Submitted:
18 August 2024
Posted:
19 August 2024
You are already at the latest version
Abstract
Keywords:
2. Methods:
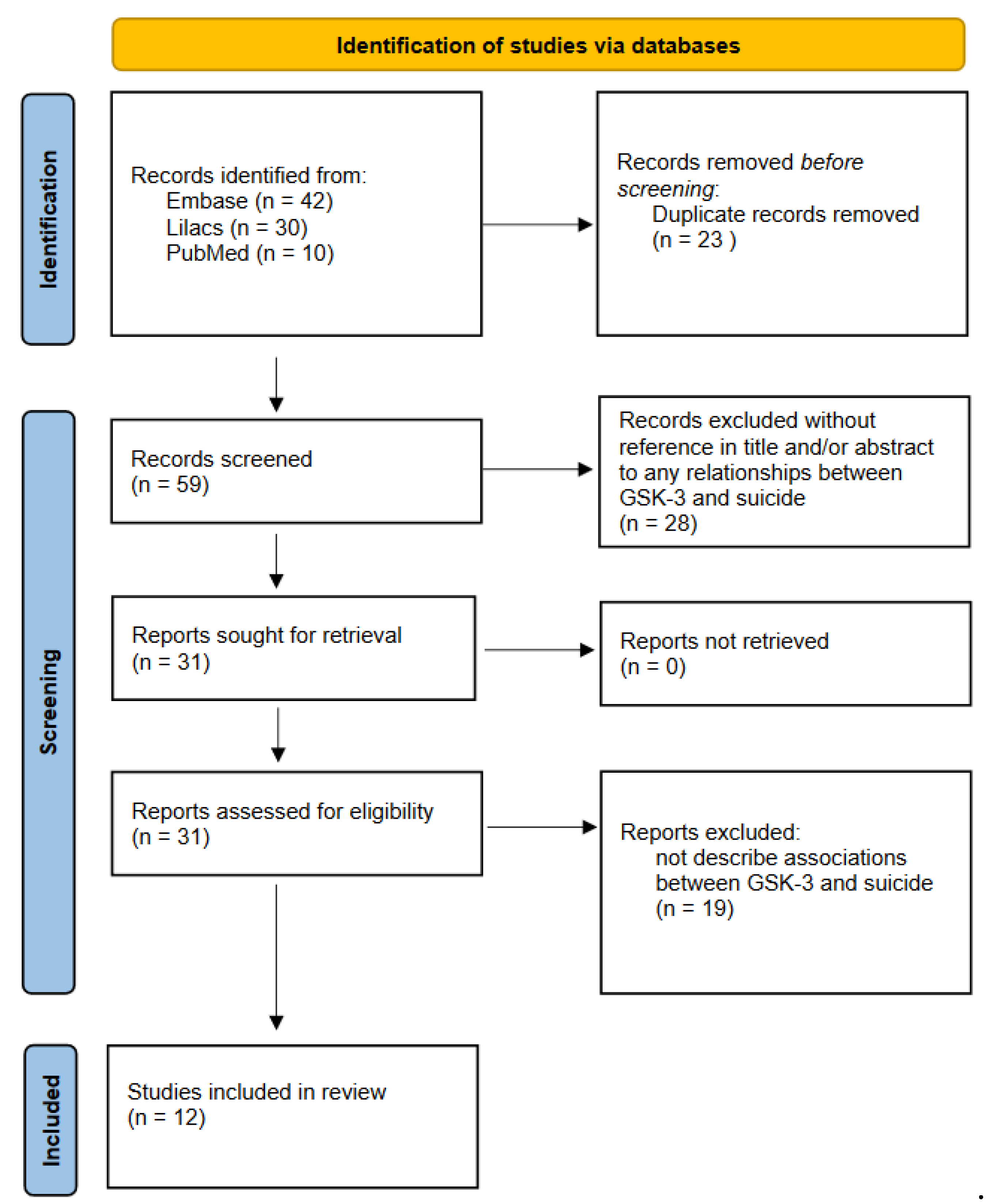
2.1. Search Strategy
2.2. Study Selection
3. Results:
3.1. GSK-3 and Inflammation
3.2. GSK-3 and Genetic Expression
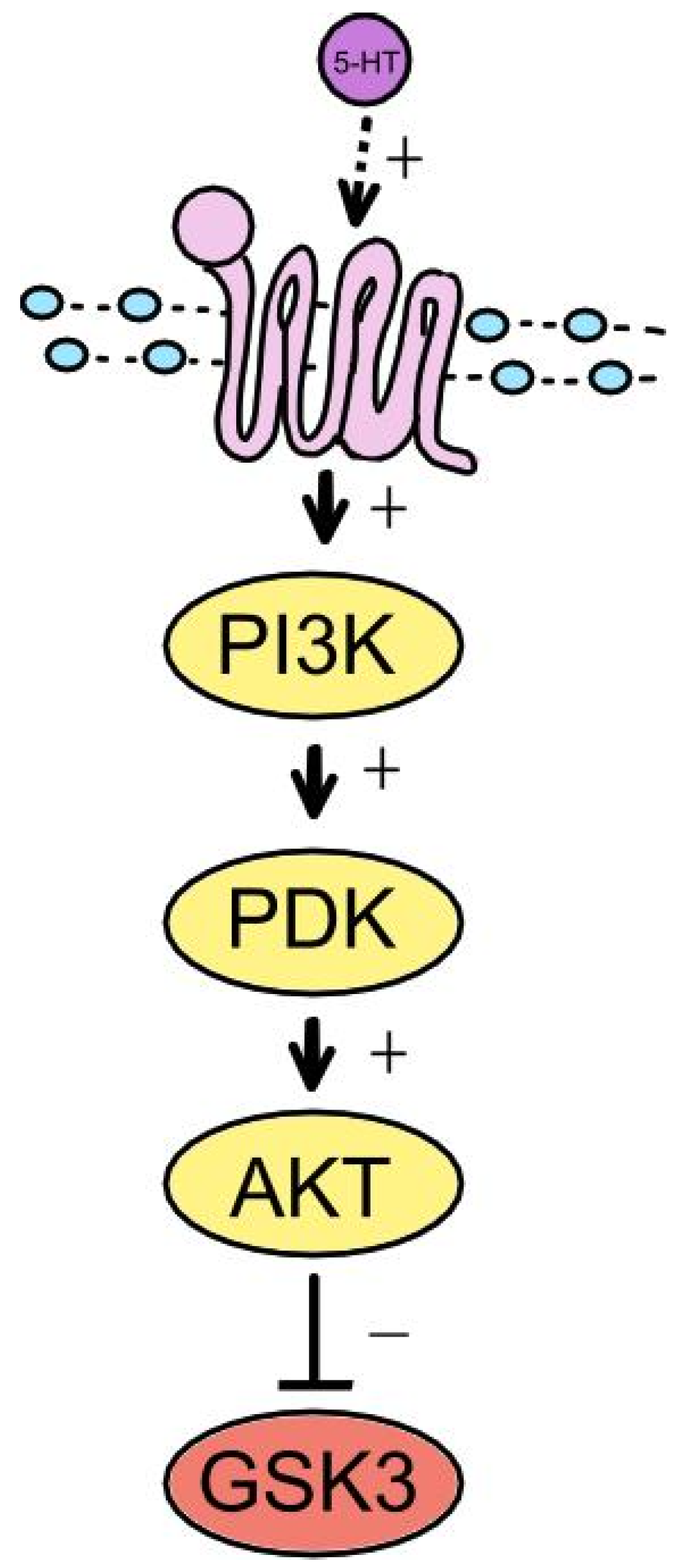
3.3. GSK-3 and Neurotransmission
3.4. GSK-3 and Impulsivity
| Citation | Design | Study aim | Relevance to GSK-3 and suicide | Suicide group(s) | Control group relevant for comparison | Relevant Result | |
|---|---|---|---|---|---|---|---|
| Karege et al, 2007[29] | Postmortem study | To examine possible abnormality in the Akt/glycogen synthase kinase-3β (GSK-3β) axis of depressed suicide victims’ brains. | Inhibition of GSK-3β might contribute to antidepressant activity, increasing our interest in the role of GSK-3β in major depression disorder (MDD) and suicidality. | n = 20 | n = 20 | There was no change either in GSK-3α/β and Akt-1 protein levels or in lithium-inhibitable total GSK-3α/β enzyme activity of the ventral prefrontal cortex. The enzyme activity of Akt decreased significantly [analysis of variance (ANOVA): F(3,36) = 5.372; p = .003], whereas GSK-3β activity increased significantly [ANOVA: F(3,36) = 8.567; p = .002] in depressed suicide victims and non-suicide subjects but not in non-depressed suicide victims. | |
| Pandey et al, 2009[28] | Postmortem study | To examine the regional distribution and compare the abundance of GSK-3β gene expression in different regions of postmortem brain samples. |
There is both direct and indirect evidence suggesting the involvement of GSK-3β in the pathophysiology of mood disorders and possibly schizophrenia. Given the observation that both mood disorders and schizophrenia may be risk factors for suicidal behavior, it was of interest to examine the role of GSK-3b in suicide. | n = 27 adults n = 29 teenage |
n = 20 adults n = 26 teenage |
There were no significant differences in the mean GSK-3β mRNA levels among the teenage suicide subjects who were diagnosed with major depressive disorders (PFC: 2,913.54 ± 983.69; hippo- campus: 2,911.71 ± 1,113.35; n = 8), with no history of mental disorders (PFC: 2,573.12 ± 1,124.47; hippocampus: 2,570.24 ± 1,110.75; n = 9), or with other psychiatric diagnoses (PFC: 3,173.46 ± 1,255.44; hippocampus: 2,849.24 ± 1,172.08; n = 10) | |
| Citation | Design | Study aim | Relevance to GSK-3 and suicide | Suicide group(s) | Control group relevant for comparison | Relevant Result | |
| Yoon et al, 2009[31] | Case-control study | Investigate GSK-3β gene for association with suicidal behavior in depressive patients. | Inhibition of GSK-3β might contribute to antidepressant activity, increasing our interest in the role of GSK-3β in major depression disorder (MDD) and suicidality. |
n = 170 | n = 164 | The genotype distribution of the -1727A/T (controls: p= 0.133; patients: p= 0.183) and -50C/T (controls: p= 0.188; patients: p= 0.430) agreed with the Hardy-Weinberg equilibrium. The results showed that allele, genotype for the two SNPs do not differ between the controls and suicide attempters. | |
| Yoon and Kim, 2010[9] | Case-control study | Investigate associations between −1727A/T and −50C/T of the GSK-3β gene and MDD and suicidal behavior. | Since mood disorders are major risk factors for suicide, it has been suggested that abnormalities in GSK-3β may also be associated with suicide. | n = 170 | n = 147 | The genotype distributions of −1727A/T and −50C/T agreed with Hardy–Weinberg equilibrium. The results showed that the alleles, genotypes, and haplotypes of the two SNPs do not differ between suicidal MDD subjects, non-suicidal MDD subjects, and normal controls. | |
| Kalkman, 2011[26] | Literature review | Propose to hypothesis of the supplementation with glutamine to reduce suicide | The GSK-3 inhibitor, lithium, reduced death by suicide | Not suicide participants | Not control group | The enzyme activity of GSK-3 was significantly increased in postmortem study of prefrontal cortex from depressed suicide victims. | |
| Karege et al, 2012[32] | Postmortem study | To examine whether there is an abnormality in this signaling axis in major depression. | Investigations of β-catenin and GSK3β activation state on human brains are therefore necessary to confirm that GSK3β is implicated in mood dysregulation. | n = 10 individuals who died by suicide with documented MDD. | n = 10 MDD who died from a different documented cause. | The tGSK3β/pGSK3β ratio was increased in MDD suicide (MDD+S; pb0.001) and non-suicide (MDD; pb0.002) subjects. | |
| Citation | Design | Study aim | Relevance to GSK-3 and suicide | Suicide group(s) | Control group relevant for comparison | Relevant Result | |
| Ren et al, 2013[33] | Postmortem study | Examined the role of Wnt signaling in teenage suicide by determining the protein and mRNA expression of GSK3β, pGSK3β-Ser9, and β-catenin in the PFC and hippocampus of teenage suicide victims and normal controls. | It is quite possible that this effect of lithium in suicidal behavior may be related to its effect on GSK3β. | n = 24 (14 male and 10 female) | n = 24 (17 male and seven female) | No significant change was observed in the GSK3β protein levels either in the PFC or hippocampus of suicide victims compared to controls. However, protein levels of pGSK3β-ser9 were significantly decreased in the PFC and hippocampus of suicide victims compared to normal controls. | |
| Jiménez el al, 2013[25] | Cohort | To investigate the association of the IMPA1 and 2, INPP1, GSK3α and β genes with suicidal behavior in BP. | A potential role of genetic variability in GSK3 comports with studies reporting that glutamine synthetase activity was significantly reduced, not only in depressed suicides, but also in suicides free of depressive symptomatology. | n = 69 | n = 130 | T-allele carriers of the rs1732170-GSK3β gene and A-allele carriers of the rs11921360-GSK3β gene had a higher risk for attempting suicide. | |
| Beurel and Jope, 2014[27] | Literature review | Inflammation may be a key factor precipitating suicidal behaviors | Lithium is an established inhibitor of GSK-3 and it may contribute to antisuicidal actions. | Not suicide participants | Not control group | Stress is established to cause activation of GSK-3 in rodent brain. Active GSK-3 promotes inflammation and was hypothesize that inflammation contributes to provoking components of suicidal behavior. | |
| Benard et al, 2016[34] | Literature review | To highlight evidence about the preventive action of Li on suicide in BD populations. | This emblematic anti-suicide effect of Li is however not well known by psychiatric physicians. | Not suicide participants | Not control group | Genetic variants of the glycogen synthase kinase 3α/ β (GSK3α and β; proteins inhibited by Li) seem to be associated with more impulsiveness in BD populations. | |
| Citation | Design | Study aim | Relevance to GSK-3 and suicide | Suicide group(s) | Control group relevant for comparison | Relevant Result | |
| Malhi et al, 2018[35] | Literature review | Investigating the effects of a medication known for its anti-suicidal properties on neurobiological and neurocognitive substrates of suicidal thinking may provide a deeper and more meaningful understanding of suicide. | A recent neuro- cognitive model of suicide in the context of bipolar disorder provides a useful developmental framework for the psychological processes that culminate in suicide. | Not suicide participants | Not control group | Via GSK3β induced changes, lithium is ultimately able to alter suicidal thinking and behaviors. | |
| Landa et al, 2021[36] | Literature review | To analyze the risk factors associated with suicidal behavior, their pathophysiological correlations, and their treatment with lithium carbonate. | A direct relationship has been demonstrated between the deregulation of GSK3 and BDNF (with an increase in the inflammatory profile) in patients at greater risk of suicidal behavior. | Not suicide participants | Not control group | Lithium carbonate has the modulation capacity of the GSK3 receptor, through direct and indirect mechanisms. A relevant point of view of the action mechanism is the modulation on the complex of beta-arrestins (ARRB). The ARRB activates GSK3 through the formation of a complex with the AKT kinase and protein phosphatase 2 (PP2A). Lithium, by blocking the formation of the ARRB-AKT-PP2A complex, indirectly inhibits the action of the GSK3. In this way, the inflammatory process at the level of the central nervous system would be reduced, and it would generate an effect on suicidal behaviors. | |
4. Discussion:
Conclusion:
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Embi, N.; Rylatt, D.B.; Cohen, P. Glycongen Synthase Kinase-3 from Rabbit Skeletal Muscle. Eur J Biochem. 1980, 107, 519–527. [Google Scholar] [CrossRef]
- Jope, R.S.; Johnson, G.V.W. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci. 2004, 29, 95–102. [Google Scholar] [CrossRef]
- Woodgett, J.R. Molecular cloning and expression of glycogen synthasekinase-3/Factor A. he EMBO Journal. 1990, 9, 2431–2438. [Google Scholar] [CrossRef]
- Doble, B.W.; Kelly, K.F.; Woodgett, J.R. Molecular Mechanisms Underlying Pluripotency and Lineage Commitment – The Role of GSK-3. 2011. In: Embryonic Stem Cells - Basic Biology to Bioengineering [Internet]. InTech. Available from: https://www.intechopen.com/chapters/19272.
- Lai, S.; Wang, P.; Gong, J.; Zhang, S. New insights into the role of GSK-3β in the brain: From neurodegenerative disease to tumorigenesis. PeerJ. 2023, 11, e16635. [Google Scholar] [CrossRef]
- Sai Varshini, M.; Aishwarya Reddy, R.; Thaggikuppe Krishnamurthy, P. Unlocking hope: GSK-3 inhibitors and Wnt pathway activation in Alzheimer's therapy. J Drug Target. 2024, 32, 909–917. [Google Scholar] [CrossRef]
- Jones, G.H.; Rong, C.; Shariq, A.S.; Mishra, A.; Machado-Vieira, R. Intracellular Signaling Cascades in Bipolar Disorder. In: Young AH, Juruena MF, editors. Bipolar Disorder: From Neuroscience to Treatment Current Topics in Behavioral Neurosciences. 48. Switzerland: Springer; 2020. p. 101-32.
- Munner, A. Wnt and GSK3 Signalling Pathways in Bipolar Disorder: Clinical and Thereapeutic Implication. Clinical Psychopharmacology and Neuroscience. 2017, 15, 100–114. [Google Scholar] [CrossRef]
- Yoon, H.-K.; Kim, Y.-K. Association between glycogen synthase kinase-3b gene polymorphisms and major depression and suicidal behavior in a Korean population. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2010, 34, 331–334. [Google Scholar]
- Musazzi L, Seguini M, Mallei A, Treccani G, Pelizzari M, Tornese P; et al. Time-dependent activation of MAPK/Erk1/2 and Akt/GSK3 cascades: Modulation by agomelatine. BMC Neuroscience. 2014; 15.
- Beaulieu, J.M.; Gainetdinov, R.R.; Caron, M.G. The Akt-GSK-3 signaling cascade in the actions of dopamine. Trends Pharmacol Sci. 2007, 28, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, J.M. A role for Akt and glycogen synthase kinase-3 as integrators of dopamine and serotonin neurotransmission in mental health. J Psychiatry Neurosci. 2012, 37, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Harkin, E.F.; Nasrallah, G.; Le François, B.; Albert, P.R. Transcriptional Regulation of the Huma 5-HT1A Receptor Gene by Lithium: Role of Deaf1 and GSK3beta. Int J Mol Sci. 2023, 24, 15620. [Google Scholar] [CrossRef] [PubMed]
- Hernandez F, Lucas JJ, Avila J. GSK3 and Tau: Two Convergence Points in Alzheimer's Disease. Journal of Alzheimer's Disease. 2013, 33, S141–S144. [Google Scholar]
- Alvarez, G.; Muñoz-Montaño, R.; Satrústegui, J.; Avila, J.; Bogónez, E.; Díaz-Nido, J. Lithium protects cultured neurons against B-amyloid-induced neurodegeneration. FEBS Letters. 1999, 453, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Giotakos, O. Is impulsivity in part a lithium deficiency state? Psychiatriki. 2018, 29, 264–270. [Google Scholar] [CrossRef]
- Kessler, R.C. ; JBE The epidemiology of depression across cultures. Annu Rev Public Health. 2013, 34, 119–138. [Google Scholar] [CrossRef] [PubMed]
- McCallum, R.T.; Perreault, M.L. Glycogen Synthase Kinase-3. Cells. 2021;10.
- Rodrigues, J.F.R. O que é o suicídio: Perfil epidemiológico de suicídio na cidade de Marília: Proposições para prevenção. São Paulo: Dialética; 2022. 312 p.
- De Berardis D, Fornaro M, Valchera A, Cavuto M, Perna G, Di Nicola M; et al. Eradicating Suicide at Its Roots: Preclinical Bases and.
- Clinical Evidence of the Efficacy of Ketamine in the.
- Treatment of Suicidal Behaviors. Int J Mol Sci. 2018; 19.
- Allen, J.P. The Debate between a Man and His Soul: A Masterpiece of Ancient Egyptian Literature. Leiden, The Netherlands: Koninklijke Brill NV; 2011. 313 p.
- Chaignet, A.E. Pythagore et la Philosophie Pythagoricenne. 12 ed. Paris: Librairie Académique; 1874. 354 p.
- Shneidman, E. Definition of suicide. Los Angelis (CA): Interscience; 1985. 272 p.
- Page MJ, McKenzie JE, Bossuyt PM; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst Rev. 2021; 10.
- Jiménez E, Arias B, Mitjans M, Goikolea JM, Roda E, Sáiz PA; et al. Genetic variability at IMPA2, INPP1 and GSK3B increases the risk of suicidal behavior in bipolar patients. European Neuropsychopharmacology. 2013, 23, 1452–1462. [Google Scholar] [CrossRef] [PubMed]
- Kalkman, H.O. Circumstantial evidence for a role of glutamine-synthetase in suicide. Medical Hypotheses. 2011; 905–907. [Google Scholar]
- Beurel, E.; Jope, R.S. Inflammation and lithium: Clues to mechanisms contributing to suicide-linked traits. Transl Psychiatry. 2014, 4, e488. [Google Scholar] [CrossRef] [PubMed]
- Pandey GN, Dwivedi Y, Rizavi HS, Teppen T, Gaszner GL, Roberts RC; et al. GSK-3b Gene Expression in Human Postmortem Brain: Regional Distribution, Effects of Age and Suicide. Neurochem Res. 2009, 34, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Karege, F.; Perroud, N.; Burkhardt, S.; Schwald, M.; Ballmann, E. Alteration in Kinase Activity But Not in Protein Levels of Protein Kinase B and Glycogen Synthase Kinase-3beta in Ventral Prefrontal Cortex of Depressed Suicide Victims. Biol Psychiatry. 2007, 61, 240–245. [Google Scholar] [CrossRef]
- Beurel, E.; Toups, M.; Nemeroff, C.B. The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron. 2020, 107, 234–256. [Google Scholar] [CrossRef]
- Yoon, H.K.; Kim, Y.K.; Lee, B.H.; Hwang, J.A. Association study of -1727 A/T and -50C/T GSK-3 beta gene polymorphism with suicidal behavior in depressed patients. European Neuropsychopharmacology. 2009, 19, S225–S226. [Google Scholar] [CrossRef]
- Karege F, Perroud N, Burkhardt S, Fernandez R, Ballmann E, La Harpe R; et al. Protein levels of B-catenin and activation of glycogen synthase kinase-3B in major depression. A study with postmortem prefontal cortex. Journal of Affective Disorders. 2012, 136, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Rizavi, H.S.; Khan, M.A.; Dwivedi, Y.; Pandey, G.N. Alterede Wnt signalling in the teenage suicide brain: Focus on glycogen synthase kinase-3B and B-catenin. International Journal of Neuropsychopharmacology. 2013, 16, 945–5. [Google Scholar] [CrossRef] [PubMed]
- Bernard, V.; Vaiva, G.; Masson, M.; Geoffroy, P.A. Lithium and suicide prevention in bipolar disorder. L'Encéphale. 2016, 42, 234–241. [Google Scholar] [CrossRef]
- Malhi GS, Das P, Outhred T, Irwin L, Morris G, Hamilton A; et al. Understanding suicide: Focusing on its mechanisms through a lithium lens. Journal of Affective Disorders. 2018, 241, 338–347. [Google Scholar] [CrossRef]
- Landa, J.I.; Duarte, J.M.; Appiani, F.J. Conductas suicidas en adolescentes: Factores de riesgo, fisiopatología y el uso de litio como terapia preventiva. Prev Méd Argent. 2021, 107, 397–405. [Google Scholar]
- Sarai, S.K.; Mekala, H.M.; Lippmann, S. Lithium Suicide Prevention: A Brief Review and Reminder. Innov Clin Neurosci. 2018, 15, 30–32. [Google Scholar]
- Can, A.; Schulze, T.G.; Gould, T.D. Molecular actions and clinical pharmacogenetics of lithium therapy. Pharmacol Biochem Behav. 2014, 123, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues JFR, Rodrigues LP, Rodrigues FCP, Rubatino FVM, Atalaia da Silva KC, Serna Rodriguez MF; et al. Tau and Depression: A Systematic Review. Preprints [Internet]. 2024. Available from: https://www.preprints.org/manuscript/202408.0925/v1.
- Rodrigues, J.F.R.; Rodrigues, L.P.; Araújo Filho, G.M. Alzheimer's Disease and Suicide: An Integrative Literature Review. Current Alzheimer Research. 2023, 20, 758–768. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





