Introduction
The emergence of effective therapeutic options for amyloid transthyretin cardiomyopathy (ATTR-CM) has contributed to increased awareness and recognition of the disease.[
1,
2] TTR-stabilizing therapy with tafamidis improves survival and quality of life, and reduces cardiovascular-related hospitalisations and functional decline.[
1] Yet, the availability of tafamidis varies, not least due to the high cost associated with this therapy.[
3] Recognition of disease prevalence[
4] and advanced cardiac diagnostics[
5] enable the detection of the disease at earlier stages with more favourable outcomes [
2], adding to the debate on the optimal timing of therapy initiation and the ideal candidacy for targeted ATTR-CM therapy.[
2]
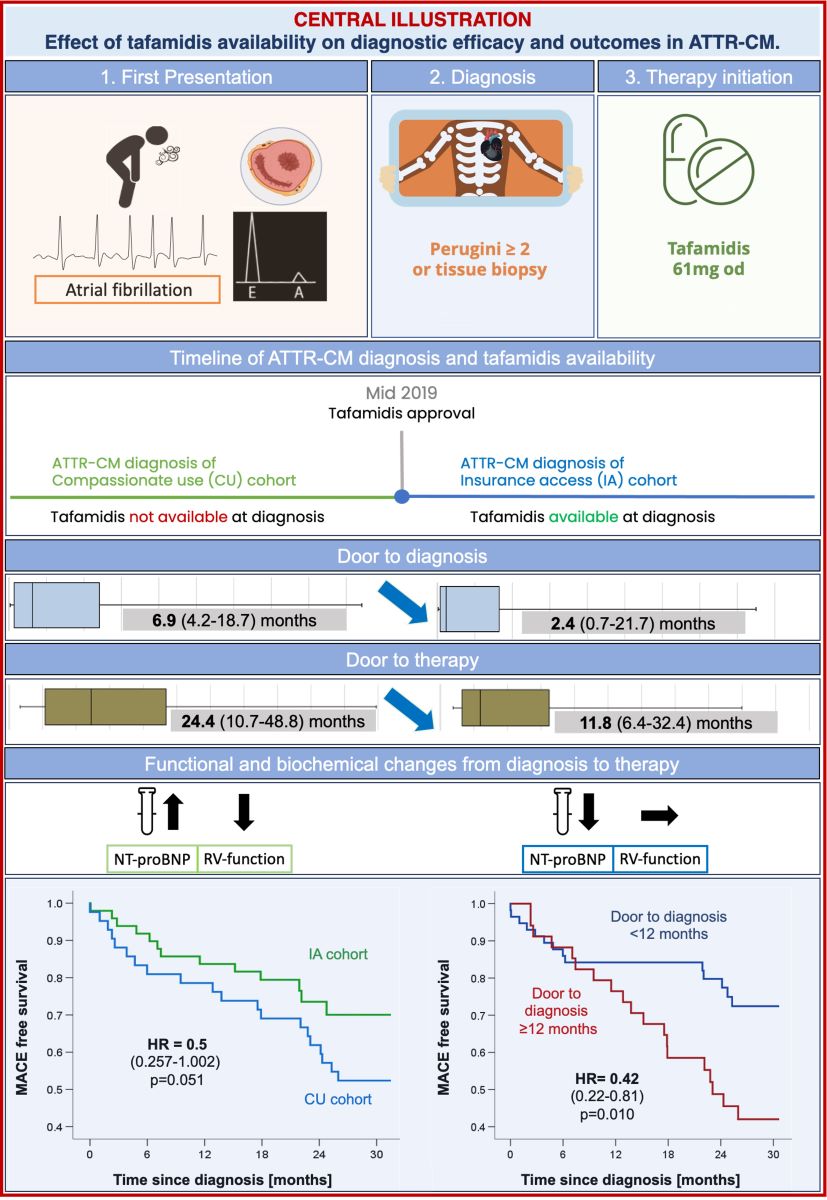
In 2019, prior to local regulatory drug approval in Europe, early tafamidis access for patients previously diagnosed with ATTR-CM was granted by the manufacturer through an expanded access program (compassionate use, CU). For patients diagnosed after regulatory drug approval in April 2020, tafamidis was more readily available, yet subject to insurance coverage of therapy costs (insurance access, IA).
The primary aim of the current study was to evaluate the effect of tafamidis availability and wider access to treatment on the diagnostic efficacy (time from first presentation to diagnosis) and therapy initiation (time from first presentation to therapy) in patients with ATTR-CM. Our secondary endpoint was to assess how these changes and delays in diagnosis and therapy influence cardiovascular outcomes in ATTR-CM patients.
Methods
Study Population
Consecutive patients referred to the Cardiac Amyloidosis Clinic at the Department of Cardiology, Bern University Hospital, Inselspital, Bern, Switzerland, and diagnosed with ATTR-CM between June 2019 and June 2021 were prospectively enrolled in the Bern Amyloidosis registry (B-CARE) (NCT04776824) upon written, informed consent. Baseline clinical and follow-up data were recorded using standardized, electronic case report forms and entered into a dedicated online database at Bern University Hospital. The study design was approved by the local ethics committee (KEK: 2021-00135) and conducted in accordance with the Declaration of Helsinki.
ATTR-CM Diagnosis
ATTR-CM was diagnosed non-invasively if bone scintigraphy detected moderate or severe myocardial
99mTc labelled 3,3-diphosphono-1,2-propanodicarboxylic acid (
99mTc-DPD) tracer uptake (Perugini≥2)[
6] after exclusion of light chain (AL) amyloidosis by a gammopathy panel consisting of serum gel electrophoresis, serum immunofixation and serum free light chain assay.[
7] If tissue biopsies confirmed TTR amyloid deposits, a biopsy-based diagnosis was made, with cardiac imaging [echocardiography or cardiac magnetic resonance imaging (CMR)] required to confirm cardiac involvement in patients with extracardiac amyloid deposits.
Time of First Presentation, Time of Diagnosis
Time of first presentation was assessed using electronic patient records and defined as first documentation of symptomatic heart failure (HF) and unexplained left ventricular (LV) wall thickness >12mm. In patients diagnosed non-invasively, time of diagnosis was defined as the date of the diagnostic bone scintigraphy or CMR. For patients undergoing biopsy, the date of diagnosis was the date of myocardial biopsy or the date of cardiac imaging (TTE or CMR) confirming cardiac involvement in patients with a TTR-positive extracardiac biopsy.
Criteria for TTR-Stabilizing Therapy with Tafamidis
Qualification for tafamidis therapy was based on the ATTR-ACT study[
1]. Patients presenting before 04/2021 were evaluated for treatment eligibility through the tafamidis expanded access program (Compassionate Use, CU) provided by the drug manufacturer. Patients evaluated after regulatory drug approval in 04/2021 were evaluated for tafamidis therapy and cost coverage through their health insurance (insurance access, IA). While the CU program extended to patients presenting with NYHA III at the time of therapy commencement, stricter prescription criteria applied after tafamidis regulatory drug approval 04/2021 for patients evaluated for IA (NYHA I-II at therapy initiation, prior heart failure hospitalization or requirement for diuretic, NT-proBNP >600pg/ml, GFR >25ml/min/1.73m2, 6-minute walk test >100m, life expectancy >2 years).
Follow-Up and Clinical Endpoints
Clinical follow-up data were obtained through standardized interviews during clinic visits, documentation from referring physicians, and hospital discharge summaries. Current mortality data was provided by the Swiss Federal Statistical Office. Adverse events were systematically collected and adjudicated by two independent board-certified cardiologists. The endpoints of the study were diagnostic efficacy (time from first presentation to diagnosis), time to therapy (time from first presentation to therapy) and major adverse cardiovascular events (MACE), which included all-cause mortality and heart failure hospitalisations (HFH).
Statistical Analysis
Statistical analysis was performed with IBM SPSS Statistics 25 (IBM Corp., Armon, New York, USA) and R software version 4.1.3 (R Foundation for Statistical Computing, Vienna, Austria). Baseline characteristics were presented as numeric frequencies (percentages), mean ± standard deviation or as median with the 25th and 75th Percentiles whenever appropriate. The study cohort was dichotomized into patients evaluated for therapy through the CU program before regulatory drug approval and those evaluated for therapy through IA after drug approval. Group characteristics were compared by Chi-square tests or Fisher’s exact tests for categorical variables, by unpaired t-tests for continuous normally-distributed variables and by Mann-Whitney U tests for highly skewed variables. Changes in numeric variables between the timepoint of diagnosis and therapy initiation were evaluated by paired t-tests. Cox proportional hazards regression analysis was conducted to investigate univariate associations with the combined endpoint of HFH and all-cause death. Time-to-event curves were plotted using the Kaplan-Meier method for 1) a composite endpoint of HFH and all-cause mortality, 2) HFH and 3) all-cause mortality, and the corresponding p-values for log-rank tests were provided. The cumulative incidence of the combined endpoint and its components was described by the cumulative annualized event rates and compared among groups by independent t-tests. Recurrent heart failure hospitalizations were visualized using a cumulative incidence function, with all-cause death considered as a competing event. A two-sided p-value was <0.05 was considered statistically significant.
Results
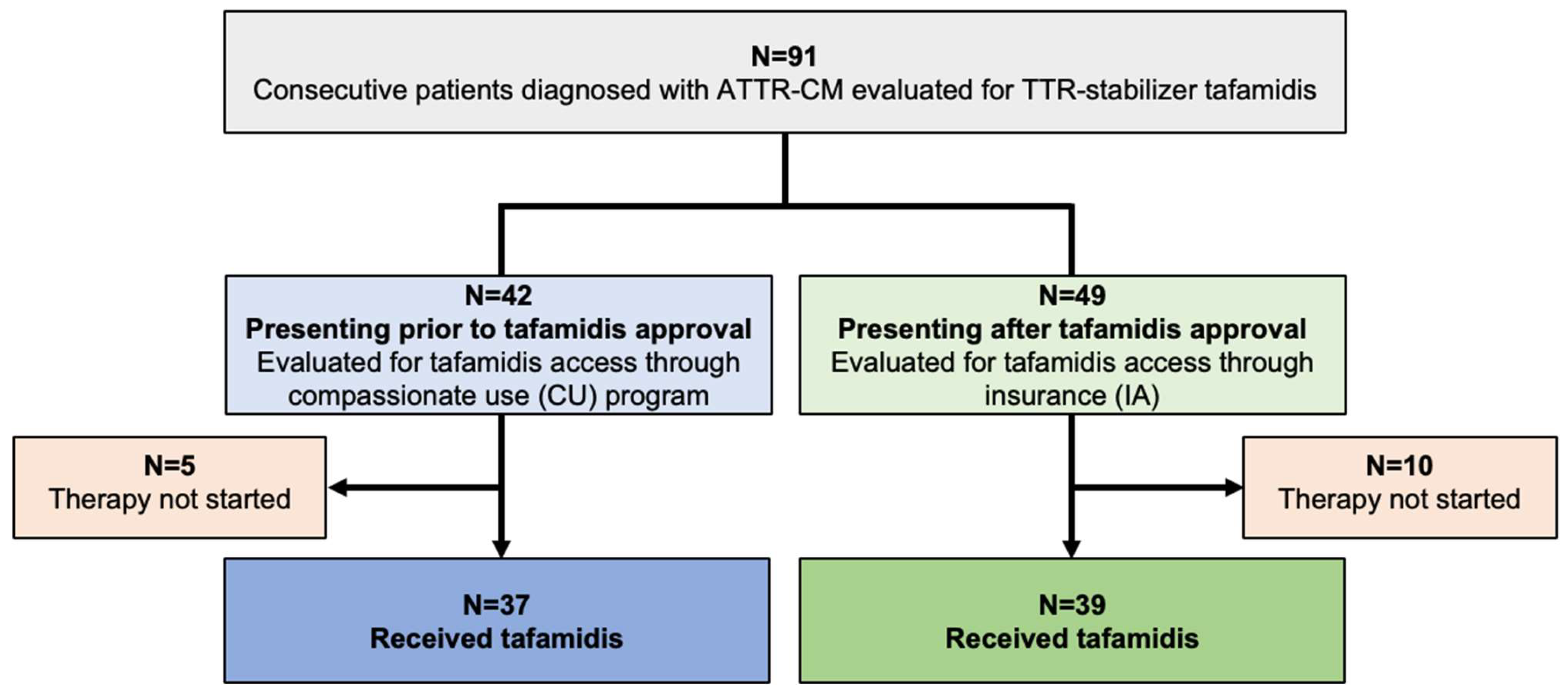
In total, 91 consecutive patients diagnosed with ATTR-CM were evaluated for TTR-stabilizing therapy with tafamidis (
Figure 1). 37 of 42 patients (88.1%) diagnosed prior to tafamidis approval fulfilled the criteria to commence therapy and tafamidis was thus provided through the drug manufacturer (CU cohort). Of the 49 patients presenting after tafamidis approval (IA cohort), 39 (79.6%) qualified for therapy, and were started on tafamidis after approval of insurance cost coverage was obtained.
Baseline Characteristics
Detailed baseline characteristics at the time of diagnosis for the CU and IA cohorts, respectively, are shown in
Table 1. Mean age of participants in the CU and IA groups was 76.3±6.4 and 77.3±6 years, respectively, with predominantly patients of male sex in both groups (95.2% and 93.9%, respectively, p>0.99). Other than a more prevalent history of atrial fibrillation (59.5% vs. 38.8%; p= 0.048) and carpal tunnel syndrome (31.0% vs. 12.2%; p=0.029) in the CU group, baseline characteristics and medical history were comparable between the groups. At diagnosis, both groups had similar levels of eGFR (59.4±18.2 vs. 57.2±34.5 ml/min) and NT-proBNP [median (IQR) 1806 (967-3104) vs 1678 (979-4226) pg/mL], and ATTR-CM disease stage was comparable between groups (p=0.92). A non-invasive scintigraphy-based diagnosis of ATTR-CM was made in 88.1% of patients in the CU-cohort, and this proportion increased to 98% in the IA-cohort, which presented after tafamidis approval, respectively. A similar proportion of patients in both groups presented with strong (89.2% vs. 89.6%) and moderate (10.8% vs. 10.4%)
99Tc-DPD-tracer uptake, also indicative of similar disease stages in the CU and IA cohort, respectively. Echocardiographic evaluation revealed comparable biventricular function in the low-normal range in both groups [53.87±11.57% (CU) vs. 52.0±10.83% (IA)], however, maximum LV wall thickness was significantly higher in the CU cohort (18.8±3.3 vs. 16.9±3.2mm; p=0.035).
Time to Diagnosis and to Therapy
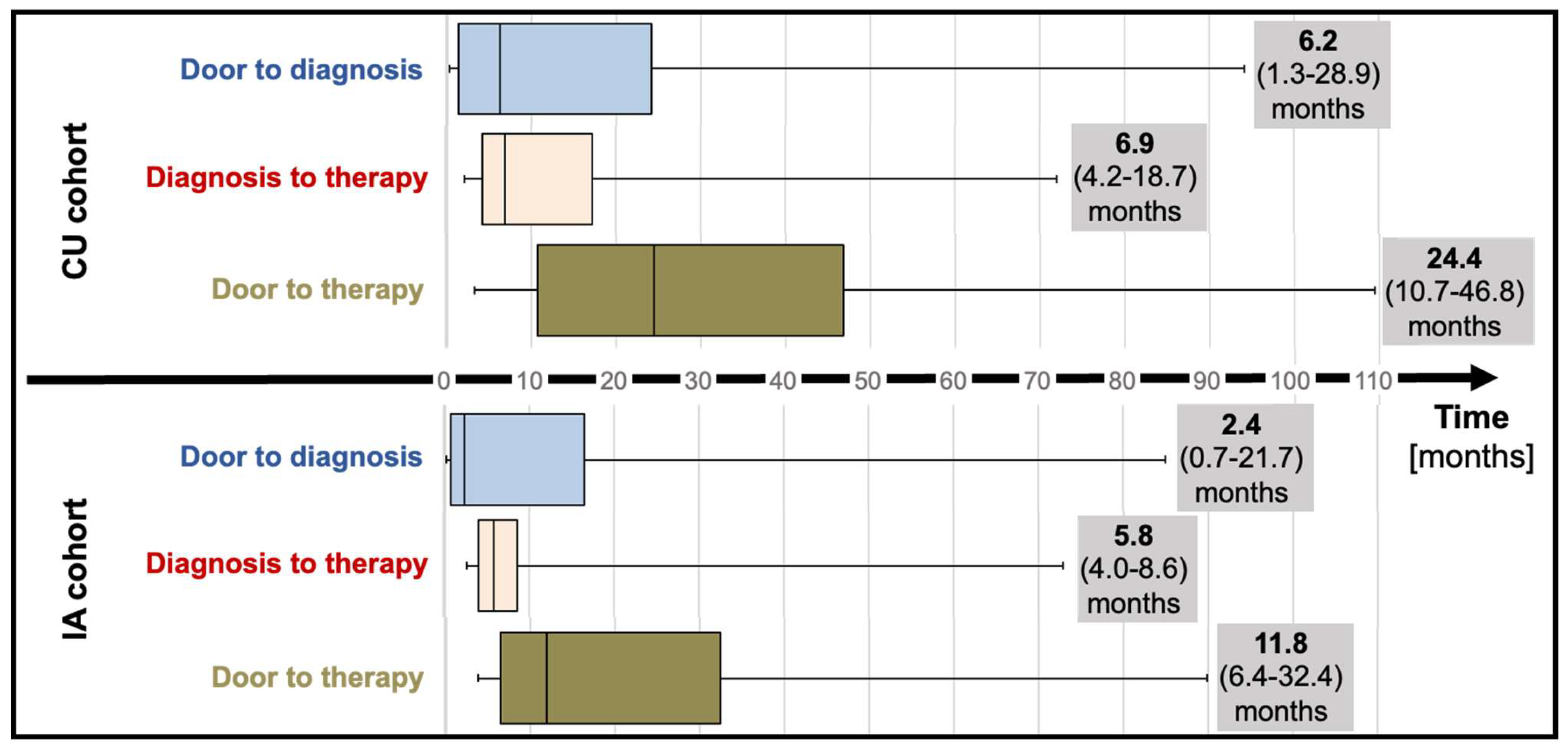
Median “door to diagnosis” (time from first presentation to diagnosis) time was 6.2 months (IQR; 1.3 to 28.9) for CU patients and numerically decreased to 2.4 months (IQR; 0.7 to 21.7, p=0.20) for the IA cohort (
Table 1,
Figure 2). After diagnosis, referral of patients to our reference center and administrative requirements to commence therapy (consultation at the reference center, electronic application for therapy for the CU cohort; letter to request insurance approval for the IA cohort) delayed therapy initiation by a median of 6.9 months (IQR; 4.2 to 18.7) and 5.8 months (IQR; 4.8 to 8.6) for CU and IA cohorts, respectively. Taken together, “door to therapy” time (time from first presentation to therapy initiation) added up to 24.4 months (IQR; 10.7 to 46.8) in CU patients. In IA patients, a reduced “door to therapy” of 11.8 months (IQR; 6.4 to 32.4, p=0.13) was observed.
Temporal Changes in Clinical ATTR-CM Disease Stage and Echocardiographic Characteristics from Time of Diagnosis to Therapy Initiation
Clinical, biochemical, and echocardiographic data at diagnosis and initiation of tafamidis therapy was available for 31 of 37 (83.8%) CU patients and 37 of 39 (94.9%) IA patients, respectively (
Table 2). ATTR-CM disease stage, cardiorenal biomarkers and NYHA functional class did not significantly change in either group. While structural and functional echocardiographic parameters remained stable in the IA cohort, RV function measured by RV S’-velocity (from 10.0±2.2 to 9.2±2.2; p=0.018) and tricuspid annular plane systolic excursion (TAPSE) (from 17.3±4.7 to 15.7±3.9; p=0.008) significantly decreased in CU patients (
Table 2).
Clinical Outcomes after ATTR-CM Diagnosis
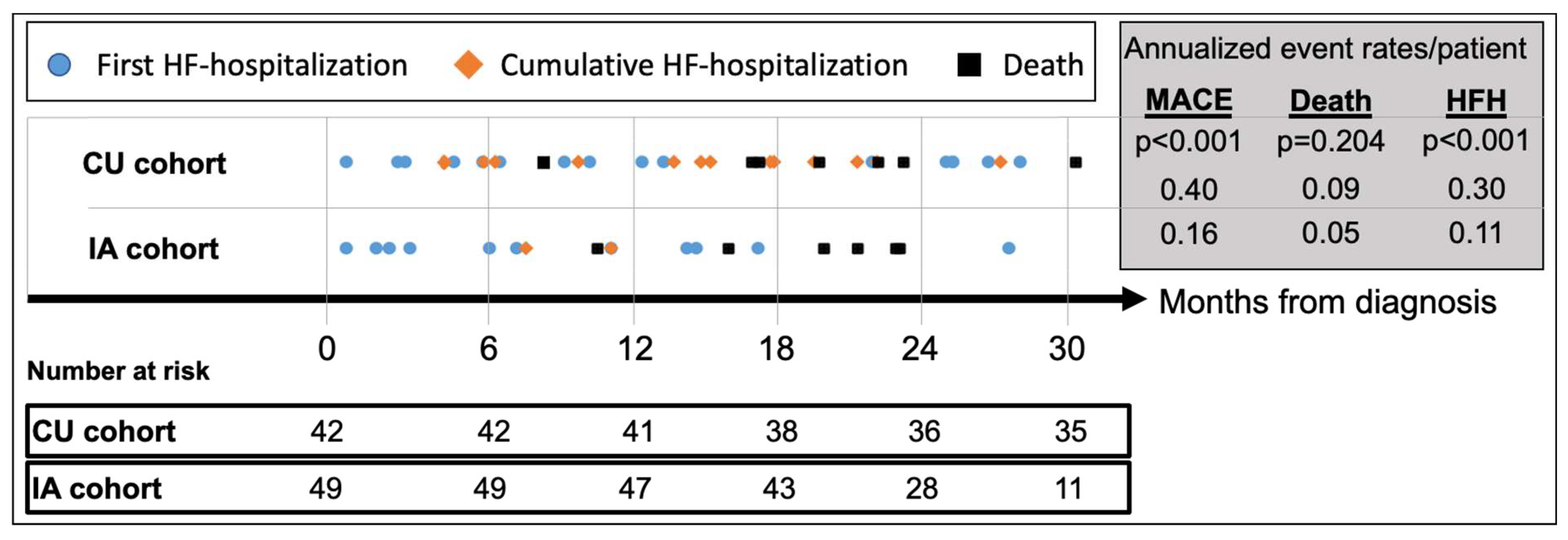
Median follow-up for the CU and IA patients was 42.3 (IQR 35.2-49.0) and 24.9 (IQR 20.1-29.8) months, respectively (
Table 3). MACE (HFH or death) occurred in 24 (57.1%) CU patients and 13 (26.5%) IA patients, respectively, translating to an annualized recurrent MACE rate of 0.40/patient (95%CI_0.30-0.51) in the CU cohort vs. 0.16/patient (95%CI_0.09-0.25) in the IA cohort (p<0.001) (
Figure 3). Compared to CU patients, annualized repeat HFH rates per patient were significantly lower in the IA cohort [CU: 0.30 (95%CI_0.23-0.41) vs. IA: 0.11 (95%CI_0.06-0.18); p<0.001] while annualized mortality rates per patient did not differ [CU: 0.09 (95%CI 0.05-0.15) vs. IA: 0.05 (95%CI 0.02-0.11); p=0.20].
After commencement of tafamidis therapy, the composite endpoint of all-cause mortality or HFH occurred in 17 (45.9%) CU patients and 6 (17.8%) IA patients, respectively, with an annualized recurrence rate of 0.38/patient (95%CI 0.28-0.52) in the CU cohort vs. 0.17/patient (95%CI 0.08-0.28) in the IA cohort (p=0.012) (
Table 3). Compared to CU patients, annualized repeat HFH rates per patient were significantly lower in the IA cohort [CU: 0.30 (95%CI 0.22-0.44) vs. IA: 0.12 (95%CI 0.05-0.23); p<0.011] while annualized mortality rates per patient again did not differ [CU: 0.09 (95%CI 0.04-0.17) vs. IA: 0.05 (95%CI 0.01-0.15); p=0.40].
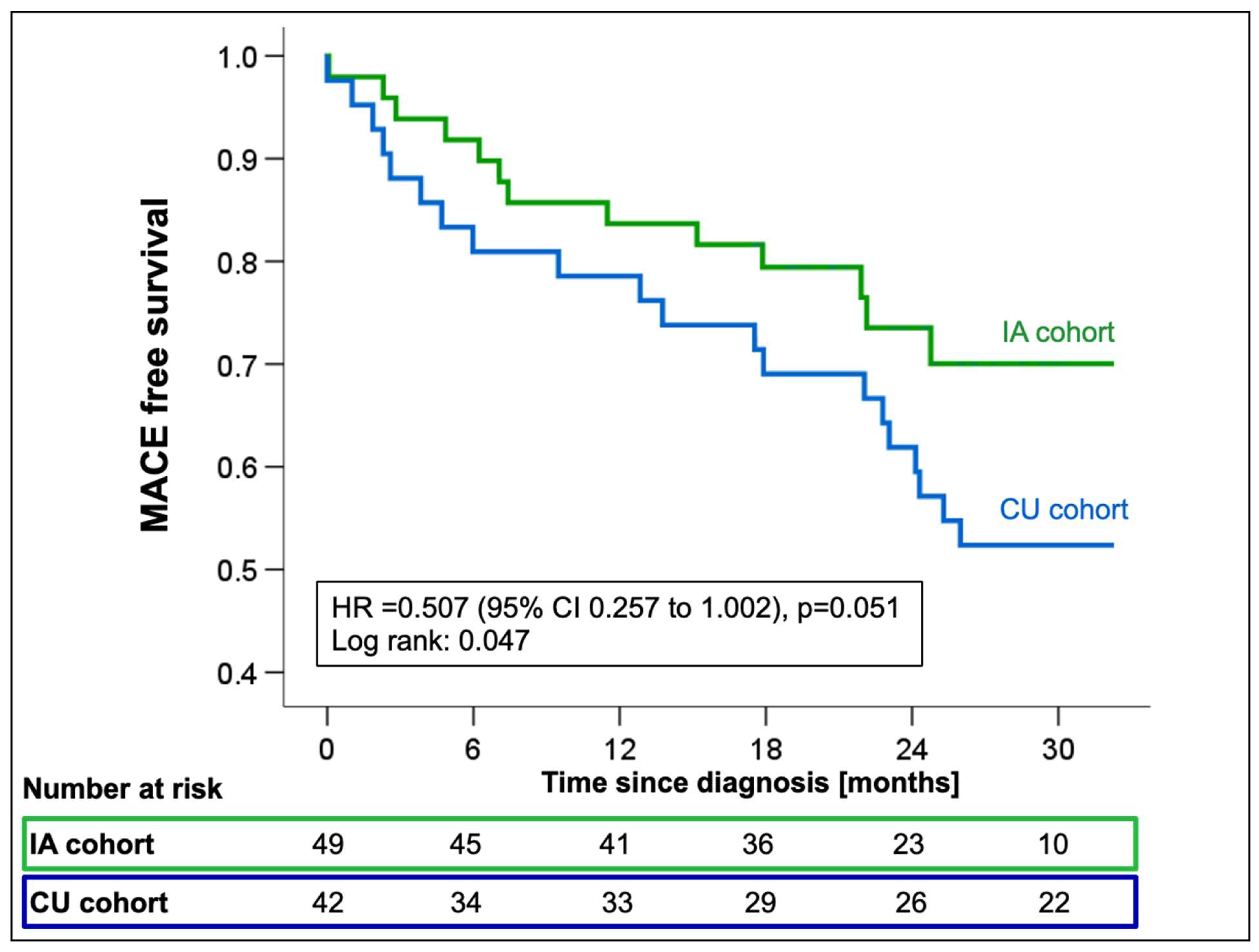
Cox-regression analysis showed a significant reduction in the incidence of all-cause mortality or first HFH in the IA cohort with a marginally non-significant hazard ratio (HR 0.51; 95%CI 0.26-1.00; p=0.051,
Figure 4) compared to CU patients. When looking at all-cause mortality and HFH individually, the risk reduction resulted mainly from a trend towards a reduction in HFH (HR 0.50; 95%CI 0.23-1.05; p=0.067), while mortality rates were comparable between groups (HR 0.58; 95%CI 0.22-1.55; p=0.28,
Supplemental Figure 1). The combined endpoint of first MACE was univariately associated with creatinine (HR 1.01; 95%CI 1.00-1.02; p=0.015), eGFR (HR 0.97; 95%CI 0.95-0.99; p=0.004), and log-transformed NT-proBNP (HR 5.64; 95%CI 2.1-15.2; p=0.001,
Supplemental Table 1) measured at the time of diagnosis.
Effect of Timely Diagnosis on Clinical Outcomes in ATTR-CM Patients
To test the predictive value of time-to-diagnosis on clinical outcomes in our cohort, we stratified patients by time from first presentation to diagnosis with a cut-off at 12 months (group 1: diagnosed<12 months vs. group 2: >12 months from presentation to diagnosis). Patients diagnosed within 12 months had significantly lower LV maximal wall thickness (16.8±2.1 vs. 18.8±3.7mm; p=0.014), lower LV mass (147.3±32.9 vs. 178.7±49.3g/m
2; p=0.003) and more preserved kidney function (eGFR: 63±17 vs. 55±17.6ml/min; p=0.033. creatinine: 104±29.6 vs. 117.3±35mmol/l; p=0.034) at the time of diagnosis (
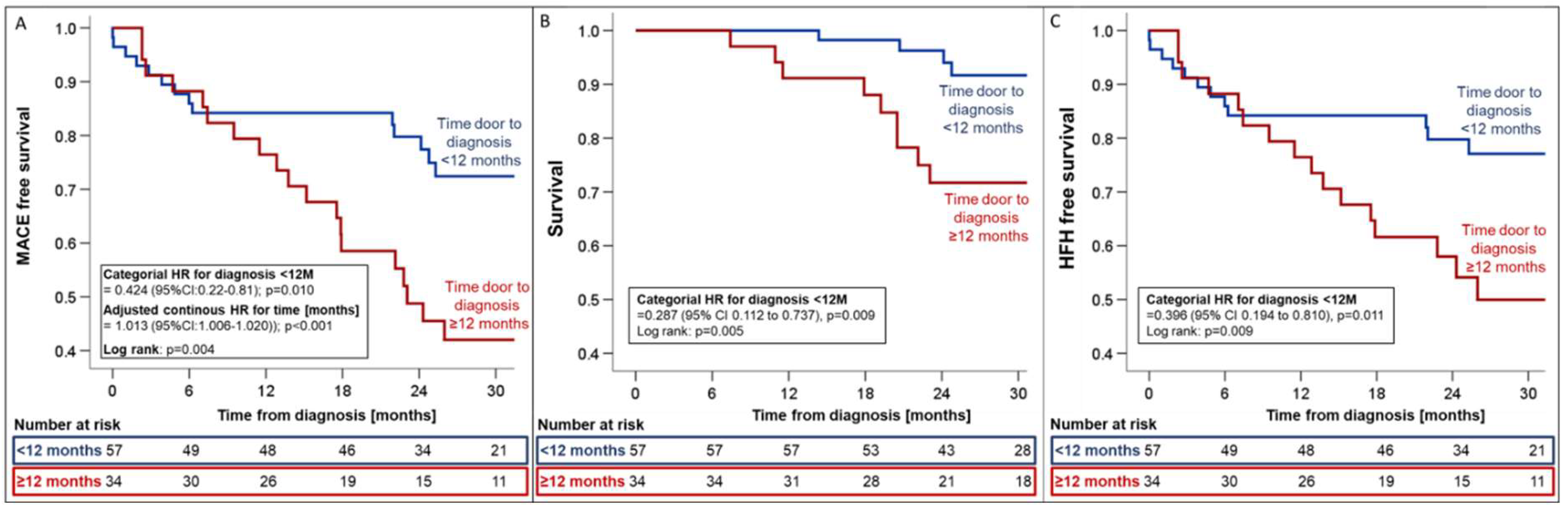
Table 4). Timely diagnosis within 12 months was associated with relative risk reductions of 57.4%, 71.3%, and 60.4% for MACE (HR 0.42; 95%CI_0.22-0.81, p=0.01,
Figure 5), all-cause mortality (HR 0.29; 95%CI_0.11-0.73; p=0.009,
Figure 5), and HFH (HR 0.40; 95%CI_0.19-0.81; p=0.011,
Figure 5), respectively. In a multivariate Cox-regression model log-transformed NT-proBNP (HR
adjusted 6.47; 95%CI_1.82-23.03; p=0.004) and delayed time to diagnosis (HR
adjusted 1.01; 95%CI_1.01-1.02; p=0.002) remained independent predictors of adverse outcomes with each additional month of delayed diagnosis increasing the relative risk of MACE by 14% (
Supplemental Table 1).
Effect of Atrial Fibrillation on Clinical Outcomes in ATTR-CM Patients
To evaluate the effect of atrial fibrillation on clinical outcomes in our cohort, we stratified patients by history of atrial fibrillation at the time of diagnosis. A history of atrial fibrillation significantly increased the risk for first MACE (HR 2.62; 95%CI:1.33-5.17, p=0.005,
Supplemental Figure 5 and 6), first HFH (HR 3.12; 95%CI:1.43-6.82, p=0.004,
Supplemental Figure 5) and cumulative HFHs (
Supplemental Figure 6). All-cause mortality (HR 1.61; 95%CI:0.64-4.04, p=0.86,
Supplemental Figure 5) was comparable irrespective of a history of atrial fibrillation.
Discussion
The availability of TTR-targeting therapeutics has changed the outlook for patients with ATTR-CM, significantly reducing heart failure hospitalisations and all-cause mortality, while preserving exercise capacity and quality of life[
1]. To gain a deeper understanding of the impact of tafamidis availability on clinical practice and patient outcomes, we compared time-to-diagnosis, time-to-therapy initiation and clinical outcomes of patients diagnosed prior to tafamidis market approval whose access to tafamidis was regulated by the manufacturers’ expanded access program (CU cohort) to patients diagnosed after market approval (IA cohort) with timely access by insurance coverage. Our results demonstrate that the increased availability of tafamidis has improved diagnostic and referral pathways, as reductions in both time-to-diagnosis and time-to-therapy were observed in the IA cohort. Increased disease awareness amongst physicians, familiarity with diagnostic modalities and therapeutic options all likely contributed to the reduction of median time-to-diagnosis from 6.2 (IQR; 1.3 to 28.9) in the CU to 2.4 months (IQR; 0.7 to 21.7, p=0.20) in the IA cohort. Together, the increased diagnostic efficacy and more timely referrals to our tertiary center for therapy evaluation reduced the time from-first-presentation-to-therapy initiation from 24.4 months (IQR 10.7-46.8) in CU patients to 11.8 months (IQR 6.4-32.4, p=0.13) in IA patients. While these reductions were not statistically significant due to large time variabilities potentially indicative of varying levels of disease awareness by treating physicians and the small sample size, the high efficacy of 18-month treatment with tafamidis with a number needed to treat (NNT) of 7.5 patients to prevent a HFH or death over 18 months as demonstrated in ATTR-ACT[
1], suggests that these median reductions of 3.8 (from-first presentation-to-diagnosis) and 12.6 months (from-first presentation-to-therapy) are clinically meaningful.
As seen in the observational long-term extension study of ATTR-ACT[
8], early access to tafamidis improved outcomes in our patients, primarily by reducing HFHs (
Figure 1). To elucidate potential mechanisms underlying improved outcomes with early adoption of tafamidis, we compared clinical, structural, and biochemical disease characteristics at diagnosis and therapy initiation, respectively. In patients diagnosed with ATTR-CM prior to market approval of tafamidis, structural and biochemical disease progression was observed as RV function declined and NT-proBNP levels increased. Both parameters, NT-proBNP as a component of the most widely used staging classification, and TAPSE as measure of RV function, have previously been identified as prognostic markers for mortality in ATTR-CM.[
9,
10] Stabilization of cardiac structural changes, thereby preserving RV function, and steadying NT-proBNP levels (
Table 2) were benefits seen in IA patients by early therapeutic intervention, and these may contribute to an amelioration of clinical disease progression.
Significantly improved clinical outcomes with a reduction both in HFH and all-cause mortality were observed when patients were stratified by time-to-diagnosis (
Figure 5). Notably, these improvements were consistent, irrespective of the availability of tafamidis at the time of diagnosis. As for NT-proBNP (HR
adjusted 6.47; 95%CI_1.82-23.03; p=0.004), uni- and multivariate Cox-regression analyses confirmed delayed time-to-diagnosis (HR
adjusted =1.01; 95%CI_1.01-1.02; p=0.002) to be an independent predictor of MACE (
Supplemental Table S1). These findings are likely attributable to a multitude of factors, including diagnosis at an earlier disease stage with less advanced structural disease and a lower prevalence of atrial fibrillation when event rates are likely lower[
2], timely access to tafamidis, but also optimization of supportive medical[
11,
12,
13], interventional[
14,
15], and device therapies[
16]. While randomized evidence for heart failure therapy in ATTR-CM remains elusive, the adverse effects of beta-blockade, particularly in more advanced disease, when chronotropic incompetence limits cardiac output in patients with a fixed stroke volume, have repeatedly been described[
17]. Thus, in line with expert opinion[
18], discontinuation of beta-blockers is typically recommended for our patients at the time of ATTR-CM diagnosis. Likewise, without evidence of a beneficial effect of neurohormonal blockers even in patients with reduced ejection fraction[
12], cessation of these drugs may help to prevent adverse events, e.g., from orthostatic hypotension or progressive kidney dysfunction caused by lower than required blood pressure targets. In line with the observation of an increased risk for MACE and HFH in ATTR-CM patients with a history of atrial fibrillation at diagnosis, we hypothesize that an aggressive and early pursuit of sinus rhythm in patients developing atrial fibrillation is yet another likely contributor to improved outcomes after the diagnosis of ATTR-CM[
19], as is the choice of a physiologic pacing modality (CRT or LBBAP)[
16], to prevent disease deteriorations more likely to be seen in ATTR-CM with high RV-only pacing burden.
Increased awareness and diagnosis at earlier ATTR-CM disease stages have called the optimal timing of therapy initiation into question[
2], particularly in light of the high economic burden of costly TTR-targeting therapies[
3]. Yet, ATTR-CM remains a progressive disease, without reliable
methods to monitor disease progression and allow for timely commencement of therapy. With promising new therapies that may allow reversal of amyloid deposition on the horizon[
20] but not yet realized, our data suggest that with delays from first presentation to diagnosis still common, early therapeutic intervention should be sought to prevent adverse clinical outcomes in ATTR-CM patients.
Limitations
The current investigation was a retrospective, unblinded, observational study and subject to multiple biases (e.g., disease awareness by physicians, referral bias, selection bias), which change over time. The study was conducted at a single tertiary reference center in Switzerland. After market approval, tafamidis became widely available to Swiss patients within 6 months. As the rate of implementation and mode of tafamidis prescription vary regionally, the improvements in diagnostic efficacy observed in our cohort may not be generalizable. The study cohort was overwhelmingly male, and suffering from wt-ATTR-CM, reflecting current screening recommendations for ATTR-CM. Extrapolation of study findings to female patients with ATTR-CM or those suffering from h-ATTR-CM may therefore not be warranted. Lastly, as enrollment into the compassionate use program was limited to 9 months, the study’s sample size was limited, increasing the likelihood of random error and chance findings.
Conclusions
Increased availability and access to tafamidis improved diagnostic efficacy for symptomatic wt-ATTR-CM patients, shortening time-to-diagnosis and time-to-therapy initiation. Timely diagnosis and early commencement of therapy were associated with a reduction of adverse cardiovascular events, providing an opportunity for treating physicians to improve patient outcomes.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Funding
Dr. Dobner received a research grant for the Bern amyloidosis registry (B-CARE) (NCT04776824) on behalf of the institution (Inselspital Bern) from Pfizer and reports speaker fees and travel grants from Boehringer Ingelheim, Alnylam and Pfizer outside of the submitted work. Dr. Gräni received a research grant for the Bern amyloidosis registry (B-CARE) (NCT04776824) on behalf of the institution (Inselspital Bern) from AstraZeneca and the GAMBIT foundation.
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
All other authors report no conflicts.
Disclosures
Dr. Dobner received a research grant for the Bern amyloidosis registry (B-CARE) (NCT04776824) on behalf of the institution (Inselspital Bern) from Pfizer. Dr. Bakula received a travel grant from Pfizer. Dr. Stortecky reports research grants to the institution from Edwards Lifesciences, Medtronic, Boston Scientific and Abbott, as well as personal fees from Boston Scientific, Teleflex and BTG. Dr. Gräni received research funding from the GAMBIT Foundation, the Swiss National Science Foundation, InnoSuisse, Center for Artificial Intelligence in Medicine University Bern, Novartis Foundation for Medical-Biological Research, and the Swiss Heart Foundation, outside of the submitted work. Dr. Bernhard reports a career development grant from the Swiss National Science Foundation.
Abbreviations
ATTR-CM Cardiac Transthyretin Amyloid Cardiomyopathy
GFR Glomerular Filtration Rate
HFH Heart failure hospitalization
HR Hazard ratio
LVEF Left Ventricular Ejection Fraction
MACE major adverse cardiovascular events
NT-proBNP N-terminal pro hormone of Brain Natriuretic Peptide
NYHA New York Heart Association functional class
TAPSE Tricuspid annular plane systolic excursion
TTR Transthyretin
99mTc-DPD Technetium99-3,3-diphosphono-1,2-propanodicarboxylic acid
References
- Maurer MS, Schwartz JH, Gundapaneni B, et al. Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy. N Engl J Med. Sep 13 2018;379(11):1007-1016. [CrossRef]
- Ioannou A, Patel RK, Razvi Y, et al. Impact of Earlier Diagnosis in Cardiac ATTR Amyloidosis Over the Course of 20 Years. Circulation. Nov 29 2022;146(22):1657-1670. [CrossRef]
- Kazi DS, Bellows BK, Baron SJ, et al. Cost-Effectiveness of Tafamidis Therapy for Transthyretin Amyloid Cardiomyopathy. Circulation. Apr 14 2020;141(15):1214-1224. [CrossRef]
- Lane T, Fontana M, Martinez-Naharro A, et al. Natural History, Quality of Life, and Outcome in Cardiac Transthyretin Amyloidosis. Circulation. Jul 2 2019;140(1):16-26. [CrossRef]
- Perugini E, Guidalotti PL, Salvi F, et al. Noninvasive etiologic diagnosis of cardiac amyloidosis using 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy. J Am Coll Cardiol. Sep 20 2005;46(6):1076-84. [CrossRef]
- Maurer MS, Bokhari S, Damy T, et al. Expert Consensus Recommendations for the Suspicion and Diagnosis of Transthyretin Cardiac Amyloidosis. Circ Heart Fail. Sep 2019;12(9):e006075. [CrossRef]
- Witteles RM, Liedtke M. Avoiding Catastrophe: Understanding Free Light Chain Testing in the Evaluation of ATTR Amyloidosis. Circ Heart Fail. Apr 2021;14(4):e008225. [CrossRef]
- Elliott P, Drachman BM, Gottlieb SS, et al. Long-Term Survival With Tafamidis in Patients With Transthyretin Amyloid Cardiomyopathy. Circ Heart Fail. Jan 2022;15(1):e008193. [CrossRef]
- Gillmore JD, Damy T, Fontana M, et al. A new staging system for cardiac transthyretin amyloidosis. Eur Heart J. Aug 7 2018;39(30):2799-2806. [CrossRef]
- Knight DS, Zumbo G, Barcella W, et al. Cardiac Structural and Functional Consequences of Amyloid Deposition by Cardiac Magnetic Resonance and Echocardiography and Their Prognostic Roles. JACC Cardiovasc Imaging. May 2019;12(5):823-833. [CrossRef]
- Dobner S, Bernhard B, Asatryan B, et al. SGLT2 inhibitor therapy for transthyretin amyloid cardiomyopathy: early tolerance and clinical response to dapagliflozin. ESC Heart Fail. Feb 2023;10(1):397-404. [CrossRef]
- Ioannou A, Massa P, Patel RK, et al. Conventional heart failure therapy in cardiac ATTR amyloidosis. Eur Heart J. May 22 2023. [CrossRef]
- Sperry BW, Hanna M, Shah SJ, Jaber WA, Spertus JA. Spironolactone in Patients With an Echocardiographic HFpEF Phenotype Suggestive of Cardiac Amyloidosis: Results From TOPCAT. JACC Heart Fail. Nov 2021;9(11):795-802. [CrossRef]
- Nitsche C, Scully PR, Patel KP, et al. Prevalence and Outcomes of Concomitant Aortic Stenosis and Cardiac Amyloidosis. J Am Coll Cardiol. Jan 19 2021;77(2):128-139. [CrossRef]
- Scully PR, Patel KP, Treibel TA, et al. Prevalence and outcome of dual aortic stenosis and cardiac amyloid pathology in patients referred for transcatheter aortic valve implantation. Eur Heart J. Aug 1 2020;41(29):2759-2767. [CrossRef]
- Donnellan E, Wazni OM, Saliba WI, et al. Cardiac devices in patients with transthyretin amyloidosis: Impact on functional class, left ventricular function, mitral regurgitation, and mortality. J Cardiovasc Electrophysiol. Nov 2019;30(11):2427-2432. [CrossRef]
- Cheng RK, Vasbinder A, Levy WC, et al. Lack of Association Between Neurohormonal Blockade and Survival in Transthyretin Cardiac Amyloidosis. J Am Heart Assoc. Dec 21 2021;10(24):e022859. [CrossRef]
- Brito D, Albrecht FC, de Arenaza DP, et al. World Heart Federation Consensus on Transthyretin Amyloidosis Cardiomyopathy (ATTR-CM). Glob Heart. 2023;18(1):59. [CrossRef]
- Donnellan E, Wazni OM, Hanna M, et al. Atrial Fibrillation in Transthyretin Cardiac Amyloidosis: Predictors, Prevalence, and Efficacy of Rhythm Control Strategies. JACC Clin Electrophysiol. Sep 2020;6(9):1118-1127. [CrossRef]
- Garcia-Pavia P, Aus dem Siepen F, Donal E, et al. Phase 1 Trial of Antibody NI006 for Depletion of Cardiac Transthyretin Amyloid. N Engl J Med. Jul 20 2023;389(3):239-250. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).