Submitted:
10 October 2024
Posted:
11 October 2024
You are already at the latest version
Abstract
Keywords:
INTRODUCTION
2. Materials and Methods
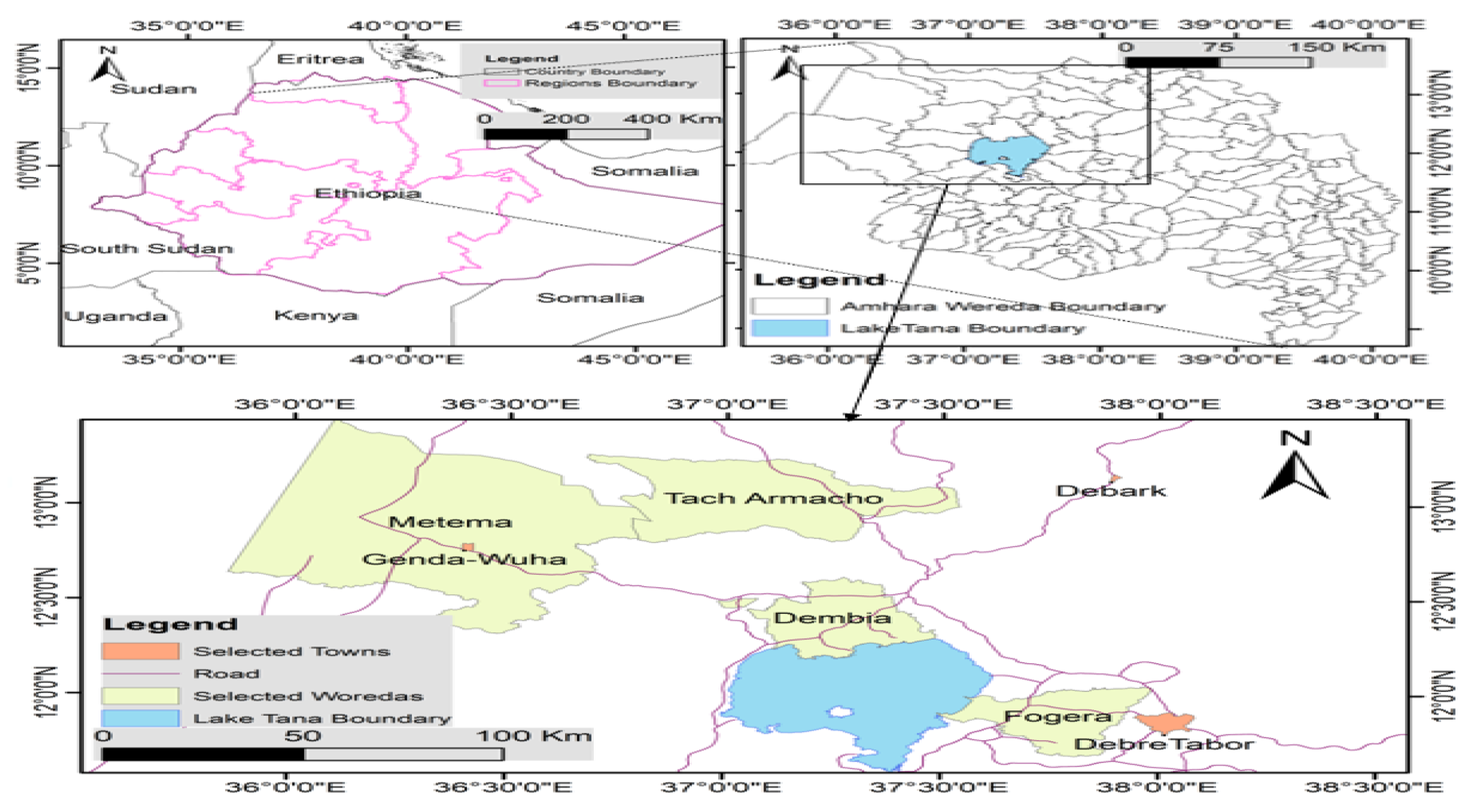
2.1. Description of the study area
2.2. Study population
2.3. Sampling methods
2.4. Design of the questionnaire and data collection
2.5. Sample size determination
2.6. Data analysis
3. Results
3.1. Respondents’ awareness and management of rabies
3.2. Prevalence of rabies exposure and associated risk factors
3.3. Sources of rabies exposure
3.4. Risk factors associated with rabies exposure.
3.5. Multivariate logistic regression model for risk association of rabies exposure
Discussion
Conclusions and Recommendations
- ➢
- Regarding, the above conclusion, we recommended the following suggestion for further study as well as prevention of rabies.
- ➢
- Public educational programs on dog behavior, dog-child interaction, and the importance of responsible dog ownership, especially reduction of free contact of the dogs.
- ➢
- Teaching the communities about the importance of the vaccine raising awareness about dog vaccination and improving access and affordability of the vaccine
- ➢
- Both the local government and the federal government take action for the availability of the dog vaccine for the especially inaccessible districts.
- ➢
- The disease is a continuing high community hazard in the study area; therefore, rabies needs continuous surveillance of dog bites to detect trends and evaluate the effect of prevention efforts.
Author Contributions
Funding
Ethical Approval and Consent to Participate
Informed Consent
Data Sharing Statement
Acknowledgments
Conflict Interest
References
- 1. Hayman DT, Johnson N, Horton DL, Hedge J, Wakeley PR, Banyard AC, Zhang S, Alhassan A, Fooks AR. Evolutionary history of rabies in Ghana. PLoS Negl Trop Dis. 2011 Apr 5;5(4): e1001. [CrossRef]
- Coetzer A, Gwenhure L, Makaya P, Markotter W, Nel L. Epidemiological aspects of the persistent transmission of rabies during an outbreak (2010 - 2017) in Harare, Zimbabwe. PLoS One. 2019 Jan 10;14(1):e0210018. [CrossRef]
- Hampson K, Coudeville L, Lembo T, Sambo M, Kieffer A, Attlan M, Barrat J, Blanton JD, Briggs DJ, Cleaveland S, Costa P, Freuling CM, Hiby E, Knopf L, Leanes F, Meslin FX, Metlin A, Miranda ME, Müller T, Nel LH, Recuenco S, Rupprecht CE, Schumacher C, Taylor L, Vigilato MA, Zinsstag J, Dushoff J; Global Alliance for Rabies Control Partners for Rabies Prevention. Estimating the global burden of endemic canine rabies. PLoS Negl Trop Dis. 2015 Apr 16;9(4): e0003709. doi: 10.1371/journal.pntd.0003709. Erratum in: PLoS Negl Trop Dis. 2015 May 11;9(5):e0003786. doi: 10.1371/journal.pntd.0003786. PMID: 25881058; PMCID: PMC4400070.
- Al-Mustapha AI, Tijani AA, Bamidele FO, Muftau O, Ibrahim A, Abdulrahim I, Osu MS, Kia G, Patrick N, Endie WN. Awareness and knowledge of canine rabies: A state-wide cross-sectional study in Nigeria. PLoS One. 2021 Mar 3;16(3):e0247523. [CrossRef]
- Jackson, A.C. Human disease and pathogenesis. In: Jackson AC, Wunner WH, (eds). Rabies. Second edition. Academic Press, London, UK. 2007, pp. 309–333 and 341-381.
- Ugolini G. Use of rabies virus as a transneuronal tracer of neuronal connections: implications for the understanding of rabies pathogenesis. Dev Biol (Basel). 2008; 131:493-506.
- Mitrabhakdi E, Shuangshoti S, Wannakrairot P, Lewis RA, Susuki K, Laothamatas J, Hemachudha T. Difference in neuropathogenetic mechanisms in human furious and paralytic rabies. J Neurol Sci. 2005 Nov 15;238(1-2):3-10.
- Suite A, Guadu T, Admassu B. Challenges of rabies. International Journal of Basic and Applied Virology. 2015;4(2):41-52.
- Ichhpujani RL, Mala C, Veena M, Singh J, Bhardwaj M, Bhattacharya D, Pattanaik SK, Balakrishnan N, Reddy AK, Samnpath G, Gandhi N. Epidemiology of animal bites and rabies cases in India. A multicentric study. The Journal of communicable diseases. 2008 Mar 1;40(1):27-36.
- Salomao C, Nacima A, Cuamba L, Gujral L, Amiel O, Baltazar C, Cliff J, Gudo ES. Epidemiology, clinical features and risk factors for human rabies and animal bites during an outbreak of rabies in Maputo and Matola cities, Mozambique, 2014: Implications for public health interventions for rabies control. PLOS Neglected Tropical Diseases. 2017 Jul 24;11(7):e0005787.
- Lembo T, Hampson K, Kaare MT, Ernest E, Knobel D, Kazwala RR, Haydon DT, Cleaveland S. The feasibility of canine rabies elimination in Africa: dispelling doubts with data. PLoS Negl Trop Dis. 2010 Feb 23;4(2):e626. [CrossRef]
- Wilde H, Khawplod P, Khamoltham T, Hemachudha T, Tepsumethanon V, Lumlerdacha B, Mitmoonpitak C, Sitprija V. Rabies control in South and Southeast Asia. Vaccine. 2005 Mar 18;23(17-18):2284-9.
- Yousaf, M.Z., Qasim, M., Zia, S. Rehman Khan, MU. Ashfaq, UA. and Khan, S, Rabies molecular virology, diagnosis, prevention and treatment. Virol J 2012 : 9(50). [CrossRef]
- Jibat T, Mourits MC, Hogeveen H. Incidence and economic impact of rabies in the cattle population of Ethiopia. Prev Vet Med. 2016 Aug 1; 130:67-76.
- Yibrah M, Damtie D. Incidence of human rabies exposure and associated factors at the Gondar Health Center, Ethiopia: a three-year retrospective study. Infect Dis Poverty. 2015 Feb 2;4(1):3. [CrossRef]
- Beyene TJ, Mourits MCM, Kidane AH, Hogeveen H. Estimating the burden of rabies in Ethiopia by tracing dog bite victims. PLoS One. 2018 Feb 21;13(2): e0192313. [CrossRef]
- Pankhurst R. The history and traditional treatment of rabies in Ethiopia. Med Hist. 1970 Oct;14(4):378-89. [CrossRef]
- Nomoto H, Yamamoto K, Kutsuna S, Asai Y, Kasamatsu Y, Shirano M, Sahara T, Nakamura F, Katsuragi Y, Yamato M, Shinohara K, Sakamoto N, Hase R, Ogawa T, Nagasaka A, Miyata N, Ohmagari N. Evaluation of potential rabies exposure among Japanese international travellers: A retrospective descriptive study. PLoS One. 2023 Aug 18;18(8):e0287838. [CrossRef]
- Central Statistics Agency (CSA). National Population Statistics. Federal Democratic Republic of Ethiopia, Central Statistical Authority, 2007 Addis Ababa, Ethiopia.
- Waktola A. Exploratory Study of two regions in Ethiopia to Identify target areas and partners for intervention. DCG; 1999 Oct.
- Yalew S, Teferi E, Van Griensven A, Uhlenbrook S, Mul M, Van Der Kwast J, Van der Zaag P. Land use change and suitability assessment in the Upper Blue Nile basin under water resources and socio-economic constraints: a drive towards a decision support system.
- Central Statistics Agency (CSA). Living Standard Measurement Study-Integrated Surveys on Agriculture((LSMS-ISA), Ethiopia Socioeconomic Survey (ESS) 2015/16, situation analysis of children and women: Amhara Region: CSA, 2017 p. 8.
- Krishna AK, Satyanarayanan M, Govil PK. Assessment of heavy metal pollution in water using multivariate statistical techniques in an industrial area: a case study from Patancheru, Medak District, Andhra Pradesh, India. Journal of hazardous materials. 2009 Aug 15;167(1-3):366-73.
- Yizengaw, L., Jemberu, W.T., Bahiru, A. Molla, W.Mekonnen, S.A. Dog Ecology and Demography with Reference to Rabies in Amhara Region, Ethiopia, University of Gondar,Ethiopia, 2021. Master’s Thesis.
- Thrusfield M. Veterinary epidemiology. John Wiley & Sons; 2018 Apr 30.
- Bennett MD, Smith JB. Nuclear DNA amounts in angiosperms. Philosophical Transactions: Biological Sciences. 1991 Dec 30:309-45.
- Ntampaka P, Nyaga PN, Niragire F, Gathumbi JK, Tukei M. Knowledge, attitudes and practices regarding rabies and its control among dog owners in Kigali city, Rwanda. PLoS One. 2019 Aug 20;14(8): e0210044. [CrossRef]
- Bahiru A, Molla W, Yizengaw L, Mekonnen SA, Jemberu WT. Knowledge, attitude and practice related to rabies among residents of Amhara region, Ethiopia. Heliyon. 2022 Nov 3;8(11): e11366. [CrossRef]
- Hampson K, Dobson A, Kaare M, Dushoff J, Magoto M, Sindoya E, Cleaveland S. Rabies exposures, post-exposure prophylaxis and deaths in a region of endemic canine rabies. PLoS Negl Trop Dis. 2008;2(11): e339. [CrossRef]
- Deressa A, Ali A, Bayene M, Selassie BN, Yimer E, Hussen K. The status of rabies in Ethiopia: A retrospective record review. Ethiopian Journal of Health Development. 2010;24(2).
- Tenzin, Wangdi K, Ward MP. Human and animal rabies prevention and control cost in Bhutan, 2001-2008: the cost-benefit of dog rabies elimination. Vaccine. 2012 Dec 17;31(1):260-70. [CrossRef]
- Acosta-Jamett G, Cleaveland S, Cunningham AA, Bronsvoort BM. Demography of domestic dogs in rural and urban areas of the Coquimbo region of Chile and implications for disease transmission. Prev Vet Med. 2010 May 1;94(3-4):272-81. [CrossRef]
- Mustiana A. Assessment of the risk for rabies introduction and establishment in Lombok, Indonesia 2013, (Master's thesis, University of Sydney).
- World Health Organization: Guidelines for dog rabies control. WHO document VPH/83.43: Rev.1. World Health Organization, Geneva, Switzerland; 1987.
- Seligsohn, D. Dog bite incidence and associated risk factors—a cross-sectional study on school children in Tamil Nadu. Degree Project, Faculty of Veterinary Medicine and Animal Science, Department of Biomedical Sciences and Veterinary Public Health, Swedish University of Agricultural Sciences 2014, http://stud. epsilon. slu. se/6622.
- Seligsohn, D. Dog bite incidence and associated risk factors—a cross-sectional study on school children in Tamil Nadu. Degree Project, Faculty of Veterinary Medicine and Animal Science, Department of Biomedical Sciences and Veterinary Public Health, Swedish University of Agricultural Sciences 2014, http://stud. epsilon. slu. se/6622.
- Seligsohn, D., (2014). Dog bite incidence and associated risk factors—a cross-sectional study on school children in Tamil Nadu. Degree Project, Faculty of Veterinary Medicine and Animal Science, Department of Biomedical Sciences and Veterinary Public Health, Swedish University of Agricultural Sciences, http://stud. epsilon. slu. se/6622.
- Mediouni S, Brisson M, Ravel A. Epidemiology of human exposure to rabies in Nunavik: incidence, the role of dog bites and their context, and victim profiles. BMC Public Health. 2020 Apr 29;20(1):584. [CrossRef]
- Tenzin, Dhand NK, Dorjee J, Ward MP. Re-emergence of rabies in dogs and other domestic animals in eastern Bhutan, 2005-2007. Epidemiol Infect. 2011 Feb;139(2):220-5. [CrossRef]
- Evangelio SA, Satur DA, Lachica ZP, Mata MA, Alviola PA. Risk Factor Analysis for Dog Bite Victims in Davao City, Southern Philippines. Philippine Journal of Science. 2020 Jun 1;149(2).
- Arias Caicedo MR, Xavier DA, Arias Caicedo CA, Andrade E, Abel I. Epidemiological scenarios for human rabies exposure notified in Colombia during ten years: A challenge to implement surveillance actions with a differential approach on vulnerable populations. PLoS One. 2019 Dec 27;14(12): e0213120. [CrossRef]
- World Health Organization (WHO). Expert consultation on rabies. First report; technical report series 918. Switzerland: Geneva;2018 p. 1–130.
- Voupawoe G, Varkpeh R, Kamara V, Sieh S, Traoré A, De Battisti C, Angot A, de J Loureiro LF, Soumaré B, Dauphin G, Abebe W. Rabies control in Liberia: Joint efforts towards zero by 30. Acta Tropica. 2021 Apr 1; 216:105787.
- Shwiff SA, Sweeney SJ, Elser JL, Miller RS, Farnsworth ML, Nol P, Shwiff SS, Anderson AM. A benefit-cost analysis decision framework for mitigation of disease transmission at the wildlife–livestock interface. Human-Wildlife Interactions. 2016 Apr 1;10(1):91-102.
- Ditsele, B. “The epidemiology of Rabies in domestic ruminants in Botswana.” (2016). https://api.semanticscholar.org/CorpusID:133353622.
- Tang X, Luo M, Zhang S, Fooks AR, Hu R, Tu C. Pivotal role of dogs in rabies transmission, China. Emerg Infect Dis. 2005 Dec;11(12):1970-2. [CrossRef]
- Fitzpatrick MC, Hampson K, Cleaveland S, Meyers LA, Townsend JP, Galvani AP. Potential for rabies control through dog vaccination in wildlife-abundant communities of Tanzania. PLoS Negl Trop Dis. 2012;6(8): e1796.
- Singh R, Singh KP, Cherian S, Saminathan M, Kapoor S, Manjunatha Reddy GB, Panda S, Dhama K. Rabies - epidemiology, pathogenesis, public health concerns and advances in diagnosis and control: a comprehensive review. Vet Q. 2017 Dec;37(1):212-251. [CrossRef]
- Kanutus, Benediktus Shiikufeni. "A household survey to assess knowledge, attitudes and practices regarding Rabies among dog owners in Omusati region." PhD diss., University of Namibia, 2020.
- Tintinalli, J. E. & Stapczynski, J. S. Tintinalli’s emergency medicine: a comprehensive study guide, 2011 McGraw-Hill New York.
- Mulipukwa, C.P., 2016. Socio-Demographic Factors Associated with Vaccination of Dogs against Rabies and Dog Bite Management in Nyimba District 2016 Doctoral dissertation, The University of Zambia. http://thesisbank.jhia.ac.ke/id/eprint/8868.
- Zinsstag J, Dürr S, Penny MA, Mindekem R, Roth F, Menendez Gonzalez S, Naissengar S, Hattendorf J. Transmission dynamics and economics of rabies control in dogs and humans in an African city. Proc Natl Acad Sci U S A. 2009 Sep 1;106(35):14996-5001. [CrossRef]
- Yalemebrat, N, Bekele T, Melaku M. Assessment of public knowledge, attitude and practices towards rabies in Debark Woreda, North Gondar, Ethiopia. Journal of Veterinary Medicine and Animal Health. 2016 Nov 30;8(11):183-92.
- McCollum AM, Blanton JD, Holman RC, Callinan LS, Baty S, Phillips R, Callahan M, Levy C, Komatsu K, Sunenshine R, Bergman DL. Community survey after rabies outbreaks, Flagstaff, Arizona, USA. Emerging infectious diseases. 2012 Jun;18(6):932.
- Overall KL, Love M. Dog bites to humans—demography, epidemiology, injury, and risk. Journal of the American Veterinary Medical Association. 2001 Jun 15;218(12):1923-34.
- Daniels DM, Ritzi RB, O'Neil J, Scherer LR. Analysis of nonfatal dog bites in children. J Trauma. 2009 Mar;66(3 Suppl): S17-22. [CrossRef]
- Auplish A, Clarke AS, Van Zanten T, Abel K, Tham C, Bhutia TN, Wilks CR, Stevenson MA, Firestone SM. Estimating the intra-cluster correlation coefficient for evaluating an educational intervention program to improve rabies awareness and dog bite prevention among children in Sikkim, India: A pilot study. Acta Trop. 2017 May; 169:62-68. [CrossRef]
| Variable | Levels |
|---|---|
| Do you know rabies exists? | No/Yes |
| Do you know rabies affects humans? | No/Yes |
| Do you know the way of transmission of rabies? | No/Yes |
| Do you have a dog(s)? | No/Yes |
| Do you know dogs as a predisposing risk factor of rabies? | No/Yes |
| Do you believe traditional medicine can treat rabies? | No/Yes |
| Do you vaccinate your dog against rabies? | No/Yes |
| Do you perceive rabies as severe/fatal? | No/Yes |
| Have you or a member of your family ever been bitten by a rabid animal | No/Yes |
| What was the species of the animal that bit you or your family? | Dog/cat/Equines/wild carnivore. |
| Do you wash the bite wound? | No/Yes |
| Do you know the presence of post-exposure prophylaxis (PEP)? | No/Yes |
| Do you or your family ever take PEP | No/Yes |
| Do your dog contact with other dogs frequently | No/Yes |
| Do you castrate your dog (s) | No/Yes |
| Is there any wild carnivore near your area? | No/Yes |
| Variables | Number of respondents | Proportion in % | |
| Zone | West Gondar | 231 | 35.98 |
| Central Gondar | 191 | 29.75 | |
| South Gondar | 166 | 25.86 | |
| North Gondar | 54 | 8.41 | |
| District | Metema | 159 | 24.76 |
| Debre tabor | 73 | 11.37 | |
| East Dembia | 138 | 21.50 | |
| Fogera | 93 | 15.42 | |
| Genda wuha | 72 | 81.94 | |
| Debark | 54 | 8.41 | |
| Tach Armachiho | 53 | 8.26 | |
| Sex of respondents | Male | 467 | 72.74 |
| Female | 175 | 27.26 | |
| Family size | 1-3 family | 107 | 16.67 |
| 6 persons | 293 | 45.63 | |
| 7 persons | 242 | 37.69 | |
| Level of education | Non educated | 280 | 43.61 |
| Basic | 215 | 33.49 | |
| Secondary | 83 | 12.93 | |
| Tertiary | 64 | 9.97 | |
| Dog ownership | Owner | 497 | 77.41 |
| Non dog owner | 145 | 22.39 | |
| Knowledge rabies exists | Yes | 635 | 98.44 |
| No | 7 | 1.56 | |
| Knowledge rabies is zoonotic | Yes | 640 | 99.69 |
| No | 2 | 0.31 | |
| Know the transmission of rabies | Yes | 603 | 93.93 |
| No | 39 | 6.07 | |
| The presence of a dog in the house is a factor in rabies exposure | Yes | 598 | 93.15 |
| No | 44 | 6.85 | |
| Knowledge of ways of rabies transmission | Bite and saliva | 574 | 89.41 |
| Salva only | 47 | 7.32 | |
| Other methods | 21 | 3.27 | |
| Treatment by a traditional healer | Treated | 515 | 80.22 |
| Not treated | 127 | 19.78 | |
| Vaccinating dogs in the last two years | Vaccinate | 265 | 41.28 |
| No vaccinate | 377 | 58.72 | |
| Rabies killer disease | Yes | 634 | 98.75 |
| No | 8 | 1.25 | |
| Washing bite wounds | Yes | 236 | 87.73 |
| No | 33 | 12.27 | |
| Knowledge of PEP | Yes | 338 | 52.65 |
| No | 304 | 47.35 | |
| Experience of taking PEP | Yes | 59 | 21.93 |
| No | 210 | 78.07 | |
| Own dog contact with other dogs | Yes | 292 | 58.75 |
| No | 205 | 41.15 | |
| Castrating/spaying dog(s) | yes | 622 | 96.88 |
| No | 20 | 3.12 | |
| Presence of wild carnivores nearby | yes | 394 | 61.37 |
| No | 248 | 38.63 | |
| Variables Levels | Number of respondents HHs | No of rabies exposed individuals | Percentage of rabies exposure | P -value | |
| Zone | West Gondar | 231 | 135 | 50.19 | 0.001 |
| Central Gondar | 191 | 66 | 24.53 | ||
| South Gondar | 166 | 44 | 16.36 | ||
| North Gondar | 54 | 24 | 8.92 | ||
| District | Metema | 159 | 93 | 58.49 | 0.001 |
| Genda wuha | 72 | 42 | 58.33 | ||
| Tach Armachiho | 53 | 26 | 49.06 | ||
| Debark | 54 | 24 | 44.44 | ||
| Fogera | 93 | 33 | 35.48 | ||
| East Dembia | 138 | 40 | 28.99 | ||
| Debre tabor | 73 | 11 | 15.07 | ||
| Sex | Male | 467 | 197 | 42.18 | 0.812 |
| Female | 175 | 72 | 41.14 | ||
| Family Size | >6 | 242 | 117 | 48.35 | 0.036 |
| 4-6 | 293 | 120 | 40.96 | ||
| 0-3 | 107 | 32 | 29.91 | ||
| Level of Education | No education | 215 | 109 | 50.70 | 0.001 |
| Basic | 280 | 118 | 42.14 | ||
| Secondary | 83 | 24 | 28.92 | ||
| Tertiary | 64 | 18 | 28.13 | ||
| Dog ownership | owner | 497 | 193 | 71.75 | 0.004 |
| Non dog owner | 145 | 76 | 28.25 | ||
| Vaccination history | Yes | 377 | 96 | 35.69 | 0.015 |
| No | 265 | 173 | 64.31 | ||
| Distance from Hospital/ Health centre | <1 day | 553 | 217 | 39.24 | 0.000 |
| 1-2days | 55 | 37 | 62.27 | ||
| >2days | 34 | 15 | 44.12 | ||
| Dogs have free contact with others | yes | 292 | 133 | 68.91 | 0.000 |
| No | 205 | 60 | 31.09 | ||
| Presence of wildlife near his/her area | yes | 248 | 93 | 34.57 | 0.073 |
| No | 394 | 176 | 65.43 | ||
| The presence of a Forest near the residence | yes | 209 | 80 | 29.74 | 0.196 |
| No | 433 | 189 | 70.26 | ||
| Presence of high-road access | Yes | 502 | 214 | 79.55 | 0.478 |
| No | 140 | 55 | 20.45 | ||
| Variables | Frequency | Percentage | |
| Species of bitten animals | Dog | 236 | 87.73 |
| Equine | 31 | 11.52 | |
| Cat | 1 | 0.37 | |
| Ownership of bite animals | Own | 131 | 48.70 |
| Stray | 71 | 26.39 | |
| Neighbours | 67 | 24.91 | |
| Wound Washing materials | Water only | 100 | 42.37 |
| Water and Soap | 85 | 36.02 | |
| Other material | 29 | 12.29 | |
| Holy water | 22 | 9.32 | |
| Variables Labels | OR | P- value | 95% CI | |
| District | Debre Tabor Ref. | |||
| Metema | 7.94 | 0.0001 | 3.89-16.23 | |
| Genda Wuha | 7.89 | 0.0001 | 3.57-17.46 | |
| Tach Armachiho | 5.43 | 0.0001 | 2.35-12.54 | |
| Debark | 4.50 | 0.0001 | 1.95-10.41 | |
| Fogera | 3.1 | 0.004 | 1.44-6.69 | |
| East Dembia | 2.30 | 0.027 | 1.10-4.82 | |
| Sex | Male Ref. | |||
| Female | 0.96 | 0.812 | 0.67-1.36 | |
| Family size | 1-3 family Ref. | |||
| 7 and above | 1.93 | 0.009 | 1.18-3.16 | |
| 4-6 Family | 1.36 | 0.210 | 0.84-2.20 | |
| Education level | Non educated Ref | |||
| Basic | 0.71 | 0.059 | 0.50-1.01 | |
| Secondary | 0.40 | 0.001 | 0.23-0.68 | |
| Tertiary | 0..38 | 0.002 | 0.21-0.69 | |
| Dog ownership | Dog owner Ref. | |||
| Non-dog owner | 0.58 | 0.004 | 0.397-0.84 | |
| The presence of wildlife nearby | yes Ref | |||
| No | 0.714 | 0.041 | 0.51-0.99 | |
| Presence of freecontactwith dogs | Yes Ref | |||
| No | 2.07 | 0.0001 | 1.42- 3.03 | |
| Forest Availability | Yes | Ref. | ||
| No | 0.80 | 0.197 | 0.57-1.12 | |
| Vaccination history | yes | Ref. | ||
| No | 0.67 | 0.015 | 0.49-0.92 | |
| Distance from Hospital/health centre | < one day | Ref. | ||
| 1-2day | 3.18 | 0.000 | 1.77-5.73 | |
| >2days | 1.22 | 0.573 | 0.61-2.46 | |
| Have you ever castrated your dog/s | No | Ref. | ||
| Yes | 0.24 | 0.022 | 0.07-0.81 | |
| Presence of high-road access | Yes Ref | |||
| No | 1.15 | 0.478 | 0.78-1.68 | |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




