabies transmission, China. Emerging infectious diseases, 11(12), p.1970.
- Fitzpatrick, M.C., Hampson, K., Cleaveland, S., Meyers, L.A., Townsend, J.P. and Galvani, A.P., 2012. Potential for rabies control through dog vaccination in wildlife-abundant communities of Tanzania.
- Singh, R., Singh, K.P., Cherian, S., Saminathan, M., Kapoor, S., Manjunatha Reddy, G.B., Panda, S. and Dhama, K., (2017). Rabies–epidemiology, pathogenesis, public health concerns and advances in diagnosis and control: a comprehensive review. Veterinary Quarterly, 37(1), pp.212-251.
- Kanutus, B.S., (2020). A household survey to assess knowledge, attitudes and practices regarding Rabies among dog owners in Omusati region (Doctoral dissertation, University of Namibia).
- Tintinalli, J. E. & Stapczynski, J. S. (2011). Tintinalli's emergency medicine: a comprehensive study guide, McGraw-Hill New York.
- Mulipukwa, C.P., 2016. Socio Demographic Factors Associated with Vaccination of Dogs against Rabies and Dog Bite Management in Nyimba District (Doctoral dissertation, The University of Zambia).
- Kitala, P.M., McDERMOTT, J.J., Coleman, P.G. and Dye, C., (2002). Comparison of vaccination strategies for the control of dog rabies in Machakos District, Kenya. Epidemiology & Infection, 129(1), pp.215-222.
- Yalemebrat, N., Bekele, T. and Melaku, M., (2016). Assessment of public knowledge, attitude and practices towards rabies in Debark Woreda, North Gondar, Ethiopia. Journal of Veterinary Medicine and Animal Health, 8(11), pp.183-192.
- McCollum, A.M., Blanton, J.D., Holman, R.C., Callinan, L.S., Baty, S., Phillips, R., Callahan, M., Levy, C., Komatsu, K., Sunenshine, R. and Bergman, D.L., (2012). Community survey after rabies outbreaks, Flagstaff, Arizona, USA. Emerging infectious diseases, 18(6), p.932.
- Overall, K.L. and Love, M., (2001). Dog bites to humans—demography, epidemiology, injury, and risk. Journal of the American Veterinary Medical Association, 218(12), pp.1923-1934.
- Daniels, D.M., Ritzi, R.B. and O’Neil, J., (2009). Analysis of nonfatal dog bites in children. Journal of Trauma and Acute Care Surgery, 66(3), pp. S17-S22.
- Auplish, A., Clarke, A.S., Van Zanten, T., Abel, K., Tham, C., Bhutia, T.N., Wilks, C.R., Stevenson, M.A., Firestone, S.M. (2017). Estimating the intra-cluster correlation coefficient for evaluating an educational intervention program to improve rabies awareness and dog bite prevention among children in Sikkim, India: A pilot study. Acta Trop., 169, 62–68.
| Variables | Number of respondents | Proportion in % | |
|---|---|---|---|
| Zone | West Gondar | 231 | 35.98 |
| Central Gondar | 191 | 29.75 | |
| South Gondar | 166 | 25.86 | |
| North Gondar | 54 | 8.41 | |
| District | Metema | 159 | 24.76 |
| Debre tabor | 73 | 11.37 | |
| East Dembia | 138 | 21.50 | |
| Fogera | 93 | 15.42 | |
| Genda wuha | 72 | 81.94 | |
| Debark | 54 | 8.41 | |
| Tach Armachiho | 53 | 8.26 | |
| Sex of respondents | Male | 467 | 72.74 |
| Female | 175 | 27.26 | |
| Family size | 1-3 family | 107 | 16.67 |
| 6 persons | 293 | 45.63 | |
| 7 persons | 242 | 37.69 | |
| Level of education | Non educated | 280 | 43.61 |
| Basic | 215 | 33.49 | |
| Secondary | 83 | 12.93 | |
| Tertiary | 64 | 9.97 | |
| Dog ownership | Owner | 497 | 77.41 |
| Non dog owner | 145 | 22.39 | |
| Knowledge rabies exists | Yes | 635 | 98.44 |
| No | 7 | 1.56 | |
| Knowledge rabies is zoonotic | Yes | 640 | 99.69 |
| No | 2 | 0.31 | |
| Know transmission of rabies | Yes | 603 | 93.93 |
| No | 39 | 6.07 | |
| Presence of dog in the house is a factor for rabies exposure | Yes | 598 | 93.15 |
| No | 44 | 6.85 | |
| Knowledge of ways of rabies transmission | Bite and saliva | 574 | 89.41 |
| Salva only | 47 | 7.32 | |
| Other methods | 21 | 3.27 | |
| Treatment by traditional healer | Treated | 515 | 80.22 |
| Not treated | 127 | 19.78 | |
| Vaccinating dogs in the last two years | Vaccinate | 265 | 41.28 |
| No vaccinate | 377 | 58.72 | |
| Rabies killer disease | Yes | 634 | 98.75 |
| No | 8 | 1.25 | |
| Washing bite wounds | Yes | 236 | 87.73 |
| No | 33 | 12.27 | |
| Knowledge of PEP | Yes | 338 | 52.65 |
| No | 304 | 47.35 | |
| Experience of taking PEP | Yes | 59 | 21.93 |
| No | 210 | 78.07 | |
| Own dog contact with other dogs | Yes | 292 | 58.75 |
| No | 205 | 41.15 | |
| Castrating/spaying dog(s) | yes | 622 | 96.88 |
| No | 20 | 3.12 | |
| Presence of wild carnivore nearby | yes | 394 | 61.37 |
| No | 248 | 38.63 | |
| Variables Levels | Number of respondents HHs | No of rabies exposed individuals | Percentage of rabies exposure | P -value | |
|---|---|---|---|---|---|
| Zone | West Gondar | 231 | 135 | 50.19 | 0.001 |
| Central Gondar | 191 | 66 | 24.53 | ||
| South Gondar | 166 | 44 | 16.36 | ||
| North Gondar | 54 | 24 | 8.92 | ||
| District | Metema | 159 | 93 | 58.49 | 0.001 |
| Genda wuha | 72 | 42 | 58.33 | ||
| Tach Armachiho | 53 | 26 | 49.06 | ||
| Debark | 54 | 24 | 44.44 | ||
| Fogera | 93 | 33 | 35.48 | ||
| East Dembia | 138 | 40 | 28.99 | ||
| Debre tabor | 73 | 11 | 15.07 | ||
| Sex | Male | 467 | 197 | 42.18 | 0.812 |
| Female | 175 | 72 | 41.14 | ||
| Family Size | >6 | 242 | 117 | 48.35 | 0.036 |
| 4-6 | 293 | 120 | 40.96 | ||
| 0-3 | 107 | 32 | 29.91 | ||
| Level of Education | No education | 215 | 109 | 50.70 | 0.001 |
| Basic | 280 | 118 | 42.14 | ||
| Secondary | 83 | 24 | 28.92 | ||
| Tertiary | 64 | 18 | 28.13 | ||
| Dog ownership | owner | 497 | 193 | 71.75 | 0.004 |
| Non dog owner | 145 | 76 | 28.25 | ||
| Vaccination history | Yes | 377 | 96 | 35.69 | 0.015 |
| No | 265 | 173 | 64.31 | ||
| Distance from Hospital/ Health center | <1 day | 553 | 217 | 39.24 | 0.000 |
| 1-2days | 55 | 37 | 62.27 | ||
| >2days | 34 | 15 | 44.12 | ||
| Dogs free contact with others | yes | 292 | 133 | 68.91 | 0.000 |
| No | 205 | 60 | 31.09 | ||
| Presence of wildlife near to his/her area | yes | 248 | 93 | 34.57 | 0.073 |
| No | 394 | 176 | 65.43 | ||
| Presence of Forest near to the residence | yes | 209 | 80 | 29.74 | 0.196 |
| No | 433 | 189 | 70.26 | ||
| Presence of high road access | Yes | 502 | 214 | 79.55 | 0.478 |
| No | 140 | 55 | 20.45 | ||
| Variables | Frequency | Percentage | |
| Species of bitten animals | Dog | 236 | 87.73 |
| Equine | 31 | 11.52 | |
| Cat | 1 | 0.37 | |
| Ownership of bite animals | Own | 131 | 48.70 |
| Stray | 71 | 26.39 | |
| Neighbors | 67 | 24.91 | |
| Wound Washing materials | Water only | 100 | 42.37 |
| Water and Soap | 85 | 36.02 | |
| Other material | 29 | 12.29 | |
| Holy water | 22 | 9.32 | |
| Variables Labels | OR | P- value | 95% CI | |
| District | Debre Tabor Ref. | |||
| Metema | 7.94 | 0.0001 | 3.89-16.23 | |
| Genda Wuha | 7.89 | 0.0001 | 3.57-17.46 | |
| Tach Armachiho | 5.43 | 0.0001 | 2.35-12.54 | |
| Debark | 4.50 | 0.0001 | 1.95-10.41 | |
| Fogera | 3.1 | 0.004 | 1.44-6.69 | |
| East Dembia | 2.30 | 0.027 | 1.10-4.82 | |
| Sex | Male Ref. | |||
| Female | 0.96 | 0.812 | 0.67-1.36 | |
| Family size | 1-3 family Ref. | |||
| 7 and above | 1.93 | 0.009 | 1.18-3.16 | |
| 4-6 Family | 1.36 | 0.210 | 0.84-2.20 | |
| Education level | Non educated Ref | |||
| Basic | 0.71 | 0.059 | 0.50-1.01 | |
| Secondary | 0.40 | 0.001 | 0.23-0.68 | |
| Tertiary | 0..38 | 0.002 | 0.21-0.69 | |
| Dog ownership | Dog owner Ref. | |||
| Non dog owner | 0.58 | 0.004 | 0.397-0.84 | |
| Presence of wildlife nearby | yes Ref | |||
| No | 0.714 | 0.041 | 0.51-0.99 | |
| Free contact of dog | Yes Ref | |||
| No | 2.07 | 0.0001 | 1.42- 3.03 | |
| Forest Availability | Yes | Ref. | ||
| No | 0.80 | 0.197 | 0.57-1.12 | |
| Vaccination history | yes | Ref. | ||
| No | 0.67 | 0.015 | 0.49-0.92 | |
| Distance from Hospital/health center | < one day | Ref. | ||
| 1-2day | 3.18 | 0.000 | 1.77-5.73 | |
| >2days | 1.22 | 0.573 | 0.61-2.46 | |
| Have you ever castrated your dog/s | No | Ref. | ||
| Yes | 0.24 | 0.022 | 0.07-0.81 | |
| Presence of high road access | Yes Ref | |||
| No | 1.15 | 0.478 | 0.78-1.68 | |
| Variables | Odds ratio | P- value | 95% CI | |
| Districts | Debre Tabor | Ref | ||
| Debark | 4.293 | 0.001 | 1.819-10.129 | |
| Fogera | 2.507 | 0.024 | 1.129-5.568 | |
| Genda wuha | 7.676 | 0.000 | 3.402-17.321 | |
| East Dembia | 2.146 | 0.052 | 0.994-4.631 | |
| Metema | 7.121 | 0.000 | 3.449-14.705 | |
| Tach Armachiho | 4.774 | 0.000 | 2.041-11.168 | |
| Family size | 1-3 families | Ref | ||
| 4-6 family | 1.577 | 0.083 | 0.942-2.639 | |
| 7 and above | 2.225 | 0.003 | 1.301-3.805 | |
| Dog ownership | Dog owner | Ref | ||
| Non dog owner | 0.59 | 0.014 | 0.388- 0.898 | |
| cons | 0.173 | 0.000 | 0.078- 0.383 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




