Submitted:
19 August 2024
Posted:
20 August 2024
You are already at the latest version
Abstract
Keywords:
Introduction
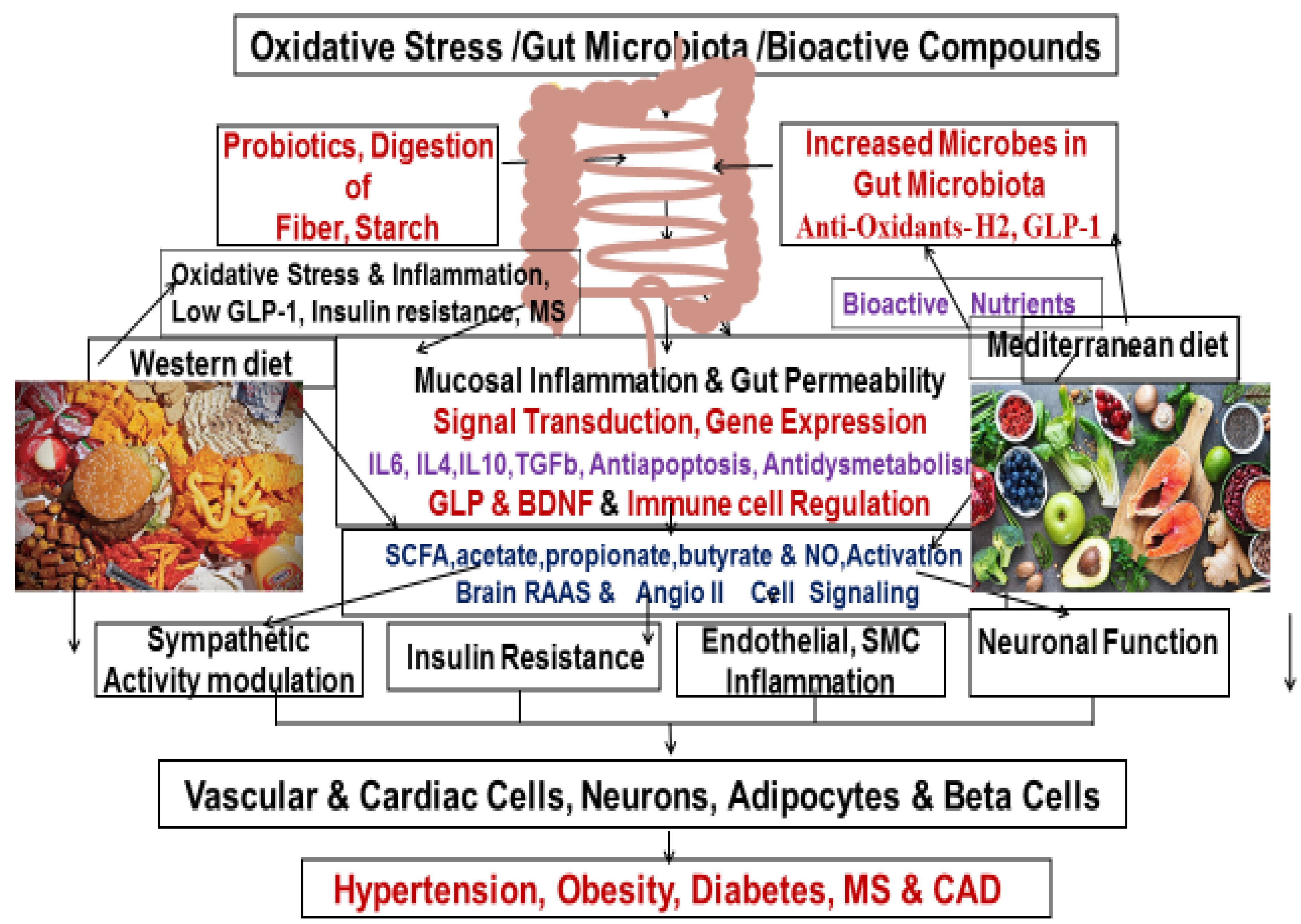
Oxidative Stress and Inflammation in the Pathophysiology of Metabolic Syndrome
Bioactive Compounds and Functional Foods
Effects of Functional Bioactive Foods on Metabolic Diseases
Effects of Polyphenols and Anthocyanins on Inflammation
Effect of the Polyphenol on Blood Pressure
Effect of Polyphenols and Anthocyanins on Blood Lipids
Effect of Polyphenols and Anthocyanins on Glycaemic Parameters
Oxidative Stress and Metabolic Diseases
Conclusions
Classification of Flavonoids
Author Contributions
Funding
Acknowledgments
Competing Interests
References
- Forman: HJ., Zhang H. Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat Rev Drug Discov 20, 689–709 (2021). [CrossRef]
- Jiang S, Liu H, Li C. Dietary Regulation of Oxidative Stress in Chronic Metabolic Diseases. Foods. 2021 Aug 11;10(8):1854. [CrossRef]
- Manna P., Jain S.K. Obesity, oxidative stress, adipose tissue dysfunction, and the associated health risks: Causes and therapeutic strategies. Metab. Syndr. Relat. Disord. 2015;13 :423–444. [CrossRef]
- Serra-Majem L., Roman-Vinas B., Sanchez-Villegas A., Guasch-Ferre M., Corella D., La Vecchia C. Benefits of the Mediterranean diet: Epidemiological and molecular aspects. Mol. Aspects Med. 2019;67:1–55. [CrossRef]
- Najjar RS, Feresin RG. Protective Role of Polyphenols in Heart Failure: MolecularTargets and Cellular Mechanisms Underlying TheirTherapeutic Potential. Int Jour Mol Sciences. 2021, 22, 1668. [CrossRef]
- Pandey KB., Rizvi, SI, & Ahmad A. Diet-induced oxidative stress. Journal of Food Science and Technology, 2019; 56(2), 686-695.
- Maurya DK, &Devasagayam, TP. Antioxidant and pro-oxidant nature of hydroxycinnamic acid derivatives ferulic and caffeic acids. Food and Chemical Toxicology, 2010;48(12), 3369-3373.
- Cooke, MS., Evans, MD., Dizdaroglu, M, &Lunec, J. Oxidative DNA damage: mechanisms, mutation, and disease. FASEB Journal, 2003;17(10), 1195-1214.
- Hotamisligil, GS. Inflammation and metabolic disorders. Nature,2006; 444(7121), 860-867. [CrossRef]
- Pryor, WA, & Stone, K.. Oxidants in cigarette smoke. Radicals, hydrogen peroxide, peroxynitrate, and peroxynitrite. Annals of the New York Academy of Sciences, 1993;686(1), 12-28.
- Martirosyan D., Miller E., Bioactive Compounds: The Key to Functional Foods. Bioactive Compounds in Health and Disease 2018; 8(7):36-39. [CrossRef]
- Martirosyan D and KasiaPisarski. Bioactive Compounds: Their Role in Functional Food and Human Health, Classifications, and Definitions. In: Bioactive Compounds and Cancer. Edited by DanikMartirosyan and Jin-Rong Zhou. San Diego: Food Science Publisher; 238-277.
- Martirosyan D and Jaishree Singh. A new definition of functional food by FFD: what makes a new definition unique? Functional Foods in Health and Disease 2015; 5(6):209-223.
- Milner JA; Functional Foods and Health Promotion, The Journal of Nutrition, 1999; 129:1395S–1397S.
- Khan, M.I., Anjum, F.M., Sohaib, M. et al. Rev EndocrMetabDisord (2013) 14: 287.
- Institute of Food Technologists. Functional foods: Opportunities and challenges. March 2005.
- del Castillo MD, Iriondo-DeHond A, Martirosyan DM. Are Functional Foods Essential for Sustainable Health? Ann Nutr Food Sci. 2018; 2(1): Article 1015.
- Alkhatib, A., Tsang, C., Tiss, A., Bahorun, T., Arefanian, H., Barake, R., … Tuomilehto, J.Functional Foods and Lifestyle Approaches for Diabetes Prevention and Management. Nutrients, 2017;9(12), Article 1310. [CrossRef]
- Isaza A, Halabi G, Yulia G, Khan S.Bioactive compounds in foods and sustainable health. Int J Clin Nutrition 2021; 21(1): 66-73.Bioactive compounds in foods and sustainable health.
- Shah K, Shah P. Effect of anthocyanin supplementations on lipid profile and inflammatory markers: a systematic review and meta-analysis of randomized controlled trials. Cholesterol. 2018;2018: Article 8450793. [CrossRef]
- Poulsen NB, Lambert MNT, Jeppesen PB. The effect of plant derived bioactive compounds on inflammation: a systematic review and meta-analysis. MolNutr Food Res. 2020; 64(18):Article e2000473. [CrossRef]
- Fallah AA, Sarmast E, Fatehi P, Jafari T. Impact of dietary anthocyanins on systemic and vascular inflammation: Systematic review and meta-analysis on randomised clinical trials. Food ChemToxicol. 2020; 135:Article 110922. [CrossRef]
- Moua ED, Hu C, Day N, Hord NG, Takata Y. Coffee consumption and c-reactive protein levels: a systematic review and meta-analysis. Nutrients. 2020;12(5): Article E1349. [CrossRef]
- Sarkhosh-Khorasani S, Hosseinzadeh M. The effect of grape products containing polyphenols on C-reactive protein levels: a systematic review and meta-analysis of randomized controlled trials. Br J Nutr. 2021;125(11):1230-1245.
- Li SH, Zhao P, Tian HB, Chen LH, Cui LQ. Effect of grape polyphenols on blood pressure: a meta-analysis of randomized controlled trials. PLoS One. 2015;10(9): Article e0137665. [CrossRef]
- Marx W, Kelly J, Marshall S, Nakos S, Campbell K, Itsiopoulos C. The effect of polyphenol-rich interventions on cardiovascular risk factors in haemodialysis: a systematic review and meta-analysis. Nutrients. 2017;9(12): Article E1345. [CrossRef]
- Weaver SR, Rendeiro C, McGettrick HM, Philp A, Lucas SJE. Fine wine or sour grapes? A systematic review and meta-analysis of the impact of red wine polyphenols on vascular health. Eur J Nutr. 2021;60(1):1-28.
- George ES, Marshall S, Mayr HL, et al. The effect of high-polyphenol extra virgin olive oil on cardiovascular risk factors: A systematic review and meta-analysis. Crit Rev Food SciNutr. 2019;59(17):2772-2795. [CrossRef]
- Liu C, Sun J, Lu Y, Bo Y. Effects of anthocyanin on serum lipids in dyslipidemia patients: a systematic review and meta-analysis. PLoS One. 2016;11(9): Article e0162089.
- Xu Lin, Tian Z, Chen H, Zhao Y, Yang Y. Anthocyanins, anthocyanin-rich berries, and cardiovascular risks: systematic review and meta-analysis of 44 randomized controlled trials and 15 prospective cohort studies. Front Nutr. 2021; 8: Article 747884.
- Wilken MR, Lambert MNT, Christensen CB, Jeppesen PB. Effects of anthocyanin-rich berries on the risk of metabolic syndrome: a systematic review and meta-analysis. Rev Diabetic Stud. 2022;18(1):42-57. [CrossRef]
- Palma-Duran SA, Vlassopoulos A, Lean M, Govan L, Combet E. Nutritional intervention and impact of polyphenol on glycohemoglobin (Hba1c) in non-diabetic and type 2 diabetic subjects: Systematic review and meta-analysis. Crit Rev Food SciNutr. 2017;57(5): Article 975-986.
- Raimundo AF, Félix F, Andrade R, et al. Combined effect of interventions with pure or enriched mixtures of (Poly)phenols and anti-diabetic medication in type 2 diabetes management: a meta-analysis of randomized controlled human trials. Eur J Nutr. 2020;59(4):1329-1343. [CrossRef]
- Yang L, Ling W, Du Z, et al. Effects of anthocyanins on cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials. AdvNutr. 2017;8(5):684-693. [CrossRef]
- Fallah AA, Sarmast E, Jafari T. Effect of dietary anthocyanins on biomarkers of glycemic control and glucose metabolism: A systematic review and meta-analysis of randomized clinical trials. Food Res Int. 2020;137: Article 109379. [CrossRef]
- Mingatto, F. E., Santos, A. C., Uyemura, S. A., Jordani, M. C., Curti, C., & Santos, R. H. (2007). Effect of fatty acid chain length on superoxide production by heart mitochondria. Biochimica et BiophysicaActa (BBA)-Bioenergetics, 2007;1767(9), Article 954-961.
- Halliwell, B, &Gutteridge, JM. Free radicals in biology and medicine. Oxford University Press.2015.
- Sies, H. Oxidative stress: a concept in redox biology and medicine. Redox biology, 2017;4, 180-183. [CrossRef]
- Harman, D. Aging: a theory based on free radical and radiation chemistry. Journal of Gerontology, 1956;11(3), 298-300. [CrossRef]
- Alberti, KG., Eckel, RH., Grundy, SM., Zimmet, P.Z., Cleeman, J. I., Donato, KA., ... & Smith Jr,SC. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation, 2009;120(16), 1640-1645.
- Grundy, SM., Cleeman, J. I., Daniels, SR., Donato, KA., Eckel, RH., Franklin, BA., ... & Costa, F. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation, 2005;112(17), 2735-2752.
- Reaven, GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes, 1988;37(12), 1595-1607.
- Choi, Y. J., Lee, D. H., Han, K. D., & Yoon, H. Metabolic risks associated with body mass index and waist circumference in older adults: a nationwide study in Korea. Obesity Research & Clinical Practice, 2013;7(3), e198-e267.
- Furukawa, S., Fujita, T., Shimabukuro, M., Iwaki, M., Yamada, Y., Nakajima, Y., ... & Kanazawa, Y. Increased oxidative stress in obesity and its impact on metabolic syndrome. The Journal of Clinical Investigation, 2004;114(12), 1752-1761.
- Hotamisligil, GS. Inflammation and metabolic disorders. Nature, 2017;444(7121), 860-867. [CrossRef]
- Katsuki, A, Sumida, Y, &Gabazza, EC. Homeostasis model assessment is a reliable indicator of insulin resistance during follow-up of patients with type 2 diabetes. Diabetes Care, 2002;25(12), 2360-2361.
- Pizzino, G., Irrera, N., Cucinotta, M., Pallio, G.,Mannino, F., Arcoraci, V, &Squadrito, F. (2017). Oxidative stress: harms and benefits for human health. Oxidative Medicine and Cellular Longevity, 2017, Article 8416763.
- Tilg H, &Moschen, AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology, 2010.52(5), 1836-1846.
- Dalle-Donne, I., Rossi, R., Colombo, R., Giustarini, D., &Milzani, A. Protein carbonyl groups as biomarkers of oxidative stress. ClinicaChimicaActa, 2006;329(1-2), 23-38.
- Furukawa, S., Fujita, T., Shimabukuro, M., Iwaki, M., Yamada, Y., Nakajima, Y,& Kanazawa, Y. Increased oxidative stress in obesity and its impact on metabolic syndrome. The Journal of Clinical Investigation, 2004;114(12), 1752-1761.
- Halliwell, B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiology, 2006;141(2), 312-322. https://doi.org/10.1104/pp.106.077073. [CrossRef]
- Valavanidis, A., Vlachogianni, T., &Fiotakis, K. Tobacco smoke: involvement of reactive oxygen species and stable free radicals in mechanisms of oxidative damage, carcinogenesis and synergistic effects with other respirable particles. International Journal of Environmental Research and Public Health, 2009;6(2), 445-462.
- Valko, M., Rhodes, C. J., Moncol, J., Izakovic, M., & Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chemico-Biological Interactions, 2008;160(1), 1-40. [CrossRef]
- Kim, JA., Montagnani, M., Koh, KK., &Quon, MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation, 2018;113(15), 1888-1904.
- Lee J, Jang M, Kim, CS., Lee, YM., Kim, KS., Kim, JS., & Kim, SG. Low-carbohydrate diets induce oxidative stress in rats. Life Sciences, 2018;204, 71-77.
- Jain M, Juneja LR, Singh RB, Takahashi T, ElmarghyO, Fedacko J, Singh R,, Tribulova N, Hristova K, De Meester F, Wilczynska A, Wilson DW, Martyrosyan D, Singh RB, Sharma R, Omidvor S. Effects of cocoa intake on risk of cardio-metabolic diseases. Int J Clin Nutrition 2021; 21: 12-27.
- Omidvar S, Pati S, Singh RB, Takahashi T, Shin HH, Lee MK, Kim SA, Fedacko J, Singh R, Tribulova N, Hristova K, De Meester F, Wilczynska A, Wilson DW, Singh RB, Sharma R, Juneja LR. Association of cocoa consumption and risk of cardiovascular diseases and other chronic diseases. World Heart J 2013; 5: 47-68.
- Fedacko J, Vargova V, Singh RB, Anjum B, Takahashi T, Tongnuka M, Dharwadkar S, Singh S, Singh V, Kulshresth SK, De Meester F, Wilson DW. Association of high w-6/w-3 fatty acid ratio diet with causes of death due to noncommunicable diseases among urban decedents in North India. The Open Nutra Jour 2012; 5:113-123.
- Li D. Effect of the vegetarian diet on non-communicable diseases. J Sci Food Agric. 2013. [CrossRef]
- Tonstad S, Stewart K, Oda K, Batech M, Herring RP, Fraser GE. Vegetarian diets and incidence of diabetes in the Adventist Health Study-2. NutrMetabCardiovasc Dis. 2013;23(4):292–9. [CrossRef]
- U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015 – 2020 Dietary Guidelines for Americans. 2015 Available from: http://health.gov/dietaryguidelines/2015/guidelines/.
- Satija A, Hu FB. Plant-based diets and cardiovascular health. Trends Cardiovasc Med. 2018 Oct;28(7):437-441. [CrossRef]
- Bruns A, Greupner T, Nebl J, Hahn A. Plant-based diets and cardiovascular risk factors: a comparison of flexitarians, vegans and omnivores in a cross-sectional study. BMC Nutr. 2024 Feb 12;10(1):29. [CrossRef]
- Willett W, Rockström J, Loken B, Springmann M, Lang T, Vermeulen S et al. Food in the Anthropocene: the EAT–Lancet Commission on healthy diets from sustainable food systems. The Lancet. 2. Februar 2019; 393(10170):447–92.
- Malek L, Umberger W. How flexible are flexitarians? Examining diversity in dietary patterns, motivations and future intentions. Clean Responsible Consum 1 Oct. 2021;3:100038. [CrossRef]
- Phillips CM, Shivappa N, Hébert JR, Perry IJ. Dietary inflammatory index and Biomarkers of Lipoprotein Metabolism, inflammation and glucose homeostasis in adults. Nutrients 8 August. 2018;10(8):1033.
| Authors | Shah, et al,2018 [23] | Sarkoshi-Khorsani, et al, 2021[24] |
| Condition | Lipid profile and inflammatory status from 17 studies. | Chronic inflammation from 17 studies. |
| Study | Systematic review and meta-analysis | Systematic review and meta-analysis |
| Study Design | Randomized controlled studies (n=1,535) | Randomized controlled trials (n=668) |
| Measured parameters | Triglycerides, low density lipoprotein, apolipoprotein B, high density lipoprotein, tumour necrosis factor, C-reactive protein, and interleukin- 6 | C-reactive protein |
| Results | Anthocyanin supplementation significantly improves lipid profile and inflammatory status | Grape products containing polyphenols decreased CRP significantly |
| Shao-hua Li, et al, 2015 [25] | Weaver, et al, 2020 [27] | |
| Condition | High blood pressure | High blood pressure |
| Study design | Meta-analysis from 10 studies. | Systematic review and meta-analysis from 37 studies |
| Criteria | Randomized controlled trials (n=543) | Randomized, placebo-controlled trials. (n=2,093) |
| Measured parameters | Systolic blood pressure | Systolic blood pressure |
| Results | Daily consumption of polyphenols from grapes could significantly reduce systolic blood pressure. | Studies indicated significant improvements in systolic blood pressure overall for polyphenols of red wine and pure resveratrol |
| Authors | George, et al,2019 [28 ] | Liu, et al,2016 [29 ] |
| Condition | Cardiovascular disease from 26 studies | Dyslipidaemia from, 6 studies |
| Study design | Systematic review and meta-analysis | Systematic review and meta-analysis |
| Criteria | Randomized controlled trials (n=925) | Randomized controlled trials (n=586) |
| Measured parameters |
Oxidized LDL, total cholesterol, and HDL cholesterol, | Total cholesterol, triglycerides, LDL-C and HDL-C |
| Results | Compared to low olive oil polyphenol, high olive oil polyphenol significantly improved blood oxidized LDL, total cholesterol, and HDL cholesterol in the body | Anthocyanin supplementation significantly reduced TC, TG and LDL-C levels in patients with dyslipidaemia. |
| Authors | Palma-Duran et al 2017 [32] | Yang, et al,2020 [34] |
| Condition | Type 2 diabetes mellitus (T2DM) from 36 studies | Cardio-metabolic diseases from 32 studies. |
| Study | Systematic review and meta-analysis | Systematic review and meta-analysis. |
| Inclusion criteria | Randomized, controlled trials (n=1954). | Randomized controlled trials (n=1491) |
| Measured parameters | HbA1c % | Fasting glucose, 2-hour glucose, HbA1c, total cholesterol and LDL cholesterol. |
| Results | Polyphenol supplementation significantly lowered HbA1c % in T2DM patients without any intervention at glycemia, and could contribute to the delay and possible, the prevention of diabetes | Anthocyanins significantly reduced fasting glucose, 2-hour postprandial glucose, glycated haemoglobin, total cholesterol, and LDL. The significant improvements in glycaemic control and lipids support the benefits of anthocyanins in the prevention and management of cardiometabolic disease. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





