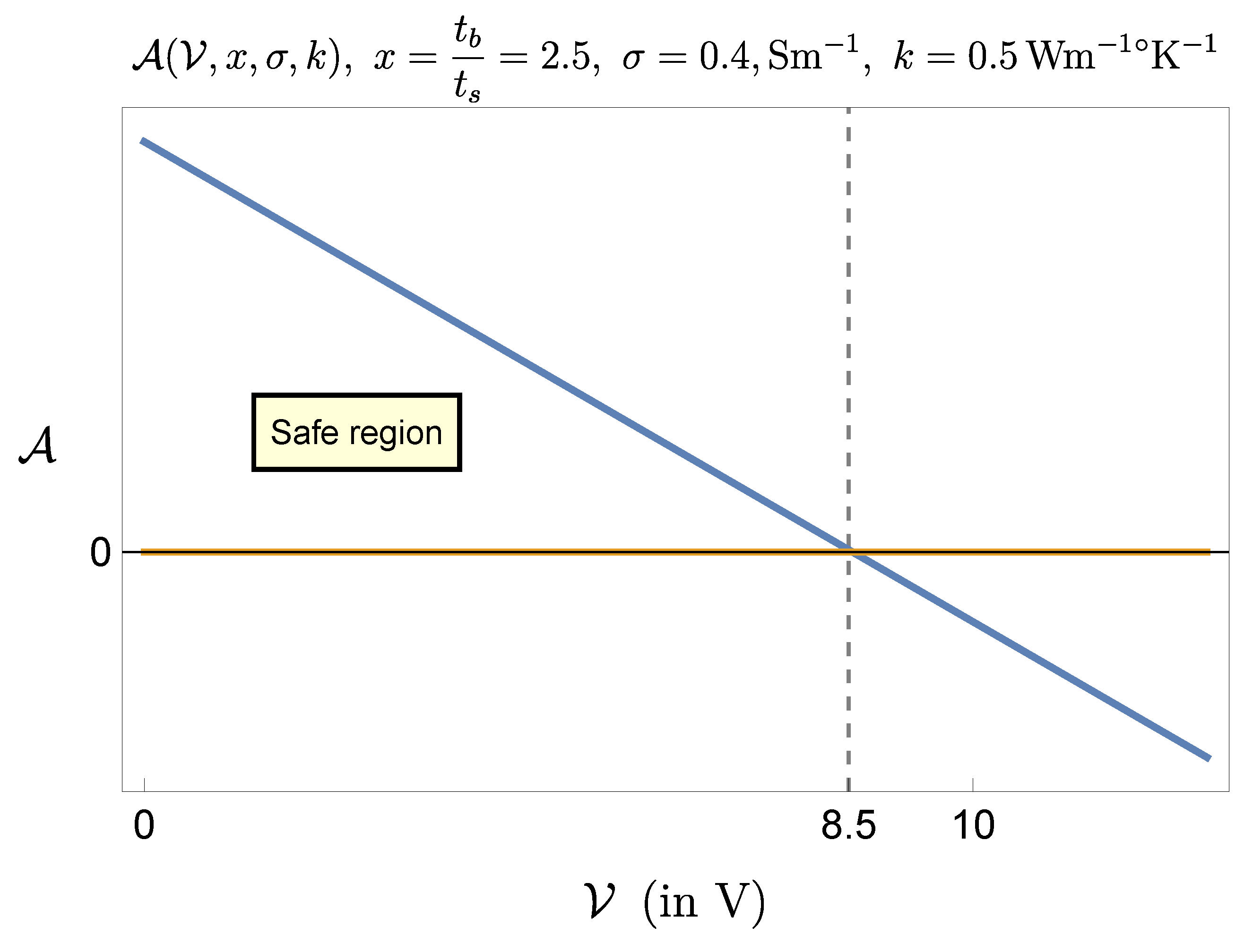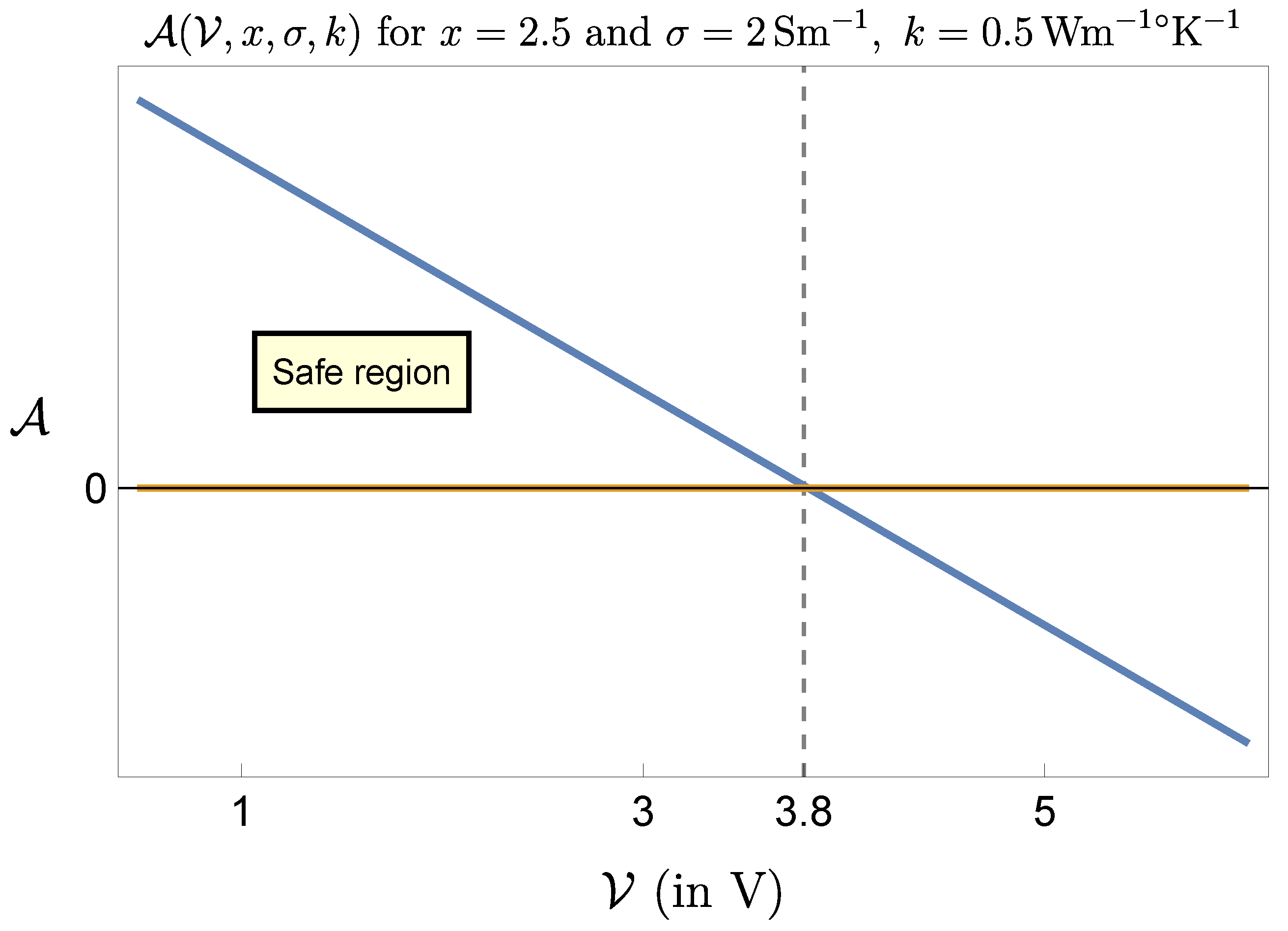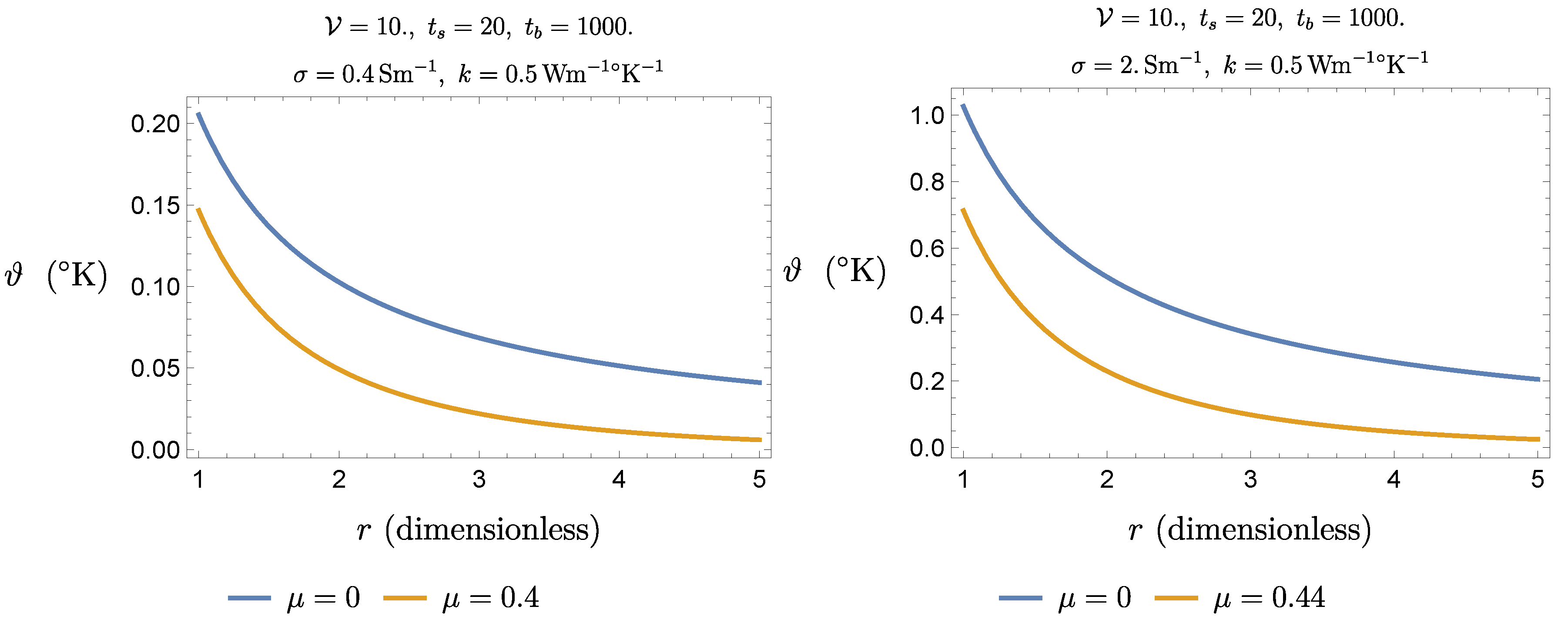2. The Physical Setting and a First Approach
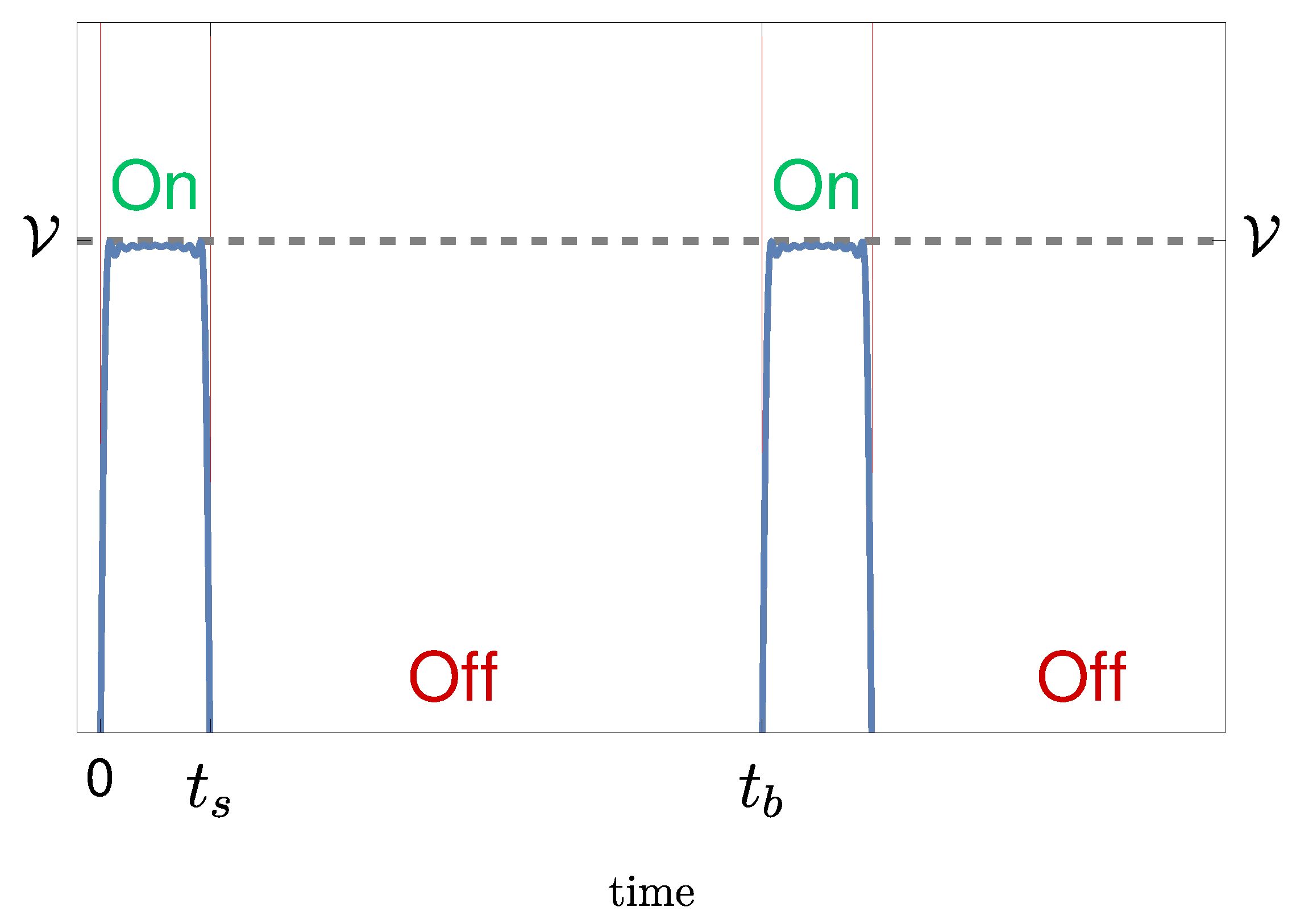
We consider the case in which the electrodes are introduced in a body cavity, generating a pulsating electric field for therapeutic purposes. Of course the electric field extends over the whole body, but it fades away rapidly far from the source. A first simplifying assumption we make is to suppose that heat is generated by a uniform pulsating current confined in a spherical region of radius R around the electrodes. The current has a duration and a period . In our approach the power delivered is considered constant, i.e. averaged over a period, considering that (<2 s) is much shorter than the application time (hours).
Figure 1.
Delivered voltage versus time for a given voltage and a given duty cycle (ratio of the pulse duration over the time it takes the signal to complete an on-off cycle).
Figure 1.
Delivered voltage versus time for a given voltage and a given duty cycle (ratio of the pulse duration over the time it takes the signal to complete an on-off cycle).
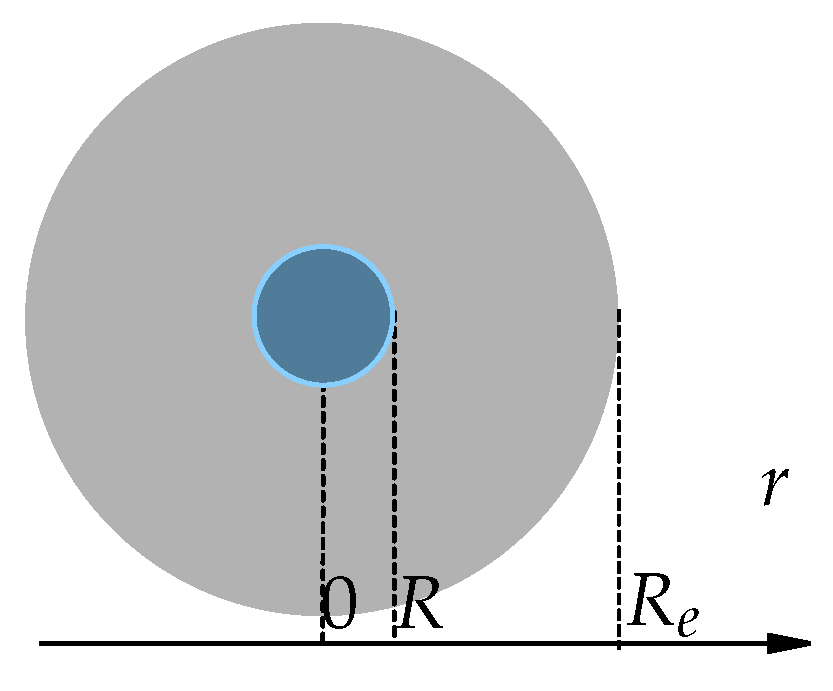
Next, we add the assumption that heat diffuses out of such a sphere through a larger sphere of radius
(for instance
; typically
mm,
cm, see
Figure 2), whose boundary has a constant temperature
, representing the patient’s basal temperature. Doing so we neglect the possible tissue thermal inhomogeneity, a fact of not great importance in the framework of the approximation we are aiming at. If
is the radial coordinate and
is time, the temperature
obeys the equation
where
denotes the power rate per unit volume, namely
The symbol Q denotes the average power delivered into the sphere because of Joule effect. We recall that is density (kgm), c is specific heat (Jkg−1), k is thermal conductivity Wm(). The equation is supplemented by the boundary conditions for and for (i.e. no heat flux through the center, by symmetry). Temperature and heat flux are continuous across the interface .
We select
as a characteristic length (in SI units) for the present problem. Then, we rescale
and
as follows
where
has to be conveniently chosen. Finally we summarize the dimensional typical values of the physical quantities involved in the case of human tissues:
Let us rewrite equation (
1) in the following form, where we have set
:
where
denotes the Heaviside function. Note that we have extended the outer domain to infinity, neglecting the influence of the boundary
, just making use of the fact that
. The solutions that will be presented are meant to be the leading order approximations when terms of the order of
are neglected. We identify
as the procedure duration, hence
Thus, (
3) entails (stationary state)
We define
measured in Kelvin degrees. During stimulation, the power supplied is
, where
is the stimulation amplitude and
is the impedance. Thus, keeping into account that the voltage is applied only during the time
during each period, we have
We may write
, where
is the medium electrical conductivity (measured in S
, S=Siemens) and
L can be taken as the side of a cubic box whose volume is equal to that of the sphere of radius
R, i. e.
. Consequently, we can rewrite (
6) as
where
The expression (
7) of the source term emphasizes the role of the three parameters
settable by the operator and of the physical properties of the medium entering as the ratio
, thus indicating that the two conductivities act in opposite ways.
Though the radius
R has eventually disappeared from our main estimates, it is interesting to check that our guess (
mm) was sensible. People working in the area of electrostimulation (see, e.g. [
2]) normally take the empirical assumption that the resistive load offered to the generator is
. So far we did not make any use of this information because it is too generic, but it can give a reasonable idea of the size of
R, using
. Setting now
(as we shall see for the normal environment), and
, we get
mm, which is of the same order of magnitude of our guess.
Taking into account the boundary and interface conditions for
, the differential system to be solved for
is
It can be easily checked that the solution writes
Notice that function
is always
and takes its maximum for
. Using
, we can rewrite (
10) as
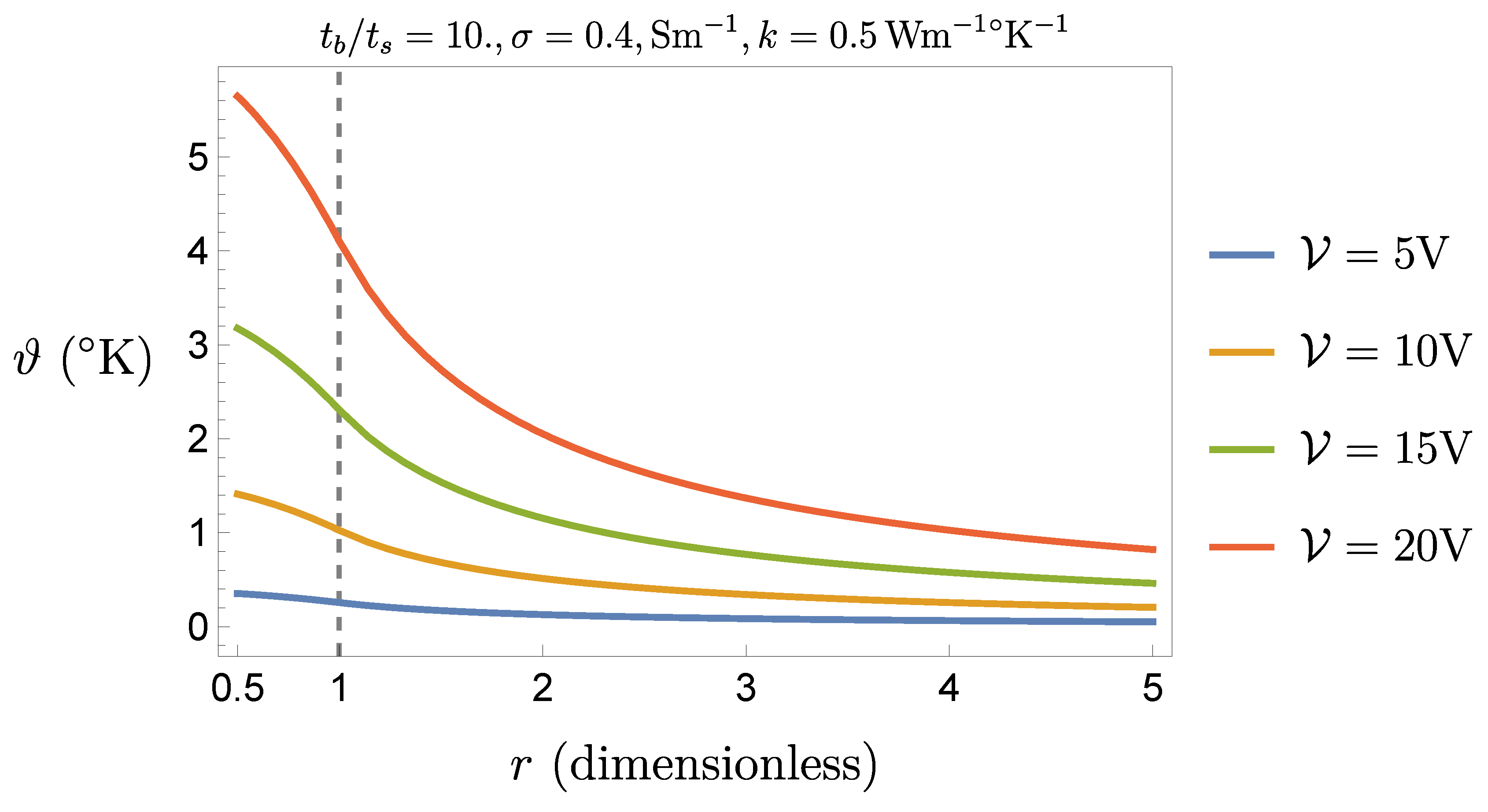
Figure 3 and
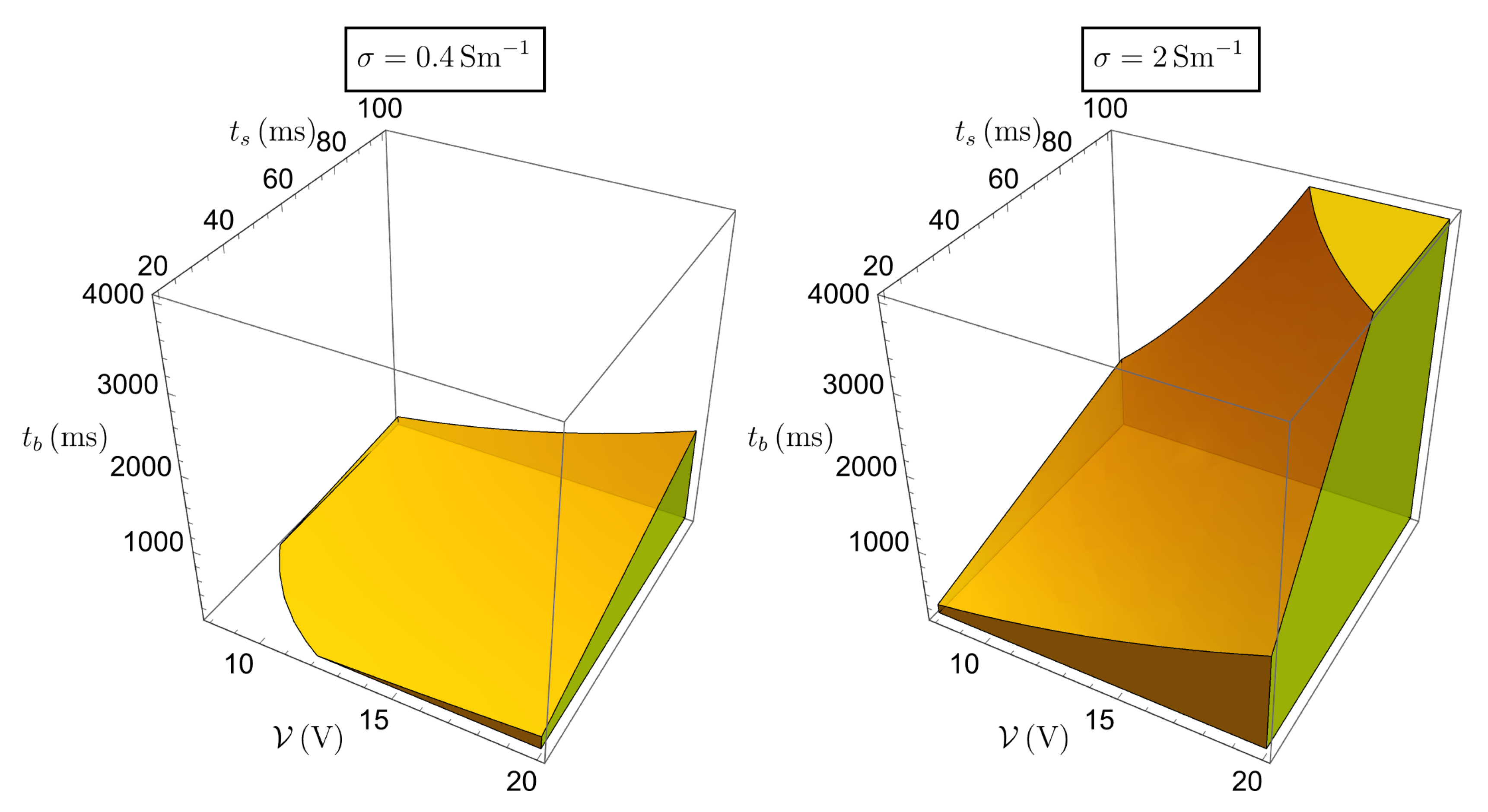
Figure 4 show how
changes by changing the operational parameters, considering a reasonable range for
(generally 0.4 but exceptionally up to 2
or more),
, and the instrument operational intervals (
V,
ms, and
ms).
Working with the temperature at the center may however be too heavy a condition, since the sphere of (dimensionless) radius
is more likely occupied by a liquid medium, the cellular tissue being more or less at the boundary of the ideal sphere in which we have confined the current. Therefore, a more interesting temperature seems to be the one calculated at
(see
Figure 3 and
Figure 4), namely, from (
10),
It is reasonable to suppose that (
12) provides the maximal temperature difference to which the tissue is exposed. Our goal now is to investigate the safety condition
, where a conservative value for
could be
.
3. Safety Stimulating Conditions
Let us define
so that the safety condition becomes
While the thermal conductivity
k for biological tissues is more or less constant with typical value
(with the exception of fat, see, e. g., [
3]), the electrical conductivity
is strongly affected by the pH of the ambient. We may first assume that the conductive medium filling the sphere of radius
R is aqueous (e.g. saliva, as it happens in the esophagus). In that case, the paper [
4] provides for
values between
and
. Passing to SI units (
=
), we take a typical value
. Thus, for
, the safety condition (
14) is to be read
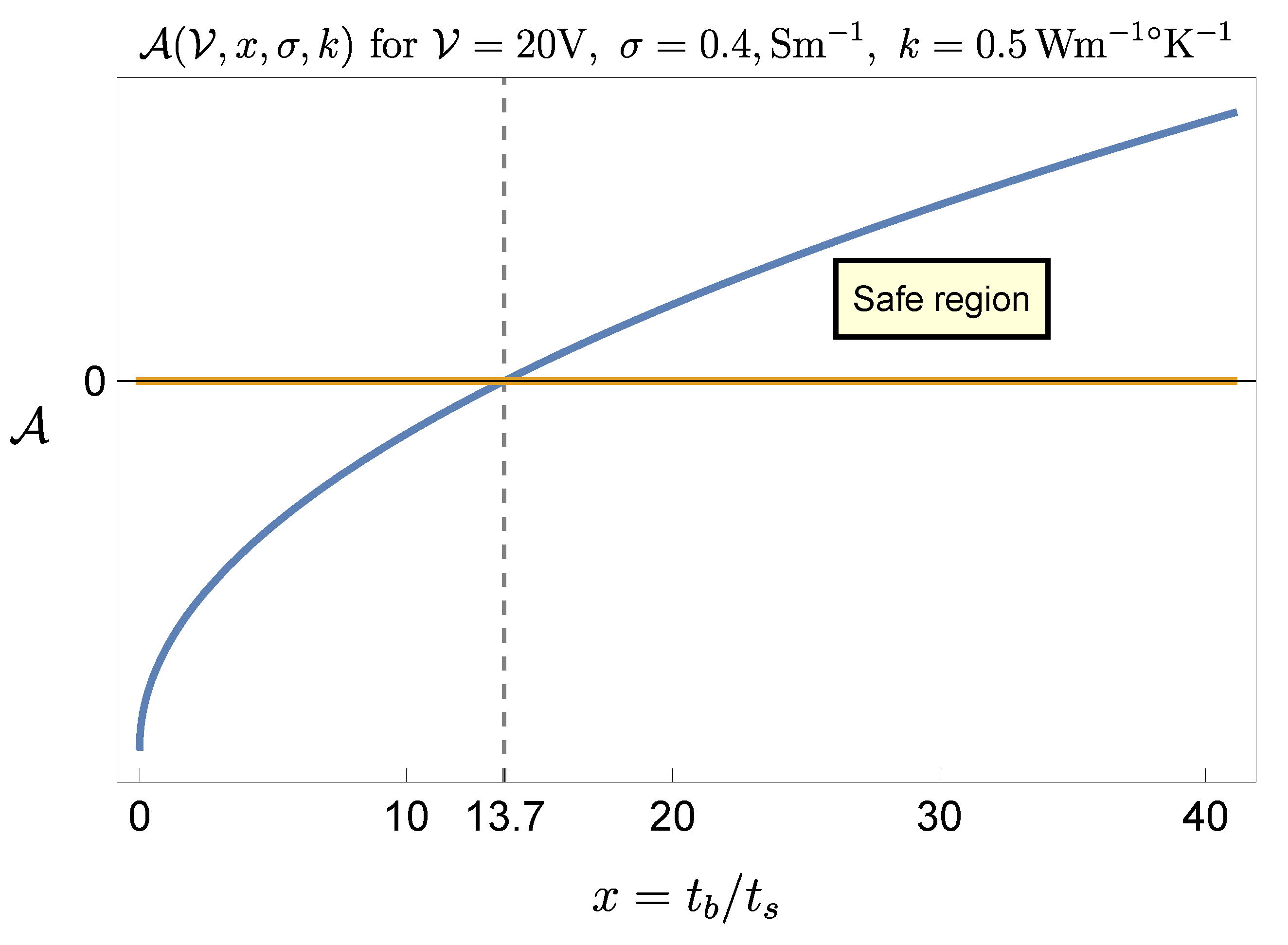
This result has to be examined in view of the range of the parameters practically available on the devices employed.
If we set
, then condition (
15) entails, approximately,
where
.
The interval of positiveness of the function
identifies the safety ratio
for any given
, or, vice versa, the safe voltage
for any given ratio
.
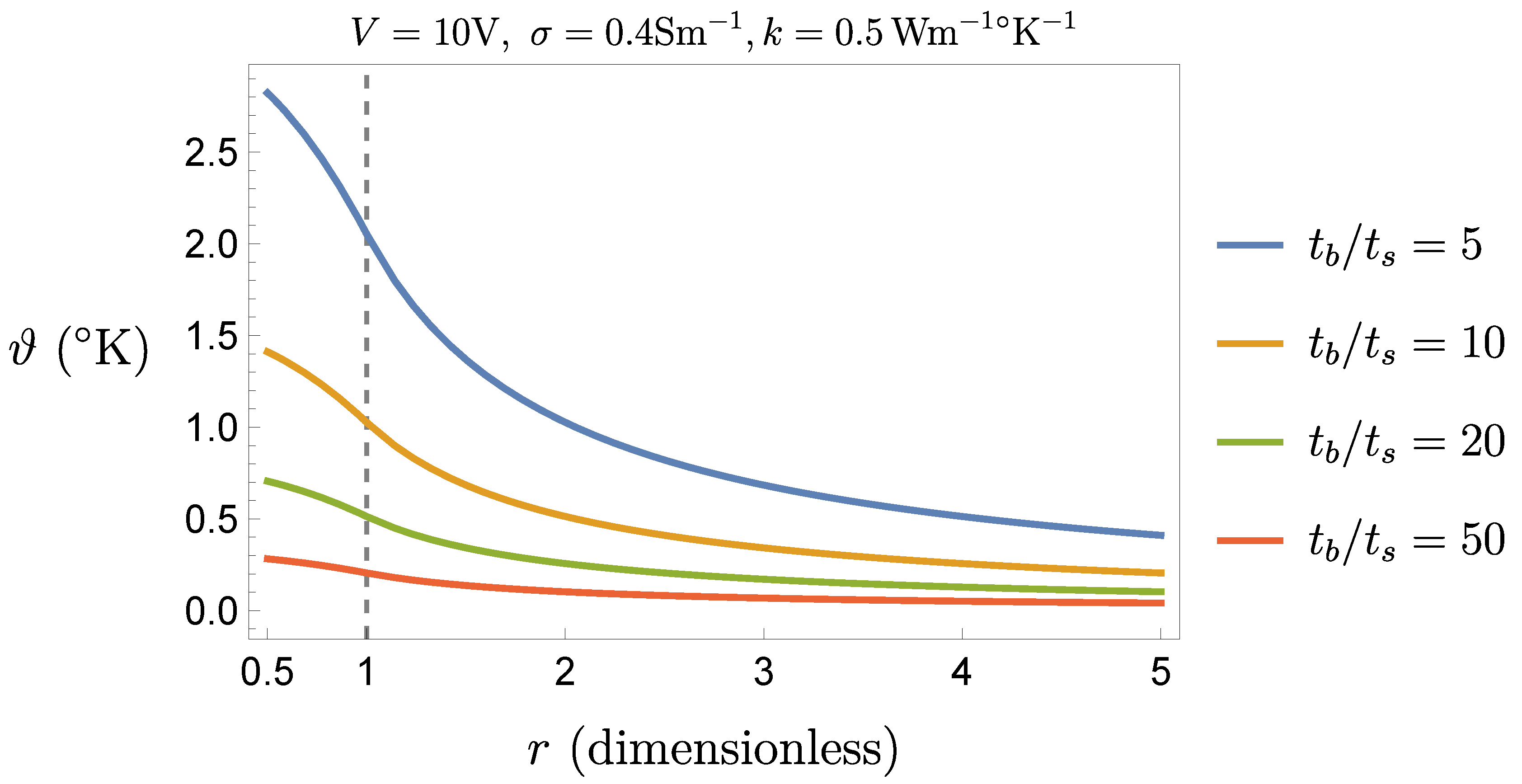
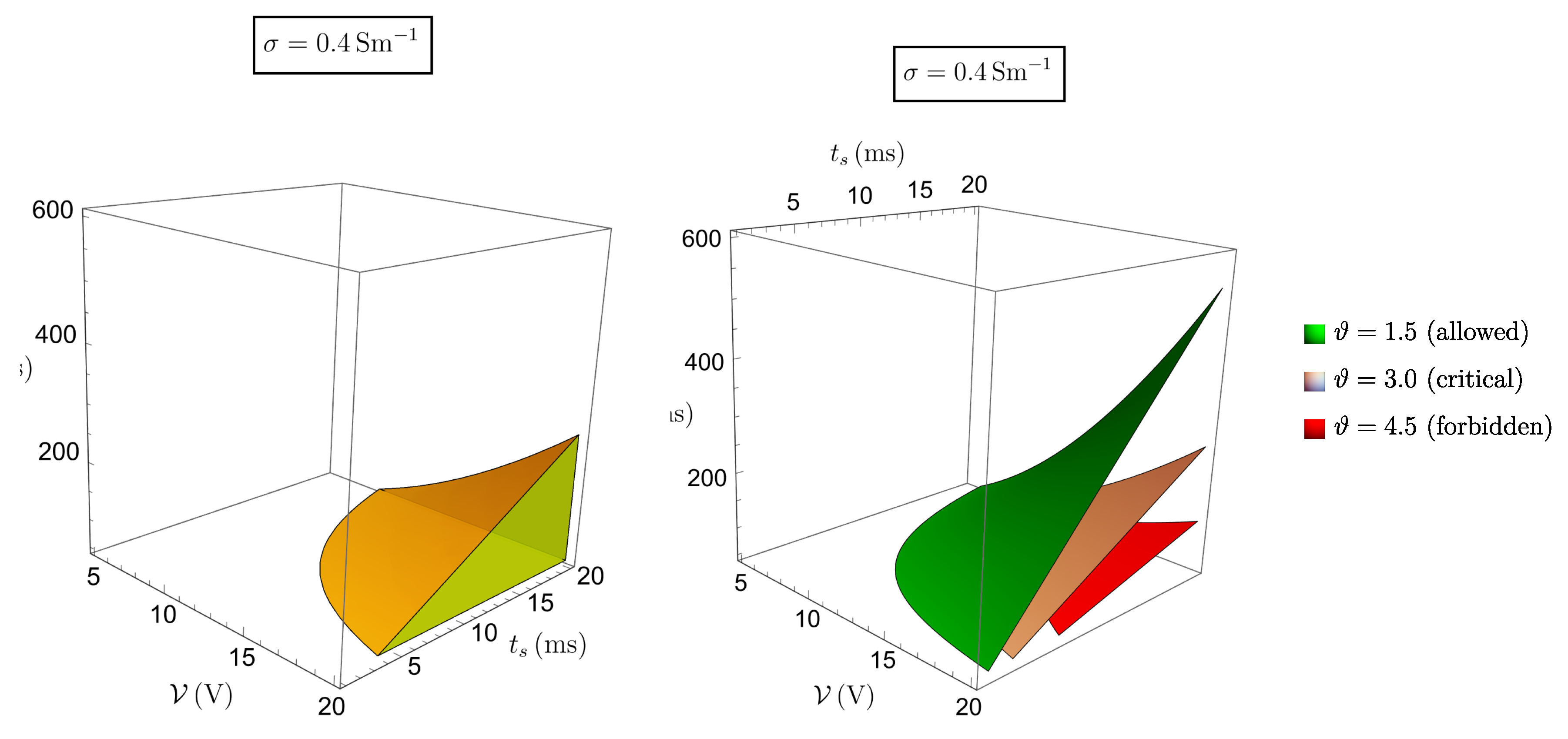
Figure 5 and
Figure 6 show, respectively, the cases
V and
.
This result indicates that the procedure is feasible even with the largest stimulation amplitude (
), but with a suitable control of the ratio
(for instance, if
s then
ms would be fine). According to (
12), the adoption of the extreme values settable on the generator (
V and
) gives a maximal temperature increase of about
, which is far beyond criticality. Therefore it is not suggestible to set the generator parameters at their extreme values, though it would be sufficient, e. g., to keep the pulse duration
sufficiently low.
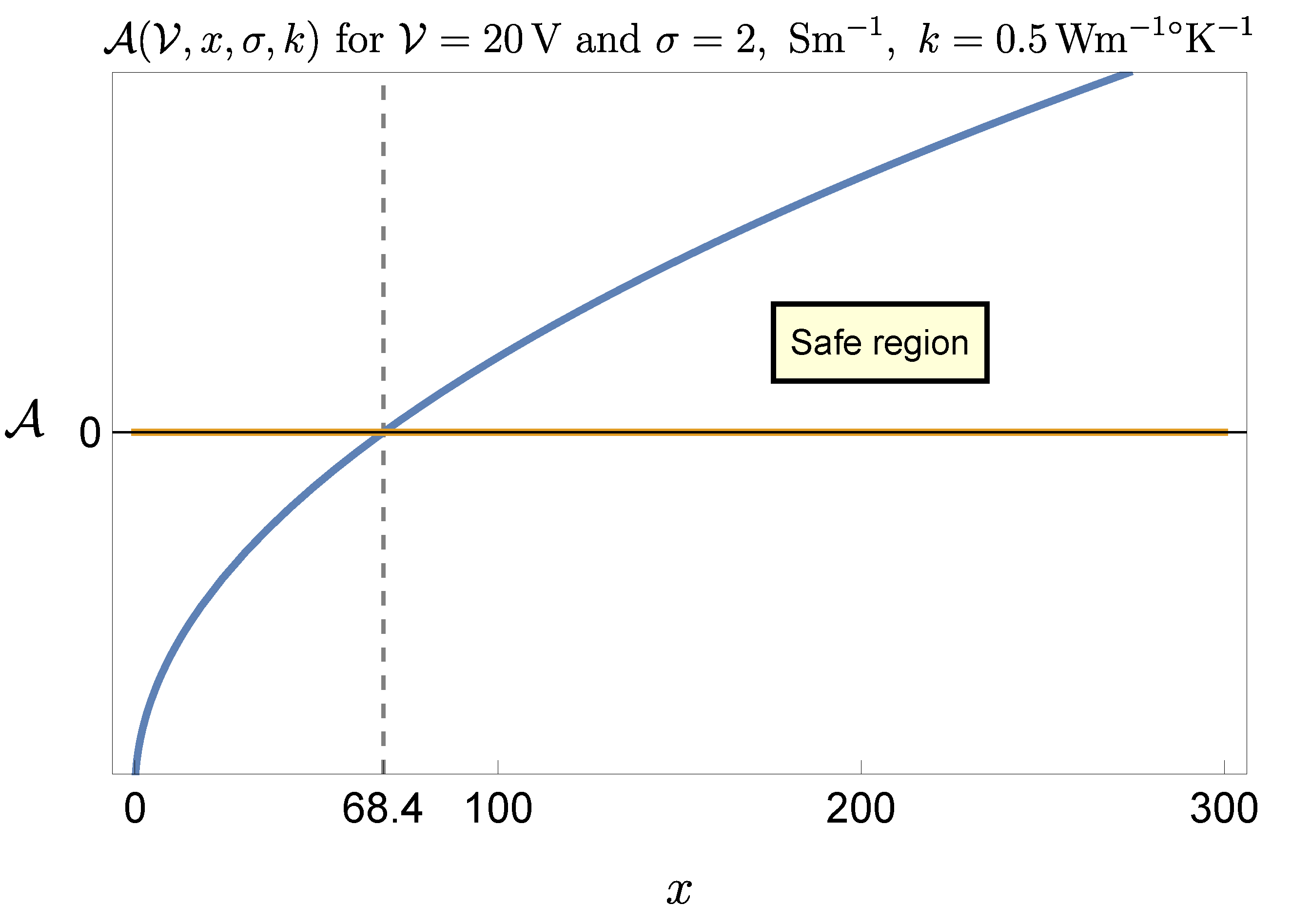
A much worse scenario is offered when current flows in an acidic medium, like gastric juice. In the paper [
4] the value of the gastric juice electrical conductivity was measured vs. pH and a typical value was found to be
, i.e. 5 times larger than the one of saliva. Clearly, the safety condition is now more severe, namely
, thus (
16) modifies (still approximately) to
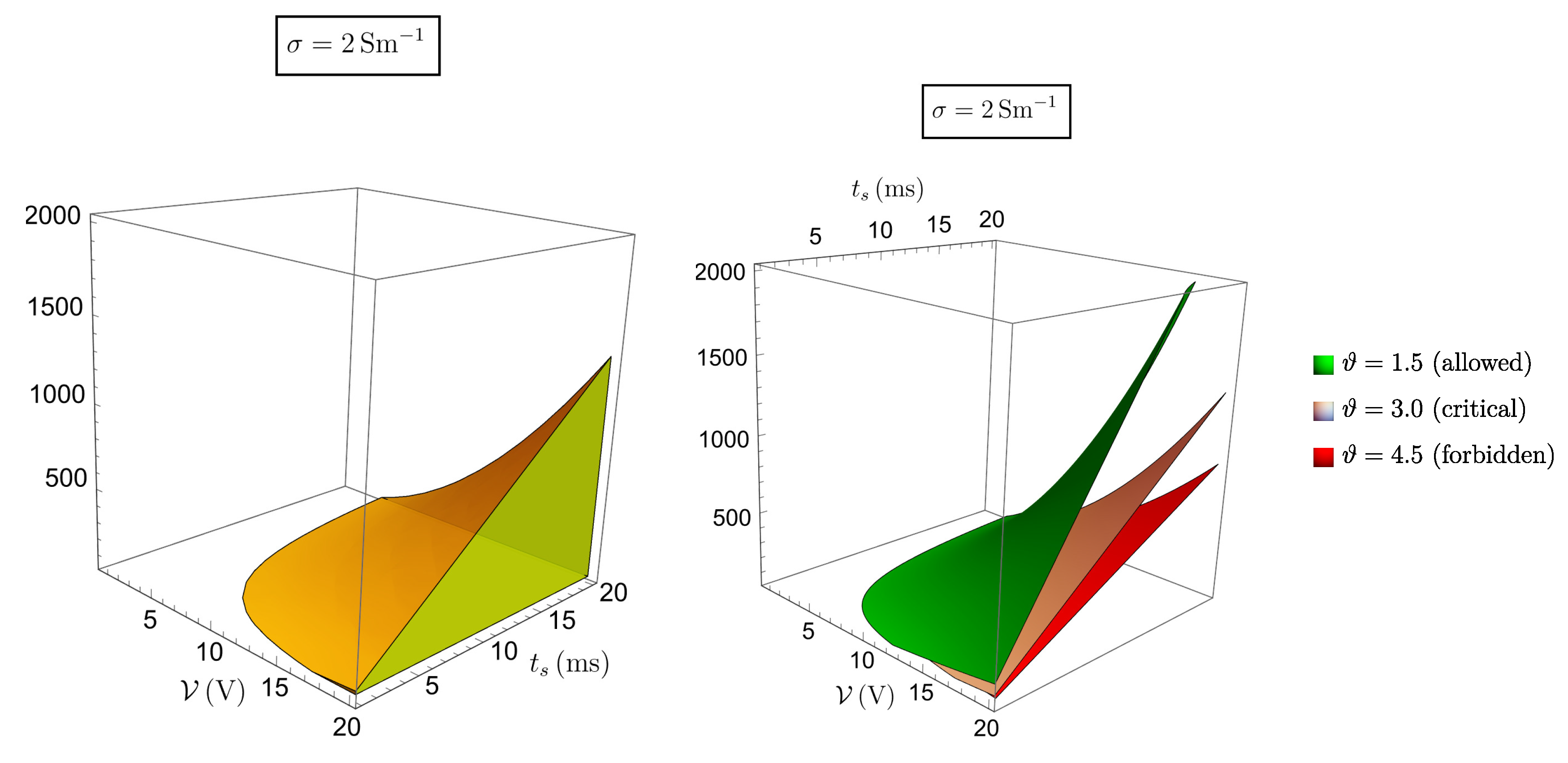
Figure 7 and
Figure 8 refer to this case, showing that the range of admissible selectable parameters is severely reduced.
For instance the lower limit for
x corresponding to
raises to 68.5, which almost nullifies the therapeutic effect on the patient. In correspondence to
the constraint on
is
, which is, in most cases, ineffective, making the procedure practically impossible. To have an idea on how to proceed in this case let us revert to the expression (
12) of the critical temperature with
, namely
With an effective value and requiring a maximum value for of, say, , the choice for the ratio must be such that , which is reasonable. Thus, still in this unfavorable situation there is a way to safely perform the procedure, but selecting the voltage and the time parameters with care. Taking the extreme values and would produce, in this case, a temperature increase greater than , which is unbearable. The conclusion is that pulsed current stimulation of biological tissues is normally safe, but it may become critical in the presence of highly conductive (i.e. acidic) media. Stimulating at high voltage and with pulse duration close to the stimulation period requires that local pH value be previously checked and the ratio x suitably selected.
The safe operating condition
can be viewed in a three dimensional setting. The two panels in
Figure 9 show, respectively, the region where
(on the left) and three level sets of
(on the right) for
when
.
Figure 10 shows the same features when
.
4. Introducing the effect of blood perfusion
Blood circulation has a stabilizing effect on temperature. In the specific case, it helps removing heat from the interested region. This feature can be introduced in the model by modifying the source term
in equation (
1) as follows:
if
(unchanged) and
(b=blood), for
, representing the heat removing rate. Clearly,
has dimension
and so
has dimension
. Indeed
is the volume of blood crossing the unit volume of tissue in one second. For soft tissues we can take
or less (see [
5,
6,
7]). Thus, the differential equation governing the temperature evolution is obviously the same, namely (
1), but now (
2) is replaced by
Proceeding as in the Sect.
Section 2, we first rescale
with
R and
with
(see (
4)), so to formulate again the problem in the steady state and in an unbounded domain. Finally, we introduce
so that the new system of differential equations to integrate is the following
Here
(dimensionless). Recalling the estimate
,
, and that
we obtain
, i. e.
. The solution of system (
19) in terms of
writes as follows
Figure 11 shows that blood perfusion entails a significant reduction of the thermal fields given by (
10). It is worth noting that if
tends to zero the no-perfusion solution is retrieved.
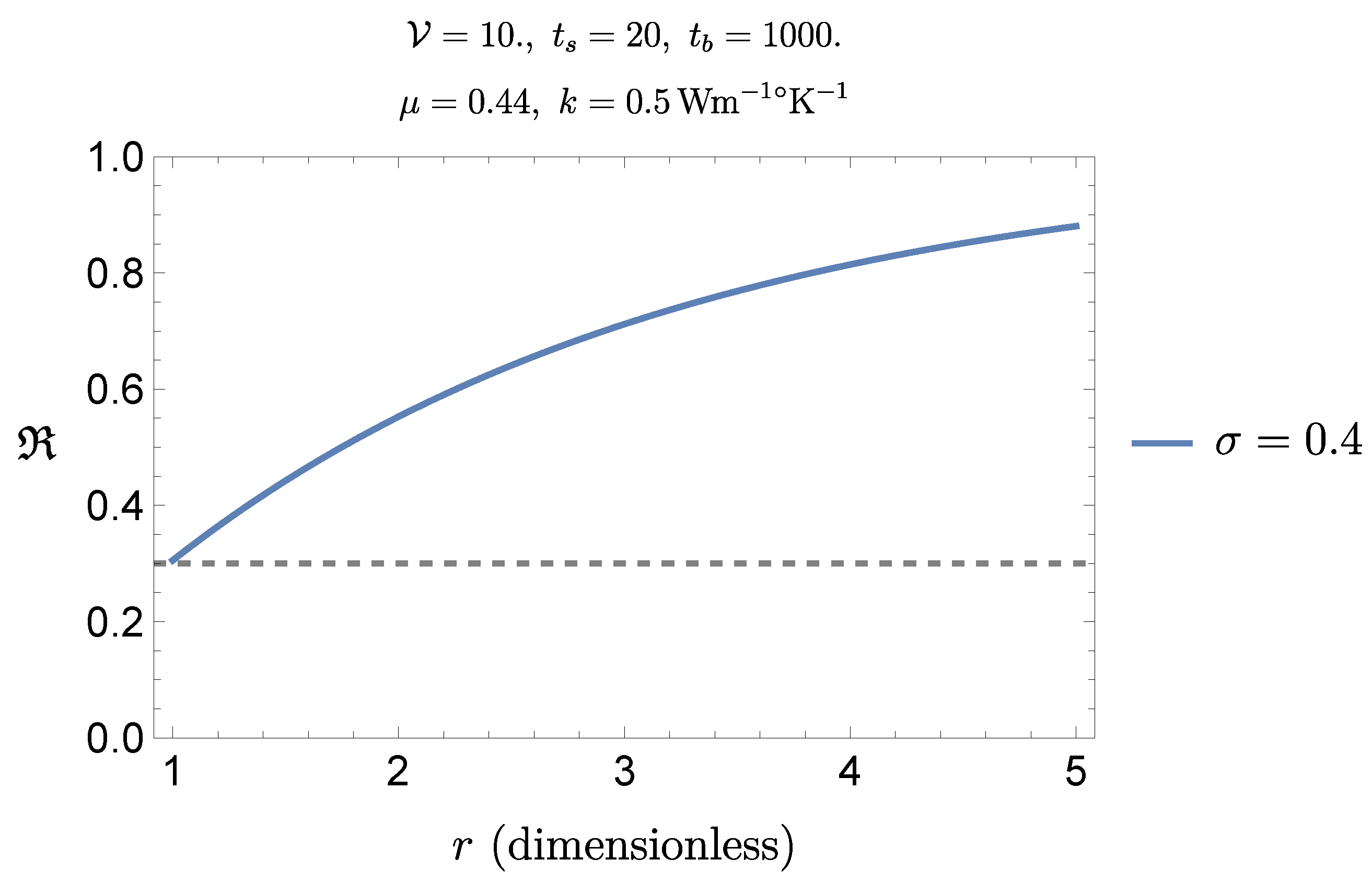
Let us analyze the influence of perfusion on the safety conditions. From (
20) we get
which clearly emphasizes the influence of perfusion: the corresponding relative change as a function or
r is
hence
(see
Figure 12).
Accordingly, the safety condition (
15) changes as follows
which, for
,
,
, and
entails
showing some improvement with respect to the no-perfusion case. For instance, for
this implies
, allowing e.g. to increase
to
when
. Similarly, for
we require
with a certain improvement with respect to the corresponding condition (
16).
In addition to safety conditions it is reasonable to add some efficacy condition, because if
is too small or
x is too large the treatment may not be effective. Thus, we impose the constraints
and
(for instance
,
) and possibly also the lower limit
. In view of these requirements, the suggestible parameters range are further reduced.
Figure 13 shows the no-efficacy region when the above conditions are imposed for the respective cases
,
.