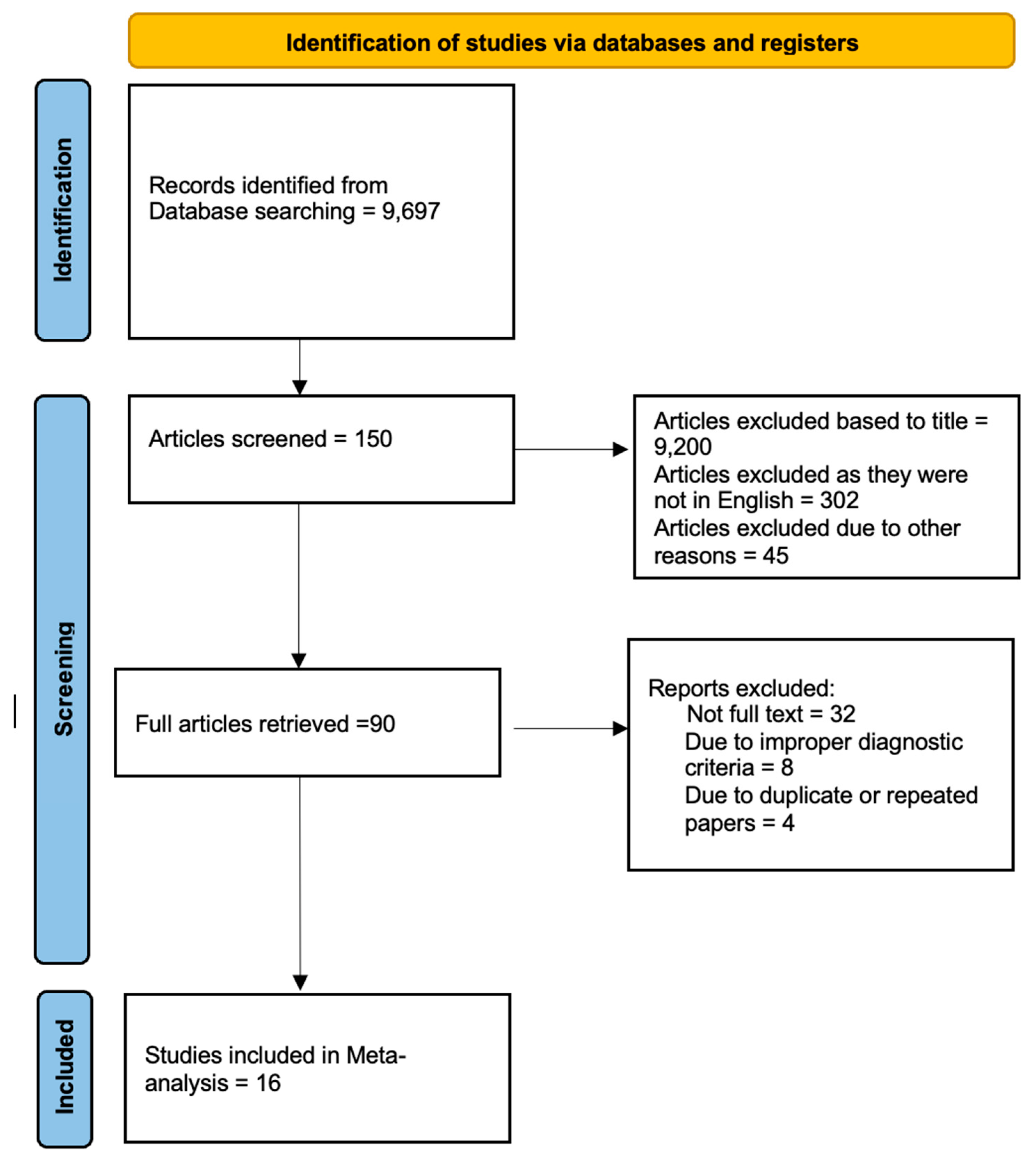
Introduction
The comprehensive clinical definition of "brain death" refers to the permanent loss of all brain functions. Because of the unique characteristics of brain disease, appropriate clinical criteria must be used before making a decision. Most of these include unexplained deep coma, absence of brain reflexes and sudden respiratory arrest or positive apnea test, the 3 main criteria for brain death. A brain injury can only be diagnosed after three criteria are met. [
1] In most countries, the clinical diagnosis of brain disease, which is very important for organ donation, should be added to the list of auxiliary tests, such as EEG, cerebral angiography, or radionuclide imaging. These confirmatory tests pose significant logistical problems, as they are time-consuming and require the transportation of critically ill patients. Therefore, there is a need to develop other methods that are simpler and less invasive to help us gain clarity and certainty.[
1,
2]
Transcranial Doppler (TCD) ultrasound is not a new technique for solving the mysterious and highly debated question of when a person dies. It was introduced into clinical practice in 1982 by Rune Aaslid. It is a non-invasive method to study the blood flow velocity of the intracranial basal artery in real time and at the patient's bedside. TCD assesses cerebral blood flow in the presence of localized cerebral hemorrhage, which may lead to cerebral circulation arrest (CCA) and brain death. Severe ischemia, hemorrhage, or cerebral edema can increase intracranial pressure (ICP). Excessive ICP can cause microcirculatory damage. The presence of smooth arteries at the TCD recording site allows blood to move backwards during the cardiac cycle. Forward flow, which occurs during systole, dilates the muscular trunk, but little or no circulation occurs in the microcirculation because of the high distal resistance. During diastole, the flow of the arteries changes. By comparing TCD patterns with angiographic criteria for cerebrovascular arrest, Hassler et al helped establish TCD as an alternative to other conventional methods for confirming cerebrovascular disease. However, the inclusion of TCD in cerebral palsy protocols requires prior validation.[
2,
3,
4,
5]
TCD would seem like an ideal test to confirm brain death, but the cessation of cerebral blood flow and clinical brain death may not temporally correspond with each other. Such cases may render the declaration of brain death difficult. A repeat TCD after the arrest of cerebral circulation may pose a problem, in that it may put potential donors at a high risk of organ dysfunction, rendering them useless. A misdiagnosis, not only has a lot of legal and ethical implications, it may be a cause of severe distress to the family of the deceased. Dire as these consequences seem, the intricacies of such matters despite lying beyond the present scope of this study, garner a mention here to shape our understanding of the importance, need, and application of TCD and similar modalities in the context of brain death. Rather than just relying on the neurological exam, the clinical utility of TCD for confirmation of brain death should be evaluated and used to scrutinize whether TCD should be used as an integral part of the protocol for the diagnosis of brain death.[
1,
5]
This study aims to substantiate the utility of TCD as a reliable ancillary test to diagnose brain death with more certainty and reliability.
Methodology
Data Collection
For the collection of the data, a search was done by two individuals using PubMed, Google Scholar, and Cochrane Library databases for all relevant literature. Full - Text Articles written only in English were considered.
The medical subject headings (MeSH) and keywords ‘Transcranial’, ‘Doppler Study’, ‘Ultrasonography’, and ‘Brain death’ were used. References, reviews, and meta-analyses were scanned for additional articles.
Inclusion and Exclusion Criteria
Screening of titles and abstracts was carried out, wherein duplicates and citations were filtered out of consideration. Reviewing references of relevant papers was done for possible additional articles, following which papers with detailed patient information and results with a strong statistical base were selected.
We searched for papers showing more accurate diagnoses, wherein the modality considered was Transcranial Doppler Ultrasonography of Intracerebral Vasculature for diagnosis of brain Death.
The inclusion criteria were as follows: (1) studies that provided information about the accurate diagnosis of brain Death with Transcranial Doppler Ultrasonography of Intracerebral Vasculature; (2) studies published in English; (3) Studies comparing Transcranial Doppler Ultrasonography of Intracerebral Vasculature to the Gold Standard (Cerebral Angiography) as a diagnostic modality for cases of brain Death.
The exclusion criteria were: (1) articles that were not full text, (2) unpublished articles, and (3) articles in other languages..
Assessment of Study Quality
Using the QualSyst tool, two authors independently assessed the efficacy of each included study. There are 10 questions in this test, each question has a score between 0 and 2, and 20 questions is the maximum mark possible. Two authors independently assessed each article based on the above criteria. Cohen's Kappa (K) weighted coefficient was used to determine interobserver agreement on study selection. We also used the Cochrane tool to assess the risk of bias in the studies. We are not responsible for missing or unclear data. No data collection or analysis was paid for.
Statistical Analysis
RevMan (Review Manager, version 5.3), SPSS (Statistics for Social Sciences, version 20), Google Sheets and Excel in Stata 14 were used to perform the statistical analysis. Data were obtained and entered into the analysis software. Fixed or random effects models were used to estimate sensitivity, specificity, positive predictive value (PPV), odds ratio (DOR) and relative risk (RR) with 95% confidence intervals. to examine clinically important outcomes (CI). Diagnostic accuracy and the Yonden index were calculated for each product. The sensitivity and specificity of individual probes were plotted on forest plots and receiver operating characteristic (ROC). A forest plot and Fagan's nomogram were used to show the sensitivity and specificity of different transcripts.
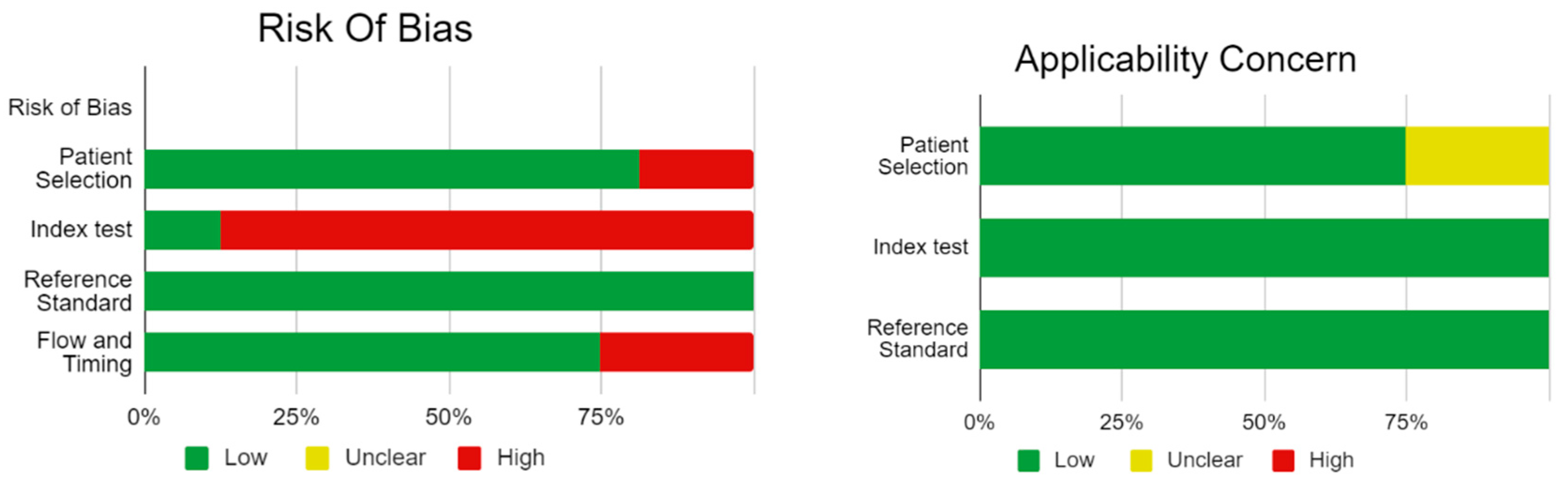
Bias Study
The risk of bias was evaluated by using QUADAS-2 analysis. This tool includes 4 domains Patient selection, Index test, Reference standard, Flow of the patients, and Timing of the Index tests.
Result
Here,
Table 1 describes all the descriptions of papers used for the study regarding the use of Transcranial Doppler Ultrasonography of Intracerebral Vasculature in the diagnosis of Prostate Cancer. In
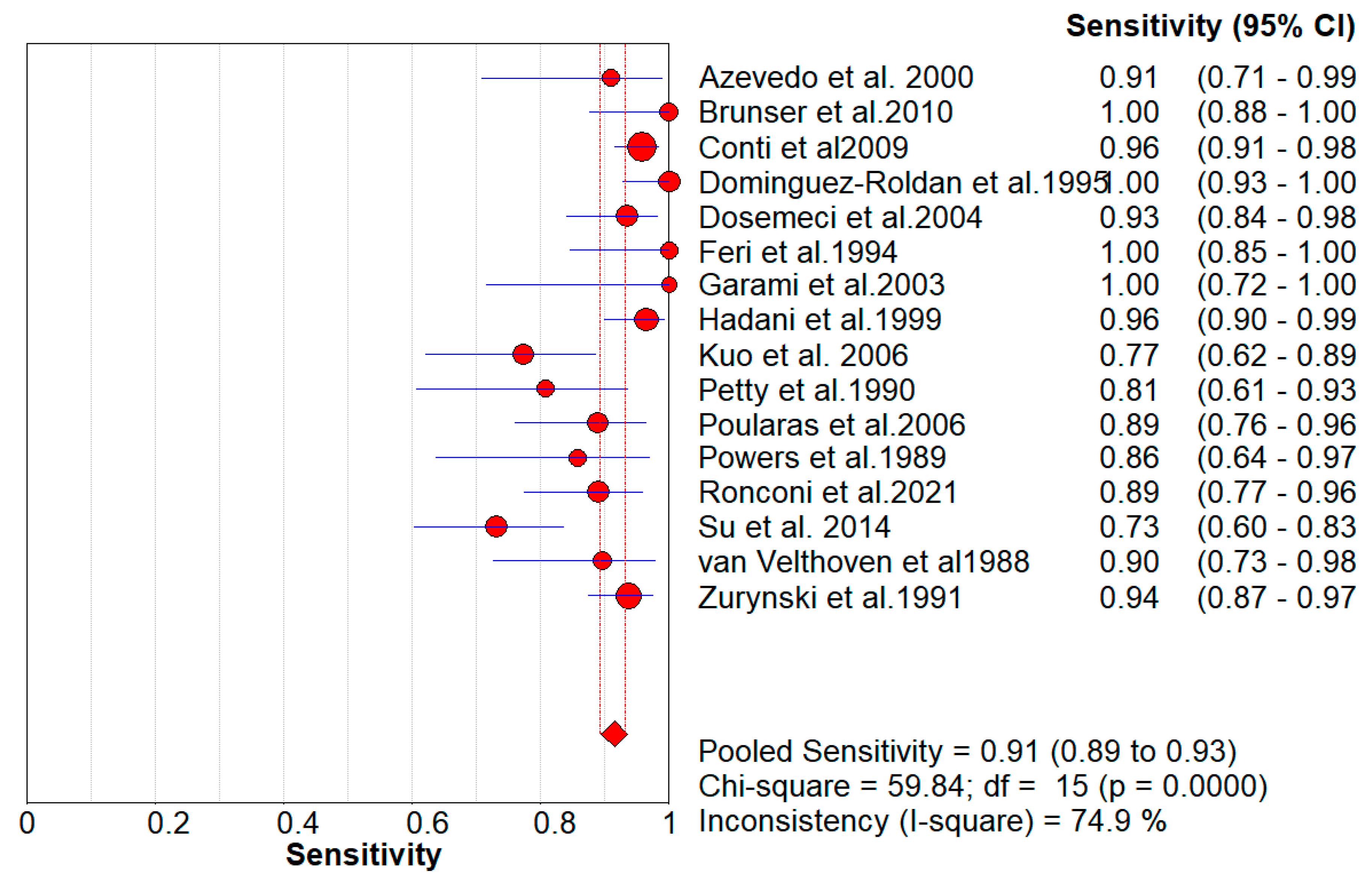
Figure 2, the Pooled Sensitivity Values for all papers being considered can be observed and compared amongst each other, while
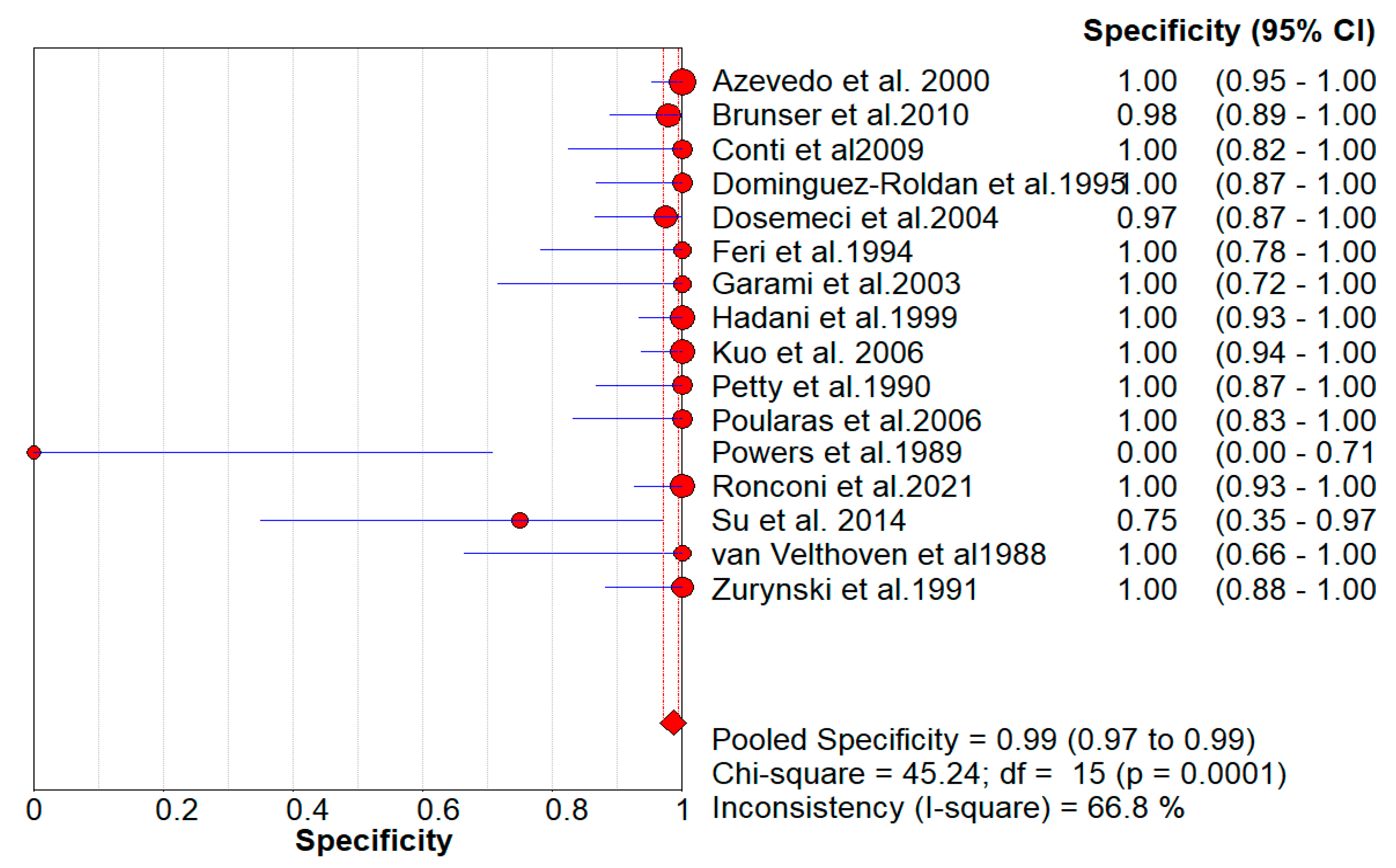
Figure 3 serves the same purpose in the context of Pooled Specificity Values.
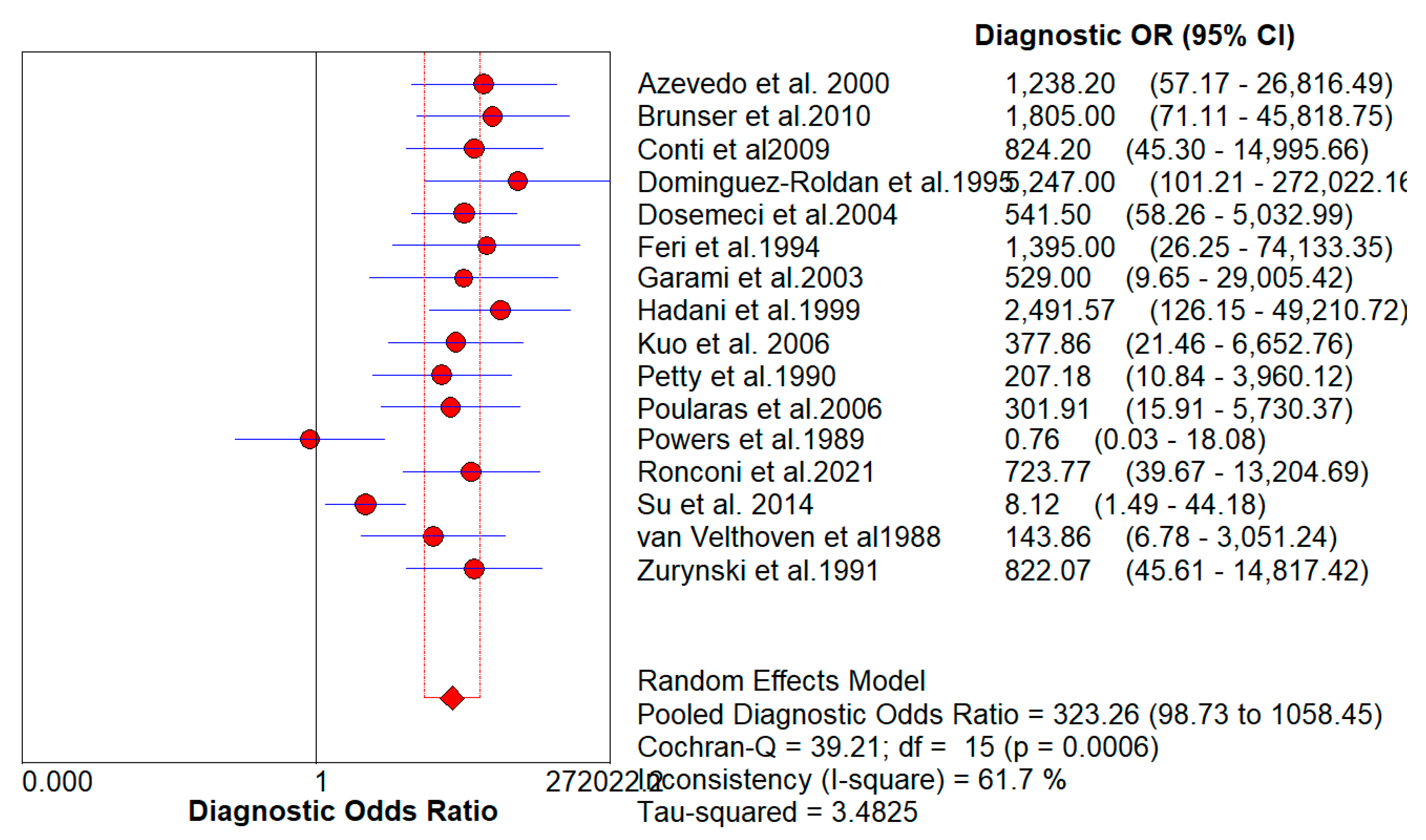
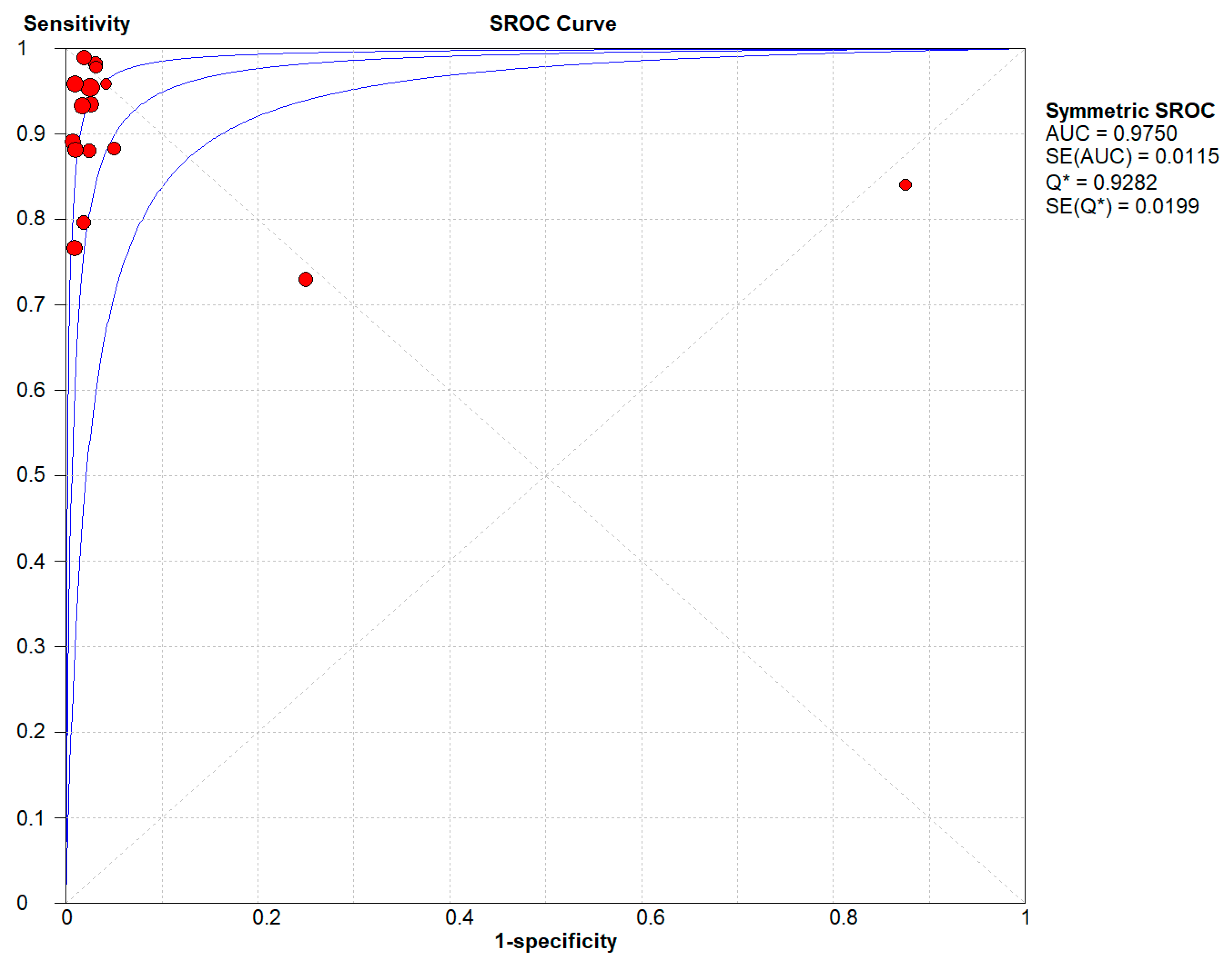
Figure 4 denotes the pooled Diagnostic Odds Ratio for the application of Transcranial Doppler Ultrasonography of Intracerebral Vasculature. The same is illustrated in the SROC curve. (
Figure 5A).
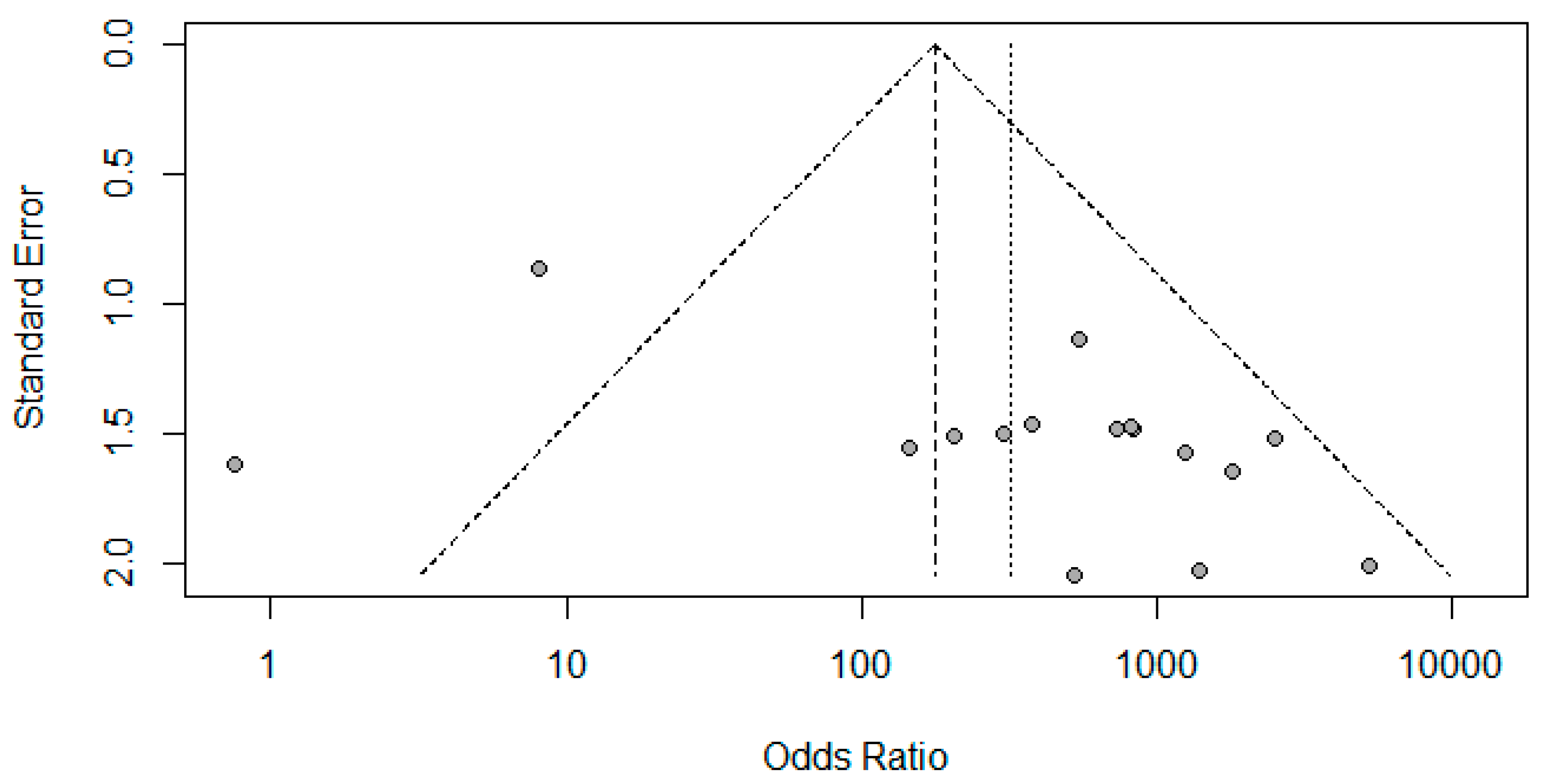
Figure 6A represents Deek’s Funnel Plot. A total of 16 studies with 1,317 subjects were selected for the study, out of which 7 studies showed sensitivity above or equal to 95%, and an astounding 14 studies showed specificity above 95%. The value of True Positive (TP) was 763, that of True Negative (TN) was 479, that of False Positive (FP) was 8, and that of False Negative (FN) was 72. With a confidence interval of 95%, sensitivity, specificity, and positive predictive values were calculated. The sensitivity of Transcranial Doppler Ultrasonography of Intracerebral Vasculature is 0.91, with a CI of 95% in a range of 0.89 to 0.93. The specificity of Transcranial Doppler Ultrasonography of Intracerebral Vasculature is 0.99, with a CI of 95% in a range of 0.97 to 0.99.
Figure 5 shows the summary of the ROC curve. It shows that the area under the curve for Transcranial Doppler Ultrasonography of Intracerebral Vasculature was 0.9750 and the overall diagnostic odds ratio (DOR) was 323.26 with Younden Index being 0.9.
Table 1.
Table of Discussion.
Table 1.
Table of Discussion.
| |
AUTHOR |
YEAR
OF PUBLISHInG |
TRUE POSITIVE (TP) |
FALSE POSITIVE
(FP) |
FALSE NEGATIVE (FN) |
TRUE NEGATIVE (TN) |
SENSITIVITY |
SPECIFICITY |
| |
|
|
|
|
|
|
|
|
| 1 |
Azevedo e.t al. [3] |
2000 |
20 |
0 |
2 |
75 |
0.91 |
1 |
| 2 |
Brunser et al. [4] |
2010 |
28 |
1 |
0 |
47 |
1 |
0.98 |
| 3 |
Conti et al. [5] |
2009 |
158 |
0 |
7 |
19 |
0.956 |
1 |
| 4 |
Dominguez-Roldan et al. [6] |
1995 |
49 |
0 |
0 |
26 |
1 |
1 |
| 5 |
Dosemeci et al. [7] |
2004 |
57 |
1 |
4 |
38 |
0.93 |
0.97 |
| 6 |
Feri et al.[8] |
1994 |
22 |
0 |
0 |
15 |
1 |
1 |
| 7 |
Garami et al. [9] |
2003 |
11 |
0 |
0 |
11 |
1 |
1 |
| 8 |
Hadani et al. [10] |
1999 |
81 |
0 |
3 |
53 |
0.96 |
1 |
| 9 |
Kuo et al. [2] |
2006 |
34 |
0 |
10 |
57 |
0.77 |
1 |
| 10 |
Petty et al. [11] |
1990 |
21 |
0 |
5 |
26 |
0.81 |
1 |
| 11 |
Poularas et al. [12] |
2006 |
40 |
0 |
5 |
20 |
0.89 |
1 |
| 12 |
Powers et al. [13] |
1989 |
18 |
3 |
3 |
0 |
0.86 |
0 |
| 13 |
Ronconi et al. [14] |
2021 |
48 |
0 |
6 |
48 |
0.896 |
1 |
| 14 |
Su et al. [15] |
2014 |
46 |
2 |
17 |
6 |
0.73 |
0.75 |
| 15 |
van Velthoven et al. [16] |
1988 |
26 |
0 |
3 |
9 |
0.9 |
1 |
| 16 |
Zurynski et al. [17] |
1991 |
104 |
0 |
7 |
29 |
0.94 |
1 |
Figure 2.
The forest chart summary for pooled sensitivity values of Transcranial Doppler Ultrasonography of Intracerebral Vasculature.
Figure 2.
The forest chart summary for pooled sensitivity values of Transcranial Doppler Ultrasonography of Intracerebral Vasculature.
Figure 3.
The forest chart summary for pooled specificity values for Transcranial Doppler Ultrasonography of Intracerebral Vasculature.
Figure 3.
The forest chart summary for pooled specificity values for Transcranial Doppler Ultrasonography of Intracerebral Vasculature.
Figure 4.
The forest chart summary for pooled Diagnostic Odds Ratio for Transcranial Doppler Ultrasonography of Intracerebral Vasculature.
Figure 4.
The forest chart summary for pooled Diagnostic Odds Ratio for Transcranial Doppler Ultrasonography of Intracerebral Vasculature.
Figure 5.
The SROC plot summary for Transcranial Doppler Ultrasonography of Intracerebral Vasculature.
Figure 5.
The SROC plot summary for Transcranial Doppler Ultrasonography of Intracerebral Vasculature.
Figure 6.
Funnel Plot for Transcranial Doppler Ultrasonography of Intracerebral Vasculature.
Figure 6.
Funnel Plot for Transcranial Doppler Ultrasonography of Intracerebral Vasculature.
Figure 7 describes the summary of Fagan plot analysis for all the studies considered for Transcranial Doppler Ultrasonography of Intracerebral Vasculature, showing a prior probability of 63% (1.7); a Positive Likelihood Ratio of 56; a probability of post-test 99% (96); a Negative likelihood ratio of 0.09, and a probability of post-test 13% (0.2).
Figure 7.
Fagan’s Analysis for Transcranial Doppler Ultrasonography of Intracerebral Vasculature Diagnosis. Prior Probability (Odds): 63% (1.7).
Figure 7.
Fagan’s Analysis for Transcranial Doppler Ultrasonography of Intracerebral Vasculature Diagnosis. Prior Probability (Odds): 63% (1.7).
Table 2.
Bias Study.
| |
|
Risk Of Bias |
Applicability Concerns |
| No. |
Author Name and Year |
Patient Selection |
Index Test |
Reference Standard |
Flow and Timing |
Patient Selection |
Index Test |
Reference Standard |
| 1 |
Azevedo et al. 2000 |
High |
High |
Low |
Low |
Low |
Low |
Low |
| 2 |
Brunser et al.2010 |
Low |
High |
Low |
Low |
Unclear |
Low |
Low |
| 3 |
Conti et al2009 |
Low |
Low |
Low |
Low |
Low |
Low |
Low |
| 4 |
Dominguez-Roldan et al.1995 |
Low |
High |
Low |
High |
Low |
Low |
Low |
| 5 |
Dosemeci et al.2004 |
Low |
High |
Low |
High |
Low |
Low |
Low |
| 6 |
Feri et al.1994 |
Low |
High |
Low |
High |
Low |
Low |
Low |
| 7 |
Garami et al.2003 |
High |
Low |
Low |
Low |
Unclear |
Low |
Low |
| 8 |
Hadani et al.1999 |
Low |
High |
Low |
Low |
Unclear |
Low |
Low |
| 9 |
Kuo et al. 2006 |
Low |
High |
Low |
Low |
Low |
Low |
Low |
| 10 |
Petty et al.1990 |
Low |
High |
Low |
Low |
Low |
Low |
Low |
| 11 |
Poularas et al.2006 |
Low |
High |
Low |
High |
Low |
Low |
Low |
| 12 |
Powers et al.1989 |
Low |
High |
Low |
Low |
Unclear |
Low |
Low |
| 13 |
Ronconi et al.2021 |
Low |
High |
Low |
Low |
Low |
Low |
Low |
| 14 |
Su et al. 2014 |
High |
High |
Low |
Low |
Low |
Low |
Low |
| 15 |
van Velthoven et al1988 |
Low |
High |
Low |
Low |
Low |
Low |
Low |
| 16 |
Zurynski et al.1991 |
Low |
High |
Low |
Low |
Low |
Low |
Low |
Discussion
In this systematic review and meta-analysis, including 31 studies, it was found that transcranial Doppler ultrasonography (TCD) of intracerebral vasculature through various routes, as it currently exists, can be used as an important and noninvasive ancillary test to aid in the clinical diagnosis of brain death. The results show that the pooled sensitivity and specificity values of TCD as a diagnostic test in the algorithm of brain death were approximately 91% and 99% respectively. These results help to establish that although the lower sensitivity of TCD rules out its use as a screening test the higher specificity can be helpful to confirm the diagnosis of brain death in certain cases where the neurological examination does not give a certain diagnosis and it is not always feasible to carry out tests like EEG, arteriography, cerebral angiography or radionuclide imaging due to their complex and invasive nature as well as the requirement of a specialized facility. Further calculations help us to derive the Younden Index to be 0.9.
The major advantages of the development of TCD techniques are the fact that they have introduced a non-invasive method that can be performed at the bedside, irrespective of whether it is done in the ICU or the general wards, and assist in those time-intensive scenarios where the diagnosis of brain death could be a crucial matter for the survival of someone who needs an organ transplant, as well as help give the relatives of the deceased, information that they would want with absolute certainty. [
14,
15,
18]
Although TCD ultrasound does not measure CBF, a positive correlation has been shown between changes in flow velocity compared to changes in CBF. In a normal person, ultrasound evaluation reveals a high current wave and a low resistance wave. After a brain injury results in complete neuronal death, many changes can be seen by TCD ultrasound that show a change in the brain's circulation to a high-resistance, low-flow system.[
17]
In some cases, TCD can show forward flow during diastole, even if brain disease is detected by other reliable methods such as angiography. The retention of forward flow during diastole in these cases was due to bypass surgery and/or ventricular leads were performed in these patients. Therefore, the manifestation of an increase in ICP leading to the arrest of cerebral circulation is not the only explanation for the loss of all cerebral functions.[
3,
5,
10,
14]
The main purpose of TCD depends on its ability, using different patterns, to attract cerebral circulation arrest (CCA) in large intracranial vessels. However, there is still a lot of blood flow in the vessels and it can be said that many factors contribute to negative results on TCD. It is probably fair to say that arteriography is considered the gold standard test for confirming cerebrovascular disease, and only a few cases of direct flow have been reported. However, a clear correlation can be drawn between CCA levels on angiography and the TCD pattern. In addition, the fact that arteriography is performed long after the diagnosis of brain disease may help us to understand this difference. We agree with Plum that this long delay [in angiography] may account for the small number of examples of continuous bleeding after BD that have been reported.[
2,
6,
7,
8,
15,
18]
A new color Doppler model can be found in patients with cerebral palsy. 2D color mode recordings show an interesting pattern as systolic and diastolic flow sometimes coexist, producing a slow lightning signal similar to a beacon. This sign was observed in all patients if cerebral blood flow was maintained on simultaneous Doppler signals in all intracranial vessels. Despite the complexity of cerebrovascular hemodynamics, cerebral perfusion pressure (CPP) can be calculated as the difference between mean arterial blood pressure (MABP) and ICP. In addition, the assessment of cerebral autoregulation is based on the assumption that changes in blood flow velocity (FV) are associated with relative changes in CBF. The CPP value at which autoregulation ceases and FV begins to decrease is called the autoregulation "threshold" or "break point" [-24]. Mathematical models for predicting CPP status and cerebral control of cerebral circulation are developed from TCD FV waveform analysis. According to these equations, when CPP decreases, FVd approaches zero, forward blood movement occurs only during systole. As the CPP decreases and the arterial resistance increases, a characteristic flow pattern is observed, where there is retrograde flow during diastole. When retrograde flow during diastole is equal to forward flow during systole, there is no net blood flow and complete cerebral tamponade in the region of the sonographic vessel. Therefore, these color mode recordings may indicate a "cerebral" cerebral circulation that is observed without autoregulation and increases in blood flow just before CBF resistance becomes permanent. stop cerebral tamponade.[
1,
6,
13,
16,
17]
Brain death cannot always be confirmed because, in a small number of patients, the lack of signal may be due to variations in the configuration of the temporal bones in terms of anatomy and ossification. However, there are several solutions to this problem, the first is as simple as the use of extracranial vessel TCD reference. Studies show that reverse diastolic flow without seeing forward diastolic flow in an extracranial internal carotid artery and undetectable flow in the middle cerebral artery can be strongly associated with interruption of cerebral circulation[
5]. A low incidence of signal loss can also be achieved by using natural aperture windows, namely the transforaminal window for VBA insonation and the transorbital window for ICA insonation, and by lowering the filtered setting. To exclude this error even more effectively, it is possible to show that the lack of flow was due to an actual interruption of circulation, rather than transmission problems: perform TCD examinations in all head trauma patients. information and, in case of subsequent development of BD, will help determine which patients had flow loss and which had signal transmission problems. The known fact that women have increased the thickness of the diploe of the temporal bone can help not only to justify why the loss of signal is more common in them but also to challenge the old saying that men are "Thick head." [
17]
It should be pointed out that TCD confirmation of BD in children and adults has been considered operator-dependent, not 100% specific, and technically demanding.
[
12]. TCD is particularly susceptible to false negative results in certain patient-related situations. To name a few, when the subject has suffered a craniotomy reduction, ventriculo-ventricular dissection, skull fracture, or severe back injury when the sutures fail to close the baby's bones. These conditions cause a difference between intracranial pressure and cessation of circulation due to lack of flow.[
12,
15,
16,
17,
18]
A series of patterns can be seen in TCD as ICP increases and cerebral blood flow decreases. This usually progresses from a diastolic backflow to a very low systolic peak, and then no signal, indicating that the neurons have reached a threshold for survival. However, it should be noted that a normal TCD current signal does not mean that the neurons are firing well. Reverse diastolic flow in the MCAs followed by a low systolic peak in the basilar artery are the most common patterns. In general, more vessels with normal flow or reduced diastole were found in the MCAs or arteries. Therefore, many waveforms can be seen in cerebral palsy. It is therefore also pointed out that the interpretation of TCD without clinical correlation may lead to more misleading results. The basilar artery is the main vessel that supplies the brain. If blood flow stops in the basilar artery, the brain stops working. Therefore, the hemodynamics of the basilar artery appear to be more involved in the timing of stroke than other vessels such as the MCA. In addition, in most people the left hemisphere is dominant, causing more problems. In summary, we should consider that the complete circle of Willis test, the MCA and/or the basilar artery, or the MCA and/or the left basilar artery, is the minimum criterion for the diagnosis of cerebral palsy, if like these patients. . Clinical criteria have been established.[
2,
5,
7,
11,
15]
Conclusion
In conclusion, this study would like to emphasize that clinical diagnosis (all requirements are met) is sufficient to declare a patient brain dead. In ambiguous cases that require additional documentation, TCD is a useful and quick method. Sound flow patterns in intracranial arteries are reliable indicators of cerebral circulation arrest. This technique shows close agreement with angiography and is effective in being non-invasive. Because it can be done at the bedside in the intensive care unit, and if the disease occurs earlier, it beats the EEG because it does not focus on cortical activity. Differences in the nature of hyperostosis that can cause loss of the Doppler signal and the continuity of blood flow are problems that need new ways to solve, but this meta-analysis shows, these are no longer the same. from the distant future.
Ethical Statement
Being a Meta-analysis, there were no ethical issues and IRB permission is not required.
Funding and Sponsorship
None of the authors are financially interested in any of the products, devices, or drugs mentioned in this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li Y, Liu S, Xun F, Liu Z, Huang X. Use of Transcranial Doppler Ultrasound for Diagnosis of Brain Death in Patients with Severe Cerebral Injury. Med Sci Monit. 2016 Jun 6;22:1910-5. [CrossRef] [PubMed] [PubMed Central]
- Kuo JR, Chen CF, Chio CC, Chang CH, Wang CC, Yang CM, Lin KC. Time-dependent validity in the diagnosis of brain death using transcranial Doppler sonography. J Neurol Neurosurg Psychiatry. 2006 May;77(5):646-9. [CrossRef] [PubMed] [PubMed Central]
- Azevedo E, Teixeira J, Neves JC, Vaz R. Transcranial Doppler and brain death. Transplant Proc. 2000 Dec;32(8):2579-81. [CrossRef] [PubMed]
- Brunser A, Hoppe A, Cárcamo DA, Lavados PM, Roldán A, Rivas R, Valenzuela M, Montes JM. Validez del Doppler transcraneal en el diagnóstico de muerte encefálica [Validation of transcranial Doppler in the diagnosis of brain death]. Rev Med Chil. 2010 Apr;138(4):406-12. Spanish. Epub 2010 Jun 30. [PubMed]
- Conti A, Iacopino DG, Spada A, Cardali SM, Giusa M, La Torre D, Campennì A, Penna O, Baldari S, Tomasello F. Transcranial Doppler ultrasonography in the assessment of cerebral circulation arrest: improving sensitivity by transcervical and transorbital carotid insonation and serial examinations. Neurocrit Care. 2009;10(3):326-35. Epub 2009 Feb 24. [CrossRef] [PubMed]
- Dominguez-Roldan JM, Murillo-Cabezas F, Muñoz-Sanchez A, Santamaria-Mifsut JL, Villen-Nieto J. Changes in the Doppler waveform of intracranial arteries in patients with brain-death status. Transplant Proc. 1995 Aug;27(4):2391-2. [PubMed]
- Dosemeci L, Dora B, Yilmaz M, Cengiz M, Balkan S, Ramazanoglu A. Utility of transcranial Doppler ultrasonography for confirmatory diagnosis of brain death: two sides of the coin. Transplantation. 2004 Jan 15;77(1):71-5. [CrossRef] [PubMed]
- Feri M, Ralli L, Felici M, Vanni D, Capria V. Transcranial Doppler and brain death diagnosis. Crit Care Med. 1994 Jul;22(7):1120-6.. [CrossRef] [PubMed]
- Estrera, A. L., Garami, Z., Miller, C. C., Sheinbaum, R., Huynh, T. T. T., Porat, E. E., Winnerkvist, A., Safi, H. J., Mavroudis, C., Cimochowski, G., & Knott-Craig, C. (2003). Determination of cerebral blood flow dynamics during retrograde cerebral perfusion using power M-mode transcranial Doppler. Annals of Thoracic Surgery, 76(3), 704-710. [CrossRef]
- Hadani M, Bruk B, Ram Z, Knoller N, Spiegelmann R, Segal E. Application of transcranial Doppler ultrasonography for the diagnosis of brain death. Intensive Care Med. 1999 Aug;25(8):822-8. [CrossRef] [PubMed]
- Petty GW, Mohr JP, Pedley TA, Tatemichi TK, Lennihan L, Duterte DI, Sacco RL. The role of transcranial Doppler in confirming brain death: sensitivity, specificity, and suggestions for performance and interpretation. Neurology. 1990 Feb;40(2):300-3. [CrossRef] [PubMed]
- Poularas J, Karakitsos D, Kouraklis G, Kostakis A, De Groot E, Kalogeromitros A, Bilalis D, Boletis J, Karabinis A. Comparison between transcranial color Doppler ultrasonography and angiography in the confirmation of brain death. Transplant Proc. 2006 Jun;38(5):1213-7. [CrossRef] [PubMed]
- Powers AD, Graeber MC, Smith RR. Doppler ultrasonography in the determination of brain death. Neurosurgery. 1989 Jun;24(6):884-9. [CrossRef] [PubMed]
- Ronconi KAL, Amorim RLO, Paschoal FM Jr, Oliveira ML, Nogueira RC, Paiva WS, Gonçalves DB, Farias SR, Brasil SP, Teixeira MJ, Bor-Seng-Shu E. Transcranial Doppler: A Useful Tool to Predict Brain Death Still Not Confirmed by Clinical Assessment. Transplant Proc. 2021 Jul-Aug;53(6):1803-1807.Epub 2021 May 4. [CrossRef] [PubMed]
- Su Y, Yang Q, Liu G, Zhang Y, Ye H, Gao D, Zhang Y, Chen W. Diagnosis of brain death: confirmatory tests after clinical test. Chin Med J (Engl). 2014;127(7):1272-7. [PubMed]
- Van Velthoven V, Calliauw L. Diagnosis of brain death. Transcranial Doppler sonography as an additional method. Acta Neurochir (Wien). 1988;95(1-2):57-60. [CrossRef] [PubMed]
- Zurynski Y, Dorsch N, Pearson I, Choong R. Transcranial Doppler ultrasound in brain death: experience in 140 patients. Neurol Res. 1991 Dec;13(4):248-52. [CrossRef] [PubMed]
- de Freitas GR, André C. Sensitivity of transcranial Doppler for confirming brain death: a prospective study of 270 cases. Acta Neurol Scand. 2006 Jun;113(6):426-32. [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).