Submitted:
17 August 2024
Posted:
20 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
Surfactant Protein B (SFTPB)
Surfactant Protein C (SFTPC)
Surfactant Proteins A and D (SFTPA and SFTPD)
NKX2.1 Gene
ABCA3 Gene
2. Materials and Methods
2.1. Molecular Analysis
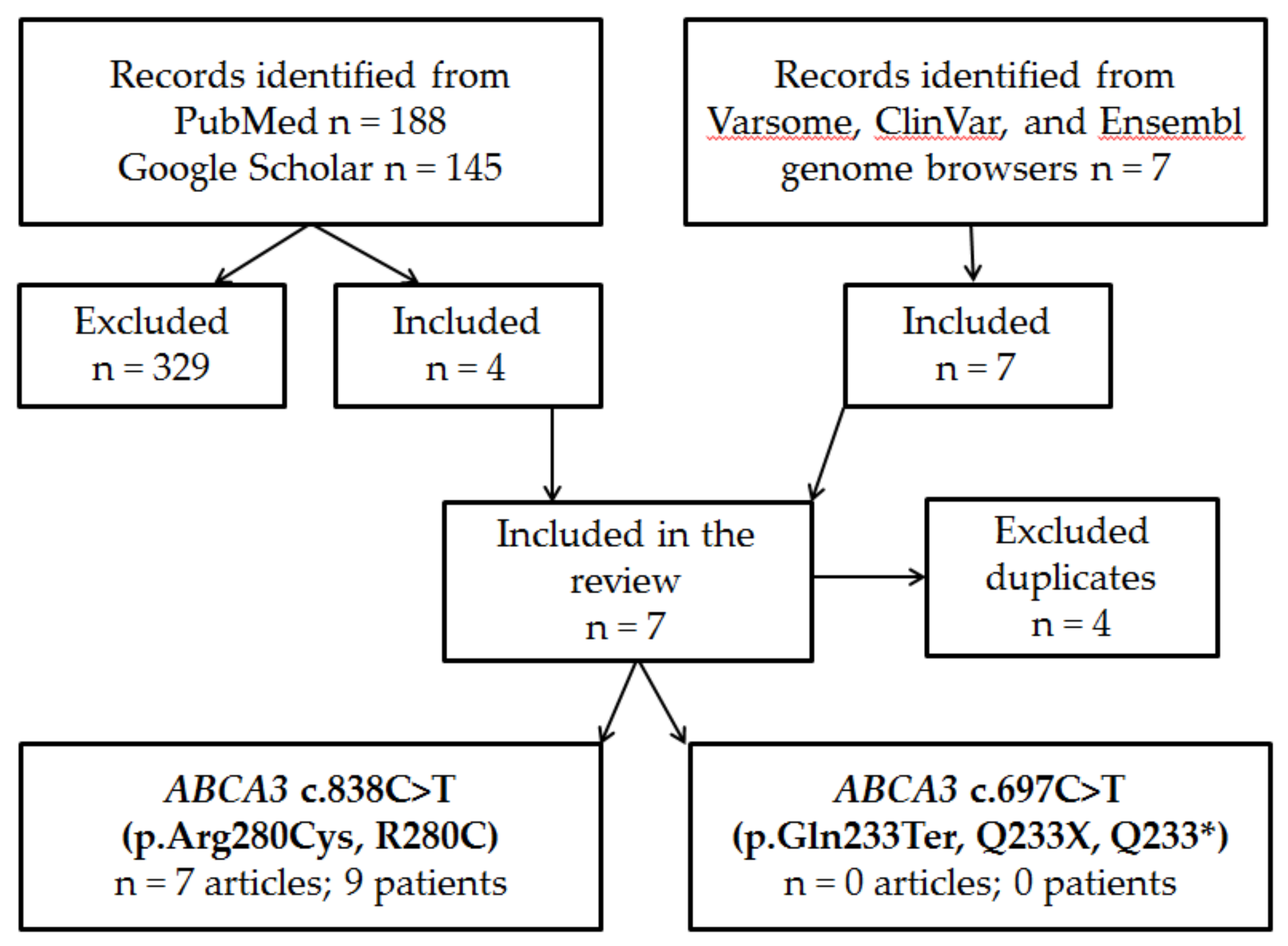
2.2. Literature Systematic Review
3. Results
3.1. Case Report
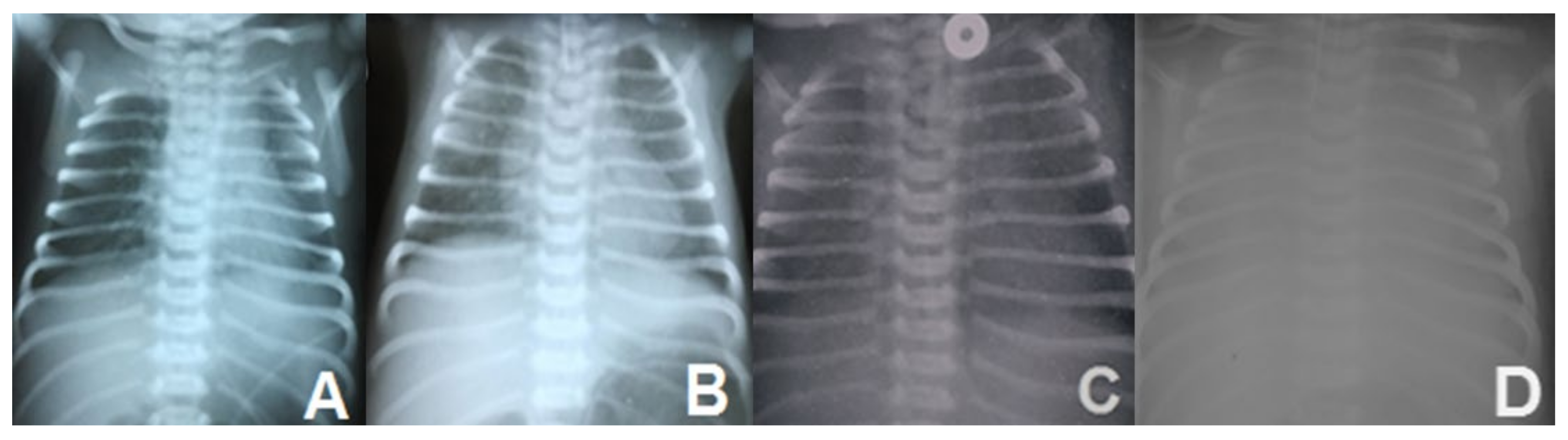
3.1.1. Clinical Aspects
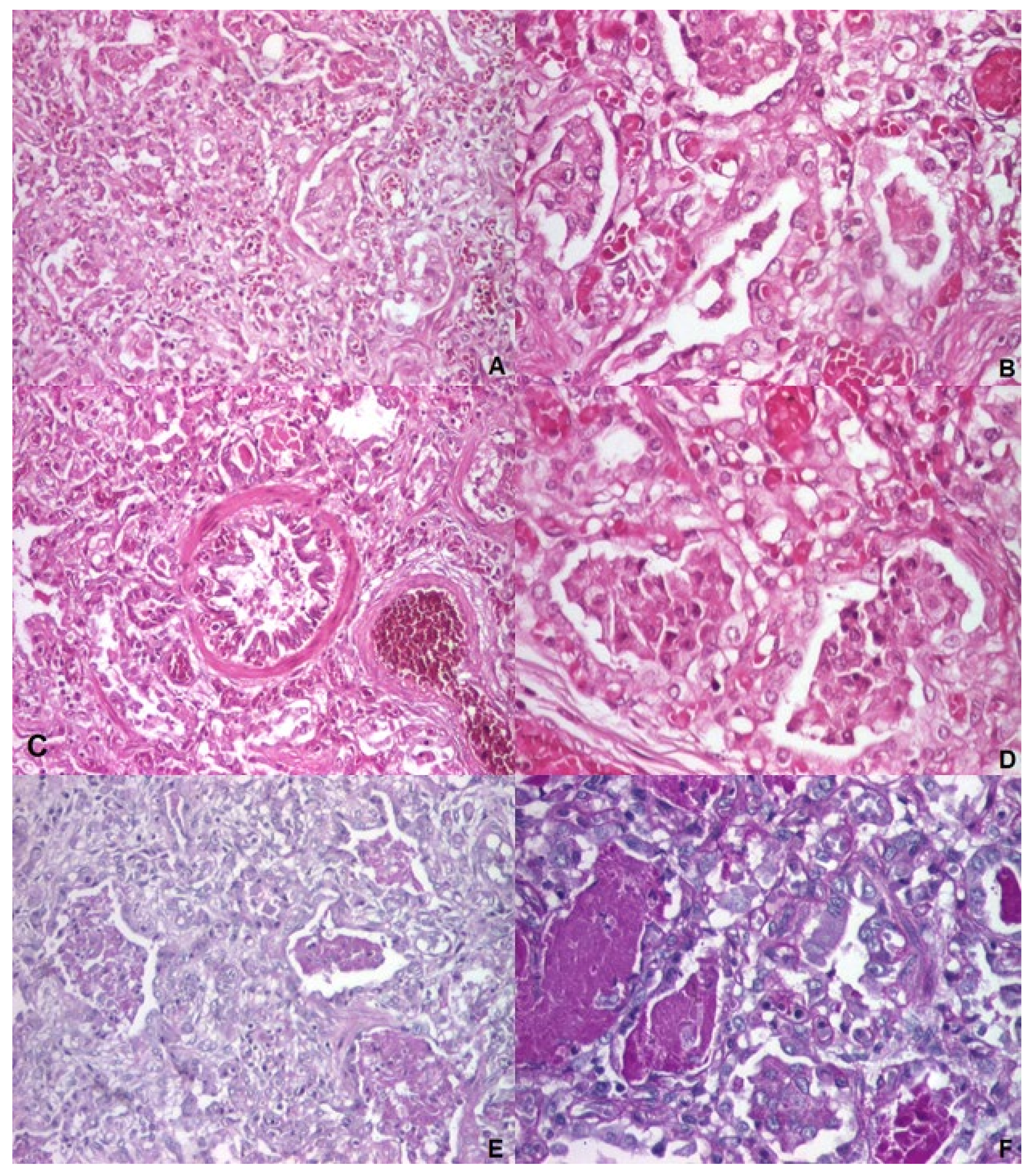
3.1.2. Autopsy and Histology
3.1.3. Pathogenic Prediction
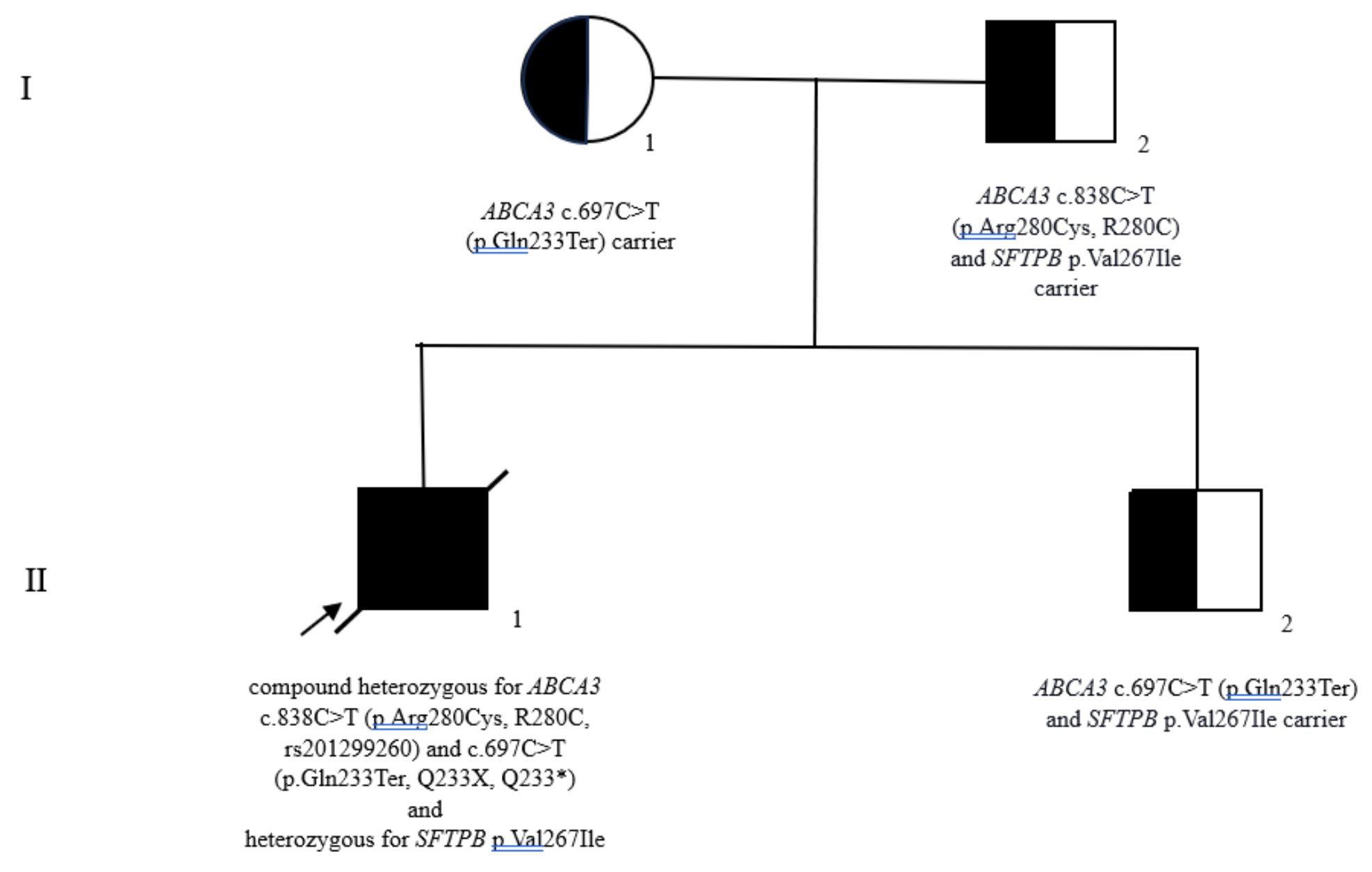
3.1.4. Genetic Counseling
3.2. Review of the Literature on ABCA3 c.838C>T (p.Arg280Cys, R280C)
4. Discussion
4.1. Neonatal Respiratory Distress Syndrome in Near-Term and Term Infants
4.2. Surfactant Protein B Variants
4.3. ABCA3 Deficiency
4.3.1. Adenosine Triphosphate-Binding Cassette Family A Member 3 (ABCA3) Protein
4.3.2. Adenosine Triphosphate-Binding Cassette Family A Member 3 (ABCA3) Gene
4.3.3. ABCA3 c.838C>T (p.Arg280Cys, R280C) Variant
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wert, S.E., Whitsett, J.A., Nogee, L.M. Genetic disorders of surfactant dysfunction. Pediatr Dev Pathol. 2009; 12(4):253-74. [CrossRef] [PubMed]
- Lei, C., Wan, C., Liu, C. ABCA3 mutation-induced congenital pulmonary surfactant deficiency: A case report. Medicine (Baltimore). 2024;103(13):e37622. [CrossRef] [PubMed]
- Winter, J., Essmann, S., Kidszun, A., Aslanidis, C., Griese, M., Poplawska, K., Bartsch, M., Schmitz, G., Mildenberger, E. Neonatal respiratory insufficiency caused by an (homozygous) ABCA3-stop mutation: a systematic evaluation of therapeutic options. Klin Padiatr. 2014;226(2):53-8. [CrossRef] [PubMed]
- Wei, M., Fu, H., Han, A., Ma, L. A Term Neonatal Case With Lethal Respiratory Failure Associated With a Novel Homozygous Mutation in ABCA3 Gene. Front Pediatr. 2020;8:138. [CrossRef] [PubMed]
- Dyer, J. Neonatal Respiratory Distress Syndrome: Tackling A Worldwide Problem. P T. 2019;44(1):12-14. [PubMed]
- Magnani, J.E., Donn, S.M. Persistent Respiratory Distress in the Term Neonate: Genetic Surfactant Deficiency Diseases. Curr Pediatr Rev. 2020;16(1):17-25. [CrossRef] [PubMed]
- Abdel-Latif, M.E., Tan, O., Fiander, M., Osborn, D.A. Non-invasive high-frequency ventilation in newborn infants with respiratory distress. Cochrane Database Syst Rev. 2024;5(5):CD012712. [CrossRef] [PubMed]
- Zhang, W., Liu, Z., Lin, Y., Wang, R., Xu, J., He, Y., Zhang, F., Wu, L., Chen. D. A novel synonymous ABCA3 variant identified in a Chinese family with lethal neonatal respiratory failure. BMC Med Genomics. 2021;14(1):256. [CrossRef] [PubMed]
- Gupta, N.P., Batra, A., Puri, R., Meena. V. Novel homozygous missense mutation in ABCA3 protein leading to severe respiratory distress in term infant. BMJ Case Rep. 2020;13(10):e235520. [CrossRef] [PubMed]
- Anciuc-Crauciuc, M., Cucerea, M.C., Tripon, F., Crauciuc, G.A., Bănescu, C.V. Descriptive and Functional Genomics in Neonatal Respiratory Distress Syndrome: From Lung Development to Targeted Therapies. Int J Mol Sci. 2024;25(1):649. [CrossRef] [PubMed]
- Bullard, J.E., Wert, S.E., Nogee, L.M. ABCA3 deficiency: neonatal respiratory failure and interstitial lung disease. Semin Perinatol. 2006;30(6):327-34. [CrossRef] [PubMed]
- Reuter, S., Moser, C., Baack, M. Respiratory distress in the newborn. Pediatr Rev. 2014;35(10):417-28; quiz 429. [CrossRef] [PubMed]
- Rogulska, J., Wróblewska-Seniuk, K., Śmigiel, R., Szydłowski, J., Szczapa, T. Diagnostic Challenges in Neonatal Respiratory Distress-Congenital Surfactant Metabolism Dysfunction Caused by ABCA3 Mutation. Diagnostics (Basel). 2022;12(5):1084 Mutation. [CrossRef] [PubMed]
- Wang, J., Fan, J., Zhang, Y., Huang, L., Shi, Y. ABCA3 gene mutations shape the clinical profiles of severe unexplained respiratory distress syndrome in late preterm and term infants. Transl Pediatr. 2021;10(2):350-358. [CrossRef] [PubMed]
- Nogee, L.M. Genetic causes of surfactant protein abnormalities. Curr Opin Pediatr. 2019;31(3):330-339. [CrossRef] [PubMed]
- Bush, A., Cunningham, S., de Blic, J., Barbato, A., Clement, A., Epaud, R., Hengst, M., Kiper, N,, Nicholson, A.G., Wetzke, M., Snijders, D., Schwerk, N., Griese, M.; chILD-EU Collaboration. European protocols for the diagnosis and initial treatment of interstitial lung disease in children. Thorax. 2015;70(11):1078-84. [CrossRef] [PubMed]
- Oltvai, Z.N., Smith, E.A., Wiens, K., Nogee, L.M., Luquette, M., Nelson, A.C., Wikenheiser-Brokamp, K.A. Neonatal respiratory failure due to novel compound heterozygous mutations in the ABCA3 lipid transporter. Cold Spring Harb Mol Case Stud. 2020;6(3):a005074. [CrossRef] [PubMed]
- Hallman, M. The surfactant system protects both fetus and newborn. Neonatology. 2013;103(4):320-6. [CrossRef] [PubMed]
- Wambach, J.A., Yang, P., Wegner, D.J., Heins, H.B., Kaliberova, L.N., Kaliberov, S.A., Curiel, D.T., White, F.V., Hamvas, A., Hackett, B.P., Cole, F.S. Functional Characterization of ATP-Binding Cassette Transporter A3 Mutations from Infants with Respiratory Distress Syndrome. Am J Respir Cell Mol Biol. 2016;55(5):716-721. [CrossRef] [PubMed]
- Malý, J., Navrátilová, M., Hornychová, H., Looman, A.C. Respiratory failure in a term newborn due to compound heterozygous ABCA3 mutation: the case report of another lethal variant. J Perinatol. 2014;34(12):951-3. [CrossRef] [PubMed]
- Griese, M., Haug, M., Brasch, F., Freihorst, A., Lohse, P., von Kries, R., Zimmermann, T., Hartl, D. Incidence and classification of pediatric diffuse parenchymal lung diseases in Germany. Orphanet J Rare Dis. 2009;4:26. [CrossRef] [PubMed]
- Mitsiakos, G., Tsakalidis, C., Karagianni, P., Gialamprinou, D., Chatziioannidis, I., Papoulidis, I., Tsanakas, I., Soubasi, V. A New ABCA3 Gene Mutation c.3445G>A (p.Asp1149Asn) as a Causative Agent of Newborn Lethal Respiratory Distress Syndrome. Medicina (Kaunas). 2019;55(7):389. [CrossRef] [PubMed]
- Nogee, L.M., de Mello, D.E., Dehner, L.P., Colten, H.R. Brief report: deficiency of pulmonary surfactant protein B in congenital alveolar proteinosis. N Engl J Med. 1993;328(6):406-10. [CrossRef] [PubMed]
- van Moorsel, C.H.M., van der Vis, J.J., Grutters, J.C. Genetic disorders of the surfactant system: focus on adult disease. Eur Respir Rev. 2021;30(159):200085. [CrossRef] [PubMed]
- Hamvas, A. Evaluation and management of inherited disorders of surfactant metabolism. Chin Med J (Engl). 2010;123(20):2943-7. [PubMed]
- Nogee, L.M., Garnier, G., Dietz, H.C., Singer, L., Murphy, A.M., deMello, D.E., Colten, H.R. A mutation in the surfactant protein B gene responsible for fatal neonatal respiratory disease in multiple kindreds. J Clin Invest. 1994;93(4):1860-3. [CrossRef] [PubMed]
- Beers, M.F., Hamvas, A., Moxley, M.A., Gonzales, L.W., Guttentag, S.H., Solarin, K.O., Longmore, W.J., Nogee, L.M., Ballard, P.L. Pulmonary surfactant metabolism in infants lacking surfactant protein B. Am J Respir Cell Mol Biol. 2000;22(3):380-91. [CrossRef] [PubMed]
- Nogee, L.M., Wert, S.E., Proffit, S.A., Hull, W.M., Whitsett, J.A. Allelic heterogeneity in hereditary surfactant protein B (SP-B) deficiency. Am J Respir Crit Care Med. 2000;161(3 Pt 1):973-81. [CrossRef] [PubMed]
- Dunbar, A.E. 3rd, Wert, S.E., Ikegami, M., Whitsett, J.A., Hamvas, A., White, F.V., Piedboeuf, B., Jobin, C., Guttentag, S., Nogee, L.M. Prolonged survival in hereditary surfactant protein B (SP-B) deficiency associated with a novel splicing mutation. Pediatr Res. 2000;48(3):275-82. [CrossRef] [PubMed]
- López-Andreu, J.A., Hidalgo-Santos, A.D., Fuentes-Castelló, M.A., Mancheño-Franch, N., Cerón-Pérez, J.A., Esteban-Ricós, M.J., Pedrola-Vidal, L., Nogee, L.M. Delayed Presentation and Prolonged Survival of a Child with Surfactant Protein B Deficiency. J Pediatr. 2017;190:268-270.e1. [CrossRef] [PubMed]
- Kurland, G., Deterding, R.R., Hagood, J.S., Young, L.R., Brody, A.S., Castile, R.G., Dell, S., Fan, L.L., Hamvas, A., Hilman, B.C., Langston, C., Nogee, L.M., Redding, G.J.; American Thoracic Society Committee on Childhood Interstitial Lung Disease (chILD) and the chILD Research Network. An official American Thoracic Society clinical practice guideline: classification, evaluation, and management of childhood interstitial lung disease in infancy. Am J Respir Crit Care Med. 2013;188(3):376-94. [CrossRef] [PubMed]
- Klein, J.M., Thompson, M.W., Snyder, J.M., George, T.N., Whitsett, J.A., Bell, E.F., McCray, P.B. Jr, Nogee, L.M. Transient surfactant protein B deficiency in a term infant with severe respiratory failure. J Pediatr. 1998;132(2):244-8. [CrossRef] [PubMed]
- Ballard, P.L., Nogee, L.M., Beers, M.F., Ballard, R.A., Planer, B.C., Polk, L., deMello, D.E., Moxley, M.A., Longmore, W.J. Partial deficiency of surfactant protein B in an infant with chronic lung disease. Pediatrics. 1995;96(6):1046-52. [PubMed]
- Lawson, W.E., Grant, S.W., Ambrosini, V., Womble, K.E., Dawson, E.P., Lane, K.B., Markin, C., Renzoni, E., Lympany, P., Thomas, A.Q., Roldan, J., Scott, T.A., Blackwell, T.S., Phillips, J.A. 3rd, Loyd, J.E., du Bois, R.M. Genetic mutations in surfactant protein C are a rare cause of sporadic cases of IPF. Thorax. 2004;59(11):977-80. [CrossRef] [PubMed]
- Tredano, M., Griese, M., Brasch, F., Schumacher, S., de Blic, J., Marque, S., Houdayer, C., Elion, J,, Couderc, R., Bahuau, M. Mutation of SFTPC in infantile pulmonary alveolar proteinosis with or without fibrosing lung disease. Am J Med Genet A. 2004;126A(1):18-26. [CrossRef] [PubMed]
- Pachajoa, H., Ruiz-Botero, F., Meza-Escobar, L.E., Villota-Delgado, V,A,, Ballesteros, A., Padilla, I., Duarte, D. Fatal respiratory disease due to a homozygous intronic ABCA3 mutation: a case report. J Med Case Rep. 2016;10(1):266. [CrossRef] [PubMed]
- Kröner, C., Reu, S., Teusch, V., Schams, A., Grimmelt, A.C., Barker, M., Brand, J., Gappa, M., Kitz, R., Kramer, B.W., Lange, L., Lau, S., Pfannenstiel, C., Proesmans, M., Seidenberg, J., Sismanlar, T., Aslan, A.T., Werner, C., Zielen, S., Zarbock, R., Brasch, F., Lohse, P., Griese, M. Genotype alone does not predict the clinical course of SFTPC deficiency in paediatric patients. Eur Respir J. 2015;46(1):197-206. [CrossRef] [PubMed]
- Wright, J.R. Host defense functions of pulmonary surfactant. Biol Neonate. 2004;85(4):326-32. [CrossRef] [PubMed]
- Kingma, P.S., Whitsett, J.A. In defense of the lung: surfactant protein A and surfactant protein D. Curr Opin Pharmacol. [CrossRef] [PubMed]
- Haagsman, H.P., Hogenkamp, A., van Eijk, M., Veldhuizen, E.J. Surfactant collectins and innate immunity. Neonatology. [CrossRef] [PubMed]
- Boggaram, V. Regulation of lung surfactant protein gene expression. Front Biosci. 2003;8:d751-64. [CrossRef] [PubMed]
- Rämet, M., Haataja, R., Marttila, R., Floros, J., Hallman, M. Association between the surfactant protein A (SP-A) gene locus and respiratory-distress syndrome in the Finnish population. Am J Hum Genet. 2000;66(5):1569-79. [CrossRef] [PubMed]
- Hilgendorff, A., Heidinger, K., Bohnert, A., Kleinsteiber, A., König, I.R., Ziegler, A., Lindner, U., Frey, G., Merz, C., Lettgen, B., Chakraborty, T., Gortner, L., Bein, G. Association of polymorphisms in the human surfactant protein-D (SFTPD) gene and postnatal pulmonary adaptation in the preterm infant. Acta Paediatr. 2009;98(1):112-7. [CrossRef] [PubMed]
- Lahti, M., Lofgren, J., Marttila, R., Renko, M., Klaavuniemi, T., Haataja, R., Ramet, M., Hallman, M. Surfactant protein D gene polymorphism associated with severe respiratory syncytial virus infection. Pediatr Res. 2002;51(6):696-9. [CrossRef] [PubMed]
- Löfgren, J., Rämet, M., Renko, M., Marttila, R., Hallman, M. Association between surfactant protein A gene locus and severe respiratory syncytial virus infection in infants. J Infect Dis. 2002;185(3):283-9. [CrossRef] [PubMed]
- Wang, Y., Kuan, P.J., Xing, C., Cronkhite, J.T., Torres, F., Rosenblatt, R.L., DiMaio, J.M., Kinch, L.N., Grishin, N.V., Garcia, C.K. Genetic defects in surfactant protein A2 are associated with pulmonary fibrosis and lung cancer. Am J Hum Genet. 2009;84(1):52-9. [CrossRef] [PubMed]
- Nattes, E., Lejeune, S., Carsin, A., Borie, R., Gibertini, I., Balinotti, J., Nathan, N., Marchand-Adam, S., Thumerelle, C., Fauroux, B., Bosdure, E., Houdouin, V., Delestrain, C., Louha, M., Couderc, R., De Becdelievre. A., Fanen, P., Funalot, B., Crestani, B., Deschildre, A., Dubus, J.C., Epaud, R. Heterogeneity of lung disease associated with NK2 homeobox 1 mutations. Respir Med. 2017;129:16-23. [CrossRef] [PubMed]
- Thorwarth, A., Schnittert-Hübener, S., Schrumpf, P., Müller, I., Jyrch, S., Dame, C., Biebermann, H., Kleinau, G., Katchanov, J., Schuelke, M., Ebert, G., Steininger, A., Bönnemann, C., Brockmann, K., Christen, H.J., Crock, P., deZegher, F., Griese, M., Hewitt, J., Ivarsson, S., Hübner, C., Kapelari. K., Plecko, B., Rating, D., Stoeva, I., Ropers, H.H., Grüters, A., Ullmann, R., Krude, H. Comprehensive genotyping and clinical characterisation reveal 27 novel NKX2-1 mutations and expand the phenotypic spectrum. J Med Genet. 2014;51(6):375-87. [CrossRef] [PubMed]
- Nevel, R.J., Garnett, E.T., Worrell, J.A., Morton, R.L., Nogee, L.M., Blackwell, T.S., Young, L.R. Persistent Lung Disease in Adults with NKX2.1 Mutation and Familial Neuroendocrine Cell Hyperplasia of Infancy. Ann Am Thorac Soc. 2016;13(8):1299-304. [CrossRef] [PubMed]
- Beers, M.F., Mulugeta, S. The biology of the ABCA3 lipid transporter in lung health and disease. Cell Tissue Res. 2017;367(3):481-493. [CrossRef] [PubMed]
- Shulenin, S., Nogee, L.M., Annilo, T., Wert, S.E., Whitsett, J.A., Dean, M. ABCA3 gene mutations in newborns with fatal surfactant deficiency. N Engl J Med. 2004;350(13):1296-303. [CrossRef] [PubMed]
- Onnée, M., Fanen, P., Callebaut, I., de Becdelièvre, A. Structure-Based Understanding of ABCA3 Variants. Int J Mol Sci. 2021;22(19):10282. [CrossRef] [PubMed]
- Gonçalves, J.P., Pinheiro, L., Costa, M., Silva, A., Gonçalves, A., Pereira, A. Novel ABCA3 mutations as a cause of respiratory distress in a term newborn. Gene. 2014;534(2):417-20. [CrossRef] [PubMed]
- Jouza, M., Jimramovsky, T., Sloukova, E., Pecl, J., Seehofnerova, A., Jezova, M., Urik, M., Kunovsky, L., Slaba, K., Stourac, P., Klincova, M., Hubacek, J.A., Jabandziev, P. A Newly Observed Mutation of the ABCA3 Gene Causing Lethal Respiratory Failure of a Full-Term Newborn: A Case Report. Front Genet. 2020;11:568303. [CrossRef] [PubMed]
- National Center for Biotechnology Information. ClinVar; [VCV000318566.16]. Available online: https://www.ncbi.nlm.nih.gov/clinvar/variation/ (accessed on 17 June 2024).
- Ensembl (2022). Available online: https://www.ensembl.org/ (accessed on 17 June 2024).
- Pejaver, V., Urresti, J., Lugo-Martinez, J., Pagel, K.A., Lin, G.N., Nam, H.J., Mort, M., Cooper, D.N., Sebat, J., Iakoucheva, L.M., Mooney, S.D., Radivojac, P. Inferring the molecular and phenotypic impact of amino acid variants with MutPred2. Nat Commun. 2020;11(1):5918. [CrossRef] [PubMed]
- Pagel, K.A., Pejaver, V., Lin, G.N., Nam, H.J., Mort, M., Cooper, D.N., Sebat, J., Iakoucheva, L.M., Mooney, S.D., Radivojac, P. When loss-of-function is loss of function: assessing mutational signatures and impact of loss-of-function genetic variants. Bioinformatics. 2017;33(14):i389-i398. [CrossRef] [PubMed]
- Somaschini, M., Nogee, L.M., Sassi, I., Danhaive, O., Presi, S., Boldrini, R., Montrasio, C., Ferrari, M., Wert, S.E., Carrera, P. Unexplained neonatal respiratory distress due to congenital surfactant deficiency. J Pediatr. 2007;150(6):649-53, 653.e1. [CrossRef] [PubMed]
- Turcu, S., Ashton, E., Jenkins, L., Gupta, A., Mok, Q. Genetic testing in children with surfactant dysfunction. Arch Dis Child. 2013;98(7):490-5. [CrossRef] [PubMed]
- Williamson, M., Wallis, C. Ten-year follow up of hydroxychloroquine treatment for ABCA3 deficiency. Pediatr Pulmonol. 2014;49(3):299-301. [CrossRef] [PubMed]
- Wambach, J.A., Casey, A.M., Fishman, M.P., Wegner, D.J., Wert, S.E., Cole, F.S., Hamvas, A., Nogee, L.M. Genotype-phenotype correlations for infants and children with ABCA3 deficiency. Am J Respir Crit Care Med. 2014;189(12):1538-43. [CrossRef] [PubMed]
- Xu, K.K., Wegner, D.J., Geurts, L.C., Heins, H.B., Yang, P., Hamvas, A., Eghtesady, P., Sweet, S.C., Sessions Cole, F., Wambach, J.A. Biologic characterization of ABCA3 variants in lung tissue from infants and children with ABCA3 deficiency. Pediatr Pulmonol. 2022;57(5):1325-1330. [CrossRef] [PubMed]
- Jackson, T., Wegner, D.J., White, F.V., Hamvas, A., Cole, F.S., Wambach, J.A. Respiratory failure in a term infant with cis and trans mutations in ABCA3. J Perinatol. 2015;35(3):231-2. [CrossRef] [PubMed]
- Wang, K., Li, M., Hakonarson, H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38(16):e164. [CrossRef] [PubMed]
- Klay, D., Platenburg, M.G.J.P., van Rijswijk, R.H.N.A.J., Grutters, J.C., van Moorselm C.H.M. ABCA3 mutations in adult pulmonary fibrosis patients: a case series and review of literature. Curr Opin Pulm Med. 2020;26(3):293-301. [CrossRef] [PubMed]
- Gjeta, I., Sala, D., Bakalli, I., Celaj, E., Kola, E. Surfactant Deficiency Causing Severe Pneumonia in a Child. Curr Health Sci J. 2023;49(1):134-138. [CrossRef] [PubMed]
- Brasch, F., Schimanski, S., Mühlfeld, C., Barlage, S., Langmann, T., Aslanidis, C., Boettcher, A., Dada, A., Schroten, H., Mildenberger, E., Prueter, E., Ballmann, M., Ochs, M., Johnen, G., Griese, M., Schmitz, G. Alteration of the pulmonary surfactant system in full-term infants with hereditary ABCA3 deficiency. Am J Respir Crit Care Med. 2006;174(5):571-80. [CrossRef] [PubMed]
- Somaschini, M., Presi, S., Ferrari, M., Vergani, B., Carrera, P. Surfactant proteins gene variants in premature newborn infants with severe respiratory distress syndrome. J Perinatol. 2018;38(4):337-344. [CrossRef] [PubMed]
- Anciuc-Crauciuc, M., Cucerea, M.C., Crauciuc, G.A., Tripon, F., Bănescu, C.V. Evaluation of the Copy Number Variants and Single-Nucleotide Polymorphisms of ABCA3 in Newborns with Respiratory Distress Syndrome-A Pilot Study. Medicina (Kaunas). 2024;60(3):419. [CrossRef] [PubMed]
- Peca, D., Cutrera, R., Masotti, A., Boldrini, R., Danhaive, O. ABCA3, a key player in neonatal respiratory transition and genetic disorders of the surfactant system. Biochem Soc Trans. 2015;43(5):913-9. [CrossRef] [PubMed]
- Xiao, G.L., Gao, Y., Hao, H., Wei, T., Hong, C., Wang, Y., Lin, Y.Y., Chi, X.F., Liu, Y., Gao, H.Y., Nie. C. Novel insights into congenital surfactant dysfunction disorders by in silico analysis of ABCA3 proteins. World J Pediatr. 2023;19(3):293-301. [CrossRef] [PubMed]
- Ciantelli, M., Ghirri, P., Presi, S., Sigali, E., Vuerich, M., Somaschini, M., Ferrari, M., Boldrini, A., Carrera, P. Fatal respiratory failure in a full-term newborn with two ABCA3 gene mutations: a case report. J Perinatol. 2011;31(1):70-2. [CrossRef] [PubMed]
- Braun, S., Ferner, M., Kronfeld, K., Griese, M. Hydroxychloroquine in children with interstitial (diffuse parenchymal) lung diseases. Pediatr Pulmonol. 2015;50(4):410-9. [CrossRef] [PubMed]
- AlAnazi, A., Epaud, R., Heena, H., de Becdelievre, A., Miqdad, A.M., Fanen, P. The most frequent ABCA3 nonsense mutation -p.Tyr1515* (Y1515X) causing lethal neonatal respiratory failure in a term neonate. Ann Thorac Med. 2017;12(3):213-215. [CrossRef] [PubMed]
- Beers, M.F., Zhao, M., Tomer, Y., Russo, S.J., Zhang, P., Gonzales, L.W., Guttentag, S.H., Mulugeta, S. Disruption of N-linked glycosylation promotes proteasomal degradation of the human ATP-binding cassette transporter ABCA3. Am J Physiol Lung Cell Mol Physiol. 2013;305(12):L970-80. [CrossRef] [PubMed]
- Li, J., Ikegami, M., Na, C.L., Hamvas A,., Espinassous, Q., Chaby, R., Nogee, L.M., Weaver, T.E., Johansson, J. N-terminally extended surfactant protein (SP) C isolated from SP-B-deficient children has reduced surface activity and inhibited lipopolysaccharide binding. Biochemistry. 2004;43(13):3891-8. [CrossRef] [PubMed]
- Clark, J.C., Wert, S.E., Bachurski, C.J., Stahlman, M.T., Stripp, B.R., Weaver, T.E., Whitsett, J.A. Targeted disruption of the surfactant protein B gene disrupts surfactant homeostasis, causing respiratory failure in newborn mice. Proc Natl Acad Sci U S A. 1995;92(17):7794-8. [CrossRef] [PubMed]
- Kitazawa, H., Moriya, K., Niizuma, H., Kawano, K., Saito-Nanjo, Y., Uchiyama, T., Rikiishi, T., Sasahara, Y., Sakamoto, O., Setoguchi, Y., Kure, S. Interstitial lung disease in two brothers with novel compound heterozygous ABCA3 mutations. Eur J Pediatr. 2013;172(7):953-7. [CrossRef] [PubMed]
- Chen, F., Xie, Z., Zhang, V.W., Chen, C., Fan, H., Zhang, D., Jiang, W., Wang, C., Wu, P. Case Report: Report of Two Cases of Interstitial Lung Disease Caused by Novel Compound Heterozygous Variants in the ABCA3 Gene. Front Genet. 2022;13:875015. [CrossRef] [PubMed]
- Zarbock, R., Kaltenborn, E., Frixel, S., Wittmann, T., Liebisch, G., Schmitz, G., Griese, M. ABCA3 protects alveolar epithelial cells against free cholesterol induced cell death. Biochim Biophys Acta. 2015;1851(7):987-95. [CrossRef] [PubMed]
- Ban, N., Matsumura, Y., Sakai, H., Takanezawa, Y., Sasaki, M., Arai, H., Inagaki, N. ABCA3 as a lipid transporter in pulmonary surfactant biogenesis. J Biol Chem. 2007;282(13):9628-9634. [CrossRef] [PubMed]
- Garmany, T.H., Wambach, J.A., Heins, H.B., Watkins-Torry. J.M., Wegner, D.J., Bennet, K., An, P., Land, G., Saugstad, O.D., Henderson, H., Nogee, L.M., Cole, F.S., Hamvas, A. Population and disease-based prevalence of the common mutations associated with surfactant deficiency. Pediatr Res. 2008;63(6):645-9. [CrossRef] [PubMed]
- Cheong, N., Zhang, H., Madesh, M., Zhao, M., Yu, K., Dodia, C., Fisher, A.B., Savani, R.C., Shuman, H. ABCA3 is critical for lamellar body biogenesis in vivo. J Biol Chem. 2007;282(33):23811-7. [CrossRef] [PubMed]
- Flamein, F., Riffault, L., Muselet-Charlier, C., Pernelle, J., Feldmann, D., Jonard, L., Durand-Schneider, A.M., Coulomb, A., Maurice, M., Nogee, L.M., Inagaki, N., Amselem, S., Dubus, J.C., Rigourd, V., Brémont, F., Marguet, C., Brouard, J., de Blic, J., Clement, A., Epaud, R., Guillot, L. Molecular and cellular characteristics of ABCA3 mutations associated with diffuse parenchymal lung diseases in children. Hum Mol Genet. 2012;21(4):765-75. [CrossRef] [PubMed]
- Weichert, N., Kaltenborn, E., Hector, A., Woischnik, M., Schams, A., Holzinger, A., Kern, S., Griese, M. Some ABCA3 mutations elevate ER stress and initiate apoptosis of lung epithelial cells. Respir Res. 2011;12(1):4. [CrossRef] [PubMed]
- Nathan, N., Borensztajn, K., Clement, A. Genetic causes and clinical management of pediatric interstitial lung diseases. Curr Opin Pulm Med. 2018;24(3):253-259. [CrossRef] [PubMed]
- Matsumura, Y., Ban, N., Ueda, K., Inagaki, N. Characterization and classification of ATP-binding cassette transporter ABCA3 mutants in fatal surfactant deficiency. J Biol Chem. 2006;281(45):34503-14. [CrossRef] [PubMed]
- Matsumura, Y., Ban, N., Inagaki, N. Aberrant catalytic cycle and impaired lipid transport into intracellular vesicles in ABCA3 mutants associated with nonfatal pediatric interstitial lung disease. Am J Physiol Lung Cell Mol Physiol. 2008;295(4):L698-707. [CrossRef] [PubMed]
- Kinting, S., Höppner, S., Schindlbeck, U., Forstner, M.E., Harfst, J., Wittmann, T., Griese, M. Functional rescue of misfolding ABCA3 mutations by small molecular correctors. Hum Mol Genet. 2018;27(6):943-953. [CrossRef] [PubMed]
- Cheong, N., Madesh, M., Gonzales, L.W., Zhao, M., Yu, K., Ballard, P.L., Shuman, H. Functional and trafficking defects in ATP binding cassette A3 mutants associated with respiratory distress syndrome. J Biol Chem. 2006;281(14):9791-800. [CrossRef] [PubMed]
- Yoshida, I., Ban, N., Inagaki, N. Expression of ABCA3, a causative gene for fatal surfactant deficiency, is up-regulated by glucocorticoids in lung alveolar type II cells. Biochem Biophys Res Commun. 2004;323(2):547-55. [CrossRef] [PubMed]
- Stahlman, M.T., Besnard, V., Wert, S.E., Weaver, T.E., Dingle, S., Xu, Y., von Zychlin, K., Olson, S.J., Whitsett, J.A. Expression of ABCA3 in developing lung and other tissues. J Histochem Cytochem. 2007;55(1):71-83. [CrossRef] [PubMed]
- Kolla, V., Gonzales, L.W., Gonzales, J., Wang, P., Angampalli, S., Feinstein, S.I., Ballard, P.L. Thyroid transcription factor in differentiating type II cells: regulation, isoforms, and target genes. Am J Respir Cell Mol Biol. 2007;36(2):213-25. [CrossRef] [PubMed]
- Wambach, J.A., Wegner, D.J., Depass, K., Heins, H., Druley, T.E., Mitra, R.D., An, P., Zhang, Q., Nogee, L.M., Cole, F.S., Hamvas, A. Single ABCA3 mutations increase risk for neonatal respiratory distress syndrome. Pediatrics. 2012;130(6):e1575-82. [CrossRef] [PubMed]
- Kröner, C., Wittmann, T., Reu, S., Teusch, V., Klemme, M., Rauch, D., Hengst, M., Kappler, M., Cobanoglu, N., Sismanlar, T., Aslan, A.T., Campo, I., Proesmans, M., Schaible, T., Terheggen-Lagro, S., Regamey, N., Eber, E., Seidenberg, J., Schwerk, N., Aslanidis, C., Lohse, P., Brasch, F., Zarbock, R., Griese, M. Lung disease caused by ABCA3 mutations. Thorax. 2017;72(3):213-220. [CrossRef] [PubMed]
- Henderson, L.B., Melton, K., Wert, S., Couriel, J., Bush, A., Ashworth, M., Nogee, L.M. Large ABCA3 and SFTPC deletions resulting in lung disease. Ann Am Thorac Soc. 2013;10(6):602-7. [CrossRef] [PubMed]
- Hamvas, A., Nogee, L.M., Wegner, D.J., Depass, K., Christodoulou, J., Bennetts, B., McQuade, L.R., Gray, P.H., Deterding, R.R., Carroll, T.R., Kammesheidt, A., Kasch, L.M., Kulkarni, S., Cole, F.S. Inherited surfactant deficiency caused by uniparental disomy of rare mutations in the surfactant protein-B and ATP binding cassette, subfamily a, member 3 genes. J Pediatr. 2009;155(6):854-859.e1. [CrossRef] [PubMed]
- Hartl, D., Griese, M. Interstitial lung disease in children -- genetic background and associated phenotypes. Respir Res. 2005;6(1):32. [CrossRef] [PubMed]
- Karjalainen, M.K., Haataja, R., Hallman, M. Haplotype analysis of ABCA3: association with respiratory distress in very premature infants. Ann Med. 2008;40(1):56-65. [CrossRef] [PubMed]
- Bullard, J.E., Wert, S.E., Whitsett, J.A., Dean, M., Nogee, L.M. ABCA3 mutations associated with pediatric interstitial lung disease. Am J Respir Crit Care Med. 2005;172(8):1026-31. [CrossRef] [PubMed]
- Crossno, P.F., Polosukhin, V.V., Blackwell, T.S., Johnson, J.E., Markin, C., Moore, P.E., Worrell, J.A., Stahlman, M.T., Phillips, J.A. 3rd, Loyd, J.E., Cogan, J.D., Lawson, W.E. Identification of early interstitial lung disease in an individual with genetic variations in ABCA3 and SFTPC. Chest. 2010;137(4):969-73. [CrossRef] [PubMed]
- Wittmann, T., Frixel, S., Höppner, S., Schindlbeck, U., Schams, A., Kappler, M., Hegermann, J., Wrede, C., Liebisch, G., Vierzig, A., Zacharasiewicz, A., Kopp, M.V., Poets, C.F., Baden, W., Hartl, D., van Kaam, A.H., Lohse, P., Aslanidis, C., Zarbock, R., Griese, M. Increased Risk of Interstitial Lung Disease in Children with a Single R288K Variant of ABCA3. Mol Med. 2016;22:183-191. [CrossRef] [PubMed]
- Kaltenborn, E., Kern, S., Frixel, S., Fragnet, L., Conzelmann, K.K., Zarbock, R., Griese, M. Respiratory syncytial virus potentiates ABCA3 mutation-induced loss of lung epithelial cell differentiation. Hum Mol Genet. 2012;21(12):2793-806. [CrossRef] [PubMed]
- Yang, X., Rapp, C.K., Li, Y., Forstner, M., Griese, M. Quantifying Functional Impairment of ABCA3 Variants Associated with Interstitial Lung Disease. Int J Mol Sci. 2023;24(8):7554. [CrossRef] [PubMed]
- Beers, M.F., Hawkins, A., Shuman, H., Zhao, M., Newitt, J.L., Maguire, J.A., Ding, W., Mulugeta, S. A novel conserved targeting motif found in ABCA transporters mediates trafficking to early post-Golgi compartments. J Lipid Res. 2011;52(8):1471-82. [CrossRef] [PubMed]
- Wambach, J.A., Yang, P., Wegner, D.J., Heins, H.B., Luke, C., Li, F., White, F.V., Cole, F.S. Functional Genomics of ABCA3 Variants. Am J Respir Cell Mol Biol. 2020;63(4):436-443. [CrossRef] [PubMed]
- Karbassi, I., Maston, G.A., Love, A., DiVincenzo, C., Braastad, C.D., Elzinga, C.D., Bright, A.R., Previte, D., Zhang, K., Rowland, C.M., McCarthy, M., Lapierre, J.L., Dubois, F., Medeiros, K.A., Batish, S.D., Jones, J., Liaquat, K., Hoffman, C.A., Jaremko, M., Wang, Z., Sun, W., Buller-Burckle, A., Strom, C.M., Keiles, S.B., Higgins, J.J. A Standardized DNA Variant Scoring System for Pathogenicity Assessments in Mendelian Disorders. Hum Mutat. 2016;37(1):127-34. [CrossRef] [PubMed]
- Richards, S., Aziz, N., Bale, S., Bick, D., Das, S., Gastier-Foster, J., Grody, W.W., Hegde, M., Lyon, E., Spector, E., Voelkerding, K., Rehm, H.L.; ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405-24. [CrossRef] [PubMed]
- Duzkale, H., Shen, J., McLaughlin, H., Alfares, A., Kelly, M.A., Pugh, T.J., Funke, B.H., Rehm, H.L., Lebo, M.S. A systematic approach to assessing the clinical significance of genetic variants. Clin Genet. 2013;84(5):453-63. [CrossRef] [PubMed]
- Thusberg, J., Olatubosun, A., Vihinen, M. Performance of mutation pathogenicity prediction methods on missense variants. Hum Mutat. 2011;32(4):358-68. [CrossRef] [PubMed]
- Thavagnanam, S., Cutz, E., Manson, D., Nogee, L.M., Dell, SD. Variable clinical outcome of ABCA3 deficiency in two siblings. Pediatr Pulmonol. 2013;48(10):1035-8. [CrossRef] [PubMed]
| Pacient number | Reference | Gender | GA | BW | Respiratory disease | Imaging aspects | Lung histology | EM | ABCA3 variant - alelle 1/parental origin | Other associated ABCA3 variant(s)/parental origin/Allele 2 | Variants of other surfactant genes/parental origin | Familial history | Outcome | Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Somaschini et al., 2007 [59] | Male | 39 weeks | 2850 g | Severe nRDS (mechanical ventilation) | N/A | N/A | N/A | R280C/wt/ | Most probably the second mutation was missed due to restrictive genetic testing protocol | Tested, none reported | No | Died at 2 days | Patient 7 in a case series of 17 cases with inherited deficiency of pulmonary surfactant |
| 2. | Turcu et al., 2013 [60] | Not reported | Term | Not reported | nRDS – Surfactant administration, ventilation, hydroxychloroquine | Interstitial changes on lung CT | CIP | N/A | c.838C>T (p.Arg280Cys)/heterozygous/parental origin not reported | c.2069A>G (p.Glu690Gly)/heterozygous/parental origin not reported | None reported | no | Alive at 13 years | Patient reported in a case serie of 323 cases analyzed for inherited deficiency of pulmonary surfactant |
| 3. | Williamson & Wallis, 2014 [61] | Female | Term | Not reported | nRDS – surfactant, mechanical ventilation, multiple corticosteroids courses, oxygen dependent at 2 years; treated with hydroxychloroquine afterwards | Extensive patchy ground glass opacification and cystic airway changes, predominantly in the upper lobes at 2 years on thoracic CT |
DIP at 2 years |
N/A | c.838C>T/heterozygous/parental origin not reported | c.2069A>G/heterozygous/parental origin not reported | Tested, no variants discovered | N/A | Final evaluation at 13 years, stable under hydroxychloroquine treatment | Case report |
| 4. | Wambach, 2014 [62] | Female | N/A | N/A | nRDS | N/A | N/A | N/A | Q1589X R280C (homozygous) |
Q1589X R280C (homozygous) |
N/A | N/A | Died < 3 months | Based on unpublished data |
| 5. | Male | N/A | N/A | nRDS | N/A | N/A | N/A | c.3997_3998delAG (null) | R280C | N/A | N/A | Alive | Based on unpublished data | |
| 6. | Female | N/A | N/A | nRDS | N/A | N/A | NA | Q1589X (p.Gln1589) R280C |
c.4195G>A (V1399M)(p.Val1399Met) | N/A | N/A | Lung transplantation at 10 months | Based on unpublished data; Reported also by Xu et al., 2022 [63] | |
| 7. | Jackson et al., 2015 [64] | Female | Term | 2870 g | Severe nRDS treated with advanced respiratory support (HFOV, iNO), weaned for respiratory support at 1 year | N/A | Diffuse interstitial fibrosis with alveolar remodeling and prominent AEC II hyperplasia | Small, dense lamellar bodies and occasional fused lamellar bodies | p.R280C (c.838C>T)/paternal (cis) | p.Val1399Met(c.4195G>A)/maternal p.Q1589X (C.4765C>T)/paternal (cis) |
Tested, none discovered | No (parents and one previous sibling without – healthy) | Alive at 3 years, mild speech and motor delay | Case report; p.V1399M is rare and has been previously reported in symptomatic infants [62]; p.Q1589X is predicted to result in a truncated protein [64] Both p.R280C and p.V1399M are predicted to be damaging to ABCA3 protein function by the majority of in silico prediction programs in ANNOVAR [65] |
| 8. | Klay et al., 2020 [66] | Female | Not reported | Not reported | ILD - onset at 19 years with dyspnea; high resolution chest CT: diffuse ground glass opacities with emphysema located in the apical regions, progressing to severe lung fibrosis |
N/A | Diffuse fibrosis with chronic inflammation, unusual interstitial pneumonia, fibroblast foci or granulomas. Mild AEC II hyperplasia, accumulation of alveolar macrophages |
N/A | c.838C>T (p.R280C, rs201299260) (trans)/one parent c.875A>T (p.E292V, rs149989682). (trans)/the other parent |
- | No report on other genetic tests | No | Proposed for lung transplantation at 25 years | Case report; Initially diagnosed with drug-induced ILD |
| 9. | Gjeta et al., 2023 [67] | Female | Not reported | Not reported | Onset at 2 years and 7 months with severe pediatric ARDS (requiring invasive respiratory support, prednisolone and azithromycin treatment), after 2 episodes of upper respiratory tract infections | Chest X-ray showed bilateral opacification suggesting interstitial bilateral pneumonia. chest CT scan showed bilateral ground-glass opacities |
N/A | N/A | c.4261 G>A p. (Gly1421Arg)/heterozygous/paternal | c.838C>T p. (Arg280Cys)/heterozygous/maternal | Tested, none reported | N/A | Alive, after prolonged oxygen therapy and on treatment with oral hydroxychloroquine and fluticasone propionate inhalations | Case report |
| 10. | Ognean et al., 2024 (this study) | Male | 37 weeks | 2700 g | Severe nRDS – advanced respiratory support (HFOV), prednison, azithromycin, hydroxychloroquine treatment | Thoracic x-ray – ground glass homogeneous opacity | CPI pattern with lobular remodeling, prominent AEC II hyperplasia, focal PAP pattern and extensive DIP-like areas, alveolar proteinosis | N/A | p.Arg280Cys (R280C, c.838C>T, rs201299260)/heterozygous/paternal origin | p.Gln233ter (Q233X, Q233*)heterozygous/maternal origin | SFTPB p.Val267Ile | No, healthy parents, one healthy sibling despite carrying p.Gln233ter and SFTPB p.Val267Ile variants | Died at 77 days of life | Current case report |
| Pros | Cons | |
| Clinical aspects | R280C mutations were reported in association with neonatal RDS and ILD, with clinical picture and course suggestive of surfactant metabolism dysfunction [59,61,62,64,66,107] | Variable phenotype, usually in association with other mutations with survival varying between 2 days and over 25 years [66]; severe phenotype was associated in 4 of the 10 cases reported in the literature (Table 1) |
| In vitro experiments | R280C mutations result in folding and trafficking defects, increased endoplasmic reticulum stress, and apoptosis induction in lung epithelial cells in vitro [86] | In vitro experiments cannot accurately represent biological functions [86] Functional impact on ABCA3 protein is less important as compared to other ABCA3 mutations [66] |
| DNA analysis | Mutations reported are associated with respiratory disease in the subjects [59,61,62,64,66,107] | Most reported inducing-disease variants are associated with other mutations in ABCA3 or SFTPB genes, some of these known as pathogenic (ex. Q1589X) [62]; co-occurrence with these mutations may suggest R280C as benign variant; Incomplete genetic testing in some patients (for example, Somaschini et al., 2007 [59] |
| Estimated allele frequency in the population | Rare, suggestive of pathogenicity (surfactant metabolic dysfunction produced by ABCA3 mutations) [108,109] | Frequency higher than for other mutations causing ABCA3 deficiency, not offering unequivocal evidence for pathogenicity [55] |
| Computational predictive tools and conservational analysis | Most indicate a damaging effect of the mutation on ABCA3 protein [55]. Calibrated prediction (examples):
|
Insufficient power for predicting pathogenicity Calibrated prediction (examples)
Bioinformatic prediction tools do not determine and or exclude pathogenicity [110]. Most of the current prediction algorithms have a 65-80% predictive accuracy for known pathogenic variants and a tendency to low specificity [111] Predictive bioinformatics seems insufficient for defining the pathogenicity of ABCA3 deficiencies [52,105] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





