1. Introduction
Species of Candida spp are important agents of healthcare-associated infections (HAIs), primarily causing catheter-associated candiduria, invasive candidiasis, and sepsis associated with central venous catheters or medical devices, especially in patients admitted to intensive care units, onco- hematological, and immunocompromised patients [
1,
2,
3].
Globally, HAIs caused by yeasts are mainly due to C. albicans, and the second most prevalent species varies by geographic area, type of candidiasis, and study period. C. parapsilosis has been reported as the second most frequent agent in candidemia in the Latin American region from the 2000s to the present, while C. glabrata is prevalent in candiduria [
2,
4,
5,
6,
7].
Azoles and echinocandins are globally used to treat deep fungal infections. However, resistance to these antifungal drugs has increased in many of the Candida species [
8]. In a recent meta-analysis, the lowest susceptibility in China was detected for the azole group; fluconazole susceptibilities were reported as follows: C. parapsilosis (93.25%), C. albicans (91.6%), Nakaseomyces glabrata (C. glabrata) (79.4%), C. tropicalis (77.95%), Meyerozyma guilliermondii (C. guilliermondii) (76%), C. pelliculosa (50%), and C. auris (0%). Amphotericin B and anidulafungin were the most susceptible drugs for all Candida species [
9]. On the other hand, Candida species can form biofilms on implanted medical devices and in the host tissue, and these Candida biofilms are inherently resistant to traditional antifungal therapies and the host immune system; therefore, biofilm-associated candidiasis are a huge clinical challenge [
10].
Candida auris is a multidrug-resistant fungal pathogen that has been reported in more than 40 countries. Most importantly, C. auris is the first fungal pathogen to show pronounced and sometimes untreatable clinical drug resistance to all known antifungal classes, including azoles, amphotericin B, and echinocandins [
11].
Candida species, mainly C. parapsilosis and C. auris, have the potential to cause outbreaks in healthcare settings, owing to their efficient transmission via skin-to-skin contact [
11,
12]. Recently, the WHO report identified 19 fungal priority pathogens to guide research, development, and public health action, placing C. albicans and C. auris as fungi from the critical priority group and C. parapsilosis and C. tropicalis as members of the high priority group according to the prioritization process focused on fungal pathogens that can cause invasive acute and subacute systemic fungal infections for which drug resistance or other treatment and management challenges exist [
3].
Candida species, mainly C. albicans, can be part of the human microbiota, colonizing more frequently the mucous membranes of the intestinal tract (50% to 70%), oral cavity (30% to 50%), and vaginal mucosa (5% to 30%). In contrast, the presence of Candida spp on the hands of community individuals is low, with reported frequencies of less than 5% [
7,
13,
14,
15]. On the other hand, yeast colonization on the hands of healthcare workers ranges from 20% to 80%, being considered an important vehicle for the transmission of these agents in healthcare settings [
16,
17,
18].
Frequent contact between healthcare workers and patients, as well as with healthcare-related items in a clinical or hospital setting, would favor colonization of the hands of healthcare workers. This was partially evidenced by a previous study from our group, which reported a 16% prevalence of yeast colonization on the hands of medical students, with significant differences between the first two years of university (basic cycle, 7%) and the last two years of study (clinical cycle, 30%). The amount of yeast and diversity of species on the hands increased as students advanced in their training, associated with longer contact time with the hospital environment. In contrast, the frequency of colonization, amount of yeast, and diversity of species remained constant in the engineering student group [
12]. These data were partially confirmed by Muango et al., (2017) in medical and nursing students, with a prevalence of hand yeast carriage of 11.2% and 9.2%, respectively [
19].
Sixty years ago, the main clinically significant Candida species was C. albicans, and its colonization frequency on hands ranged from less than 1% in community individuals to 17% on the hands of healthcare workers [
13,
14]. Later studies reported a broader spectrum of yeast species on the hands of healthcare workers and students, predominantly C. parapsilosis, followed by other species such as C. tropicalis, C. famata, Nakaseomyces glabrata (C. glabrata), Meyerozyma guilliermondii (C. guilliermondii), Rhodotorula spp., and Cryptococcus albidus, among others [
5,
12,
15,
17,
19,
20].
Both permanent and transient yeast colonization on hands is a potential vehicle for transmission to patients, both directly via skin-to-skin contact and indirectly through the contamination of medical devices, contributing to increased HAIs and outbreaks [
4,
7,
17,
18]. Handwashing can reduce the presence of yeasts by 50%, but if an antiseptic such as chlorhexidine, povidone-iodine, or alcohol gel is added, colonization decreases significantly, reaching 10.5%, 18.7%, and 21.1%, respectively [
21].
Considering that the hands of healthcare workers play an important role as a vehicle for the exogenous transmission of yeasts to patients, and the constant reporting of HAIs caused by yeasts, it is essential to know the frequency of yeast carriage on the hands of individuals associated with hospital services. Therefore, we conducted this study to determine the prevalence of yeast carriage on the hands of students in the fields of medicine, nursing, and medical laboratory scientist, in relation to variables such as study cycle, frequency of application of biosecurity and infection control measures, as well as the prevalence of yeast species and antifungal susceptibility patterns, to obtain a background information that supports future recommendations in the prevention and control of cross-transmission of these opportunistic agents.
2. Materials and Methods
We conducted a descriptive, prospective, and analytical study to determine the prevalence of hand yeast carriage of healthcare students and the impact of infection control measures, as well as the prevalence of yeast species and the antifungal susceptibility profile of the isolated strains. Students from medicine, nursing, and medical laboratory scientist programs at a private university in Santiago de Chile were informed and invited to participate in the study through a consent letter.
A survey was administered to each volunteer, which included questions about age, gender, the field of study, the university study cycle: basic (1st and 2nd year), preclinical (3rd and 4th year), or clinical (5th year), the average daily frequency of clinical handwashing, alcohol gel use, moisturizing cream use, among others.
For sample collection, each student was asked to perform a simple hand wash for 1 minute using 10 mL of sterile saline solution kept in Falcon tubes. The saline solution was placed in the students' palms, who then performed handwashing for 20 seconds. The sample was collected using a swab, which was vigorously rubbed on the palms of both hands. The swab was then placed in a conical tube with 0.5 mL of sterile saline for transportation to the laboratory, at 4° to 6°C. The sample was received in the laboratory, where the quality of the sample, transportation conditions, and documents such as the signed consent form, completed survey, and time of sample collection were verified. The samples were plated on 2 Petri dishes one with Sabouraud dextrose agar plus chloramphenicol and the other with chromogenic agar (Chrom-agar Candida) within 4 hours of collection. The plates were incubated at 36 ± 1°C for 72 hours.
Yeast identification was performed using standard methodology. Briefly, after observing and analyzing the colony characteristics, a germ tube test in fresh human plasma incubated at 36 ± 1°C for 2.5 hours was conducted for suspected Candida spp. strains, and microcultures in rice agar with 1% tween 80 incubated at 30°C for 72 hours were conducted for suspected Candida spp. and Trichosporon spp. Biochemical tests were performed on all yeast strains using API 32C galleries (Biomerieux) incubated at 30°C for 24 or 48 hours, with the Api-WebTM software version 4.0 used for interpreting the bio-code [
7].
Identification of species in the C. parapsilosis complex (C. parapsilosis sensu stricto, C. methapsilosis, and C. orthopsilosis) was performed by genotyping using the secondary alcohol dehydrogenase (SADH) gene polymerase chain reaction (PCR) amplification strategy, which generates a 716 bp fragment in the C. parapsilosis complex species. This fragment was digested with the BanI restriction enzyme to molecularly discriminate between C. parapsilosis sensu stricto, which generates two bands of 521 bp and 196 bp, C. orthopsilosis, which presents a 716 bp band, and C. methapsilosis, which generates 4 bands of 370 bp, 188 bp, 93 bp, and 60 bp [
22,
23]. Briefly, primers S1F (5′-GTTGATGCTGTTGGATTGT-3′) and SIR (5′-CAATGCCAAATCTCCCAA-3′) were used for the SADH gene. Amplified products were electrophoresed on 1.5% agarose gels, visualized under ultraviolet light, and analyzed on a photo documenter (Bio-Rad). The 716 bp PCR fragments were purified with a specialized kit (Qiagen), and digestion of the purified PCR product was carried out for 120 minutes at 37°C using the restriction enzyme BanI (Thermo Scientific, USA). Digestion products were subjected to electrophoresis on 2% agarose gels (Invitrogen Life Technologies).
Confirmation of C. albicans or C. dubliniensis was performed by PCR with specific primers designed previously by our group from 18S, ITS1, and ITS2 of the main 6 clinically relevant Candida species, as indicated in
Table 1 [
7]. In summary, DNA was extracted with the Wizard Genomic DNA kit (Promega) from the culture of the strain identified as C. albicans by phenotypic methods. The PCR reaction included 25 pmol of primers and 3 μL of yeast DNA in a total volume of 25 μL. The PCR conditions were one cycle at 95°C for 3 minutes, followed by 40 cycles at 94°C for 1 minute, 60°C for 2 minutes, and 72°C for 3 minutes, with a final cycle at 72°C for 7 minutes. Amplification was performed in a T100 thermal cycler (Bio-Rad), and the amplified products were loaded onto 2% agarose gels containing ethidium bromide (0.5 mg/mL). A 100-bp DNA ladder was used as a molecular size (Invitrogen Life Technologies, USA).
The antifungal susceptibility test was performed using the agar diffusion method following CLSI M44-A2 [
24] recommendations. Briefly, from each yeast strain isolated on Sabouraud dextrose agar at 36 ± 1°C for 24h, a 0.5 McFarland inoculum was prepared using a turbidimeter to seed 1-5 x 105 CFU/mL on Müller-Hinton agar plus 2% glucose and 0.5 µg/mL Methylene Blue for gradient strip testing (E-Test). The plates were incubated at 36 ± 1°C for 20 to 24 hours, and the reading of the inhibition ellipse was visual. The in vitro interpretative criteria were analyzed according to CLSI M27M44S [
25].
The reference strains used for quality control were: C. albicans ATCC 90028, C. dubliniensis, Pichia kudriavzeveii (C. krusei) 6258, and C. parapsilosis ATCC 22019.
Statistical analysis was performed using Fisher's test, relative risk, and odds ratio with a 95% confidence level or power for significance.
3. Results
We studied hand yeast carriage in 260 students (167 or 64.2% female) from medicine (78), nursing (89), and medical laboratory scientist (93) programs.
Table 2 shows that the prevalence of the hand yeast carriage was detected in 27 students (10.4%), with no significant differences among the three healthcare programs, although, nursing students showed a slightly higher carriage (13.5%), while medical laboratory scientist students showed the lowest carriage (7.5%). The distribution of hand yeast carriage by gender was slightly higher in women (20 – 12%) than in men (7 – 7.5%), with no statistical difference (data not shown).
Table 3 shows a significant difference in yeast carriage on hands among students is evident according to the university study cycle, being less prevalent in the clinical cycle with 2 carriers (2.7%) compared to 12 (13.5%) and 13 (13.5%) in the basic (p=0.022) and preclinical (p=0.014) cycles, respectively. No yeasts were detected on the hands of students in the clinical cycle of the medicine and medical laboratory scientist programs.
In
Table 4, we show a tendency towards a decrease in the frequency of yeast carriage on hands according to handwashing frequency, decreasing from 15.8% in students who wash their hands 3 or fewer times a day to 7% in students who wash their hands 7 or more times a day, with a relative risk and odds ratio of 2.13 and 2.5, respectively. This tendency is similar with the use of antiseptic soap, as a 6.8% yeast carriage rate is observed in individuals who use this product compared to 14.2% in those who do not use it (p>0.05).
Table 5 shows thar there is a tendency towards a decrease in yeast carriage frequency among students who use hand sanitizer 5 or more times a day (3.2%), compared to students who do not use hand sanitizer (14.4%), with a relative risk of 2.2 and an odds ratio of 5.1, but without a significant difference (p>0.05).
In
Table 6, we show a significant decrease in yeast carriage is evident (P=0.016) among students who apply hand moisturizing cream with a frequency of 4 or more times a day (0%) or 2 to 3 times a day (3.4%), compared to those who do not use it or apply it only once a day (16.5%), with a relative risk of 2.6 and an odds ratio of 5.5 and p=0.016.
The yeast species most predominantly on the hands of nursing, medicine, and medical laboratory scientist students was
C. parapsilosis, recovered in 22 out of 27 students (81.5%), followed by
Meyerozyma guilliermondii (C. guilliermondii guilliermondii) in 2 students, one from nursing and one from medicine,
Trichosporon mucoides in 2 medical laboratory scientist students, and
R. mucilagenosa in one nursing student. One medical laboratory scientist student had simultaneous carriage of
C. parapsilosis and
C. albicans on their hands (
Table 7).
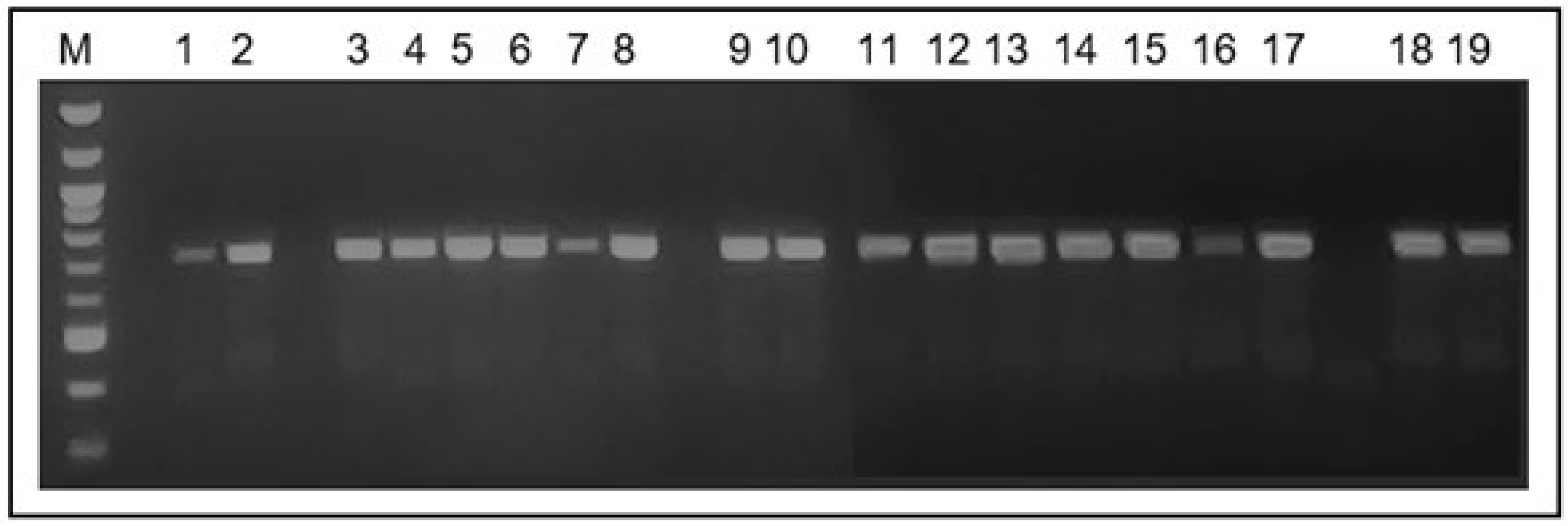
The 22 strains identified as part of the
C. parapsilosis complex, using the conventional method, amplified the characteristic 716 bp fragment of the secondary alcohol dehydrogenase gene -SADH (
Figure 1).
M: 100 bp Ladder. Lanes 1 to 18: C. parapsilosis strains isolated from the hands of students; lane 19: C. parapsilosis ATCC 22019 strains. All strains amplified the 716 bp fragment of the SADH gene.
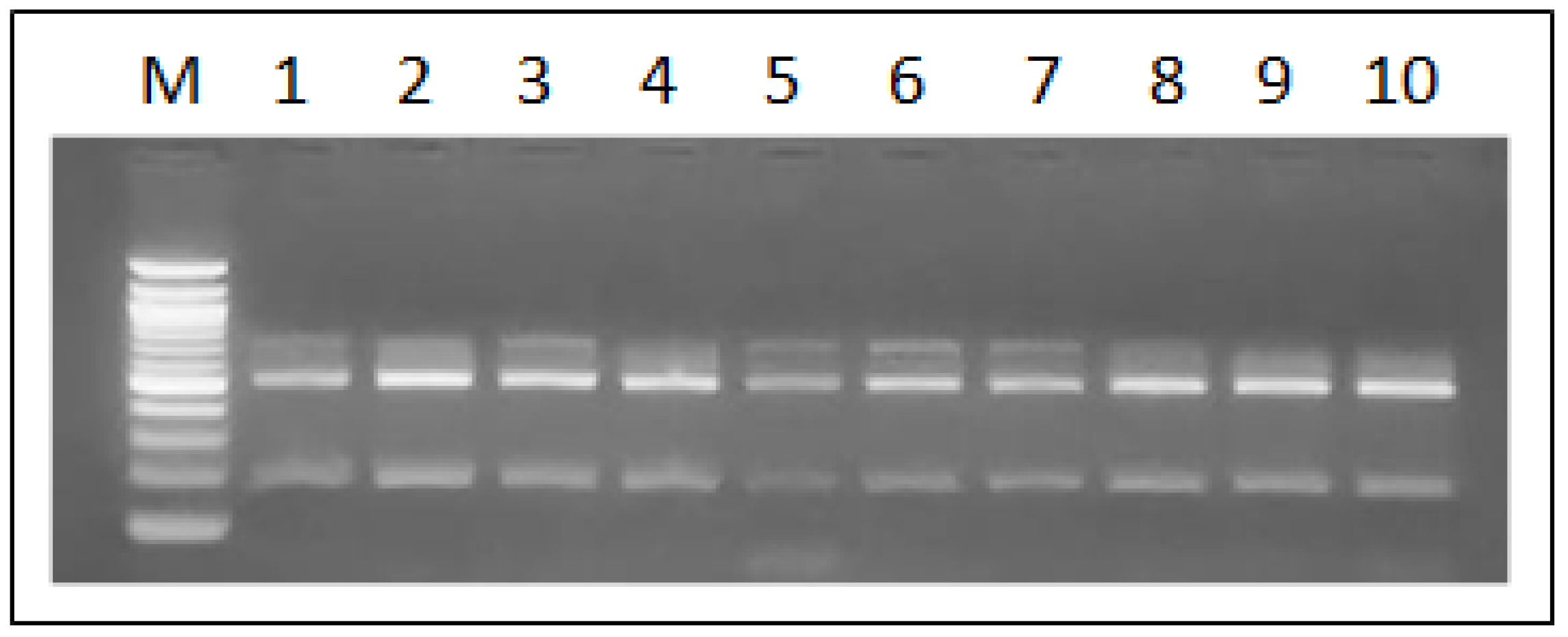
According to the Ban I restriction pattern of the amplified SADH gene fragment, all isolates of the
C. parapsilosis complex strains were molecularly discriminated as
C. parapsilosis sensu stricto, as evidenced by the two bands of 521 and 196 bp that characterize this species (
Figure 2). No profiles associated with
C. methapsilosis or
C. orthopsilosis were detected.
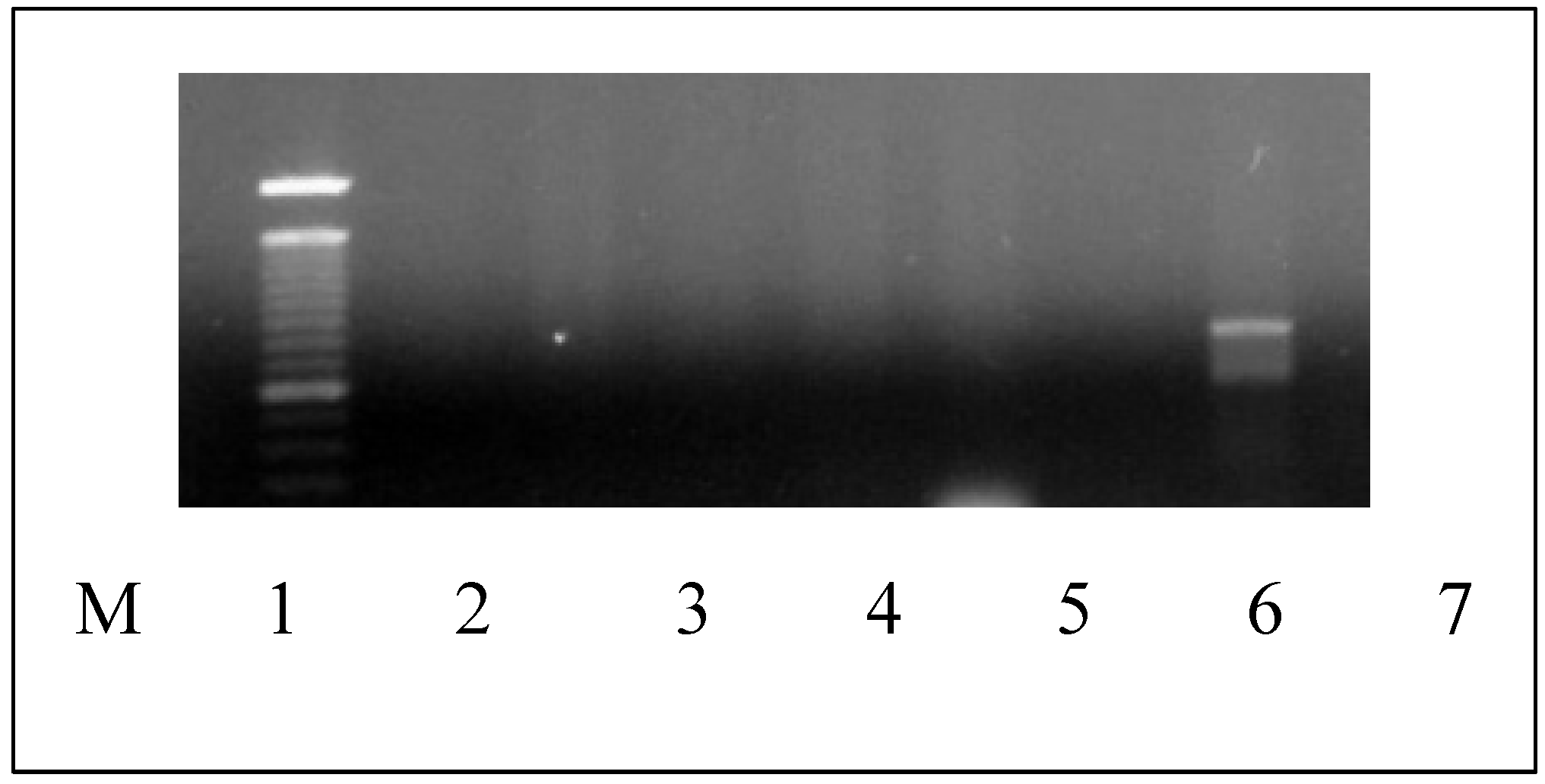
The species-specific PCR for 6 Candida species confirmed the identification of
C. albicans by generating a 724 bp fragment (
C. dubliniensis generates a 354 bp amplicon) (
Figure 3).
As shown in
Table 8, all isolated yeasts were sensitive to voriconazole, caspofungin, and anidulafungin, with MIC90 values of 1 µg/mL, 2 µg/mL, and 1 µg/mL, respectively.
4. Discussion
Our study shows that the prevalence of yeast carriage on the hands of nursing, medicine, and medical laboratory scientist students was 10.4%, being slightly higher in nursing students at 13.5% and lower in medical laboratory scientist students at 7.5%. The distribution of yeast carriage by sex was slightly higher in women (12%) than in men (7.5%), with no statistical difference. These data are similar to those previously published for medicine students at 16% [
12] and for nursing and medicine students at 9.9% and 11.2%, respectively [
19]. The presence of yeasts on the hands of healthcare students is higher than reported for the general community [
13,
14,
15], but lower than detected among hospital personnel, with carriage rates ranging from 20% to 80% [
16,
17]. Yildirim et al. [
18] reported that 34.1% of the people analyzed carried
Candida spp. on their hands: 30.7% were nurses, 25.8% were resident doctors, 28.6% were laboratory workers, 84.6% were dining room personnel, and 43.3% were officers.
In this study, the hand yeast carriage in healthcare students was significantly less prevalent among clinical cycle students (2.7%) compared to younger students in the basic (13.5%) and preclinical (13.5%) cycles. These results differ from previous reports, which indicated that the prevalence of yeast carriage in medical and nursing students increases progressively as they advance to the clinical cycle and spend more hours in the hospital environment [
12,
19]. Our results showing lower yeast carriage prevalence in the clinical cycle group may be explained by the greater adherence to clinical handwashing and infection control measures among advanced students. We found that the frequency of yeast carriage decreased from 15.8% in students who wash their hands 3 or fewer times a day to 7% in students who wash their hands 7 or more times a day. This tendency in controlling yeast carriage is reinforced with the use of antiseptic soap, as yeast carriage in students using this product is 6.8% compared to 14.2% in those who do not use it, along with frequent use of hand sanitizer (3.2%) compared to students who do not use hand sanitizer (14.4%). Our results confirm the findings previously reported by Yildirim et al., who indicated that handwashing can reduce yeast presence by 50%, while the use of antiseptics such as chlorhexidine, iodine povacrylate, or hand sanitizer significantly decreases yeast carriage on hands to frequencies of 10.5%, 18.7%, and 21.1%, respectively [
21], as well as chlorhexidine soap, which reduces microbial load when cleaning the skin [
26].
We also detected that frequent use of moisturizing hand cream significantly decreases yeast carriage in students who apply this product 2 to 3 times (3.4%) and 4 or more times a day (0%) compared to those who do not use it or apply it only once a day (16.5%). This result can be partly explained by the protective role of the moisturizing cream on the keratinocytes of the skin surface, thus contributing to the integrity of the skin’s mechanical barrier, which can be affected by the use of latex or vinyl gloves and chemical antiseptics [
16,
27].
The predominant isolated species from the hands of nursing, medicine, and medical laboratory scientist students was
C. parapsilosis sensu stricto (81.5%), confirmed molecularly by the restriction pattern with Ban I enzyme of the amplified SADH gene fragment, affirming the usefulness of this genotyping technique by PCR [
22,
23]. Additionally,
Meyerozyma guilliermondii (C. guilliermondii),
Trichosporon mucoides, and
R. mucilagenosa were isolated less frequently. The predominance of
C. parapsilosis on the hands of healthcare students observed in this study agree with various previous studies on hand yeast colonization in healthcare-related individuals [
5,
12,
15,
17,
18,
19,
20].
In many countries, especially in Latin America,
C. parapsilosis has been reported as the second most frequently isolated fungal agent causing candidemia in both pediatric and adult patients over the past two decades [
2,
6], indicating the high level of circulation in hospital environments. The hands of healthcare personnel are a potential vehicle for transmitting this agent, highlighting the importance of understanding its carriage among healthcare-related individuals and implementing appropriate control measures to prevent infections caused by
C. parapsilosis.
Only one student presented simultaneous carriage of
C. parapsilosis and
C. albicans, confirmed by specific PCR, thus ruling out
C. dubliniensis, which has very similar phenotypic and biochemical characteristics [
28]. The low frequency of
C. albicans on the hands of community and healthcare personnel has been previously documented [
12,
13,
14,
15,
19].
All yeasts isolated from the hands of students showed MIC values indicating sensitivity to voriconazole, caspofungin, and anidulafungin, consistent with other studies that have reported high sensitivity in
Candida strains isolated from invasive infections [
29,
30]. However, this differs from the gradual increase in resistance to certain antifungals reported by some researchers [
8,
9]. It is important to note that in vitro sensitivity tests are performed with vegetative yeasts, whereas their condition can change radically when colonizing and forming biofilms on implanted medical devices such as catheters. These
Candida biofilms are inherently resistant to traditional antifungal therapies and the host immune system [
10].
5. Conclusions
This study highlights yeast carriage on the hands of nursing, medicine, and medical laboratory scientist students, with C. parapsilosis sensu stricto being the most prevalent species and no resistance detected among the analyzed strains. The frequency of yeast colonization on hands significantly decreased in clinical cycle students, since they show high adherence and greater frequency of application of biosafety and infection control measures.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Conceptualization, Victor Silva and Ceidy Silva; methodology, V. Silva and Ceidy Silva; validation, Victor Silva, Rodrigo Gacitúa and Viviana Silva; formal analysis, Ceidy Silva and Claudio Alburquenque; investigation, Ceidy Silva, Coral Silva, Rodrigo Gacitúa and Hernán Salas; data curation, Ceidy Silva, Coral Silva, Hernan Salas and Rodrigo Gacitúa; writing—original draft preparation, Víctor Silva; writing—review and editing, all authors.; supervision, Víctor Silva and Viviana Silva; project administration, Victor Silva and Ceidy Silva; funding acquisition, Víctor Silva. All authors have read and agreed to the published version of the manuscript”.
Funding
This research was funded by Universidad Mayor, Santiago, Chile, grant number 2017-2018.
Data Availability Statement
Acknowledgments
We acknowledge the support given by the laboratory technician and volunteers student from medicine, nursing and medical laboratory scientist for participating in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Febré, N.; Silva, V.; Medeiros, E.A.S.; Wey, S.B.; Colombo, A.L.; Fischman, O. Microbiological characteristics of yeast isolated from urinary tracts of intensive care unit patients undergoing urinary catheterization. J Clin Microbiol 1999, 37, 1584–1586. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Díaz, M.C.; Febré, N.; the Chilean Invasive Fungal Group. Chilean Invasive Fungal Group. Invasive fungal infections in Chile: A multicenter study of fungal prevalence and susceptibility during a 1-year period. Med Mycol 2004, 42, 333–339. [Google Scholar] [CrossRef] [PubMed]
- WHO fungal priority pathogens list to guide research, development and public health actions. Geneva: World Health Organization; 2022. Licence: CC BY-NC-SA 3.0 IGO.
- Sanchez, V.; Vazquez, J.A.; Barth-Jones, D.; Dembry, L.; Sobel, J.D.; Zervos, M.J. Nosocomial acquisition of Candida parapsilosis: an epidemiologic study. Am J Med 1993, 94, 557–582. [Google Scholar] [CrossRef] [PubMed]
- Hedderwick, S.A.; Lyons, M.J.; Liu, M.; Vazquez, J.A.; Kauffman, C.A. Epidemiology of yeast colonization in the intensive care unit. Eur J Clin Microbiol Infect Dis 2000, 19, 663–670. [Google Scholar] [CrossRef]
- Nucci, M.; Queiroz-Telles, F.; Alvarado-Matute, T.; Tiraboschi, I.N.; Cortes, J.; Zurita, J.; et al. Epidemiology of candidemia in Latin America: A Laboratory- Based Survey. PloS ONE 2013, 8, e59373. [Google Scholar] [CrossRef]
- Silva, V.; Zaror, L. Diagnóstico Micológico en el Laboratorio Clínico, 1st ed.; Universidad Mayor: Santiago, Chile, 2015; p. 233. [Google Scholar]
- Pristov, K.E.; Ghannoum, M.A. Resistance of Candida to azoles and echinocandins worldwide. Clin Microbiol Infect. 2019, 25, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Bilal, H.; Shafiq, M.; Hou, B.; Islam, R.; Khan, M.N.; Khan, R.U.; Zeng, Y. Distribution and antifungal susceptibility pattern of Candida species from mainland China: A systematic analysis. Virulence 2022, 13, 1573–1589. [Google Scholar] [CrossRef]
- Fan, F.; Liu, Y.; Liu, Y.; Lv, R.; Sun, W.; Ding, W.; Cai, Y.; Li, W.; Liu, X.; Qu, W. Candida albicans biofilm: antifungal resistance, immune evasion, and emerging therapeutic strategies. Int J Antimicrob agents 2022, 60, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, A.; Jain, K.; Chauhan, M. Candida auris genetics and emergence. Annu Rev Microbiol 2023, 77, 583–602. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Zepeda, G.; Rybak, M.E.; Febre, N. Yeast carriage on the hands of Medicine students. Rev Iberoam Micol 2003, 20, 41–45. [Google Scholar] [PubMed]
- Clayton, Y.M.; Noble, W.C. Observations on the epidemiology of Candida albicans. J Clinic Pathol 1966, 19, 76–78. [Google Scholar] [CrossRef]
- Marples, M.J.; Somerville, D.A. The oral and cutaneous distribution of Candida albicans and other yeasts in Rarotonga, Cook Islands. Trans R Soc Trop Med Hyg 1968, 62, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Horn, W.A.; Larson, E.L.; McGinley, K.J.; Leyden, J.J. Microbial flora on the hands of health care personnel: Differences in composition and antibacterial resistance. Infect Control Hosp Epidemiol 1988, 9, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Strausbaugh, L.J.; Sewell, D.L.; Tjoelker, R. Comparison of three methods for recovery of yeasts from hands of health-care workers. J Clin Microbiol 1996, 34, 471–473. [Google Scholar] [CrossRef]
- Kumar, S.; Batra, R. A study of yeast carriage on hands of hospital personnel. Indian J Pathol Microbiol 2000, 43, 65–67. [Google Scholar] [PubMed]
- Yildirim, M.; Sahin, I.; Kucukbayrak, A.; Ozdemir, D.; Tevfik-Yavuz, M.; Oksuz, S.; Cakir, S. Hand carriage of Candida species and risk factors in hospital personnel. Mycoses 2007, 50, 189–92. [Google Scholar] [CrossRef]
- Muango, A.; Brevis, P.; Mora, C. Carriage of yeasts in the hands of the students of medicine and nursing careers at the University of Talca, Chile. Bol Micol 2017, 32, 8–14. [Google Scholar]
- Huang, Y.C.; Lin, T.Y.; Leu, H.S.; Wu, J.L.; Wu, J.H. Yeast carriage on hands of hospital personnel working in intensive care units. J Hosp Infect 1998, 39, 47–51. [Google Scholar] [CrossRef]
- Yildirim, M.; Sahim, I.; Oksuz, S.; Sencan, I.; Kucukbayrak, A.; Cakir, S.; Ozaydin, C. Hand carriage of Candida occurs at lesser rates in hospital personnel who use antimicrobial hand disinfectant. Scand J Infect Dis 2014, 49, 633–636. [Google Scholar] [CrossRef]
- Tavanti, A.; Davidson, A.D.; Gow, N.A.; Maiden, M.C.; Odds, F.C. Candida orthopsilosis and Candida metapsilosis spp. nov. to replace Candida parapsilosis groups II and III. J Clin Microbiol 2005, 43, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Ataides, F.S.; Costa, C.R.; Souza, L.K.H.; Fernández, O.L.; Jesuino, R.S.A.; Silva, M.R.R. Molecular identification and antifungal susceptibility profiles of Candida parapsilosis complex species isolated from culture collection of clinical samples. Rev Soc Bras Med Trop 2015, 48, 454–459. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Method for Antifungal Disk Diffusion Susceptibility Testing of Yeasts, Approved Guideline, CLSI document M44-A2, 2nd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2009. [Google Scholar]
- CLSI. Performance Standards for Antifungal Susceptibility Testing of Yeasts, CLSI supplement M27M44S, 2nd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2022. [Google Scholar]
- Aburto, I.; Espinoza, G.; Silva, V.; Rodríguez, H.; Moreno, G. Costo efectividad en protocolos de limpieza de piel en pacientes con úlceras. Rev Chil Heridas & Ostomias 2017, 8, 33–42. [Google Scholar]
- Schliemann, S. Limitations of skin protection. Curr Problem Dermatol 2007, 34, 171–177. [Google Scholar] [CrossRef]
- Silva, V.; Cabrera, M.; Díaz, M.C.; Abarca, C.; Hermosilla, G. Prevalencia de serotipos de Candida albicans en aislamientos de hemocultivo en Chile y primer caso de candidemia por C. dubliniensis. Rev Iberoam Micol 2003, 20, 46–51. [Google Scholar] [PubMed]
- Alburquenque, C.; Silva, V.A.; Fuentes, M.; Tapia, C.; Silva, V. Susceptibilidad in vitro a anidulafungina en 100 cepas de especies de Candida aisladas de muestras clínicas previo a la introducción de esta equinocandina en Chile. Rev Chil Infect 2011, 28, 399–403. [Google Scholar] [CrossRef]
- Badiee, P.; Boekhout, T.; Haddadi, P.; Mohammadi, R.; Ghadimi-Moghadam, A.; Soltani, J.; Zarei-Mahmoudabadi, A.; Ayatollahi-Mousavi, S.A.; Najafzadeh, M.J.; Diba, K.; Salimi-Khorashad., A.R.; Amin-Shahidi, M.; Ghasemi, F.; Jafarian, H. Epidemiology and Antifungal Susceptibility of Candida Species Isolated from 10 Tertiary Care Hospitals in Iran. Microbiol Spectr 2022, 10, e0245322. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).