Introduction
Unmanaged diabetes can result in severe complications. It is a persistent ailment that impedes the body's ability to process and retain dietary sugars, causing excessive levels of glucose in the blood (known as hyperglycemia). This can occur when the pancreas fails to generate sufficient insulin, a hormone required to assimilate glucose into cells. Regrettably, diabetes remains an incurable condition that arises when the body cannot effectively utilize glucose or insulin [
1]. Type 2 diabetes is primarily associated with lifestyle factors, including poor diet, lack of physical activity, and obesity. Genetics also play a role, as individuals with a family history of diabetes are at a higher risk. One of the key features of Type 2 diabetes is insulin resistance. In this condition, the body's cells become less responsive to the effects of insulin, making it difficult for glucose to enter cells and be used for energy. This results in elevated blood sugar levels. While insulin resistance is a major factor, many people with Type 2 diabetes also experience a decline in insulin production by the pancreas over time. This contributes to the overall inability to regulate blood sugar effectively [
2].
Morocco is home to a thriving PAM industry, recognized as one of the most prolific in the world. The country's rich ecosystems nurture a wide variety of around 400 species, each with distinct and valuable therapeutic and aromatic properties. Of the 4200 vascular species found here, 382 (9% of the total Moroccan flora) are specifically employed for their medicinal benefits. These plants show great promise for further development, particularly for export [
3].
Centaurium erythraea, commonly referred to as European centaury, is a diminutive herbaceous plant exhibiting either an annual or biennial life cycle. It typically attains a stature ranging from 10 to 50 centimeters. This botanical species is notably recognized for its conspicuous star-shaped flowers, which manifest in vibrant hues spanning from vivid pink to deep crimson, each characterized by the presence of five distinct petals. While indigenous to Europe, Centaurium erythraea has demonstrated adaptability to diverse ecological niches outside its native habitat like Mediterranean countries such as Morocco [
4]. It tends to thrive in varied ecosystems, frequently occupying open grasslands, meadows, and the fringes of woodland regions.
Centaurium erythraea boasts an extensive historical legacy within traditional herbal medicine, primarily revered for its well-documented bitter profile. Over successive generations, it has earned a distinguished status as a venerable digestive tonic and a potent appetite stimulant. The plant's pharmacological attributes are underpinned by its intricate phytochemical composition, encompassing a range of bioactive constituents such as iridoids, flavonoids, and xanthones. These chemical entities collectively account for their multifaceted medicinal properties [
2,
3,
4,
5].
Within the sphere of scientific inquiry, several publications and scientific studies have mentioned the plant Centaurium erythraea which has garnered substantial attention for its potential therapeutic implications, notably in the context of enhancing gastrointestinal well-being and addressing prevalent ailments like dyspepsia and anorexia. Consequently, it is frequently harnessed as a pivotal constituent in a plethora of herbal remedies and natural healthcare products which is utilized in traditional medicine and has been shown to have anti-diabetic properties as well as the potential to treat allergies and digestive problems. [
6,
7,
8,
9]
The aims of this study: i) Chemical characterization of Centaurium erythraea’s extracts and ii) Identification of the antioxidant and antidiabetic potential of the plant in STZ-inducted diabetic rats in vitro and in vivo.
Materials and Methods
The aerial portion of Centaurium erythraea was the plant material used in this investigation, collected from the Ouezzane region (GPS: 35.16878, -5.2636), by the agricultural cooperative Ghzawa. The drying was carried out naturally on paper for 15 days, away from light and humidity, at ambient temperature (about 25°C). To produce an aqueous extract, 30g of plant material (powder) was dissolved in 600 ml of distillate water on a magnetic agitator for one hour at a temperature of 80°C while being mixed with the aid of a barometer at a rate of 500 revolutions per minute. The decoct was then filtered twice, peeled, and put through the oven using silicone molds for 24 hours at the same temperature. the decocted extract is kept in appropriate vials with a yield of 21.3%.
- 2.
Total phenol content quantification
Polyphenols Contents
To determine the polyphenol levels in various plant extracts, the Folin-Ciocalteu reagent is used. This method is a modified version of the one described by [
10]. 0.1 mL of each extract is taken and mixed with 2 mL of a freshly prepared 2% sodium carbonate solution. The mixture is then stirred using a vortex and left to incubate for 5 minutes. 100 μl of the Folin Ciocalteu 0.1N reagent is added to the mixture and incubated at room temperature, while protecting it from light, for 30 minutes. a 760 nm spectrophotometer is used to measure the absorbance against a white reference. The amount of phenolic compounds in extracts can be determined by measuring the maximum absorbance at 760 nm. To create a calibration curve, gallic acid is used as a positive control under the same conditions. The final results are reported as the amount of gallic acid equivalent per gram of dry vegetable matter in milligrams (
Figure 1).
Flavonoids Contents
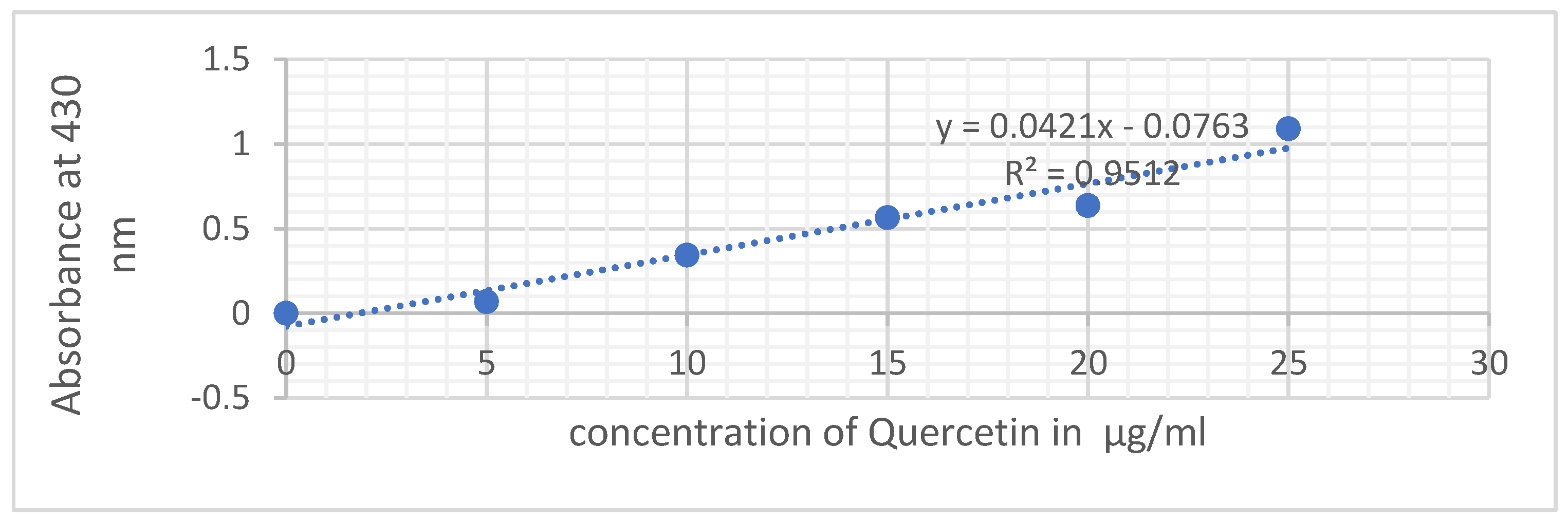
The determination of total flavonoid content in the crude extracts was conducted using the aluminum trichloride colorimetric method described by Chang et al. (2002). This method utilizes the reaction between aluminum chloride and the oxygen atoms present in carbons 4 and 5 of flavonoids, resulting in the formation of yellowish complexes. Each extract, appropriately diluted in its original solvent, was mixed with 1 ml of AlCl3 (2% methanol solution). Following a 10-minute reaction period, absorbance was measured at 430nm utilizing a UV-VIS spectrophotometer. The flavonoid concentrations were then expressed in micrograms of quercetin equivalent per milligram of dry extract (μg EQ/mg extract).
Figure 2
Tannins Contents
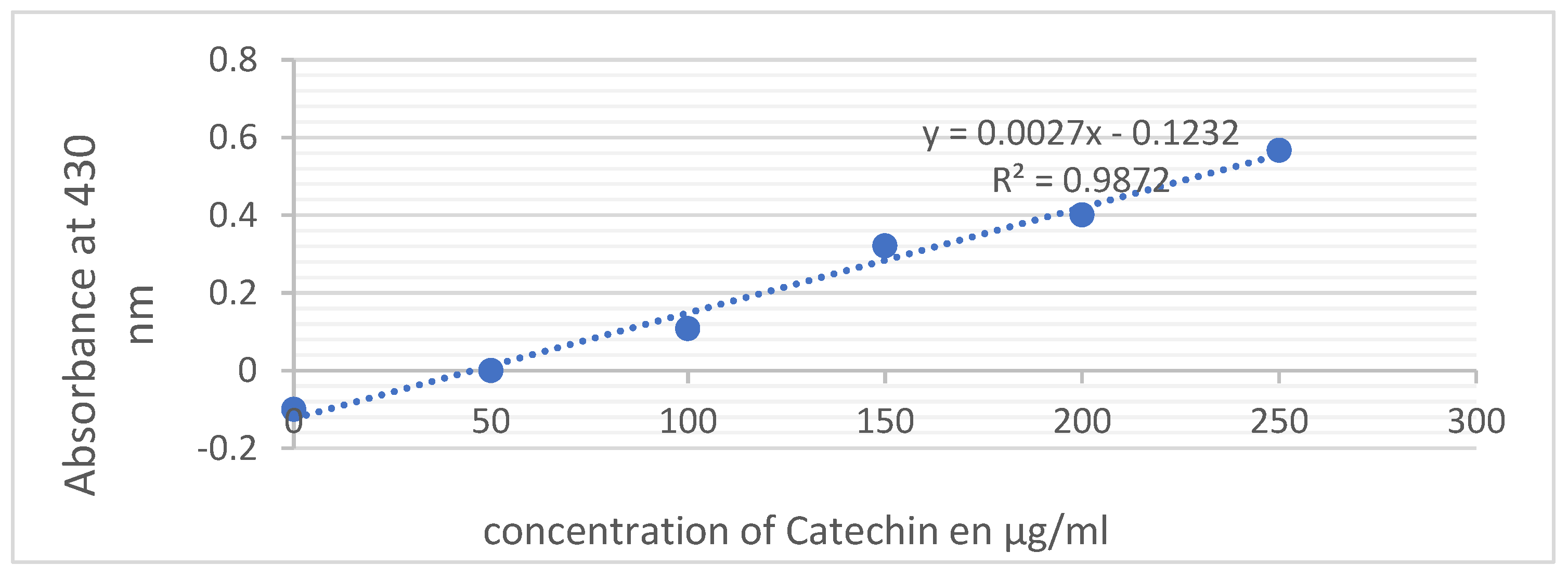
To measure the amount of condensed tannins in a sample, the vanillin method is used. This involves reacting the sample with vanillin in an acidic solution. To do this, 400μl of the sample or standard is mixed with a solution of vanillin (4% in methanol) and concentrated hydrochloric acid. The mixture is left for 15 minutes and the absorbance is measured at 500nm against a white background. The amount of condensed tannins is calculated using calibration ranges established with catechin (ranging from 0-300μg/ml) and is expressed in micrograms of catechin equivalent per milligram of extract (μg ECT/mg extract).
Figure 3.
Anthocyanin Contents
Many plants contain anthocyanins, which are colorants found in their leaves and fruits. Like other polyphenols, anthocyanins have phenolic hydroxyl groups (Ar-OH) that can neutralize free radicals by providing them with H. The spectrophotometric method of differential pH is a quick and accurate way to measure total anthocyanins, even when there are degraded polymerized pigments and other interfering compounds present. Anthocyanins can change structure and color depending on the pH level. At pH 1, the colored form (oxonium) is dominant, while at pH 4.5, the colorless form (hemiacetic) is dominant.
- 3.
Antioxidant activity determination
Total Antioxidant Activity
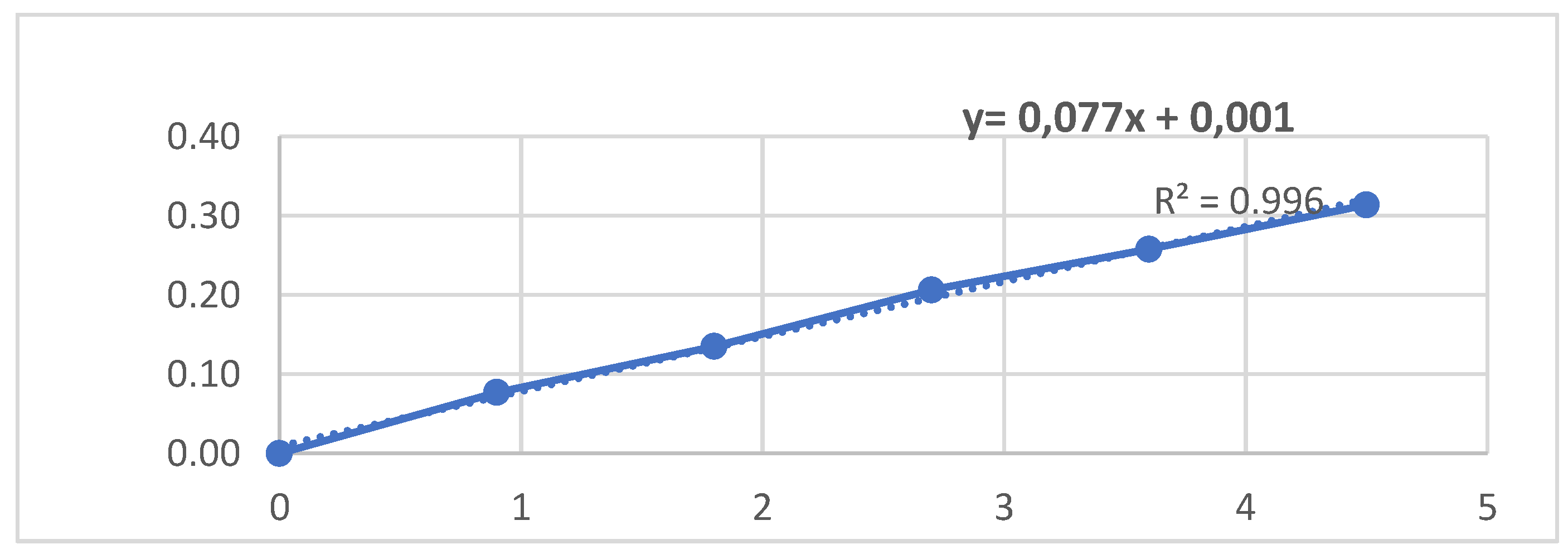
The measurement of the total antioxidant activity is conducted using a method that involves the ability of the extracts to reduce the molybdenum Mo. The first step in this process is to activate the water bath and set the temperature to 99°C. Following this, a volume of extract (either 5 or 10 µl) (mother C = 25 mg/ml) is transferred to a test tube. 1 ml of ammonium molybdate (4mM), 1 ml of sodium phosphate (28), and 1 ml of sulphuryl acid (0.6M) are then added to the test tube. The mixture is then stirred and covered with a lid without using food film. The mixture is incubated at 95°C for 90 minutes and then normalized at room temperature for 20-30 minutes. The final step is to measure the absorbance at 270 nm against a white that contains only the reagent. The total antioxidant capacity of the analyzed extracts is expressed using the number of equivalents of ascorbic acid in a gram of dry extract (mg EAA/1 g DE). To calculate this value, we use the calibration curve (y = 0.0413x + 0.0209: R² = 0.9971). This method is a reliable way to determine the antioxidant activity of extracts and is widely used in both academic and business settings.
DPPH Scavenging Activity Assay
The DPPH+ compound is a stable and bright purple radical cation that has its maximum absorbance at 517 nm. The DPPH test measures the antiradical power of plant extracts or pure molecules in a model system that can be organic solvent or water. [
11,
12]
To begin the experiment, 2.4 mg of DPPH is measured and placed onto an aluminum foil. Next, a 2.4% DPPH solution is prepared by stirring 2.4 mg of DPPH into 100 ml of EtOH for 30 minutes. Once the DPPH solution is ready, pre-calculated extracts are added to the tubes, and 200 µl of pure EtOH is added to each tube to complete the volume. Using a burette that has been rinsed with pure ethanol, 2.8 ml of the DPPH ethanolic solution is added to each tube. After stirring and incubating the prepared DPPH solution for 30 minutes, the reading is taken at 517 nm against a control that contains 200 µl of EtOH and 2.8 ml of DPPH that has been prepared under the same conditions.
Ferric-Reducing Power Assay
This method involves using an antioxidant to convert ferric iron (Fe3+) in the potassium ferrocyanide complex (K3Fe (CN)6) to ferrous iron (Fe2+), as seen by the change from yellow to blue-green color. The intensity of this color change is measured using spectrophotometry at 700 nm. To perform the test, 10 μl of the sample is placed in triplicate on a 96-well microplate, followed by the addition of 40 μl of phosphate buffer (pH 6.6) and 50 μl of potassium ferricyanide (1%) K3Fe (CN)6 (1g K3Fe (CN)6 in 100ml of water) to each well. The plate is then incubated at 50°C for 20 minutes. Next, 50 μl of 10% trichloroacetic acid (TCA), 40 μl of water, and 10 μl of 0.1% ferric chloride (FeCl3) are added to each well. The microplate is then inserted into a spectrophotometer to measure the absorption at 700nm.
- 4.
Statistic study
Data were reported as mean±SD. The results were compared using ANOVA analysis of variance, followed by a test to compare means among the groups. Differences were considered statistically significant at p < 0.05.
- 5.
Determination of antidiabetic activity
Acute Toxicity Test
Test Objective: The purpose of this test is to demonstrate that the therapeutic dose does not have short-term toxicity in normal mice.
Animals: A study was carried out on male and female albino mice. These mice were bred and raised at the pet store of the Faculty of Medicine and Pharmacy Rabat. The mice were kept in optimal breeding conditions with a photoperiod of 12 hours of light and 12 hours of darkness, as well as a temperature of 22 2°C. Additionally, the mice had free access to both food and water.
Mouse Distribution: To test the effects of a plant, 4 groups of fasting albino mice (20-35 g) were randomly created, with 6 mice in each group and equal male-to-female ratios. The control group received 2,4 mL/kg of ED. The other three groups received the plant decocted at 0.5 g/kg, 1 g/kg, and 2 g/kg, respectively.
Test Flow: Mice receive one dose of decoct extract and are observed for 10 hours for toxicity. They are monitored for 14 days for any additional signs of toxicity.
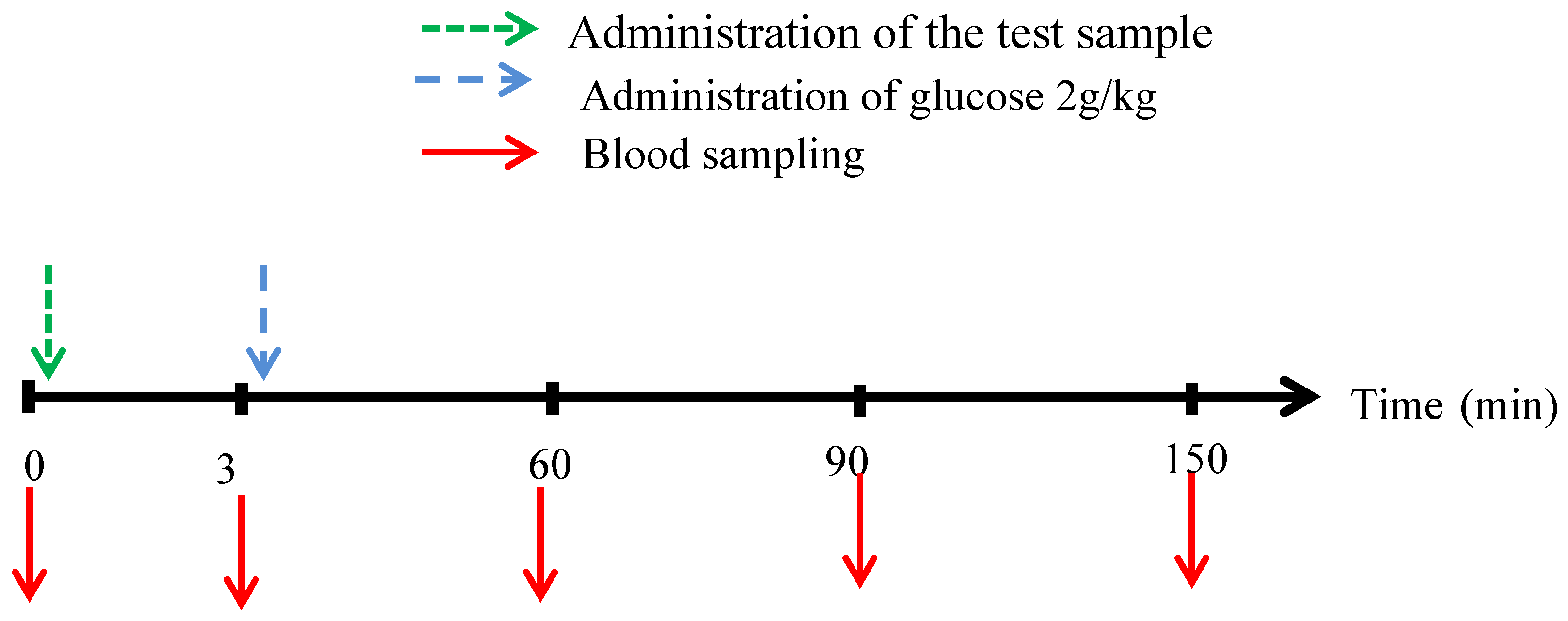
Purpose of the Study: The study aims to explore the postprandial hypoglycemic impact of the extracts on normal rats overloaded with D-glucose.
Animals: The study was conducted on both male and female Wistar rats. These animals were bred and raised at the pet shop located in the Faculty of Medicine and Pharmacy of Rabat. They were subjected to a 12-hour light/12-hour dark photoperiod and a temperature controlled at 22 2°C. The rats were maintained in optimal breeding conditions with unlimited access to water and food.
Oral Glucose Tolerance Test (OGTT)
To determine the antihyperglycemic effect (postprandial glycemia) of these extracts in vivo, the oral glucose tolerance test was conducted. The normal rats were segregated into three groups for each plant (n=6; ♂/♀=1):
Control: Normal rats that were given distilled water (2,5 mL/kg).
Decocted: Normal rats that were given extract (50 mg/kg).
Glib: Normal rats that were given Glibenclamide (2 mg/mL).
Results and Discussion
This study aims to evaluate the biological potential of Centaurium erythraea and identify the secondary metabolites contained in its aerial part that may act against Diabetes type II. Furthermore, to test two techniques (simple decoction and Soxhlet extraction) for extracting bioactive molecules from EC using water and ethanol as solvents. This choice may be explained by the analysis of the results of the ethnobotanical survey of [
13,
14,
15] presume that the majority of the Moroccan population uses decoction or extraction with only water to extract the active ingredients of medicinal plants. In addition, water is non-toxic and can be simply used to prepare a future drug. Ethanol is also known for its ability to separate polar and nonpolar compounds. The decocted and aqueous extracts were 21.3% and 18.03%, respectively, in terms of yield. While the yield of the hydroalcoholic extract was the lowest at 17.43%. For decoction extraction, the solvent can become saturated with extracted compounds in the initial few minutes and cannot be regenerated.
Chemical Characterization
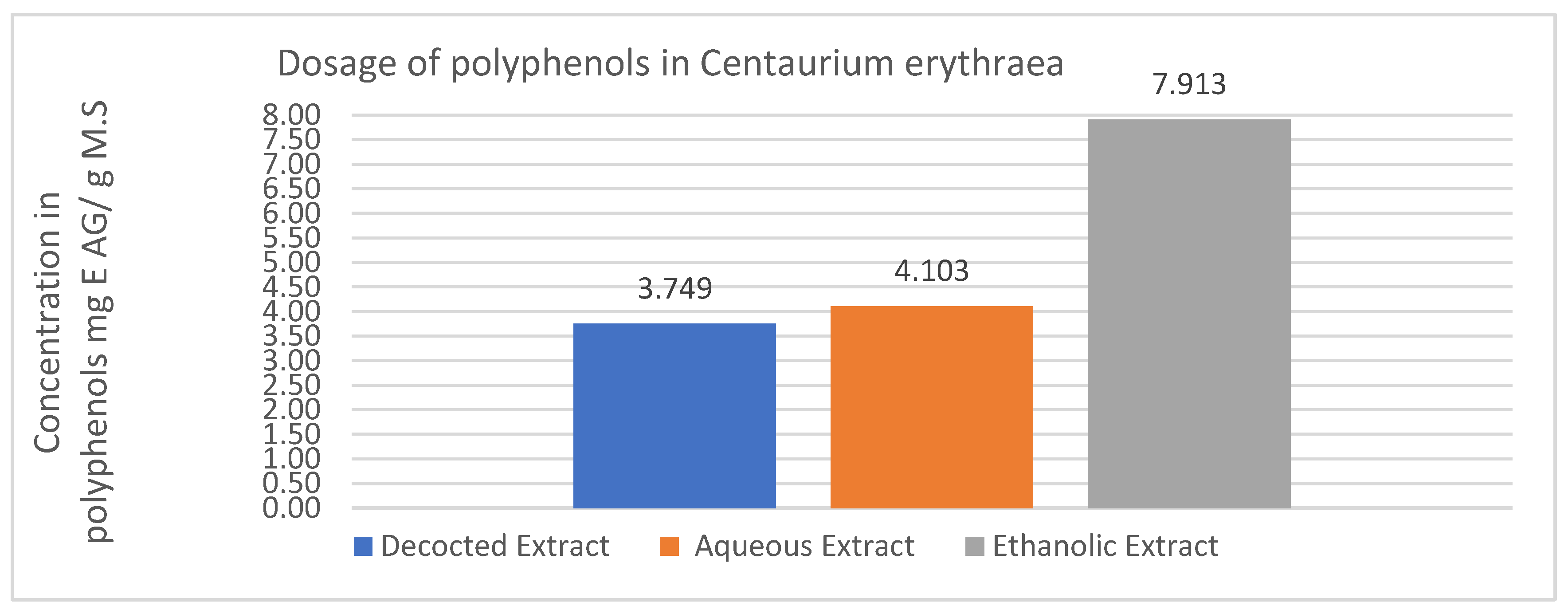
The determination of polyphenols in the crude extract was performed using the Folin Ciocalteu method, employing a calibration range established with varying gallic acid concentrations.
Figure 5 illustrates the range of gallic acid concentrations utilized for polyphenol determination and the respective absorbances measured at 760nm.
In the ethanolic extract, the polyphenol content is 7.913 mg GA equivalents per gram of dry mass (mg GA equivalents/g MS), whereas the aqueous extract contains 4.103 mg GA equivalents/g MS. Notably, the ethanolic extract exhibits the highest polyphenol content when compared to the decocted extract, which contains 3.749 mg GA equivalents/g MS.
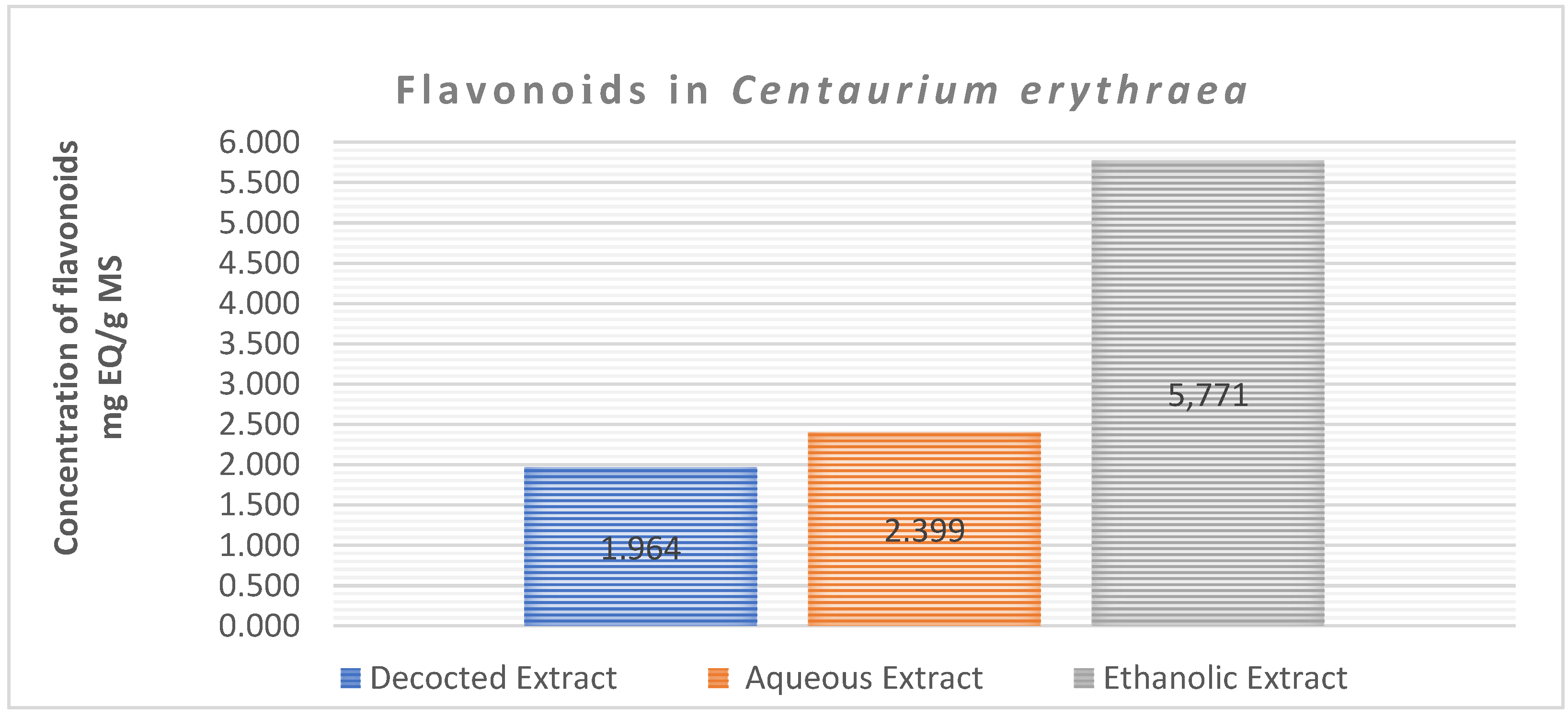
The determination of flavonoids in the crude extract was carried out using a calibration range established with various concentrations of quercetin. The range of quercetin concentrations used for flavonoid determination and their respective absorbances measured at 430 nm are as follows: 0,5,10,15,20 and 25 µg /ml.
The enclosed results in
Figure 6 illustrate the flavonoid content in the three extracts of Centaurium erythraea. Notably, the hydroalcoholic extract stands out with a notably high concentration of 5.771 mg EQ/g MS, followed by the aqueous extract at 2.399 mg EQ/g MS. In comparison, the decocted extract displays a lower flavonoid content, measuring 1.964 mg EQ/g MS
Both the aqueous and hydroalcoholic extracts exhibit elevated levels of tannins, with concentrations of 2.46 mg EC/g MS and 1.28 mg EC/g MS, respectively. In contrast, the decocted extract demonstrates a comparatively lower tannin content, measuring 0.31 mg EC/g MS. In terms of anthocyanin content, it's noteworthy that both the decocted and ethanolic extracts exhibit higher levels, measuring 1.31 ± 0.016 mg/mL each. In contrast, the aqueous extract contains a comparatively lower amount of anthocyanins, registering at 0.46 ± 0.03 mg/mL.
Furthermore, our findings reinforce the notion of C. erythraea being a rich source of polyphenols. This observation aligns with previous research, including the work of [
16], which reported diverse pharmacological effects associated with the decoction of C. erythraea. Additionally, studies by [
17] and [
18] have emphasized the significance of various chemical compounds, especially xanthones, found in C. erythraea [
19]. Collectively, these studies contribute to our understanding of the potential health benefits and pharmacological properties of C. erythraea and underscore the importance of solvent choice in polyphenol extraction processes. Additional studies have corroborated the presence of flavonoids in the aqueous extract of Centaurium erythraea. For instance, [
20] identified twenty-two flavonoid compounds, including derivatives of quercetin and kaempferol, within the aqueous extract.
Furthermore, a separate investigation of C. erythraea's aerial parts in a study conducted in Romania by [
21] determined the total phenolic content in the ethanol extract to be 271.613 mg/L, expressed as gallic acid equivalents. These findings further affirm the rich phytochemical composition of Centaurium erythraea and its potential health-promoting properties.
Tannins and anthocyanins are not commonly associated with Centaurium erythraea, which is in line with our study's findings and corroborated by another study [
22,
23], in this broader investigation covering various plant species, the analysis revealed that tannin content ranged from 4.4549 g/100g to 89.5833 g/100g, and anthocyanin content ranged from 1.2 mg/100g to 109.7 mg/100g across different plants. Notably, Centaurium erythraea exhibited one of the lower levels of tannins and anthocyanins among the tested plants.
Total Antioxidant Activity
The total antioxidant activity of Centaurium erythraea (C. erythraea) can vary depending on several factors, including the plant's growth conditions, the part of the plant used, and the method of extraction. Antioxidant activity is typically assessed through various assays, such as the DPPH (2,2-diphenyl-1-picrylhydrazyl) assay, FRAP (Ferric Reducing Antioxidant Power) assay, or ORAC (Oxygen Radical Absorbance Capacity) assay, among others.
To determine the total antioxidant activity of C. erythraea, researchers typically conduct these assays and express the results in terms of gallic acid equivalents, which is a common reference compound for antioxidant capacity. A calibration curve for ascorbic acid is prepared from a series of standard solutions of ascorbic acid with concentrations (0, 4, 8,12, 16, 20, 24, and 28 µg/ml), using a spectrophotometer, the absorbance of each standard solution is measured at 270 nm. The outcomes are displayed in
Table 1, revealing distinct degrees of antioxidant efficacy among the various extracts. Notably, the aqueous extract exhibits potent antioxidant activity, as evidenced by its IC50 value of 0.5483 ± 0.0084 mg AA/mg MS. In a closely ranked position is the ethanolic extract, demonstrating an IC50 value of 0.2485 ± 0.002 mg AA/mg MS. In contrast, the decocted extract displays the least pronounced antioxidant activity, with an IC50 value of 0.1311 ± 0.001 mg AA/mg MS. These findings underscore the variability in antioxidant potential across the different extracts, with the aqueous extract standing out as the most potent in this context [
24,
25].
DPPH Scavenging Activity Assay
The results of the DPPH test in
Table 2 showed less powerful antioxidant activity with a very similar IC50 for the aqueous (IC50 = 0,063±0,005 mg/ml) and ethanolic (0,09± 0,007 mg/ml) extracts than the decocted extract (0,186±0,103 mg/ml) comparing to standards.
The current results align harmoniously with the findings of a previous study conducted by [
23]. In their research, they also identified significant antioxidant potential in both methanolic and aqueous extracts of Centaurium erythraea's aerial parts using the DPPH test. Their recorded IC50 values were 0.232 ± 0.002 mg/mL and 0.208 ± 0.002 mg/mL, respectively.
Interestingly, these values closely resemble those obtained in a study conducted by Bouyahya and their research team in 2019, where the ethanolic extract displayed an IC50 value of 382.25 ± 5.59 μg/mL. This consistency in results across different studies reinforces the robust antioxidant potential of Centaurium erythraea's extracts and highlights their valuable role in scavenging free radicals, this bioactivity can likely be attributed to the presence of xanthones and phenolic compounds in Centaurium erythraea's extracts reported by [
26].
Ferric-Reducing Power Assay
The reducing power of the extract is presented in
Table 3. The decocted extract has a higher reducing power (IC50= 0.4210 ± 0.001 mg AA/mg MS) compared to the Aqueous extract (IC50= 0.0989 ± 0.001 mg AA/mg MS) and the ethanolic extract (IC50 = 0,0439 ± 0,002 mg AA/mg MS). In the realm of antioxidant research, flavonoids, and phenolic acids have emerged as prominent subjects of study [
27]. These compounds are well-regarded for their proficiency in neutralizing free radicals and exhibiting potent antioxidant properties, this antioxidative prowess can be attributed to the presence of several phenolic compounds within the extract, including esters of p-coumaric acid, ferulic acid, sinapic acid, and kaempferol [
28]
Acute Toxicity Test
The test does not show any adverse effects or lethality in the tested organisms.
Oral Glucose Tolerance Test (OGTT)
The Oral Glucose Tolerance Test (OGTT) is a diagnostic tool used to evaluate how an individual's body processes glucose. It plays a critical role in diagnosing various glucose metabolism disorders, including diabetes and prediabetes, by monitoring blood glucose levels before and after the consumption of a standardized glucose solution. It provides valuable information about how the body handles glucose, which is essential for effective diabetes management and prevention. The OGTT is valuable because it can detect glucose metabolism abnormalities that may not be apparent through fasting blood glucose tests alone.
This study explored the ability of the aqueous extract of Centaurium erythraea to combat diabetes. The investigation employed in vitro assays to evaluate their impact on the inhibition of two key enzymes, α-amylase and α-glucosidase. The results revealed that aqueous extract possessed anti-hyperglycemic properties compared with Glibenclamide. This implies that the extract has the potential to lower elevated blood glucose levels, a critical aspect of diabetes management, a previous study on Centaurium’s aqueous extract declared the presence of a hypoglycemic effect by inhibition of the intestinal enzymes [
29].
Conclusions
Our research is centered on investigating bioactive molecules derived from medicinal plants that hold potential for the treatment of diabetes. We conducted three extractions, employing water (decoction) and aqueous and ethanol extraction using the Soxhlet method. Additionally, we performed chemical characterization of the medicinal plant Centaurium erythraea through experimental techniques, quantifying the concentrations of secondary metabolites including polyphenols, flavonoids, tannins, and anthocyanins in the three extracts. Our findings revealed that our extracts are abundant in secondary metabolites. Furthermore, we conducted assessments to evaluate total antioxidant activity, DPPH (2,2-diphenyl-1-picrylhydrazyl) scavenging activity, and iron-reducing power to comprehend Centaurium erythraea's capability to inhibit or mitigate oxidative molecules. The results underscored the significant antioxidant potential of Centaurium erythraea extracts. Regarding the anti-diabetic properties of the Centaurium erythraea extracts, our findings demonstrated the presence of a hypoglycemic effect in the decoction extract obtained from Centaurium erythraea. To develop an effective drug for diabetes management, it is imperative to expand our research by exploring other extracts utilizing various solvents and extraction methodologies.
Authors Contributions
The authors hereby declare that the work presented in this article is original and that any liability for claims relating to the content of this article will be borne by them.
Acknowledgments
Financial support from Mohamed V University (Laboratory of Toxicology, and Pharmacology of Faculty of Medicine and Pharmacy Rabat) regarding this research is gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Singab, A. N., Youssef, F. S., & Ashour, M. L. Medicinal plants with potential antidiabetic activity and their assessment. Med Aromat Plants. 2014; 3(151): 2167-0412.
- Bouyahya, A., El Omari, N., Elmenyiy, N., Guaouguaou, F. E., Balahbib, A., Belmehdi, O., ... & Bakri, Y. Moroccan antidiabetic medicinal plants: Ethnobotanical studies, phytochemical bioactive compounds, preclinical investigations, toxicological validations and clinical evidences; challenges, guidance and perspectives for future management of diabetes worldwide. Trends in Food Science & Technology. 2021 ;115 : 147-254. [CrossRef]
- Aafi, A. A., El Kadmiri, A. A., Benabid, A., & Rochdi, M. Richesse et diversité floristique de la suberaie de la Mamora (Maroc). Acta Botanica Malacitana. 2005 ; 30 :127-138. [CrossRef]
- Đorđević, M., Grdović, N., Mihailović, M., Jovanović, J. A., Uskoković, A., Rajić, J., ... & Dinić, S. Centaurium erythraea extract improves survival and functionality of pancreatic beta-cells in diabetes through multiple routes of action. Journal of Ethnopharmacology. 2019; 242 : 112043. [CrossRef]
- Barešová, H. "Centaurium erythraea Rafn: micropropagation and the production of secoiridoid glucosides." Medicinal and Aromatic Plants I. Berlin, Heidelberg: Springer Berlin Heidelberg. 1988; 350-366.
- Uskoković, A., Jovanović, J. A., Dinić, S., Vidaković, M., Mihailović, M., Poznanović, G., & Grdović, N. Mushroom and plant extracts as potential intervention supplements in diabetes management. Biodiversity and Biomedicine. 2020 ; 247-256.
- Bouyahya, A., Belmehdi, O., El Jemli, M., Marmouzi, I., Bourais, I., Abrini, J., ... & Bakri, Y. Chemical variability of Centaurium erythraea essential oils at three developmental stages and investigation of their in vitro antioxidant, antidiabetic, dermatoprotective and antibacterial activities. Industrial Crops and Products. 2019; 132 : 111-117. [CrossRef]
- Zahrae Redouan, F., Benitez, G., Aboubakr, B., Bassma, E. B., Picone, R. M., Crisafulli, A., ... & Merzouki, A. The status and perception of medicinal plants by local population of Talassemtane National Park (Northern Morocco). Caspian Journal of Environmental Sciences. 2020 ; 18(2) : 131-147.
- El-Hilaly, J., Hmammouchi, M., & Lyoussi, B. Ethnobotanical studies and economic evaluation of medicinal plants in Taounate province (Northern Morocco). Journal of Ethnopharmacology. 2003; 86(2-3): 149-158. [CrossRef]
- Singleton, V. L., & Rossi, J. A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American journal of Enology and Viticulture. 1965; 16(3): 144-158. [CrossRef]
- Sefi, M., Fetoui, H., Lachkar, N., Tahraoui, A., Lyoussi, B., Boudawara, T., & Zeghal, N. Centaurium erythrea (Gentianaceae) leaf extract alleviates streptozotocin-induced oxidative stress and β-cell damage in rat pancreas. Journal of Ethnopharmacology. 2011 ; 135(2) : 243-250. [CrossRef]
- Blois, M. S. Antioxidant determinations by the use of a stable free radical. Nature. 1958; 181(4617): 1199-1200. [CrossRef]
- Bondet, V., Brand-Williams, W., & Berset, C. L. W. T. Kinetics and mechanisms of antioxidant activity using the DPPH. free radical method. LWT-Food Science and Technology. 1997 ; 30(6) : 609-615. [CrossRef]
- Boutahiri, S., Bouhrim, M., Abidi, C., Mechchate, H., Alqahtani, A. S., Noman, O. M., ... & Eto, B. Antihyperglycemic effect of lavandula pedunculata: in vivo, in vitro and ex vivo approaches. Pharmaceutics. 2021 ; 13(12) :2019.
- Benkhnigue, O., Akka, F. B., Salhi, S., Fadli, M., Douira, A., & Zidane, L. Catalogue des plantes médicinales utilisées dans le traitement du diabète dans la région d’Al Haouz-Rhamna (Maroc). J Anim Plant Sci. 2014 ; 23(1) : 3539-68.
- Mustafa, B., Hajdari, A., Krasniqi, F., Hoxha, E., Ademi, H., Quave, C. L., & Pieroni, A. Medical ethnobotany of the Albanian Alps in Kosovo. Journal of Ethnobiology and Ethnomedicine. 2012; 8: 1-14. [CrossRef]
- Gaspar, N., Godinho, J., Vasconcelos, T., Caldas, D., Mendes, P., & Barros, O. Ethnobotany in the center of Portugal (Santarém). Natural products in the new millennium: prospects and industrial application. 2002; 271-284.
- Guedes, L., Reis, P. B., Machuqueiro, M., Ressaissi, A., Pacheco, R., & Serralheiro, M. L. Bioactivities of Centaurium erythraea (Gentianaceae) decoctions: Antioxidant activity, enzyme inhibition and docking studies. Molecules. 2019 ; 24(20) : 3795.
- Pedraza-Chaverri, J., Cárdenas-Rodríguez, N., Orozco-Ibarra, M., & Pérez-Rojas, J. M. (2008). Medicinal properties of mangosteen (Garcinia mangostana). Food and chemical toxicology. 2008; 46(10): 3227-3239. [CrossRef]
- Negi, J. S., Bisht, V. K., Singh, P., Rawat, M. S. M., & Joshi, G. P. Naturally occurring xanthones: chemistry and biology. Journal of Applied Chemistry. 2013. [CrossRef]
- Aberham, A., Pieri, V., Croom Jr, E. M., Ellmerer, E., & Stuppner, H. Analysis of iridoids, secoiridoids and xanthones in Centaurium erythraea, Frasera caroliniensis and Gentiana lutea using LC–MS and RP-HPLC. Journal of pharmaceutical and biomedical analysis. 2011; 54(3) : 517-525.
- Sandru, D., Niculescu, V., Lengyel, E., & Tita, O. Identification and quantification of total polyphenols in plants with bioactive potentially. Cancer. 2016; 1: 2. [CrossRef]
- Ciucure, C. T., Șandru, D., Lengyel, E., Iancu, R., & Tiț, O. IDENTIFICATION AND QUANTIFICATION OF TANNINS AND ANTHOCYANINS FROM PLANTS WITH BIOACTIVE POTENTIAL. International Multidisciplinary Scientific GeoConference: SGEM. 2016; 1: 575-581.
- Merghem, M., & Dahamna, S. Antioxidant activity of Centaurium erythraea extracts. Journal of Drug Delivery and Therapeutics. 2020 ; 10(2) : 171-174. [CrossRef]
- Pereira, E. M. R., Gomes, R. T., Freire, N. R., Aguiar, E. G., Brandão, M. D. G. L., & Santos, V. R. In vitro antimicrobial activity of Brazilian medicinal plant extracts against pathogenic microorganisms of interest to dentistry. Planta medica. 2011 ; 77(04) : 401-404. [CrossRef]
- Mihaylova, D., Vrancheva, R., & Popova, A. Phytochemical profile and in vitro antioxidant activity of Centaurium erythraea Rafn. Bulgarian Chemical Communications. 2019; 51 : 95-100.
- Chamkhi, I., Benali, T., Aanniz, T., El Menyiy, N., Guaouguaou, F. E., El Omari, N., ... & Bouyahya, A. Plant-microbial interaction: The mechanism and the application of microbial elicitor induced secondary metabolites biosynthesis in medicinal plants. Plant Physiology and Biochemistry. 2021; 167: 269-295. [CrossRef]
- Mansar-Benhamza, L., & Djerrou, Z. Evaluation of anti-hyperglycemic activity and side effects of Erythraea centaurium (L.) Pers. in rats. African Journal of Biotechnology. 2013; 12(50): 6980.
- Stefkov, G., Miova, B., Dinevska-Kjovkarovska, S., Stanoeva, J. P., Stefova, M., Petrusevska, G., & Kulevanova, S. Chemical characterization of Centaurium erythrea L. and its effects on carbohydrate and lipid metabolism in experimental diabetes. Journal of ethnopharmacology. 2014; 152(1): 71-77. [CrossRef]
- J. Pedraza-Chaverri, N. Cárdenas-Rodríguez, M. Orozco-Ibarra, and J. M. Pérez-Rojas, “Medicinal properties of mangosteen (Garcinia mangostana),” Food and Chemical Toxicology, vol. 46, no. 10, pp. 3227–3239, 2008. [CrossRef]
- M. Saxena, M. B. Bajpai, P. S. R. Murthy, and S. K. Mukherjee, “Mechanism of blood sugar lowering by a swerchirin-containing hexane fraction (SWI) of Swertia chirayita,” Indian Journal of Experimental Biology, vol. 31, no. 2, pp. 178–181, 1993.
- P. Basnet, S. Kadota, M. Shimizu, Y. Takata, M. Kobayashi, and T. Namba, “Bellidifolin stimulates glucose uptake in rat 1 fibroblasts and ameliorates hyperglycemia in streptozotocin (STZ)-induced diabetic rats,” Planta Medica, vol. 61, no. 5, pp. 402–405, 1995. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).