Submitted:
18 August 2024
Posted:
21 August 2024
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
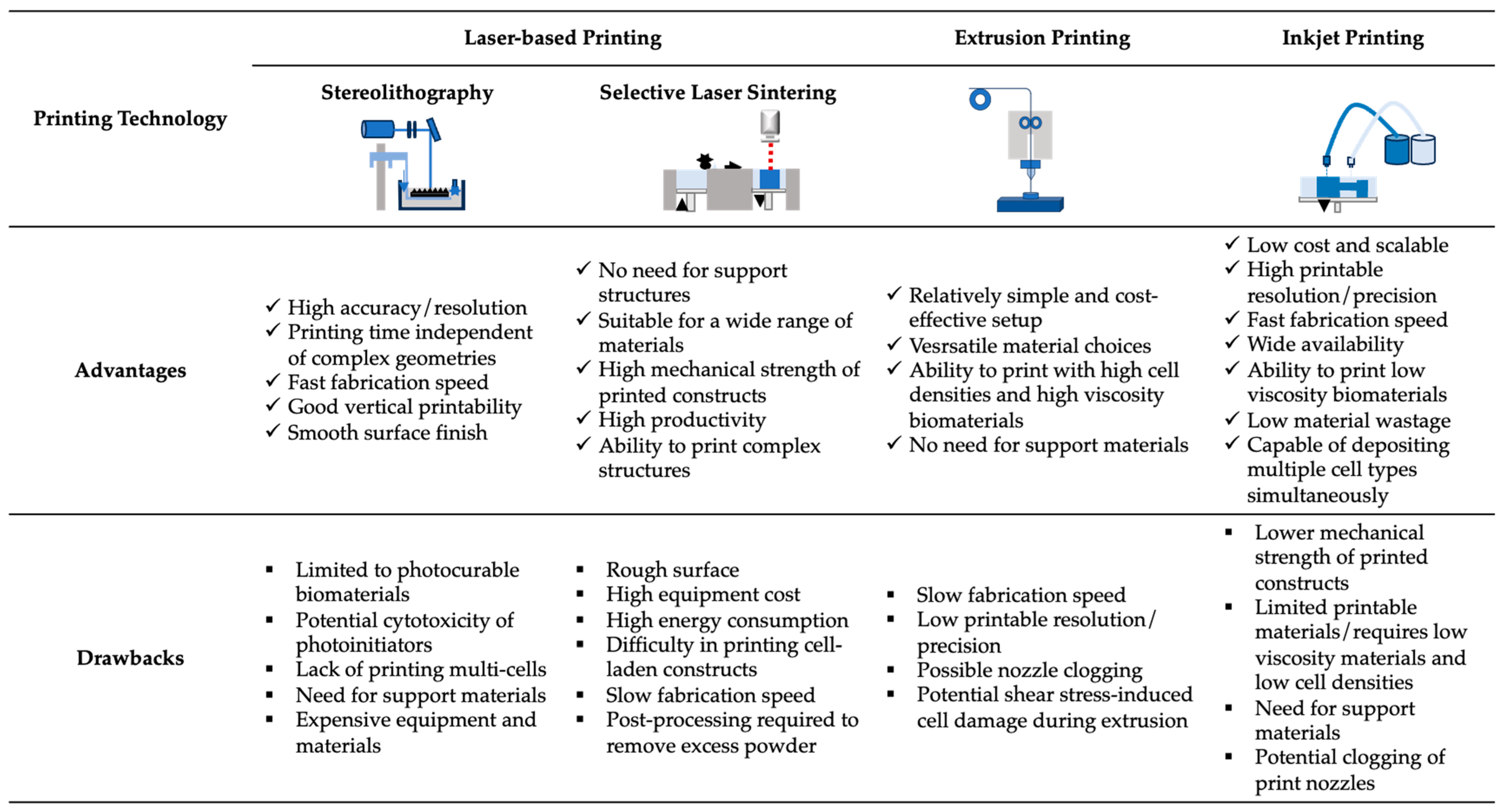
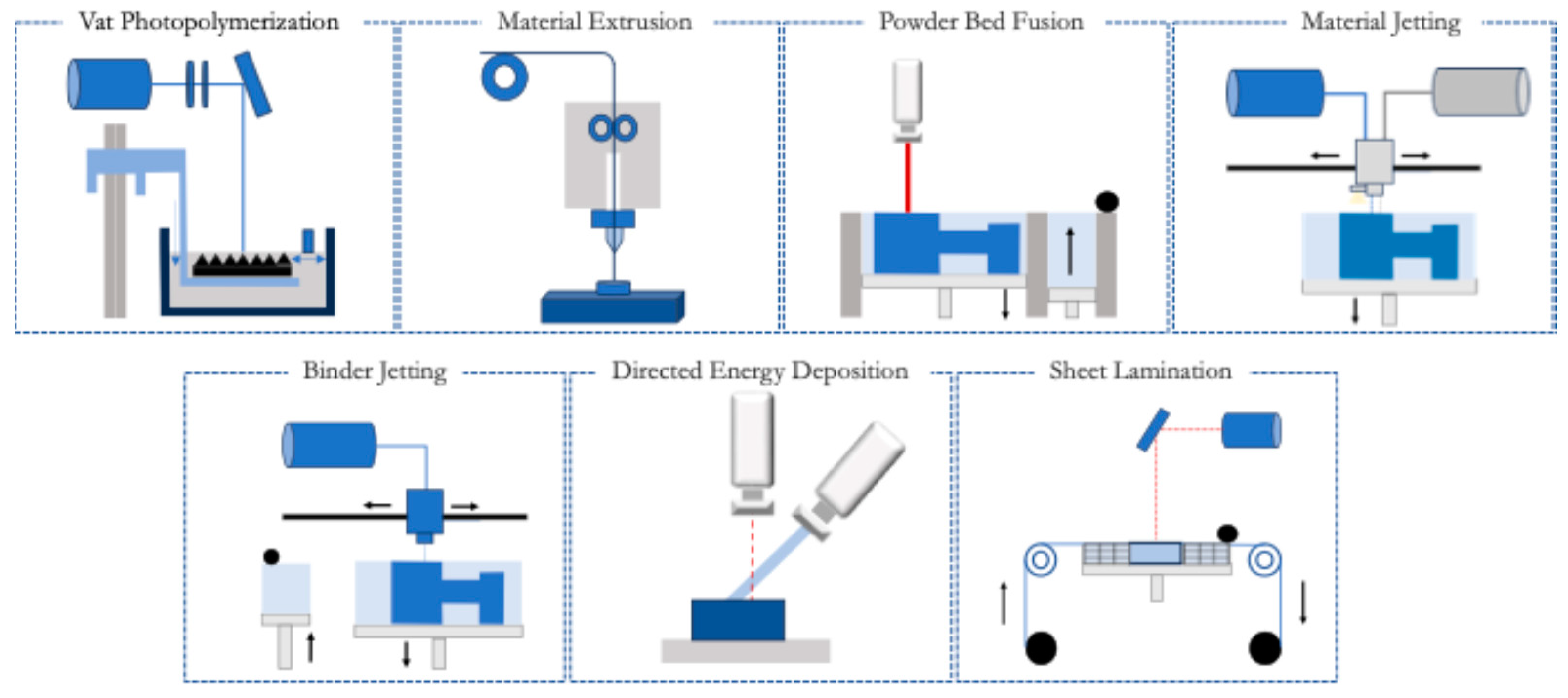
2. Three-Dimensional Printing
2.1. Vat Photopolymerization
2.2. Material Extrusion
2.3. Powder Bed Fusion
2.4. Material Jetting
2.5. Binder Jetting
2.6. Directed Energy Deposition
2.7. Sheet Lamination
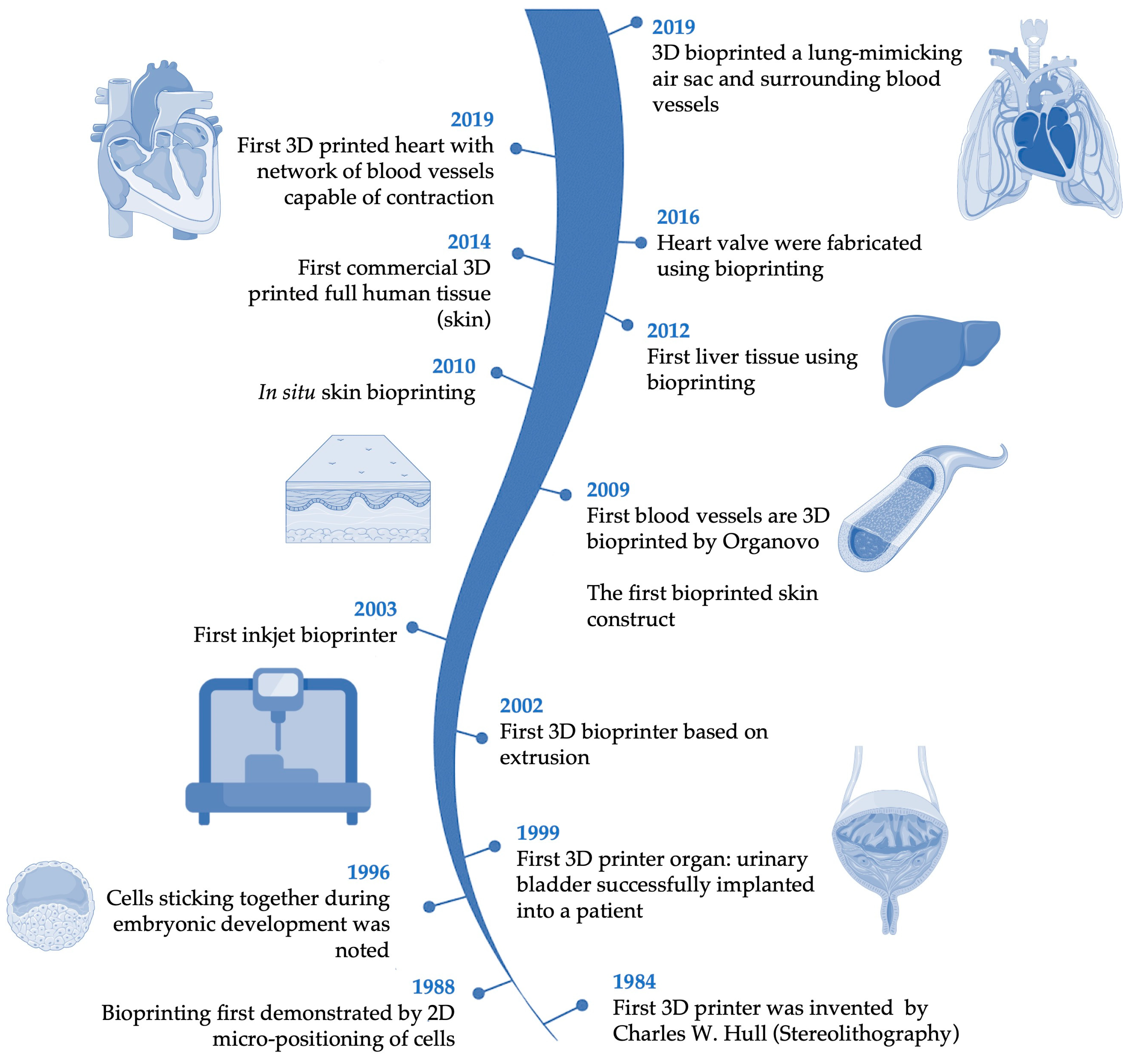
3. Bioprinting
3.1. Techniques
3.2. Materials Used in 3D Bioprinting
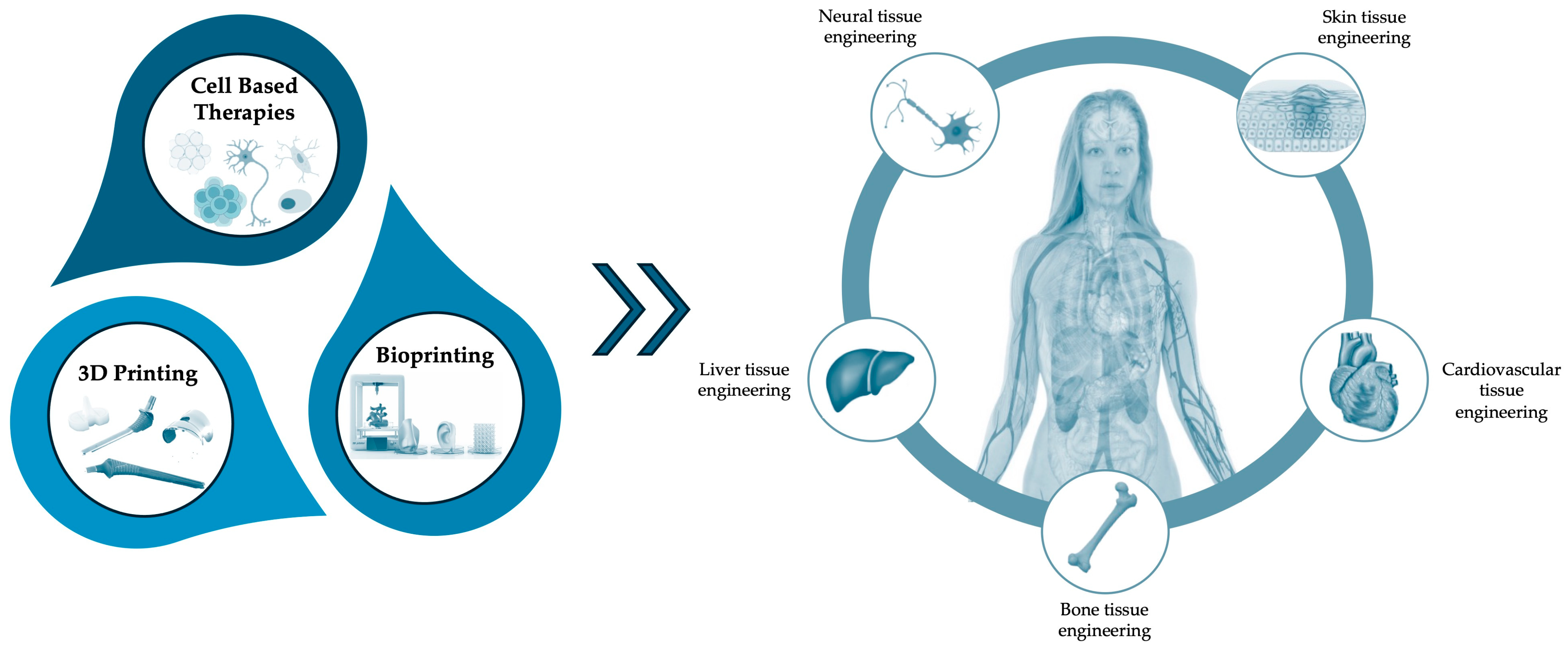
4. Cellular Therapies
4.1. Stem Cell-Based Therapies
4.2. Non-Stem Cell-Based Therapies
4.3. Multicellular Therapies
5. Advancements in 3D Printed/ Bioprinting and Cellular Therapies for Regenerative Medicine
5.1. Cardiovascular Tissue Engineering
5.2. Bone Tissue Engineering
5.3. Liver Tissue Engineering
5.4. Skin Tissue Engineering
5.5. Neural Tissue Engineering
6. Limitations and Challenges
7. Current State and Future Outlook
8. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2D | Two-dimensional |
| 132ACG | Bioink with alginate (1%), cellulose nanocrystal (3%), and gelatin methacryloyl (5%) |
| 2PP | Two-photon polymerization |
| 3D | Three-dimensional |
| 3DP-HOs | Three-dimensional bioprinted hepatorganoids |
| 4D | Four-dimensional |
| ACP | Amorphous calcium phosphate |
| AD | Additive manufacturing |
| ADM | Acellular dermal matrix |
| aHSC | Primary fetal activated hepatic stellate cells |
| ALP | Alkaline phosphatase |
| AMX | Amoxicillin |
| ASTM | American Society for Testing and Materials |
| BFGF | Basic fibroblast growth factor |
| BJ | Binder jetting |
| BL | Bi-layer |
| BMP-2 | Bone morphogenetic protein 2 |
| BMSCs | bone marrow-derived mesenchymal stem/stromal cells |
| C17.2 | Murine neural stem cells |
| Ca | Calcium |
| CAD | Computer-aided design |
| CBER | Center for Biologics Evaluation and Research |
| cECM | decellularized cardiac extracellular |
| CFs | Human cardiac fibroblasts |
| CLIP | Continuous light interface production |
| CNC | Cellulose nanocrystal |
| CSMA | Chondroitin sulfate methacrylate |
| dECM | Decellularized extracellular matrix |
| DEP | Directed energy deposition |
| Dex | Dextran |
| DFs | Dermal fibroblasts |
| DLP | Digital light processing |
| DMLS | Direct metal laser sintering |
| DO | Diamond |
| DPSCs | Dental Pulp stem/stromal cells |
| EBM | Electron beam melting |
| ECM | Extracellular matrix |
| EMA | European medicines agency |
| EPCs | Endothelial progenitor cells |
| ESCs | Embryonic stem cells |
| ESCs | Epidermal stem cells |
| EVCs | Early vascular cells |
| FDA | Food and Drug Administration |
| FDM | Fused deposition modeling |
| Fe | Iron |
| FFF | Fused filament fabrication |
| GAM | Matrix hydrogel with 2.8% of gellan gum, 1.6% of alginate, and 2.8% of methyl cellulose |
| Gel | Gelatin |
| GelMA | Gelatin methacrylate |
| H9c2 | Cardiomyocytes |
| HA | Hyaluronic acid |
| HAGM | Hyaluronic acid-gelatin methacrylate |
| HAp | Hydroxyapatite |
| Hap/β-TCP | Biphasic calcium phosphate system |
| hCAECs | Human coronary artery endothelial cells |
| HCC | Hepatocellular carcinoma |
| hCMPCs | Human cardiac-derived cardiomyocyte progenitor cells |
| hCPCs | Human cardiac progenitor cells |
| hdECM | Heart tissue-derived extracellular matrix |
| hDFs | Human dermal fibroblasts |
| hECM | Human extracellular matrix |
| hECs | Human endothelial cells |
| hepG2 | Human hepatocellular carcinoma |
| hESCs | Human embryonic stem cells |
| hiHep | Human-induced hepatocyte |
| hiPSCs | Human induced pluripotent stem cells |
| hKCs | Human keratinocytes |
| hLFs | Human lung fibroblasts |
| hMVECs | Human microvascular endothelial cells |
| hnDFs | Human neonatal dermal fibroblasts |
| hPCs | Human placental pericytes |
| hPSCs | Human pluripotent stem cells |
| hSFs | Human skin fibroblasts |
| hUCMSCs | Human umbilical cord mesenchymal stem cells |
| HUH7 | Undifferentiated hepatocyte cell line |
| hUVECs | Human umbilical vein/vascular endothelial cells |
| iCMs | Induced pluripotent stem cell-derived cardiomyocytes |
| IFN-γ | Interferon-gamma |
| iPSC-CMs | Induced pluripotent stem cell-derived cardiomyocytes |
| iPSCs | Induced pluripotent stem cells |
| ISO | International Standard Organization |
| Kr | Keratin |
| L x 2 | Human hepatic stellate cell line |
| LAP | Lithium phenyl-2,4,6-trimethylbenzoylphosphinate |
| LOM | Laminated object manufacturing |
| mEFs | Mouse embryonic fibroblasts |
| MFDS | Ministry of Food and Drug Safety |
| Mg | Magnesium |
| MJ | Material jetting |
| Mn | Manganese |
| MSCs | Mesenchymal stem/stromal cells |
| MUVECs | Murine umbilical vein endothelial cells |
| n/a | Not applicable |
| Nb | Niobium |
| NB | N-(2-aminoethyl)-4-(4-(hydroxymethyl)-2-methoxy-5-nitrosophenoxy) butanamide |
| NPCs | Neural progenitor cells |
| NRCMs | Neonatal rat cardiomyocytes |
| NSCs | Neural stem cells |
| NSPCs | Neural stem/progenitor cells |
| OMT | Oxymatrine |
| PBF | Powder bed fusion |
| pc-12 | Rat adrenal medullary pheochromocytoma |
| PCL | Poly (ℇ-caprolactone) |
| PecMA | Pectin methacrylate |
| PEDOT | Poly(3,4-ethylenedioxythiophene) |
| PEEK | Polyether ether ketone |
| PEG | Polyethylene glycol |
| PEG4A | 4-arm polyethylene glycol acrylate |
| PEGDA | Diacrylate poly (ethylene glycol) |
| PEGDMA | Poly (ethylene glycol) dimethacrylate |
| PEGMA | Poly (ethylene glycol) methacrylate |
| PF | PEG-Fibrinogen |
| PF | Poly (ethylene glycol)-fibrinogen |
| PGA | Poly (glycolic acid) |
| PGS | Poly (glycerol sebacate) |
| phDFs | Primary human dermal fibroblasts |
| PLA | Poly(l-lactic) acid |
| PLGA | Poly (lactic-co-glycolic) acid |
| PMDA | Pharmaceuticals and Medical Devices Agency |
| PMHs | Primary mouse hepatocytes |
| PrHCs | Primary rat hepatocytes cells |
| PRP | Platelet-rich plasma |
| PSC | Phosphosilicate calcium bioglass |
| PSCs | Phosphosilicate calcium bioglasses |
| PU | Polyurethane |
| PVP | Polyvinylpyrrolidone |
| r-BMSCs | Rat bone marrow mesenchymal stem cells |
| RD | Rhombic dodecahedron |
| rGO | Reduced graphene oxide |
| rhEGF | External human epidermal growth factor |
| SCAPs | Stromal cells from apical papilla |
| SF | Silk fibroin |
| SilMA | Methacrylated silk fibroin |
| SLA | Stereolithography |
| SLM | Selective laser melting |
| SLS | Selective laser sintering |
| Sr-CSH | Xonotlite |
| SR2+ | Strontium |
| SS | Strontium silicate |
| Ta | Tantalum |
| Ti | Titanium |
| Ti6AI4V | Titanium alloy |
| UAM | Ultrasonic additive manufacturing |
| UV | Ultraviolet |
| VEGF | Vascular endothelial |
| XG | Xanthan gum |
| Zn2+ | Zinc |
| Zr | Zirconium |
| β-TCP | Beta-tricalcium phosphate |
References
- Jessop, Z.M.; Al-Sabah, A.; Francis, W.R.; Whitaker, I.S. Transforming healthcare through regenerative medicine. BMC Medicine 2016, 14, 115. [CrossRef]
- Damaser, M.S.; Sievert, K.D. Tissue engineering and regenerative medicine: bench to bedside in urology. Preface. Adv Drug Deliv Rev 2015, 82-83, v-vii. [CrossRef]
- Jacques, E.; Suuronen, E.J. The Progression of Regenerative Medicine and its Impact on Therapy Translation. Clinical and Translational Science 2020, 13, 440-450. [CrossRef]
- Edwards, J.; Thomas, R.; Guilliatt, R. Regenerative medicine: from the laboratory looking out. Palgrave Communications 2017, 3, 27. [CrossRef]
- Pathak, K.; Saikia, R.; Das, A.; Das, D.; Islam, M.A.; Pramanik, P.; Parasar, A.; Borthakur, P.P.; Sarmah, P.; Saikia, M.; et al. 3D printing in biomedicine: advancing personalized care through additive manufacturing. Exploration of Medicine 2023, 4, 1135-1167. [CrossRef]
- Huang, G.; Zhao, Y.; Chen, D.; Hu, Z.; Li, J.; Zhou, X.; Yang, B.; Chen, Z. Applications, advancements, and challenges of 3D bioprinting in organ transplantation. Biomaterials science 2024, 12. [CrossRef]
- Bakhtiar, S.M.; Butt, H.A.; Zeb, S.; Quddusi, D.M.; Gul, S.; Dilshad, E. Chapter 10 - 3D Printing Technologies and Their Applications in Biomedical Science. In Omics Technologies and Bio-Engineering, Barh, D., Azevedo, V., Eds.; Academic Press: 2018; pp. 167-189.
- dos Santos, J.; de Oliveira, R.S.; de Oliveira, T.V.; Velho, M.C.; Konrad, M.V.; da Silva, G.S.; Deon, M.; Beck, R.C.R. 3D Printing and Nanotechnology: A Multiscale Alliance in Personalized Medicine. Adv. Funct. Mater. 2021. [CrossRef]
- Li, J.; He, L.; Zhou, C.; Zhou, Y.; Bai, Y.Y.; Lee, F.Y.; Mao, J.J. 3D printing for regenerative medicine: From bench to bedside. Mrs Bull 2015, 40, 145-153. [CrossRef]
- Amoyav, B.; Goldstein, Y.; Steinberg, E.; Benny, O. 3D Printed Microfluidic Devices for Drug Release Assays. Pharmaceutics 2021, 13. [CrossRef]
- Ong, C.S.; Yesantharao, P.; Huang, C.Y.; Mattson, G.; Boktor, J.; Fukunishi, T.; Zhang, H.T.; Hibino, N. 3D bioprinting using stem cells. Pediatr Res 2018, 83, 223-231. [CrossRef]
- Liu, K.; Hu, N.; Yu, Z.; Zhang, X.; Ma, H.; Qu, H.; Ruan, C. 3D printing and bioprinting in urology. IJB 2023, 9. [CrossRef]
- Jain, P.; Kathuria, H.; Dubey, N. Advances in 3D bioprinting of tissues/organs for regenerative medicine and in-vitro models. Biomaterials 2022, 287, 121639. [CrossRef]
- Administration, U.S.F.D. Medical Applications of 3D Printing. Available online: https://www.fda.gov/medical-devices/3d-printing-medical-devices/medical-applications-3d-printing (accessed on.
- Martins, J.P.; Ferreira, M.P.A.; Ezazi, N.Z.; Hirvonen, J.T.; Santos, H.A.; Thrivikraman, G.; França, C.M.; Athirasala, A.; Tahayeri, A.; Bertassoni, L.E. Chapter 4 - 3D printing: prospects and challenges. In Nanotechnologies in Preventive and Regenerative Medicine, Uskoković, V., Uskoković, D.P., Eds.; Elsevier: 2018; pp. 299-379.
- Chung, J.J.; Im, H.; Kim, S.H.; Park, J.W.; Jung, Y. Toward Biomimetic Scaffolds for Tissue Engineering: 3D Printing Techniques in Regenerative Medicine. Frontiers in Bioengineering and Biotechnology 2020, 8. [CrossRef]
- ASTM. ASTM International Committee F42 on Additive Manufacturing Technologies ASTM F2792–10. Standard Terminology for Additive Manufacturing Technologies 2009.
- Ng, W.L.; Lee, J.M.; Zhou, M.; Chen, Y.W.; Lee, K.A.; Yeong, W.Y.; Shen, Y.F. Vat polymerization-based bioprinting-process, materials, applications and regulatory challenges. Biofabrication 2020, 12, 022001. [CrossRef]
- Robles Martinez, P.; Basit, A.W.; Gaisford, S. The History, Developments and Opportunities of Stereolithography. In 3D Printing of Pharmaceuticals, Basit, A.W., Gaisford, S., Eds.; Springer International Publishing: Cham, 2018; pp. 55-79.
- Xu, X.; Awad, A.; Robles-Martinez, P.; Gaisford, S.; Goyanes, A.; Basit, A.W. Vat photopolymerization 3D printing for advanced drug delivery and medical device applications. Journal of Controlled Release 2021, 329, 743-757. [CrossRef]
- Al Rashid, A.; Ahmed, W.; Khalid, M.Y.; Koç, M. Vat photopolymerization of polymers and polymer composites: Processes and applications. Additive Manufacturing 2021, 47, 102279. [CrossRef]
- Rajan, K.; Samykano, M.; Kadirgama, K.; Harun, W.S.W.; Rahman, M.M. Fused deposition modeling: process, materials, parameters, properties, and applications. The International Journal of Advanced Manufacturing Technology 2022, 120, 1531-1570. [CrossRef]
- Marwah, O.M.F.; Shukri, M.; Mohamad, E.J.; Johar, M.; Haq, R.; Khirotdin, R.K. Direct Investment Casting For Pattern Developed By Desktop 3D Printer. MATEC Web of Conferences 2017, 135, 00036. [CrossRef]
- Siddique, S.H.; Hazell, P.J.; Wang, H.; Escobedo, J.P.; Ameri, A.A.H. Lessons from nature: 3D printed bio-inspired porous structures for impact energy absorption – A review. Additive Manufacturing 2022, 58, 103051. [CrossRef]
- Chartrain, N.A.; Gilchrist, K.H.; Ho, V.B.; Klarmann, G.J. 3D bioprinting for the repair of articular cartilage and osteochondral tissue. Bioprinting 2022, 28, e00239. [CrossRef]
- Gibson, I.; Rosen, D.W.; Stucker, B.; Khorasani, M.; Rosen, D.; Stucker, B.; Khorasani, M. Additive manufacturing technologies; Springer: 2021; Volume 17.
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chemical Reviews 2017, 117, 10212-10290. [CrossRef]
- Alami, A.H.; Ghani Olabi, A.; Alashkar, A.; Alasad, S.; Aljaghoub, H.; Rezk, H.; Abdelkareem, M.A. Additive manufacturing in the aerospace and automotive industries: Recent trends and role in achieving sustainable development goals. Ain Shams Engineering Journal 2023, 14, 102516. [CrossRef]
- Zhang, X.; Liou, F. Chapter 1 - Introduction to additive manufacturing. In Additive Manufacturing, Pou, J., Riveiro, A., Davim, J.P., Eds.; Elsevier: 2021; pp. 1-31.
- University, L. About Additive Manufacturing: Powder Bed Fusion. Available online: https://www.lboro.ac.uk/research/amrg/about/the7categoriesofadditivemanufacturing/powderbedfusion/ (accessed on.
- Singh, R.; Gupta, A.; Tripathi, O.; Srivastava, S.; Singh, B.; Awasthi, A.; Rajput, S.K.; Sonia, P.; Singhal, P.; Saxena, K.K. Powder bed fusion process in additive manufacturing: An overview. Materials Today: Proceedings 2020, 26, 3058-3070. [CrossRef]
- Thakur, V.; Singh, R.; Kumar, R. Chapter Two - Materials for additive manufacturing in clinical podiatry. In 3D Printing in Podiatric Medicine, Sandhu, K., Singh, S., Prakash, C., Subburaj, K., Ramakrishna, S., Eds.; Academic Press: 2023; pp. 35-50.
- Bourell, D.; Kruth, J.P.; Leu, M.; Levy, G.; Rosen, D.; Beese, A.M.; Clare, A. Materials for additive manufacturing. CIRP Annals 2017, 66, 659-681. [CrossRef]
- University, L. About Additive Manufacturing: Material Jetting Available online: https://www.lboro.ac.uk/research/amrg/about/the7categoriesofadditivemanufacturing/materialjetting/ (accessed on.
- Bourell, D.L. Perspectives on Additive Manufacturing. Annual Review of Materials Research 2016, 46, 1-18. [CrossRef]
- Mostafaei, A.; Elliott, A.M.; Barnes, J.E.; Li, F.; Tan, W.; Cramer, C.L.; Nandwana, P.; Chmielus, M. Binder jet 3D printing—Process parameters, materials, properties, modeling, and challenges. Progress in Materials Science 2021, 119, 100707. [CrossRef]
- Naghieh, S.; Chen, X. Printability–A key issue in extrusion-based bioprinting. Journal of Pharmaceutical Analysis 2021, 11, 564-579. [CrossRef]
- Tuominen, J. Directed energy deposition–Review of materials, properties and applications. In Proceedings of the conference seminar, 3D Boosti ja Invest, Tampere, Finland, 2017.
- University, L. About Additive Manufacturing: Directed Energy Deposition. Available online: https://www.lboro.ac.uk/research/amrg/about/the7categoriesofadditivemanufacturing/directedenergydeposition/ (accessed on.
- Gibson, I.; Rosen, D.; Stucker, B.; Khorasani, M.; Gibson, I.; Rosen, D.; Stucker, B.; Khorasani, M. Sheet lamination. Additive manufacturing technologies 2021, 253-283.
- University, L. About Additive Manufacturing: Sheet Lamination. Available online: https://www.lboro.ac.uk/research/amrg/about/the7categoriesofadditivemanufacturing/sheetlamination/ (accessed on.
- Pantermehl, S.; Emmert, S.; Foth, A.; Grabow, N.; Alkildani, S.; Bader, R.; Barbeck, M.; Jung, O. 3D Printing for Soft Tissue Regeneration and Applications in Medicine. Biomedicines 2021, 9. [CrossRef]
- Xing, F.; Xu, J.; Yu, P.; Zhou, Y.; Zhe, M.; Luo, R.; Liu, M.; Xiang, Z.; Duan, X.; Ritz, U. Recent advances in biofabrication strategies based on bioprinting for vascularized tissue repair and regeneration. Materials & Design 2023, 229, 111885. [CrossRef]
- Gu, Z.; Fu, J.; Lin, H.; He, Y. Development of 3D bioprinting: From printing methods to biomedical applications. Asian Journal of Pharmaceutical Sciences 2020, 15, 529-557. [CrossRef]
- Sufaru, I.-G.; Macovei, G.; Stoleriu, S.; Martu, M.-A.; Luchian, I.; Kappenberg-Nitescu, D.-C.; Solomon, S.M. 3D Printed and Bioprinted Membranes and Scaffolds for the Periodontal Tissue Regeneration: A Narrative Review. Membranes 2022, 12, 902. [CrossRef]
- Ayran, M.; Bulut, B.; Ulag, S. Bioprinting. In Biomaterials and Tissue Engineering, Gunduz, O., Egles, C., Pérez, R.A., Ficai, D., Ustundag, C.B., Eds.; Springer International Publishing: Cham, 2023; pp. 357-384.
- Mironov, V.; Boland, T.; Trusk, T.; Forgacs, G.; Markwald, R.R. Organ printing: computer-aided jet-based 3D tissue engineering. TRENDS in Biotechnology 2003, 21, 157-161. [CrossRef]
- Roth, E.A.; Xu, T.; Das, M.; Gregory, C.; Hickman, J.J.; Boland, T. Inkjet printing for high-throughput cell patterning. Biomaterials 2004, 25, 3707-3715. [CrossRef]
- Norotte, C.; Marga, F.S.; Niklason, L.E.; Forgacs, G. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials 2009, 30, 5910-5917. [CrossRef]
- Roque, R.; Barbosa, G.F.; Guastaldi, A.C. Design and 3D bioprinting of interconnected porous scaffolds for bone regeneration. An additive manufacturing approach. Journal of Manufacturing Processes 2021, 64, 655-663. [CrossRef]
- Dababneh, A.B.; Ozbolat, I.T. Bioprinting technology: a current state-of-the-art review. Journal of Manufacturing Science and Engineering 2014, 136, 061016. [CrossRef]
- Rider, P.; Kačarević, Ž.P.; Alkildani, S.; Retnasingh, S.; Schnettler, R.; Barbeck, M. Additive Manufacturing for Guided Bone Regeneration: A Perspective for Alveolar Ridge Augmentation. International Journal of Molecular Sciences 2018, 19, 3308. [CrossRef]
- Bartolo, P.; Kruth, J.-P.; Silva, J.; Levy, G.; Malshe, A.; Rajurkar, K.; Mitsuishi, M.; Ciurana, J.; Leu, M. Biomedical production of implants by additive electro-chemical and physical processes. CIRP Annals 2012, 61, 635-655. [CrossRef]
- Yi, H.-G.; Kim, H.; Kwon, J.; Choi, Y.-J.; Jang, J.; Cho, D.-W. Application of 3D bioprinting in the prevention and the therapy for human diseases. Signal Transduction and Targeted Therapy 2021, 6, 177. [CrossRef]
- Ovsianikov, A.; Farsari, M.; Chichkov, B.N. Photonic and Biomedical Applications of the Two-Photon Polymerization Technique. In Stereolithography: Materials, Processes and Applications, Bártolo, P.J., Ed.; Springer US: Boston, MA, 2011; pp. 257-297.
- Gu, B.K.; Choi, D.J.; Park, S.J.; Kim, M.S.; Kang, C.M.; Kim, C.H. 3-dimensional bioprinting for tissue engineering applications. Biomater Res 2016, 20, 12. [CrossRef]
- Song, Y.; Ghafari, Y.; Asefnejad, A.; Toghraie, D. An overview of selective laser sintering 3D printing technology for biomedical and sports device applications: Processes, materials, and applications. Optics & Laser Technology 2024, 171, 110459. [CrossRef]
- Xie, Z.; Gao, M.; Lobo, A.O.; Webster, T.J. 3D Bioprinting in Tissue Engineering for Medical Applications: The Classic and the Hybrid. Polymers 2020, 12, 1717. [CrossRef]
- Ramesh, S.; Harrysson, O.L.A.; Rao, P.K.; Tamayol, A.; Cormier, D.R.; Zhang, Y.; Rivero, I.V. Extrusion bioprinting: Recent progress, challenges, and future opportunities. Bioprinting 2021, 21, e00116. [CrossRef]
- Arefin, A.M.E.; Khatri, N.R.; Kulkarni, N.; Egan, P.F. Polymer 3D Printing Review: Materials, Process, and Design Strategies for Medical Applications. Polymers 2021, 13, 1499. [CrossRef]
- Dong, G.; Wijaya, G.; Tang, Y.; Zhao, Y.F. Optimizing process parameters of fused deposition modeling by Taguchi method for the fabrication of lattice structures. Additive Manufacturing 2018, 19, 62-72. [CrossRef]
- Carneiro, O.S.; Silva, A.F.; Gomes, R. Fused deposition modeling with polypropylene. Materials & Design 2015, 83, 768-776. [CrossRef]
- Dong, C.; Petrovic, M.; Davies, I.J. Applications of 3D printing in medicine: A review. Annals of 3D Printed Medicine 2024, 14, 100149. [CrossRef]
- Barbeck, M.; Jung, O.; Smeets, R.; Koržinskas, T. Biomaterial-Supported Tissue Reconstruction or Regeneration; BoD–Books on Demand: 2019.
- Rossi, A.; Pescara, T.; Gambelli, A.M.; Gaggia, F.; Asthana, A.; Perrier, Q.; Basta, G.; Moretti, M.; Senin, N.; Rossi, F.; et al. Biomaterials for extrusion-based bioprinting and biomedical applications. Frontiers in Bioengineering and Biotechnology 2024, 12. [CrossRef]
- Saunders, R.E.; Derby, B. Inkjet printing biomaterials for tissue engineering: bioprinting. International Materials Reviews 2014, 59, 430-448. [CrossRef]
- Barui, S. 3D inkjet printing of biomaterials: Principles and applications. MEDICAL DEVICES & SENSORS 2021, 4, e10143. [CrossRef]
- Saygili, E.; Dogan-Gurbuz, A.A.; Yesil-Celiktas, O.; Draz, M.S. 3D bioprinting: A powerful tool to leverage tissue engineering and microbial systems. Bioprinting 2020, 18, e00071. [CrossRef]
- Derakhshanfar, S.; Mbeleck, R.; Xu, K.; Zhang, X.; Zhong, W.; Xing, M. 3D bioprinting for biomedical devices and tissue engineering: A review of recent trends and advances. Bioactive Materials 2018, 3, 144-156. [CrossRef]
- Li, J.; Chen, M.; Fan, X.; Zhou, H. Recent advances in bioprinting techniques: approaches, applications and future prospects. Journal of Translational Medicine 2016, 14, 271. [CrossRef]
- Ng, W.L.; Lee, J.M.; Yeong, W.Y.; Win Naing, M. Microvalve-based bioprinting – process, bio-inks and applications. Biomater. Sci. 2017, 5. [CrossRef]
- Vanaei, S.; Parizi, M.S.; Vanaei, S.; Salemizadehparizi, F.; Vanaei, H.R. An Overview on Materials and Techniques in 3D Bioprinting Toward Biomedical Application. Engineered Regeneration 2021, 2, 1-18. [CrossRef]
- Ouyang, L.; Yao, R.; Zhao, Y.; Sun, W. Effect of bioink properties on printability and cell viability for 3D bioplotting of embryonic stem cells. Biofabrication 2016, 8, 035020. [CrossRef]
- Lozano, R.; Stevens, L.; Thompson, B.C.; Gilmore, K.J.; Gorkin, R., 3rd; Stewart, E.M.; in het Panhuis, M.; Romero-Ortega, M.; Wallace, G.G. 3D printing of layered brain-like structures using peptide modified gellan gum substrates. Biomaterials 2015, 67, 264-273. [CrossRef]
- Su, C.; Chen, Y.; Tian, S.; Lu, C.; Lv, Q. Natural Materials for 3D Printing and Their Applications. Gels 2022, 8. [CrossRef]
- Marques, C.F.; Diogo, G.S.; Pina, S.; Oliveira, J.M.; Silva, T.H.; Reis, R.L. Collagen-based bioinks for hard tissue engineering applications: a comprehensive review. J Mater Sci Mater Med 2019, 30, 32. [CrossRef]
- Suo, H.; Zhang, J.; Xu, M.; Wang, L. Low-temperature 3D printing of collagen and chitosan composite for tissue engineering. Mater Sci Eng C Mater Biol Appl 2021, 123, 111963. [CrossRef]
- Dai, M.; Belaïdi, J.P.; Fleury, G.; Garanger, E.; Rielland, M.; Schultze, X.; Lecommandoux, S. Elastin-like Polypeptide-Based Bioink: A Promising Alternative for 3D Bioprinting. Biomacromolecules 2021, 22, 4956-4966. [CrossRef]
- Aiyelabegan, H.T.; Zaidi, S.S.Z.; Fanuel, S.; Eatemadi, A.; Ebadi, M.T.K.; Sadroddiny, E. Albumin-based biomaterial for lung tissue engineering applications. International Journal of Polymeric Materials and Polymeric Biomaterials 2016, 65, 853-861. [CrossRef]
- Placone, J.K.; Navarro, J.; Laslo, G.W.; Lerman, M.J.; Gabard, A.R.; Herendeen, G.J.; Falco, E.E.; Tomblyn, S.; Burnett, L.; Fisher, J.P. Development and Characterization of a 3D Printed, Keratin-Based Hydrogel. Ann Biomed Eng 2017, 45, 237-248. [CrossRef]
- Srinivasan, B.; Kumar, R.; Shanmugam, K.; Sivagnam, U.T.; Reddy, N.P.; Sehgal, P.K. Porous keratin scaffold-promising biomaterial for tissue engineering and drug delivery. J Biomed Mater Res B Appl Biomater 2010, 92, 5-12. [CrossRef]
- Khanarian, N.T.; Haney, N.M.; Burga, R.A.; Lu, H.H. A functional agarose-hydroxyapatite scaffold for osteochondral interface regeneration. Biomaterials 2012, 33, 5247-5258. [CrossRef]
- Axpe, E.; Oyen, M.L. Applications of Alginate-Based Bioinks in 3D Bioprinting. Int J Mol Sci 2016, 17. [CrossRef]
- Petta, D.; D'Amora, U.; Ambrosio, L.; Grijpma, D.W.; Eglin, D.; D'Este, M. Hyaluronic acid as a bioink for extrusion-based 3D printing. Biofabrication 2020, 12, 032001. [CrossRef]
- Dutta, P.; Rinki, K.; Dutta, J. Chitosan: A promising biomaterial for tissue engineering scaffolds. Chitosan for biomaterials II 2011, 45-79. [CrossRef]
- Kumar, A.; Rao, K.M.; Han, S.S. Application of xanthan gum as polysaccharide in tissue engineering: A review. Carbohydr Polym 2018, 180, 128-144. [CrossRef]
- Jena, S.R.; Dalei, G.; Das, S.; Nayak, J.; Pradhan, M.; Samanta, L. Harnessing the potential of dialdehyde alginate-xanthan gum hydrogels as niche bioscaffolds for tissue engineering. Int J Biol Macromol 2022, 207, 493-506. [CrossRef]
- Kumar, P.T.; Ramya, C.; Jayakumar, R.; Nair, S.; Lakshmanan, V.K. Drug delivery and tissue engineering applications of biocompatible pectin-chitin/nano CaCO3 composite scaffolds. Colloids Surf B Biointerfaces 2013, 106, 109-116. [CrossRef]
- Akkineni, A.R.; Elci, B.S.; Lode, A.; Gelinsky, M. Addition of High Acyl Gellan Gum to Low Acyl Gellan Gum Enables the Blends 3D Bioprintable. Gels 2022, 8. [CrossRef]
- Li, S.; Tian, X.; Fan, J.; Tong, H.; Ao, Q.; Wang, X. Chitosans for tissue repair and organ three-dimensional (3D) bioprinting. Micromachines 2019, 10, 765. [CrossRef]
- Wu, Q.; Therriault, D.; Heuzey, M.-C. Processing and properties of chitosan inks for 3D printing of hydrogel microstructures. ACS Biomaterials Science & Engineering 2018, 4, 2643-2652. [CrossRef]
- Rastogi, P.; Kandasubramanian, B. Review of alginate-based hydrogel bioprinting for application in tissue engineering. Biofabrication 2019, 11, 042001.
- Gaetani, R.; Feyen, D.A.; Verhage, V.; Slaats, R.; Messina, E.; Christman, K.L.; Giacomello, A.; Doevendans, P.A.; Sluijter, J.P. Epicardial application of cardiac progenitor cells in a 3D-printed gelatin/hyaluronic acid patch preserves cardiac function after myocardial infarction. Biomaterials 2015, 61, 339-348. [CrossRef]
- Negrini, N.C.; Celikkin, N.; Tarsini, P.; Farè, S.; Święszkowski, W. Three-dimensional printing of chemically crosslinked gelatin hydrogels for adipose tissue engineering. Biofabrication 2020, 12, 025001. [CrossRef]
- Abelseth, E.; Abelseth, L.; De la Vega, L.; Beyer, S.T.; Wadsworth, S.J.; Willerth, S.M. 3D printing of neural tissues derived from human induced pluripotent stem cells using a fibrin-based bioink. ACS Biomaterials Science & Engineering 2018, 5, 234-243. [CrossRef]
- Khoeini, R.; Nosrati, H.; Akbarzadeh, A.; Eftekhari, A.; Kavetskyy, T.; Khalilov, R.; Ahmadian, E.; Nasibova, A.; Datta, P.; Roshangar, L.; et al. Natural and Synthetic Bioinks for 3D Bioprinting. Advanced NanoBiomed Research 2021, 1, 2000097. [CrossRef]
- Xin, S.; Chimene, D.; Garza, J.E.; Gaharwar, A.K.; Alge, D.L. Clickable PEG hydrogel microspheres as building blocks for 3D bioprinting. Biomater Sci 2019, 7, 1179-1187. [CrossRef]
- Izgordu, M.S.; Uzgur, E.I.; Ulag, S.; Sahin, A.; Karademir Yilmaz, B.; Kilic, B.; Ekren, N.; Oktar, F.N.; Gunduz, O. Investigation of 3D-printed polycaprolactone-/polyvinylpyrrolidone-based constructs. Cartilage 2021, 13, 626S-635S.
- Hospodiuk, M.; Dey, M.; Sosnoski, D.; Ozbolat, I.T. The bioink: A comprehensive review on bioprintable materials. Biotechnology advances 2017, 35, 217-239. [CrossRef]
- Diomede, F.; Gugliandolo, A.; Cardelli, P.; Merciaro, I.; Ettorre, V.; Traini, T.; Bedini, R.; Scionti, D.; Bramanti, A.; Nanci, A. Three-dimensional printed PLA scaffold and human gingival stem cell-derived extracellular vesicles: a new tool for bone defect repair. Stem Cell Research & Therapy 2018, 9, 1-21. [CrossRef]
- Wang, P.; Zou, B.; Xiao, H.; Ding, S.; Huang, C. Effects of printing parameters of fused deposition modeling on mechanical properties, surface quality, and microstructure of PEEK. Journal of Materials Processing Technology 2019, 271, 62-74. [CrossRef]
- Lee, J.; Chae, S.; Lee, H.; Kim, G.H. A 3D printing strategy for fabricating in situ topographical scaffolds using pluronic F-127. Additive Manufacturing 2020, 32, 101023. [CrossRef]
- Liu, F.; Wang, X. Synthetic Polymers for Organ 3D Printing. Polymers (Basel) 2020, 12. [CrossRef]
- Clapper, J.D.; Skeie, J.M.; Mullins, R.F.; Guymon, C.A. Development and characterization of photopolymerizable biodegradable materials from PEG–PLA–PEG block macromonomers. Polymer 2007, 48, 6554-6564. [CrossRef]
- Shan Wong, Y.; Yong Tay, C.; Wen, F.; S Venkatraman, S.; Poh Tan, L. Engineered polymeric biomaterials for tissue engineering. Current Tissue Engineering (Discontinued) 2012, 1, 41-53. [CrossRef]
- S, S.; R G, A.P.; Bajaj, G.; John, A.E.; Chandran, S.; Kumar, V.V.; Ramakrishna, S. A review on the recent applications of synthetic biopolymers in 3D printing for biomedical applications. Journal of Materials Science: Materials in Medicine 2023, 34, 62. [CrossRef]
- Im, S.H.; Im, D.H.; Park, S.J.; Chung, J.J.; Jung, Y.; Kim, S.H. Stereocomplex Polylactide for Drug Delivery and Biomedical Applications: A Review. Molecules 2021, 26, 2846. [CrossRef]
- Alqurashi, H.; Khurshid, Z.; Syed, A.U.Y.; Rashid Habib, S.; Rokaya, D.; Zafar, M.S. Polyetherketoneketone (PEKK): An emerging biomaterial for oral implants and dental prostheses. Journal of Advanced Research 2021, 28, 87-95. [CrossRef]
- Elmowafy, E.M.; Tiboni, M.; Soliman, M.E. Biocompatibility, biodegradation and biomedical applications of poly(lactic acid)/poly(lactic-co-glycolic acid) micro and nanoparticles. Journal of Pharmaceutical Investigation 2019, 49, 347-380. [CrossRef]
- Rutz, A.L.; Hyland, K.E.; Jakus, A.E.; Burghardt, W.R.; Shah, R.N. A multi-material bioink method for 3D printing tunable, cell-compatible hydrogels. Advanced materials (Deerfield Beach, Fla.) 2015, 27, 1607.
- Mao, H.; Yang, L.; Zhu, H.; Wu, L.; Ji, P.; Yang, J.; Gu, Z. Recent advances and challenges in materials for 3D bioprinting. Progress in Natural Science: Materials International 2020, 30, 618-634. [CrossRef]
- Lui, Y.S.; Sow, W.T.; Tan, L.P.; Wu, Y.; Lai, Y.; Li, H. 4D printing and stimuli-responsive materials in biomedical aspects. Acta biomaterialia 2019, 92, 19-36. [CrossRef]
- Li, Y.-C.; Zhang, Y.S.; Akpek, A.; Shin, S.R.; Khademhosseini, A. 4D bioprinting: the next-generation technology for biofabrication enabled by stimuli-responsive materials. Biofabrication 2016, 9, 012001. [CrossRef]
- El-Kadiry, A.E.; Rafei, M.; Shammaa, R. Cell Therapy: Types, Regulation, and Clinical Benefits. Front Med (Lausanne) 2021, 8, 756029. [CrossRef]
- Kim, I. A brief overview of cell therapy and its product. J Korean Assoc Oral Maxillofac Surg 2013, 39, 201-202. [CrossRef]
- Facklam, A.L.; Volpatti, L.R.; Anderson, D.G. Biomaterials for Personalized Cell Therapy. Advanced Materials 2020, 32, 1902005. [CrossRef]
- Xue, J.; Qin, C.; Wu, C. 3D printing of cell-delivery scaffolds for tissue regeneration. Regenerative Biomaterials 2023, 10, rbad032. [CrossRef]
- Urciuolo, A.; Poli, I.; Brandolino, L.; Raffa, P.; Scattolini, V.; Laterza, C.; Giobbe, G.G.; Zambaiti, E.; Selmin, G.; Magnussen, M.; et al. Intravital three-dimensional bioprinting. Nat Biomed Eng 2020, 4, 901-915. [CrossRef]
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 2020, 226, 119536. [CrossRef]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.H. 3D bioprinting for engineering complex tissues. Biotechnol Adv 2016, 34, 422-434. [CrossRef]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D bioprinting: an overview. Biomater Sci 2018, 6, 915-946. [CrossRef]
- Ventura, R.D. An Overview of Laser-assisted Bioprinting (LAB) in Tissue Engineering Applications. Medical Lasers 2021, 10, 76-81. [CrossRef]
- Fahimipour, F.; Dashtimoghadam, E.; Mahdi Hasani-Sadrabadi, M.; Vargas, J.; Vashaee, D.; Lobner, D.C.; Jafarzadeh Kashi, T.S.; Ghasemzadeh, B.; Tayebi, L. Enhancing cell seeding and osteogenesis of MSCs on 3D printed scaffolds through injectable BMP2 immobilized ECM-Mimetic gel. Dent Mater 2019, 35, 990-1006. [CrossRef]
- Jang, C.H.; Koo, Y.; Kim, G. ASC/chondrocyte-laden alginate hydrogel/PCL hybrid scaffold fabricated using 3D printing for auricle regeneration. Carbohydr Polym 2020, 248, 116776. [CrossRef]
- Polak, J.M.; Mantalaris, S. Stem cells bioprocessing: an important milestone to move regenerative medicine research into the clinical arena. Pediatr Res 2008, 63, 461-466. [CrossRef]
- Saini, G.; Segaran, N.; Mayer, J.L.; Saini, A.; Albadawi, H.; Oklu, R. Applications of 3D Bioprinting in Tissue Engineering and Regenerative Medicine. J Clin Med 2021, 10. [CrossRef]
- Jaenisch, R.; Young, R. Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell 2008, 132, 567-582. [CrossRef]
- Poliwoda, S.; Noor, N.; Downs, E.; Schaaf, A.; Cantwell, A.; Ganti, L.; Kaye, A.D.; Mosel, L.I.; Carroll, C.B.; Viswanath, O.; et al. Stem cells: a comprehensive review of origins and emerging clinical roles in medical practice. Orthop Rev (Pavia) 2022, 14, 37498. [CrossRef]
- Caddeo, S.; Boffito, M.; Sartori, S. Tissue Engineering Approaches in the Design of Healthy and Pathological In Vitro Tissue Models. Front Bioeng Biotechnol 2017, 5, 40. [CrossRef]
- Irvine, S.A.; Venkatraman, S.S. Bioprinting and Differentiation of Stem Cells. Molecules 2016, 21, 1188. [CrossRef]
- Kariminekoo, S.; Movassaghpour, A.; Rahimzadeh, A.; Talebi, M.; Shamsasenjan, K.; Akbarzadeh, A. Implications of mesenchymal stem cells in regenerative medicine. Artif Cells Nanomed Biotechnol 2016, 44, 749-757. [CrossRef]
- Huang, N.F.; Li, S. Mesenchymal stem cells for vascular regeneration. Regen Med 2008, 3, 877-892. [CrossRef]
- Alvites, R.D.; Branquinho, M.V.; Caseiro, A.R.; Amorim, I.; Santos Pedrosa, S.; Rêma, A.; Faria, F.; Porto, B.; Oliveira, C.; Teixeira, P.; et al. Rat Olfactory Mucosa Mesenchymal Stem/Stromal Cells (OM-MSCs): A Characterization Study. International Journal of Cell Biology 2020, 2020, 2938258. [CrossRef]
- Zhou, T.; Yuan, Z.; Weng, J.; Pei, D.; Du, X.; He, C.; Lai, P. Challenges and advances in clinical applications of mesenchymal stromal cells. Journal of Hematology & Oncology 2021, 14, 24. [CrossRef]
- Conrad, C.; Niess, H.; Huss, R.; Huber, S.; von Luettichau, I.; Nelson, P.J.; Ott, H.C.; Jauch, K.W.; Bruns, C.J. Multipotent mesenchymal stem cells acquire a lymphendothelial phenotype and enhance lymphatic regeneration in vivo. Circulation 2009, 119, 281-289. [CrossRef]
- Paulo Zambon, J.; Atala, A.; Yoo, J.J. Methods to generate tissue-derived constructs for regenerative medicine applications. Methods 2020, 171, 3-10. [CrossRef]
- Kaebisch, C.; Schipper, D.; Babczyk, P.; Tobiasch, E. The role of purinergic receptors in stem cell differentiation. Computational and Structural Biotechnology Journal 2015, 13, 75-84. [CrossRef]
- Ntege, E.H.; Sunami, H.; Shimizu, Y. Advances in regenerative therapy: A review of the literature and future directions. Regenerative Therapy 2020, 14, 136-153. [CrossRef]
- Feyen, D.A.M.; Gaetani, R.; Doevendans, P.A.; Sluijter, J.P.G. Stem cell-based therapy: Improving myocardial cell delivery. Advanced Drug Delivery Reviews 2016, 106, 104-115. [CrossRef]
- Cui, H.; Miao, S.; Esworthy, T.; Zhou, X.; Lee, S.-j.; Liu, C.; Yu, Z.-x.; Fisher, J.P.; Mohiuddin, M.; Zhang, L.G. 3D bioprinting for cardiovascular regeneration and pharmacology. Advanced Drug Delivery Reviews 2018, 132, 252-269. [CrossRef]
- Ye, L.; Swingen, C.; Zhang, J. Induced pluripotent stem cells and their potential for basic and clinical sciences. Curr Cardiol Rev 2013, 9, 63-72. [CrossRef]
- Golchin, A.; Farahany, T.Z. Biological Products: Cellular Therapy and FDA Approved Products. Stem Cell Rev Rep 2019, 15, 166-175. [CrossRef]
- De Pieri, A.; Rochev, Y.; Zeugolis, D.I. Scaffold-free cell-based tissue engineering therapies: advances, shortfalls and forecast. NPJ Regen Med 2021, 6, 18. [CrossRef]
- Sánchez, A.; Schimmang, T.; García-Sancho, J. Cell and tissue therapy in regenerative medicine. Adv Exp Med Biol 2012, 741, 89-102. [CrossRef]
- Gyorgypal, A. An Introduction to Cell Therapy. Available online: https://www.technologynetworks.com/biopharma/webinars/strategies-for-error-free-chromatographic-analyses-388826 (accessed on 28-07-2024).
- Dainichi, T.; Kabashima, K.; Ivanov, I.I.; Goto, Y. Editorial: Regulation of Immunity by Non-Immune Cells. Frontiers in Immunology 2021, 12. [CrossRef]
- Guidance for human somatic cell therapy and gene therapy. Hum Gene Ther 2001, 12, 303-314. [CrossRef]
- Bartel, R.L.; Cramer, C.; Ledford, K.; Longcore, A.; Parrish, C.; Stern, T.; Watling, S.; Zeigler, F. The Aastrom experience. Stem Cell Research & Therapy 2012, 3, 26. [CrossRef]
- Wolff, M.; Shillington, J.M.; Rathbone, C.; Piasecki, S.K.; Barnes, B. Injections of concentrated bone marrow aspirate as treatment for Discogenic pain: a retrospective analysis. BMC Musculoskelet Disord 2020, 21, 135. [CrossRef]
- Buisseret, L.; Garaud, S.; de Wind, A.; Van den Eynden, G.; Boisson, A.; Solinas, C.; Gu-Trantien, C.; Naveaux, C.; Lodewyckx, J.N.; Duvillier, H.; et al. Tumor-infiltrating lymphocyte composition, organization and PD-1/ PD-L1 expression are linked in breast cancer. Oncoimmunology 2017, 6, e1257452. [CrossRef]
- Guo, J.; Nguyen, A.; Banyard, D.A.; Fadavi, D.; Toranto, J.D.; Wirth, G.A.; Paydar, K.Z.; Evans, G.R.; Widgerow, A.D. Stromal vascular fraction: A regenerative reality? Part 2: Mechanisms of regenerative action. J Plast Reconstr Aesthet Surg 2016, 69, 180-188. [CrossRef]
- Heo, D.N.; Alioglu, M.A.; Wu, Y.; Ozbolat, V.; Ayan, B.; Dey, M.; Kang, Y.; Ozbolat, I.T. 3D bioprinting of carbohydrazide-modified gelatin into microparticle-suspended oxidized alginate for the fabrication of complex-shaped tissue constructs. ACS applied materials & interfaces 2020, 12, 20295-20306. [CrossRef]
- Dey, M.; Ozbolat, I.T. 3D bioprinting of cells, tissues and organs. Scientific Reports 2020, 10, 14023. [CrossRef]
- Alonzo, M.; AnilKumar, S.; Roman, B.; Tasnim, N.; Joddar, B. 3D Bioprinting of cardiac tissue and cardiac stem cell therapy. Transl Res 2019, 211, 64-83. [CrossRef]
- Qasim, M.; Haq, F.; Kang, M.H.; Kim, J.H. 3D printing approaches for cardiac tissue engineering and role of immune modulation in tissue regeneration. Int J Nanomedicine 2019, 14, 1311-1333. [CrossRef]
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J Am Coll Cardiol 2017, 70, 1-25. [CrossRef]
- Bhandari, S.; Yadav, V.; Ishaq, A.; Sanipini, S.; Ekhator, C.; Khleif, R.; Beheshtaein, A.; Jhajj, L.K.; Khan, A.W.; Al Khalifa, A.; et al. Trends and Challenges in the Development of 3D-Printed Heart Valves and Other Cardiac Implants: A Review of Current Advances. Cureus 2023, 15, e43204. [CrossRef]
- Itier, R.; Roncalli, J. New therapies for acute myocardial infarction: current state of research and future promise. Future Cardiology 2018, 14, 329-342. [CrossRef]
- Shi, W.Y.; Smith, J.A.; Shi, W.Y.; Smith, J.A. Role of coronary artery bypass surgery in acute myocardial infarction. Primary Angioplasty: A Practical Guide 2018, 211-221.
- Kwon, Y.-W.; Yang, H.-M.; Cho, H.-J. Cell therapy for myocardial infarction. International journal of stem cells 2010, 3, 8-15.
- Das, S.; Nam, H.; Jang, J. 3D bioprinting of stem cell-laden cardiac patch: A promising alternative for myocardial repair. APL bioengineering 2021, 5. [CrossRef]
- Fernandes, S.; Kuklok, S.; McGonigle, J.; Reinecke, H.; Murry, C.E. Synthetic matrices to serve as niches for muscle cell transplantation. Cells Tissues Organs 2011, 195, 48-59. [CrossRef]
- Vukicevic, M.; Mosadegh, B.; Min, J.K.; Little, S.H. Cardiac 3D printing and its future directions. JACC: Cardiovascular Imaging 2017, 10, 171-184. [CrossRef]
- Hinderer, S.; Brauchle, E.; Schenke-Layland, K. Generation and assessment of functional biomaterial scaffolds for applications in cardiovascular tissue engineering and regenerative medicine. Advanced healthcare materials 2015, 4, 2326-2341. [CrossRef]
- Zhu, J.; Marchant, R.E. Design properties of hydrogel tissue-engineering scaffolds. Expert review of medical devices 2011, 8, 607-626. [CrossRef]
- Maiullari, F.; Costantini, M.; Milan, M.; Pace, V.; Chirivì, M.; Maiullari, S.; Rainer, A.; Baci, D.; Marei, H.E.-S.; Seliktar, D.; et al. A multi-cellular 3D bioprinting approach for vascularized heart tissue engineering based on HUVECs and iPSC-derived cardiomyocytes. Scientific Reports 2018, 8, 13532. [CrossRef]
- Bejleri, D.; Streeter, B.W.; Nachlas, A.L.Y.; Brown, M.E.; Gaetani, R.; Christman, K.L.; Davis, M.E. A Bioprinted Cardiac Patch Composed of Cardiac-Specific Extracellular Matrix and Progenitor Cells for Heart Repair. Advanced Healthcare Materials 2018, 7, 1800672. [CrossRef]
- Melhem, M.R.; Park, J.; Knapp, L.; Reinkensmeyer, L.; Cvetkovic, C.; Flewellyn, J.; Lee, M.K.; Jensen, T.W.; Bashir, R.; Kong, H.; et al. 3D Printed Stem-Cell-Laden, Microchanneled Hydrogel Patch for the Enhanced Release of Cell-Secreting Factors and Treatment of Myocardial Infarctions. ACS Biomaterials Science & Engineering 2017, 3, 1980-1987. [CrossRef]
- Noor, N.; Shapira, A.; Edri, R.; Gal, I.; Wertheim, L.; Dvir, T. 3D Printing of Personalized Thick and Perfusable Cardiac Patches and Hearts. Advanced Science 2019, 6, 1900344. [CrossRef]
- Cui, X.; Boland, T. Human microvasculature fabrication using thermal inkjet printing technology. Biomaterials 2009, 30, 6221-6227. [CrossRef]
- Kucukgul, C.; Ozler, S.B.; Inci, I.; Karakas, E.; Irmak, S.; Gozuacik, D.; Taralp, A.; Koc, B. 3D bioprinting of biomimetic aortic vascular constructs with self-supporting cells. Biotechnology and Bioengineering 2015, 112, 811-821. [CrossRef]
- Gaetani, R.; Doevendans, P.A.; Metz, C.H.G.; Alblas, J.; Messina, E.; Giacomello, A.; Sluijter, J.P.G. Cardiac tissue engineering using tissue printing technology and human cardiac progenitor cells. Biomaterials 2012, 33, 1782-1790. [CrossRef]
- Ketabat, F.; Maris, T.; Duan, X.; Yazdanpanah, Z.; Kelly, M.E.; Badea, I.; Chen, X. Optimization of 3D printing and in vitro characterization of alginate/gelatin lattice and angular scaffolds for potential cardiac tissue engineering. Frontiers in Bioengineering and Biotechnology 2023, 11. [CrossRef]
- Roche, C.D.; Lin, H.; Huang, Y.; de Bock, C.E.; Beck, D.; Xue, M.; Gentile, C. 3D bioprinted alginate-gelatin hydrogel patches containing cardiac spheroids recover heart function in a mouse model of myocardial infarction. Bioprinting 2023, 30, e00263. [CrossRef]
- Zhang, Y.S.; Arneri, A.; Bersini, S.; Shin, S.-R.; Zhu, K.; Goli-Malekabadi, Z.; Aleman, J.; Colosi, C.; Busignani, F.; Dell'Erba, V.; et al. Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials 2016, 110, 45-59. [CrossRef]
- Das, S.; Kim, S.-W.; Choi, Y.-J.; Lee, S.; Lee, S.-H.; Kong, J.-S.; Park, H.-J.; Cho, D.-W.; Jang, J. Decellularized extracellular matrix bioinks and the external stimuli to enhance cardiac tissue development in vitro. Acta Biomaterialia 2019, 95, 188-200. [CrossRef]
- Atari, M.; Labbaf, S.; Javanmard, S.H. Fabrication and characterization of a 3D scaffold based on elastomeric poly-glycerol Sebacate polymer for heart valve applications. Journal of Manufacturing Processes 2023, 102, 350-364. [CrossRef]
- Yang, Y.; Lei, D.; Huang, S.; Yang, Q.; Song, B.; Guo, Y.; Shen, A.; Yuan, Z.; Li, S.; Qing, F.-L.; et al. Elastic 3D-Printed Hybrid Polymeric Scaffold Improves Cardiac Remodeling after Myocardial Infarction. Advanced Healthcare Materials 2019, 8, 1900065. [CrossRef]
- Liu, Y.; Zhang, Y.; Mei, T.; Cao, H.; Hu, Y.; Jia, W.; Wang, J.; Zhang, Z.; Wang, Z.; Le, W.; et al. hESCs-Derived Early Vascular Cell Spheroids for Cardiac Tissue Vascular Engineering and Myocardial Infarction Treatment. Adv Sci (Weinh) 2022, 9, e2104299. [CrossRef]
- Miller, K.L.; Xiang, Y.; Yu, C.; Pustelnik, J.; Wu, J.; Ma, X.; Matsui, T.; Imahashi, K.; Chen, S. Rapid 3D BioPrinting of a human iPSC-derived cardiac micro-tissue for high-throughput drug testing. Organs-on-a-Chip 2021, 3, 100007. [CrossRef]
- Wang, Z.; Lee, S.J.; Cheng, H.-J.; Yoo, J.J.; Atala, A. 3D bioprinted functional and contractile cardiac tissue constructs. Acta Biomaterialia 2018, 70, 48-56. [CrossRef]
- Cieza, A.; Causey, K.; Kamenov, K.; Hanson, S.W.; Chatterji, S.; Vos, T. Global estimates of the need for rehabilitation based on the Global Burden of Disease study 2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet 2020, 396, 2006-2017. [CrossRef]
- Bauso, L.V.; La Fauci, V.; Longo, C.; Calabrese, G. Bone Tissue Engineering and Nanotechnology: A Promising Combination for Bone Regeneration. Biology 2024, 13, 237. [CrossRef]
- Smrke, D.; Rožman, P.; Veselko, M.; Gubina, B. Treatment of bone defects—allogenic platelet gel and autologous bone technique. In Regenerative medicine and tissue engineering; IntechOpen: 2013.
- Schlickewei, C.W.; Kleinertz, H.; Thiesen, D.M.; Mader, K.; Priemel, M.; Frosch, K.-H.; Keller, J. Current and future concepts for the treatment of impaired fracture healing. Int J Mol Sci 2019, 20, 5805. [CrossRef]
- Zhang, Y.; Lei, T.; Tang, C.; Chen, Y.; Liao, Y.; Ju, W.; Zhang, H.; Zhou, B.; Liang, R.; Zhang, T. 3D printing of chemical-empowered tendon stem/progenitor cells for functional tissue repair. Biomaterials 2021, 271, 120722. [CrossRef]
- Aytac, Z.; Dubey, N.; Daghrery, A.; Ferreira, J.A.; de Souza Araújo, I.J.; Castilho, M.; Malda, J.; Bottino, M.C. Innovations in craniofacial bone and periodontal tissue engineering–from electrospinning to converged biofabrication. International materials reviews 2022, 67, 347-384. [CrossRef]
- Sousa, A.C.; Biscaia, S.; Alvites, R.; Branquinho, M.; Lopes, B.; Sousa, P.; Valente, J.; Franco, M.; Santos, J.D.; Mendonça, C.; et al. Assessment of 3D-Printed Polycaprolactone, Hydroxyapatite Nanoparticles and Diacrylate Poly(ethylene glycol) Scaffolds for Bone Regeneration. Pharmaceutics 2022, 14. [CrossRef]
- Dubey, N.; Ferreira, J.A.; Daghrery, A.; Aytac, Z.; Malda, J.; Bhaduri, S.B.; Bottino, M.C. Highly tunable bioactive fiber-reinforced hydrogel for guided bone regeneration. Acta biomaterialia 2020, 113, 164-176. [CrossRef]
- Chung, J.J.; Im, H.; Kim, S.H.; Park, J.W.; Jung, Y. Toward biomimetic scaffolds for tissue engineering: 3D printing techniques in regenerative medicine. Frontiers in Bioengineering and Biotechnology 2020, 8, 586406. [CrossRef]
- Lei, P.; Qian, H.; Zhang, T.; Lei, T.; Hu, Y.; Chen, C.; Zhou, K. Porous tantalum structure integrated on Ti6Al4V base by Laser Powder Bed Fusion for enhanced bony-ingrowth implants: In vitro and in vivo validation. Bioactive Materials 2022, 7, 3-13. [CrossRef]
- Yu, L.; Wu, Y.; Liu, J.; Li, B.; Ma, B.; Li, Y.; Huang, Z.; He, Y.; Wang, H.; Wu, Z.; et al. 3D Culture of Bone Marrow-Derived Mesenchymal Stem Cells (BMSCs) Could Improve Bone Regeneration in 3D-Printed Porous Ti6Al4V Scaffolds. Stem Cells International 2018, 2018, 2074021. [CrossRef]
- Wu, Y.-F.; Wen, Y.-T.; Salamanca, E.; Moe Aung, L.; Chao, Y.-Q.; Chen, C.-Y.; Sun, Y.-S.; Chang, W.-J. 3D-bioprinted alginate-based bioink scaffolds with β-tricalcium phosphate for bone regeneration applications. Journal of Dental Sciences 2024, 19, 1116-1125. [CrossRef]
- Ressler, A.; Zakeri, S.; Dias, J.; Hannula, M.; Hyttinen, J.; Ivanković, H.; Ivanković, M.; Miettinen, S.; Schwentenwein, M.; Levänen, E.; et al. Vat photopolymerization of biomimetic bone scaffolds based on Mg, Sr, Zn-substituted hydroxyapatite: Effect of sintering temperature. Ceramics International 2024, 50, 27403-27415. [CrossRef]
- Yu, X.; Jiang, S.; Li, D.; Shen, S.G.F.; Wang, X.; Lin, K. Osteoimmunomodulatory bioinks for 3D bioprinting achieve complete regeneration of critical-sized bone defects. Composites Part B: Engineering 2024, 273, 111256. [CrossRef]
- Choe, G.; Lee, M.; Oh, S.; Seok, J.M.; Kim, J.; Im, S.; Park, S.A.; Lee, J.Y. Three-dimensional bioprinting of mesenchymal stem cells using an osteoinductive bioink containing alginate and BMP-2-loaded PLGA nanoparticles for bone tissue engineering. Biomaterials Advances 2022, 136, 212789. [CrossRef]
- Wang, W.; Zhu, Y.; Liu, Y.; Chen, B.; Li, M.; Yuan, C.; Wang, P. 3D bioprinting of DPSCs with GelMA hydrogel of various concentrations for bone regeneration. Tissue and Cell 2024, 88, 102418. [CrossRef]
- Tao, J.; Zhu, S.; Liao, X.; Wang, Y.; Zhou, N.; Li, Z.; Wan, H.; Tang, Y.; Yang, S.; Du, T.; et al. DLP-based bioprinting of void-forming hydrogels for enhanced stem-cell-mediated bone regeneration. Materials Today Bio 2022, 17, 100487. [CrossRef]
- Gatto, M.L.; Furlani, M.; Giuliani, A.; Bloise, N.; Fassina, L.; Visai, L.; Mengucci, P. Biomechanical performances of PCL/HA micro- and macro-porous lattice scaffolds fabricated via laser powder bed fusion for bone tissue engineering. Materials Science and Engineering: C 2021, 128, 112300. [CrossRef]
- Hong, D.; Chou, D.-T.; Velikokhatnyi, O.I.; Roy, A.; Lee, B.; Swink, I.; Issaev, I.; Kuhn, H.A.; Kumta, P.N. Binder-jetting 3D printing and alloy development of new biodegradable Fe-Mn-Ca/Mg alloys. Acta Biomaterialia 2016, 45, 375-386. [CrossRef]
- Barro, Ó.; Arias-González, F.; Lusquiños, F.; Comesaña, R.; del Val, J.; Riveiro, A.; Badaoui, A.; Gómez-Baño, F.; Pou, J. Improved Commercially Pure Titanium Obtained by Laser Directed Energy Deposition for Dental Prosthetic Applications. Metals 2021, 11. [CrossRef]
- Arias-González, F.; Rodríguez-Contreras, A.; Punset, M.; Manero, J.M.; Barro, Ó.; Fernández-Arias, M.; Lusquiños, F.; Gil, F.J.; Pou, J. In-Situ Laser Directed Energy Deposition of Biomedical Ti-Nb and Ti-Zr-Nb Alloys from Elemental Powders. Metals 2021, 11. [CrossRef]
- Cedeño-Viveros, L.D.; Vázquez-Lepe, E.; Rodríguez, C.A.; García-López, E. Influence of process parameters for sheet lamination based on laser micro-spot welding of austenitic stainless steel sheets for bone tissue applications. The International Journal of Advanced Manufacturing Technology 2021, 115, 247-262. [CrossRef]
- Touya, N.; Devun, M.; Handschin, C.; Casenave, S.; Ahmed Omar, N.; Gaubert, A.; Dusserre, N.; De Oliveira, H.; Kérourédan, O.; Devillard, R. In vitro and in vivo characterization of a novel tricalcium silicate-based ink for bone regeneration using laser-assisted bioprinting. Biofabrication 2022, 14, 024104. [CrossRef]
- Li, W.; Liu, Z.; Tang, F.; Jiang, H.; Zhou, Z.; Hao, X.; Zhang, J.M. Application of 3D Bioprinting in Liver Diseases. Micromachines (Basel) 2023, 14. [CrossRef]
- Kasturi, M.; Mathur, V.; Gadre, M.; Srinivasan, V.; Vasanthan, K.S. Three Dimensional Bioprinting for Hepatic Tissue Engineering: From In Vitro Models to Clinical Applications. Tissue Eng Regen Med 2024, 21, 21-52. [CrossRef]
- Palakkan, A.A.; Hay, D.C.; Anil Kumar, P.R.; Kumary, T.V.; Ross, J.A. Liver tissue engineering and cell sources: issues and challenges. Liver Int 2013, 33, 666-676. [CrossRef]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. Journal of hepatology 2019, 70, 151-171. [CrossRef]
- Singh, S.; Osna, N.A.; Kharbanda, K.K. Treatment options for alcoholic and non-alcoholic fatty liver disease: A review. World journal of gastroenterology 2017, 23, 6549. [CrossRef]
- Robbins, J.B.; Gorgen, V.; Min, P.; Shepherd, B.R.; Presnell, S.C. A novel in vitro three-dimensional bioprinted liver tissue system for drug development. The FASEB Journal 2013, 27, 872.812-872.812. [CrossRef]
- Chang, R.; Emami, K.; Wu, H.; Sun, W. Biofabrication of a three-dimensional liver micro-organ as an in vitro drug metabolism model. Biofabrication 2010, 2, 045004. [CrossRef]
- Lee, S.Y.; Kim, H.J.; Choi, D. Cell sources, liver support systems and liver tissue engineering: alternatives to liver transplantation. International journal of stem cells 2015, 8, 36-47. [CrossRef]
- Iqbal, S.; Sohail, M.; Fang, S.; Ding, J.; Shen, L.; Chen, M.; Shu, G.; Du, Y.-Z.; Ji, J. Biomaterials evolution: from inert to instructive. Biomaterials Science 2023, 11, 6109-6115. [CrossRef]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D bioprinting: an overview. Biomaterials science 2018, 6, 915-946. [CrossRef]
- Allu, I.; Sahi, A.K.; Koppadi, M.; Gundu, S.; Sionkowska, A. Decellularization techniques for tissue engineering: towards replicating native extracellular matrix architecture in liver regeneration. Journal of Functional Biomaterials 2023, 14, 518. [CrossRef]
- Khati, V.; Turkki, J.A.; Ramachandraiah, H.; Pati, F.; Gaudenzi, G.; Russom, A. Indirect 3D bioprinting of a robust trilobular hepatic construct with decellularized liver matrix hydrogel. Bioengineering 2022, 9, 603. [CrossRef]
- Wang, Q.; Feng, Y.; Wang, A.; Hu, Y.; Cao, Y.; Zheng, J.; Le, Y.; Liu, J. Innovations in 3D bioprinting and biomaterials for liver tissue engineering: Paving the way for tissue-engineered liver. iLIVER 2024, 3, 100080. [CrossRef]
- Yang, H.; Sun, L.; Pang, Y.; Hu, D.; Xu, H.; Mao, S.; Peng, W.; Wang, Y.; Xu, Y.; Zheng, Y.-C.; et al. Three-dimensional bioprinted hepatorganoids prolong survival of mice with liver failure. Gut 2021, 70, 567. [CrossRef]
- Xie, F.; Sun, L.; Pang, Y.; Xu, G.; Jin, B.; Xu, H.; Lu, X.; Xu, Y.; Du, S.; Wang, Y.; et al. Three-dimensional bio-printing of primary human hepatocellular carcinoma for personalized medicine. Biomaterials 2021, 265, 120416. [CrossRef]
- Lewis, P.L.; Green, R.M.; Shah, R.N. 3D-printed gelatin scaffolds of differing pore geometry modulate hepatocyte function and gene expression. Acta Biomaterialia 2018, 69, 63-70. [CrossRef]
- Jeon, H.; Kang, K.; Park, S.A.; Kim, W.D.; Paik, S.S.; Lee, S.H.; Jeong, J.; Choi, D. Generation of Multilayered 3D Structures of HepG2 Cells Using a Bio-printing Technique. Gut Liver 2017, 11, 121-128. [CrossRef]
- Wu, Y.; Wenger, A.; Golzar, H.; Tang, X.S. 3D bioprinting of bicellular liver lobule-mimetic structures via microextrusion of cellulose nanocrystal-incorporated shear-thinning bioink. Sci Rep 2020, 10, 20648. [CrossRef]
- Mao, Q.; Wang, Y.; Li, Y.; Juengpanich, S.; Li, W.; Chen, M.; Yin, J.; Fu, J.; Cai, X. Fabrication of liver microtissue with liver decellularized extracellular matrix (dECM) bioink by digital light processing (DLP) bioprinting. Mater Sci Eng C Mater Biol Appl 2020, 109, 110625. [CrossRef]
- Kim, M.K.; Jeong, W.; Jeon, S.; Kang, H.-W. 3D bioprinting of dECM-incorporated hepatocyte spheroid for simultaneous promotion of cell-cell and -ECM interactions. Frontiers in Bioengineering and Biotechnology 2023, 11. [CrossRef]
- Faulkner-Jones, A.; Fyfe, C.; Cornelissen, D.J.; Gardner, J.; King, J.; Courtney, A.; Shu, W. Bioprinting of human pluripotent stem cells and their directed differentiation into hepatocyte-like cells for the generation of mini-livers in 3D. Biofabrication 2015, 7, 044102. [CrossRef]
- Lee, H.; Han, W.; Kim, H.; Ha, D.-H.; Jang, J.; Kim, B.S.; Cho, D.-W. Development of Liver Decellularized Extracellular Matrix Bioink for Three-Dimensional Cell Printing-Based Liver Tissue Engineering. Biomacromolecules 2017, 18, 1229-1237. [CrossRef]
- Lee, J.W.; Choi, Y.J.; Yong, W.J.; Pati, F.; Shim, J.H.; Kang, K.S.; Kang, I.H.; Park, J.; Cho, D.W. Development of a 3D cell printed construct considering angiogenesis for liver tissue engineering. Biofabrication 2016, 8, 015007. [CrossRef]
- Hiller, T.; Berg, J.; Elomaa, L.; Röhrs, V.; Ullah, I.; Schaar, K.; Dietrich, A.-C.; Al-Zeer, M.A.; Kurtz, A.; Hocke, A.C.; et al. Generation of a 3D Liver Model Comprising Human Extracellular Matrix in an Alginate/Gelatin-Based Bioink by Extrusion Bioprinting for Infection and Transduction Studies. Int J Mol Sci 2018, 19, 3129. [CrossRef]
- Kang, D.; Hong, G.; An, S.; Jang, I.; Yun, W.-S.; Shim, J.-H.; Jin, S. Bioprinting of Multiscaled Hepatic Lobules within a Highly Vascularized Construct. Small 2020, 16, 1905505. [CrossRef]
- Mazzocchi, A.; Devarasetty, M.; Huntwork, R.; Soker, S.; Skardal, A. Optimization of collagen type I-hyaluronan hybrid bioink for 3D bioprinted liver microenvironments. Biofabrication 2018, 11, 015003. [CrossRef]
- Wu, Y.; Lin, Z.Y.; Wenger, A.C.; Tam, K.C.; Tang, X. 3D bioprinting of liver-mimetic construct with alginate/cellulose nanocrystal hybrid bioink. Bioprinting 2018, 9, 1-6. [CrossRef]
- Calonje, J.E.; Brenn, T.; Lazar, A.J.; Billings, S.D. McKee's Pathology of the Skin, 2 Volume Set E-Book; Elsevier Health Sciences: 2018.
- Liu, Y.; Liu, X.; Guo, H.; Wang, X.; Li, A.; Qiu, D.; Gu, Q. 3D bioprinting bioglass to construct vascularized full-thickness skin substitutes for wound healing. Materials Today Bio 2024, 24, 100899. [CrossRef]
- Zöller, N.; Valesky, E.; Butting, M.; Hofmann, M.; Kippenberger, S.; Bereiter-Hahn, J.; Bernd, A.; Kaufmann, R. Clinical application of a tissue-cultured skin autograft: an alternative for the treatment of non-healing or slowly healing wounds? Dermatology 2014, 229, 190-198.
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nature biotechnology 2014, 32, 773-785. [CrossRef]
- Tabriz, A.G.; Douroumis, D. Recent advances in 3D printing for wound healing: A systematic review. Journal of Drug Delivery Science and Technology 2022, 74, 103564. [CrossRef]
- Hong, N.; Yang, G.H.; Lee, J.; Kim, G. 3D bioprinting and its in vivo applications. Journal of Biomedical Materials Research Part B: Applied Biomaterials 2018, 106, 444-459.
- Vijayavenkataraman, S.; Lu, W.; Fuh, J. 3D bioprinting of skin: a state-of-the-art review on modelling, materials, and processes. Biofabrication 2016, 8, 032001. [CrossRef]
- Admane, P.; Gupta, A.C.; Jois, P.; Roy, S.; Chandrasekharan Lakshmanan, C.; Kalsi, G.; Bandyopadhyay, B.; Ghosh, S. Direct 3D bioprinted full-thickness skin constructs recapitulate regulatory signaling pathways and physiology of human skin. Bioprinting 2019, 15, e00051. [CrossRef]
- Jin, R.; Cui, Y.; Chen, H.; Zhang, Z.; Weng, T.; Xia, S.; Yu, M.; Zhang, W.; Shao, J.; Yang, M.; et al. Three-dimensional bioprinting of a full-thickness functional skin model using acellular dermal matrix and gelatin methacrylamide bioink. Acta Biomaterialia 2021, 131, 248-261. [CrossRef]
- Song, Y.; Hu, Q.; Liu, S.; Wang, Y.; Zhang, H.; Chen, J.; Yao, G. Electrospinning/3D printing drug-loaded antibacterial polycaprolactone nanofiber/sodium alginate-gelatin hydrogel bilayer scaffold for skin wound repair. International Journal of Biological Macromolecules 2024, 129705. [CrossRef]
- Ma, J.; Qin, C.; Wu, J.; Zhang, H.; Zhuang, H.; Zhang, M.; Zhang, Z.; Ma, L.; Wang, X.; Ma, B.; et al. 3D Printing of Strontium Silicate Microcylinder-Containing Multicellular Biomaterial Inks for Vascularized Skin Regeneration. Advanced Healthcare Materials 2021, 10, 2100523. [CrossRef]
- Pajooh, A.M.D.; Tavakoli, M.; Al-Musawi, M.H.; Karimi, A.; Salehi, E.; Nasiri-Harchegani, S.; Sharifianjazi, F.; Tavamaishvili, K.; Mehrjoo, M.; Najafinezhad, A.; et al. Biomimetic VEGF-loaded bilayer scaffold fabricated by 3D printing and electrospinning techniques for skin regeneration. Materials & Design 2024, 238, 112714. [CrossRef]
- Zhou, F.; Hong, Y.; Liang, R.; Zhang, X.; Liao, Y.; Jiang, D.; Zhang, J.; Sheng, Z.; Xie, C.; Peng, Z.; et al. Rapid printing of bio-inspired 3D tissue constructs for skin regeneration. Biomaterials 2020, 258, 120287. [CrossRef]
- Kwak, H.; Shin, S.; Lee, H.; Hyun, J. Formation of a keratin layer with silk fibroin-polyethylene glycol composite hydrogel fabricated by digital light processing 3D printing. Journal of Industrial and Engineering Chemistry 2019, 72, 232-240. [CrossRef]
- Zhao, M.; Wang, J.; Zhang, J.; Huang, J.; Luo, L.; Yang, Y.; Shen, K.; Jiao, T.; Jia, Y.; Lian, W.; et al. Functionalizing multi-component bioink with platelet-rich plasma for customized in-situ bilayer bioprinting for wound healing. Materials Today Bio 2022, 16, 100334. [CrossRef]
- Wu, S.-D.; Dai, N.-T.; Liao, C.-Y.; Kang, L.-Y.; Tseng, Y.-W.; Hsu, S.-h. Planar-/Curvilinear-Bioprinted Tri-Cell-Laden Hydrogel for Healing Irregular Chronic Wounds. Advanced Healthcare Materials 2022, 11, 2201021. [CrossRef]
- Datta, S.; Sarkar, R.; Vyas, V.; Bhutoria, S.; Barui, A.; Roy Chowdhury, A.; Datta, P. Alginate-honey bioinks with improved cell responses for applications as bioprinted tissue engineered constructs. Journal of Materials Research 2018, 33, 2029-2039. [CrossRef]
- Pereira, R.F.; Sousa, A.; Barrias, C.C.; Bártolo, P.J.; Granja, P.L. A single-component hydrogel bioink for bioprinting of bioengineered 3D constructs for dermal tissue engineering. Materials Horizons 2018, 5, 1100-1111. [CrossRef]
- Baltazar, T.; Merola, J.; Catarino, C.; Xie, C.B.; Kirkiles-Smith, N.C.; Lee, V.; Hotta, S.; Dai, G.; Xu, X.; Ferreira, F.C.; et al. Three Dimensional Bioprinting of a Vascularized and Perfusable Skin Graft Using Human Keratinocytes, Fibroblasts, Pericytes, and Endothelial Cells. Tissue Eng Part A 2020, 26, 227-238. [CrossRef]
- Shi, L.; Xiong, L.; Hu, Y.; Li, W.; Chen, Z.; Liu, K.; Zhang, X. Three-dimensional printing alginate/gelatin scaffolds as dermal substitutes for skin tissue engineering. Polymer Engineering & Science 2018, 58, 1782-1790. [CrossRef]
- Fligge, M.; Letofsky-Papst, I.; Bäumers, M.; Zimmer, A.; Breitkreutz, J. Personalized dermal patches – Inkjet printing of prednisolone nanosuspensions for individualized treatment of skin diseases. International Journal of Pharmaceutics 2023, 630, 122382. [CrossRef]
- Cadena, M.; Ning, L.; King, A.; Hwang, B.; Jin, L.; Serpooshan, V.; Sloan, S.A. 3D Bioprinting of Neural Tissues. Adv Healthc Mater 2021, 10, e2001600. [CrossRef]
- Joung, D.; Lavoie, N.S.; Guo, S.Z.; Park, S.H.; Parr, A.M.; McAlpine, M.C. 3D Printed Neural Regeneration Devices. Adv Funct Mater 2020, 30. [CrossRef]
- Yi, S.; Zhang, Y.; Gu, X.; Huang, L.; Zhang, K.; Qian, T.; Gu, X. Application of stem cells in peripheral nerve regeneration. Burns & trauma 2020, 8, tkaa002. [CrossRef]
- Han, Y.; Yin, J. Industry news: the additive manufacturing of nerve conduits for the treatment of peripheral nerve injury. 2022, 1-3. [CrossRef]
- Zhu, H.; Yao, C.; Wei, B.; Xu, C.; Huang, X.; Liu, Y.; He, J.; Zhang, J.; Li, D. 3D printing of functional bioengineered constructs for neural regeneration: a review. International Journal of Extreme Manufacturing 2023, 5, 042004. [CrossRef]
- Hsieh, F.-Y.; Hsu, S.-h. 3D bioprinting: A new insight into the therapeutic strategy of neural tissue regeneration. Organogenesis 2015, 11, 153-158. [CrossRef]
- Lee, H.-W.; Chen, K.-T.; Li, Y.-C.E.; Yeh, Y.-C.; Chiang, C.-Y.; Lee, I.C. Dual crosslinking silk fibroin/pectin-based bioink development and the application on neural stem/progenitor cells spheroid laden 3D bioprinting. International Journal of Biological Macromolecules 2024, 269, 131720. [CrossRef]
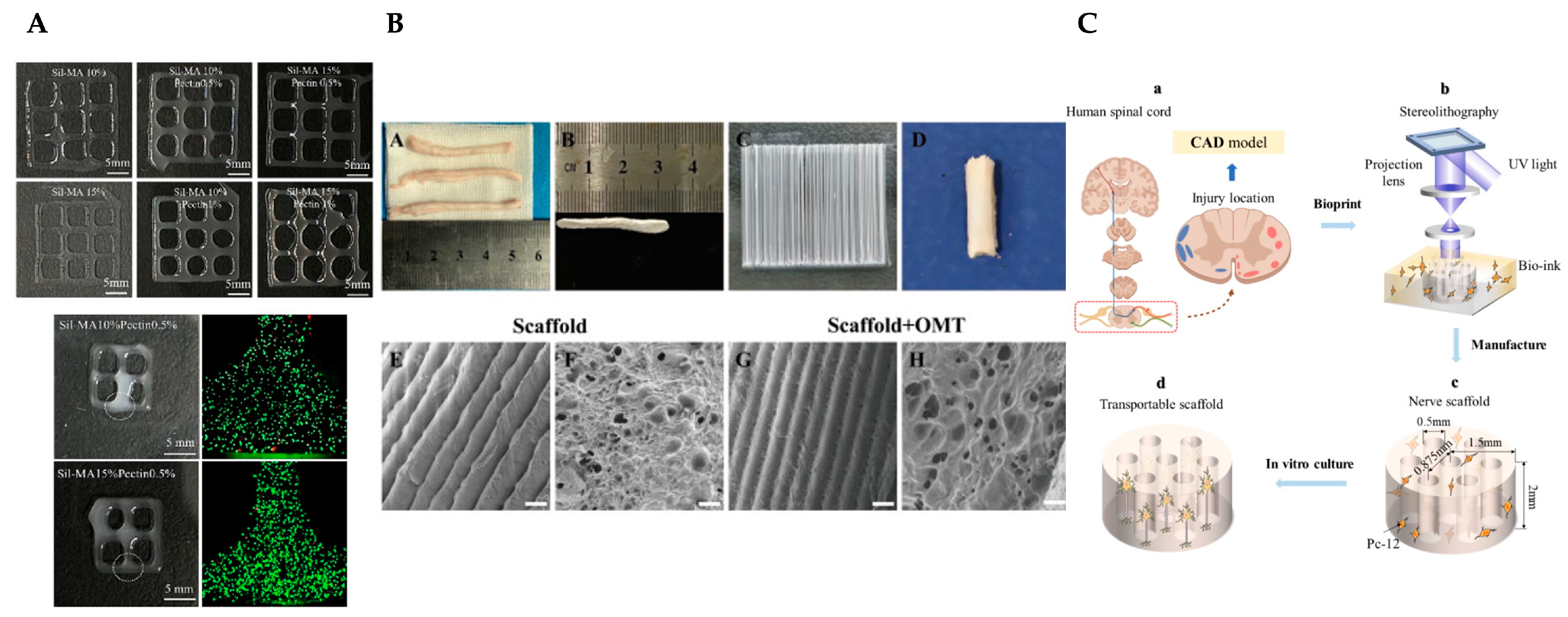
- Song, S.; Zhou, J.; Wan, J.; Zhao, X.; Li, K.; Yang, C.; Zheng, C.; Wang, L.; Tang, Y.; Wang, C.; et al. Three-dimensional printing of microfiber- reinforced hydrogel loaded with oxymatrine for treating spinal cord injury. Int J Bioprint 2023, 9, 692. [CrossRef]
- Li, J.; He, N.; Chen, F.; Han, X. The development of the multi-physical model for cell-laden nerve scaffolds and its bioprinting by stereolithography. Materials Today: Proceedings 2022, 70, 388-394. [CrossRef]
- Hsieh, F.-Y.; Lin, H.-H.; Hsu, S.-h. 3D bioprinting of neural stem cell-laden thermoresponsive biodegradable polyurethane hydrogel and potential in central nervous system repair. Biomaterials 2015, 71, 48-57. [CrossRef]
- Yan, Y.; Li, X.; Gao, Y.; Mathivanan, S.; Kong, L.; Tao, Y.; Dong, Y.; Li, X.; Bhattacharyya, A.; Zhao, X.; et al. 3D bioprinting of human neural tissues with functional connectivity. Cell Stem Cell 2024, 31, 260-274.e267. [CrossRef]
- Tortorella, S.; Greco, P.; Valle, F.; Barbalinardo, M.; Foschi, G.; Lugli, F.; Dallavalle, M.; Zerbetto, F.; Bortolotti, C.A.; Biscarini, F. Laser Assisted Bioprinting of laminin on biodegradable PLGA substrates: Effect on neural stem cell adhesion and differentiation. Bioprinting 2022, 26, e00194. [CrossRef]
- Ho, L.; Hsu, S.-h. Cell reprogramming by 3D bioprinting of human fibroblasts in polyurethane hydrogel for fabrication of neural-like constructs. Acta Biomaterialia 2018, 70, 57-70. [CrossRef]
- Lee, Y.-B.; Polio, S.; Lee, W.; Dai, G.; Menon, L.; Carroll, R.S.; Yoo, S.-S. Bio-printing of collagen and VEGF-releasing fibrin gel scaffolds for neural stem cell culture. Experimental Neurology 2010, 223, 645-652. [CrossRef]
- Chen, C.; Chang, Z.-H.; Yao, B.; Liu, X.-Y.; Zhang, X.-W.; Liang, J.; Wang, J.-J.; Bao, S.-Q.; Chen, M.-M.; Zhu, P.; et al. 3D printing of interferon γ-preconditioned NSC-derived exosomes/collagen/chitosan biological scaffolds for neurological recovery after TBI. Bioactive Materials 2024, 39, 375-391. [CrossRef]
- Han, Y.; Sun, M.; Lu, X.; Xu, K.; Yu, M.; Yang, H.; Yin, J. A 3D printable gelatin methacryloyl/chitosan hydrogel assembled with conductive PEDOT for neural tissue engineering. Composites Part B: Engineering 2024, 273, 111241. [CrossRef]
- Song, S.; Liu, X.; Huang, J.; Zhang, Z. Neural stem cell-laden 3D bioprinting of polyphenol-doped electroconductive hydrogel scaffolds for enhanced neuronal differentiation. Biomaterials Advances 2022, 133, 112639. [CrossRef]
- Liu, J.; Zhang, B.; Li, L.; Yin, J.; Fu, J. Additive-lathe 3D bioprinting of bilayered nerve conduits incorporated with supportive cells. Bioactive Materials 2021, 6, 219-229. [CrossRef]
- Vijayavenkataraman, S.; Thaharah, S.; Zhang, S.; Lu, W.F.; Fuh, J.Y.H. 3D-Printed PCL/rGO Conductive Scaffolds for Peripheral Nerve Injury Repair. Artificial Organs 2019, 43, 515-523. [CrossRef]
- Heinrich, M.A.; Liu, W.; Jimenez, A.; Yang, J.; Akpek, A.; Liu, X.; Pi, Q.; Mu, X.; Hu, N.; Schiffelers, R.M. 3D bioprinting: from benches to translational applications. Small 2019, 15, 1805510. [CrossRef]
- Stanco, D.; Urbán, P.; Tirendi, S.; Ciardelli, G.; Barrero, J. 3D bioprinting for orthopaedic applications: Current advances, challenges and regulatory considerations. Bioprinting 2020, 20, e00103. [CrossRef]
- Agarwal, K.; Srinivasan, V.; Lather, V.; Pandita, D.; Vasanthan, K.S. Insights of 3D bioprinting and focusing the paradigm shift towards 4D printing for biomedical applications. Journal of Materials Research 2023, 38, 112-141. [CrossRef]
- Jafarkhani, M.; Salehi, Z.; Aidun, A.; Shokrgozar, M.A. Bioprinting in Vascularization Strategies. Iran Biomed J 2019, 23, 9-20.
- Jain, R.K.; Au, P.; Tam, J.; Duda, D.G.; Fukumura, D. Engineering vascularized tissue. Nat Biotechnol 2005, 23, 821-823. [CrossRef]
- Zheng, K.; Chai, M.; Luo, B.; Cheng, K.; Wang, Z.; Li, N.; Shi, X. Recent progress of 3D printed vascularized tissues and organs. Smart Materials in Medicine 2024, 5, 183-195. [CrossRef]
- Ma, Y.; Deng, B.; He, R.; Huang, P. Advancements of 3D bioprinting in regenerative medicine: Exploring cell sources for organ fabrication. Heliyon 2024, 10, e24593. [CrossRef]
- Inglis, S.; Christensen, D.; Wilson, D.I.; Kanczler, J.M.; Oreffo, R.O. Human endothelial and foetal femur-derived stem cell co-cultures modulate osteogenesis and angiogenesis. Stem cell research & therapy 2016, 7, 1-16. [CrossRef]
- Lovett, M.; Lee, K.; Edwards, A.; Kaplan, D.L. Vascularization strategies for tissue engineering. Tissue Eng Part B Rev 2009, 15, 353-370. [CrossRef]
- Bishop, E.S.; Mostafa, S.; Pakvasa, M.; Luu, H.H.; Lee, M.J.; Wolf, J.M.; Ameer, G.A.; He, T.-C.; Reid, R.R. 3-D bioprinting technologies in tissue engineering and regenerative medicine: Current and future trends. Genes & Diseases 2017, 4, 185-195. [CrossRef]
- Persaud, A.; Maus, A.; Strait, L.; Zhu, D. 3D Bioprinting with Live Cells. Engineered Regeneration 2022, 3, 292-309. [CrossRef]
- Martin, I.; Simmons, P.J.; Williams, D.F. Manufacturing challenges in regenerative medicine. Science translational medicine 2014, 6, 232fs216-232fs216. [CrossRef]
- Jin, Z.; Li, Y.; Yu, K.; Liu, L.; Fu, J.; Yao, X.; Zhang, A.; He, Y. 3D Printing of Physical Organ Models: Recent Developments and Challenges. Advanced Science 2021, 8, 2101394. [CrossRef]
- Hinton, T.J.; Jallerat, Q.; Palchesko, R.N.; Park, J.H.; Grodzicki, M.S.; Shue, H.-J.; Ramadan, M.H.; Hudson, A.R.; Feinberg, A.W. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Science advances 2015, 1, e1500758. [CrossRef]
- Kang, H.-W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nature biotechnology 2016, 34, 312-319. [CrossRef]
- Murphy, S.V.; De Coppi, P.; Atala, A. Opportunities and challenges of translational 3D bioprinting. Nature Biomedical Engineering 2020, 4, 370-380. [CrossRef]
- Zhang, Y.S.; Oklu, R.; Dokmeci, M.R.; Khademhosseini, A. Three-Dimensional Bioprinting Strategies for Tissue Engineering. Cold Spring Harb Perspect Med 2018, 8. [CrossRef]
- Jones, N. Science in three dimensions: The print revolution. Nature 2012, 487, 22-23. [CrossRef]
- Liang, K. Tissue Bioprinting: Promise and Challenges. Bioengineering (Basel) 2023, 10. [CrossRef]
- Ramadan, Q.; Zourob, M. 3D Bioprinting at the Frontier of Regenerative Medicine, Pharmaceutical, and Food Industries. Frontiers in Medical Technology 2021, 2. [CrossRef]
- Muskan; Gupta, D.; Negi, N.P. 3D bioprinting: Printing the future and recent advances. Bioprinting 2022, 27, e00211. [CrossRef]
- Cui, H.; Nowicki, M.; Fisher, J.P.; Zhang, L.G. 3D bioprinting for organ regeneration. Advanced healthcare materials 2017, 6, 1601118. [CrossRef]
- Ghosh, R.; Pin, K.Y.; Reddy, V.S.; Jayathilaka, W.; Ji, D.; Serrano-García, W.; Bhargava, S.K.; Ramakrishna, S.; Chinnappan, A. Micro/nanofiber-based noninvasive devices for health monitoring diagnosis and rehabilitation. Applied Physics Reviews 2020, 7. [CrossRef]
- Yilmaz, B.; Al Rashid, A.; Mou, Y.A.; Evis, Z.; Koç, M. Bioprinting: A review of processes, materials and applications. Bioprinting 2021, 23, e00148. [CrossRef]
- Koons, G.L.; Mikos, A.G. Progress in three-dimensional printing with growth factors. J Control Release 2019, 295, 50-59. [CrossRef]
- Zhang, Y.S.; Haghiashtiani, G.; Hübscher, T.; Kelly, D.J.; Lee, J.M.; Lutolf, M.; McAlpine, M.C.; Yeong, W.Y.; Zenobi-Wong, M.; Malda, J. 3D extrusion bioprinting. Nature Reviews Methods Primers 2021, 1, 75. [CrossRef]
- Abbadessa, A.; Ronca, A.; Salerno, A. Integrating bioprinting, cell therapies and drug delivery towards in vivo regeneration of cartilage, bone and osteochondral tissue. Drug Deliv Transl Res 2024, 14, 858-894. [CrossRef]
- Reddy, V.S.; Tian, Y.; Zhang, C.; Ye, Z.; Roy, K.; Chinnappan, A.; Ramakrishna, S.; Liu, W.; Ghosh, R. A review on electrospun nanofibers based advanced applications: From health care to energy devices. Polymers 2021, 13, 3746. [CrossRef]
- Bejoy, A.M.; Makkithaya, K.N.; Hunakunti, B.B.; Hegde, A.; Krishnamurthy, K.; Sarkar, A.; Lobo, C.F.; Keshav, D.; Dharshini, G.; Mascarenhas, S. An insight on advances and applications of 3d bioprinting: A review. Bioprinting 2021, 24, e00176. [CrossRef]
- Ozbolat, I.T.; Peng, W.; Ozbolat, V. Application areas of 3D bioprinting. Drug discovery today 2016, 21, 1257-1271. [CrossRef]
- De Santis, M.M.; Alsafadi, H.N.; Tas, S.; Bölükbas, D.A.; Prithiviraj, S.; Da Silva, I.A.; Mittendorfer, M.; Ota, C.; Stegmayr, J.; Daoud, F. Extracellular-matrix-reinforced bioinks for 3D bioprinting human tissue. Advanced materials 2021, 33, 2005476.
- Reddy, V.S.; Ramasubramanian, B.; Telrandhe, V.M.; Ramakrishna, S. Contemporary standpoint and future of 3D bioprinting in tissue/organs printing. Current Opinion in Biomedical Engineering 2023, 27, 100461. [CrossRef]
- Veiga, A.; Silva, I.V.; Duarte, M.M.; Oliveira, A.L. Current Trends on Protein Driven Bioinks for 3D Printing. Pharmaceutics 2021, 13, 1444. [CrossRef]
- Gao, B.; Yang, Q.; Zhao, X.; Jin, G.; Ma, Y.; Xu, F. 4D Bioprinting for Biomedical Applications. Trends in Biotechnology 2016, 34, 746-756. [CrossRef]
- An, J.; Chua, C.K.; Mironov, V. A perspective on 4D bioprinting. IJB 2. [CrossRef]
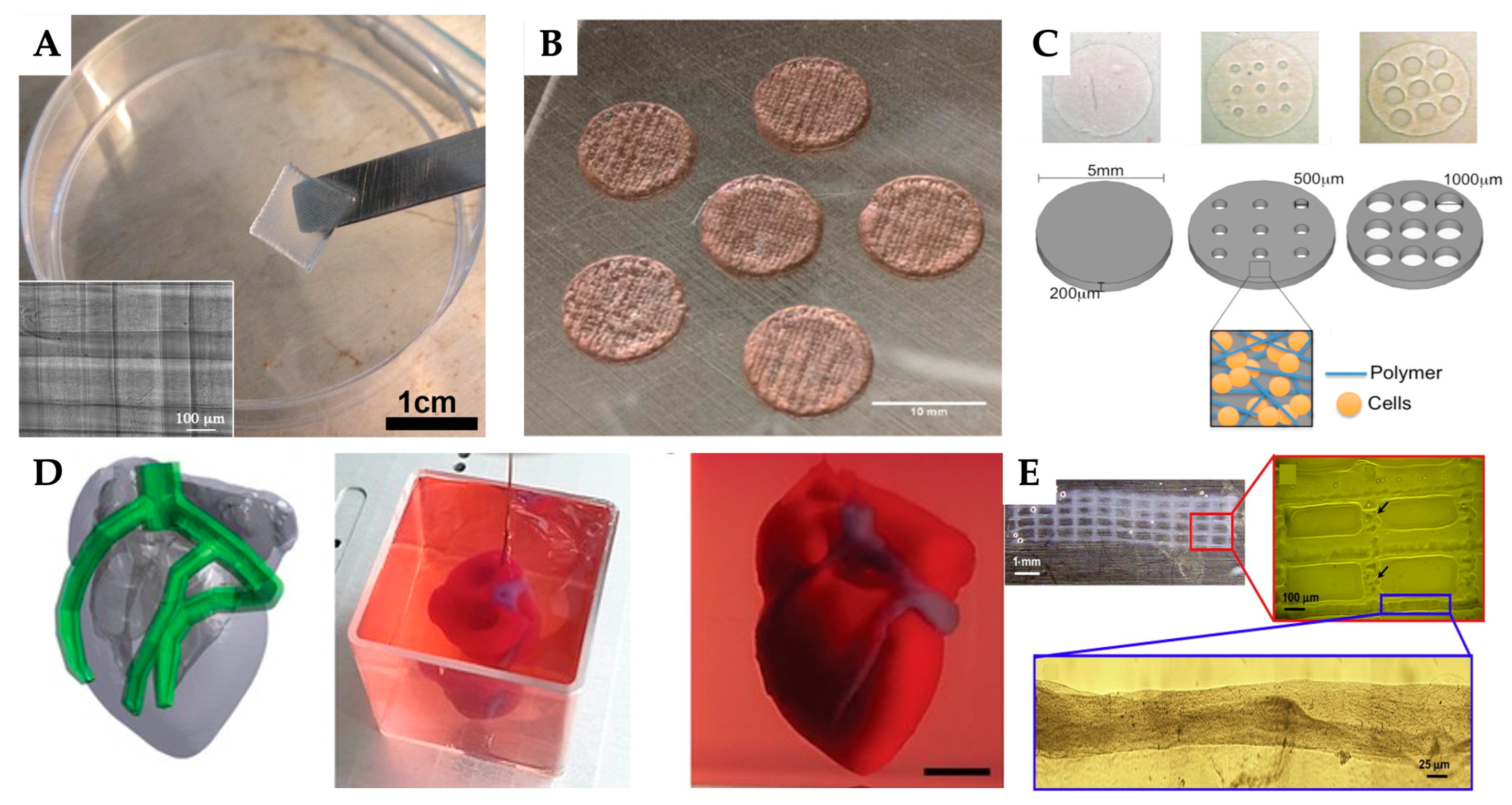
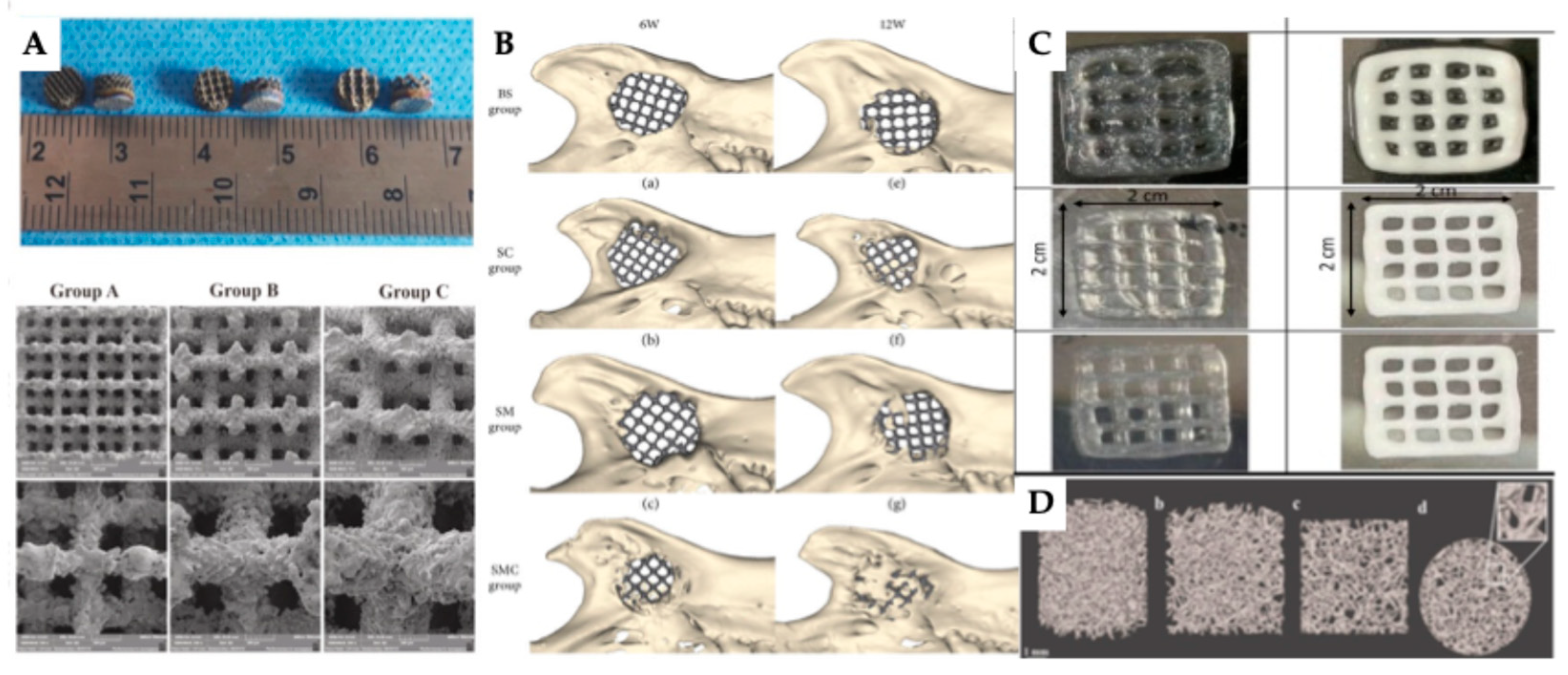
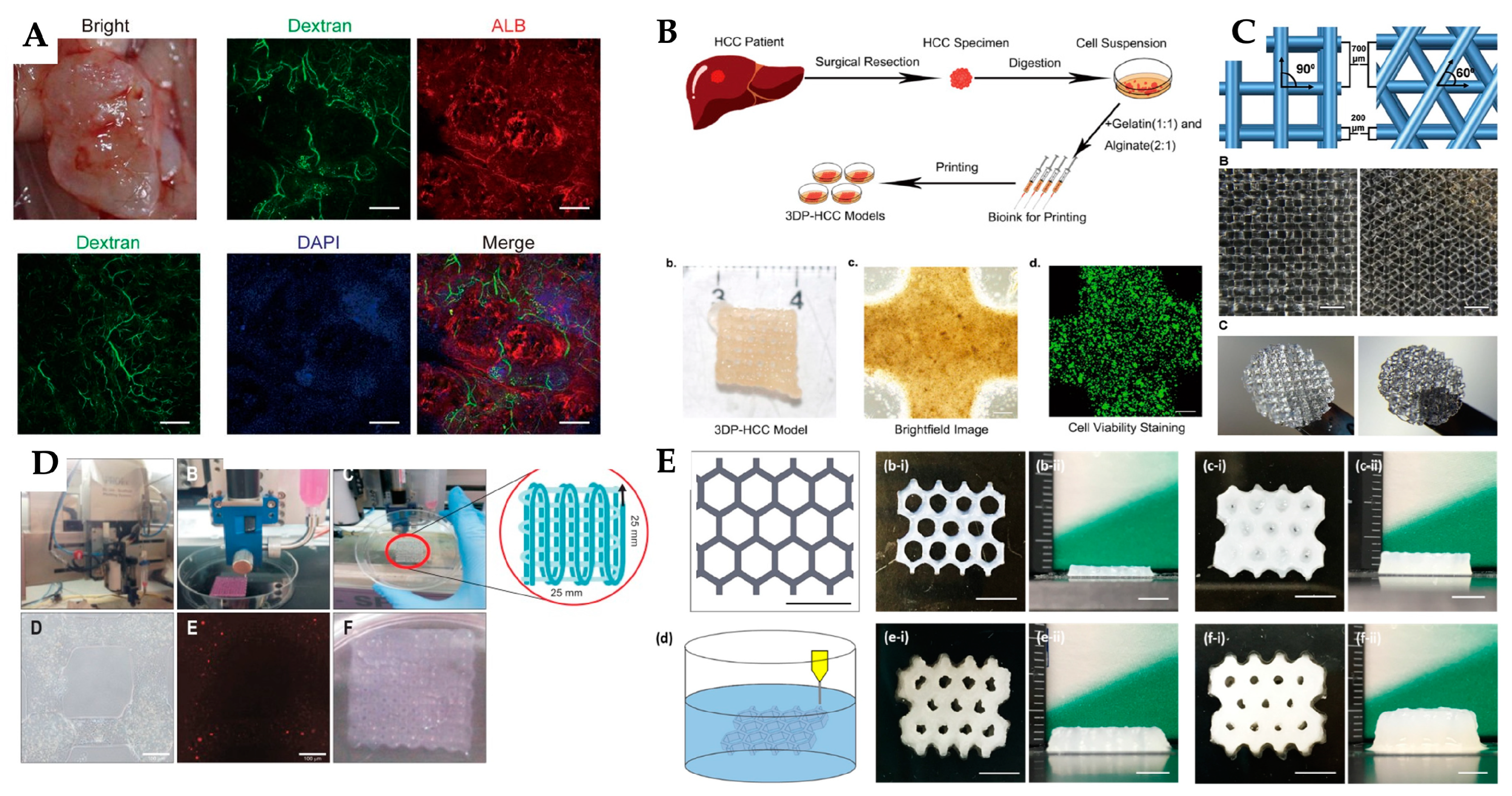
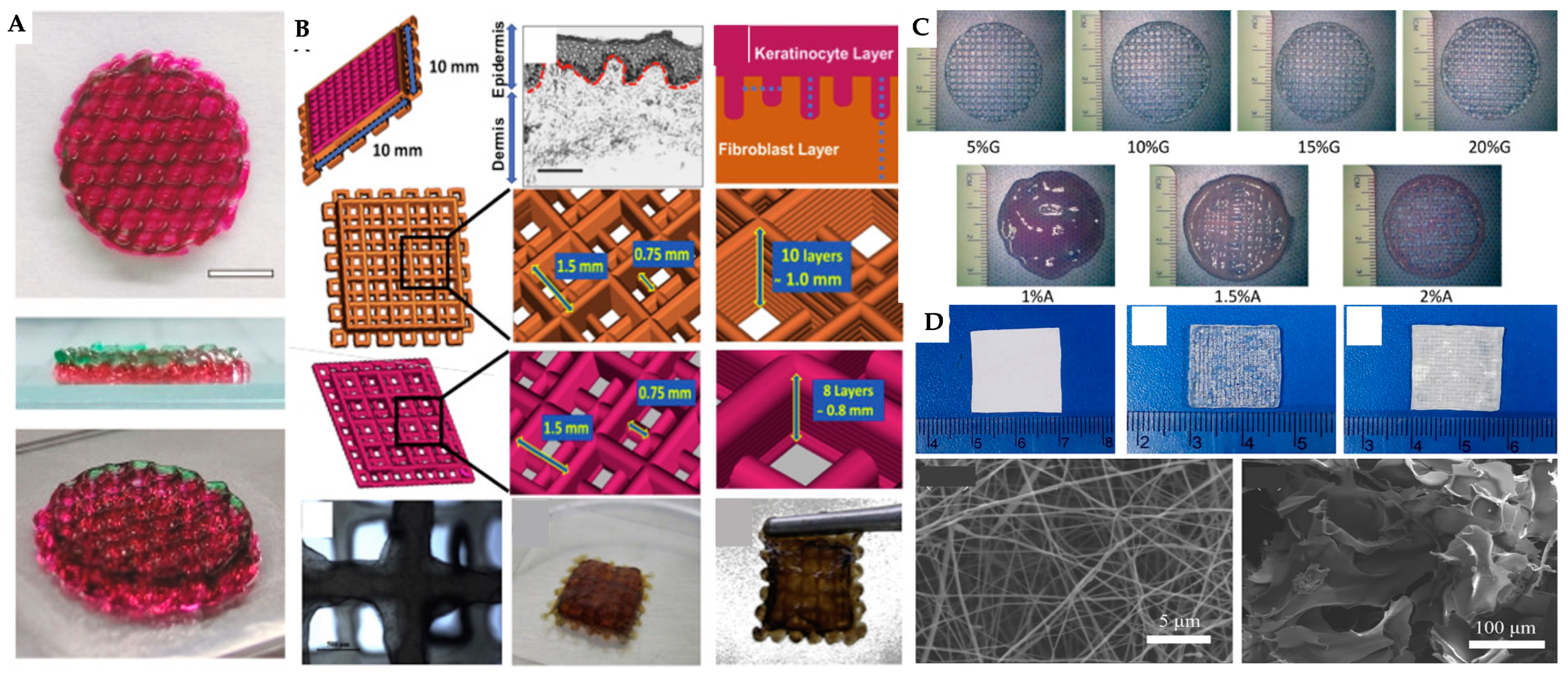
| 3D printing technology | Biomaterials | Cellular Therapies | Application | Outcomes | Reference |
|---|---|---|---|---|---|
| Inkjet Printing | Fibrin gel | hMVECs | Microvasculature construction | The construction promoted hMVEC proliferation and microvasculature formation. | [170] |
| Extrusion 3D Bioprinting |
NovoGel | mEFs | Aortic tissue construct | Support structure and mEF were successfully printed with the self-supporting approach. | [171] |
| Alginate | hCMPCs | Construction with cardiogenic potential for use in vitro and in vivo | The printed hCMPCs were able to migrate in the alginate matrix while maintaining their functional properties. | [172] | |
| Alginate/ Gelatin | hUVECs, H9c2 cells | Cardiac tissue engineering | A novel angular structure mimicking the orientation of the heart fibers presents high cell viability (for both hUVECs and H9c2 cells), high mechanical strength, and suitable dilation and degradation properties. | [173] | |
| Alginate/Gelatin | iCMs, CFs, hCAECs | Cardiac tissue patches | 3D bioprinted AlgGel-based patches for epicardial transplantation improved cardiac function. | [174] | |
| GelMA, Alginate | hUVECs | Endothelialized myocardial tissues | New method for generating endothelialised fabricated organoids. | [175] | |
| GelMA | cECM, hCPCs | Cardiac tissue patches | Cardiac patches printed with cECM and GelMA demonstrated significantly higher viability of hCPCs and exhibited a 30-fold increase in the expression of cardiogenic genes compared to patches composed solely of GelMA. In vivo studies on rats revealed vascularization over a period of 14 days. | [167] | |
| Collagen | hdECM, NRCMs | Cardiac tissue model | It promoted early differentiation and improved the maturation of cardiomyocytes in hdECM. | [176] | |
| 3D bioprinting with microfluidic printing head | Alginate, PF | iPSC-CMs, hUVECs | Vascularized cardiac tissue | A 3D cardiac tissue comprising iPSC-CMs with a high orientation index caused by the different defined geometries and blood vessel-like shapes produced by hUVECs. | [166] |
| DLP-based 3D printing | PGS/PCL/Gelatin | hUVECs | Heart valve substitute | A crosslinked 3D valve analog with elastomeric characteristics. | [177] |
| FDM 3D printing | PGS/PCL | n/a | Myocardial remodeling | It improved and preserved heart function. |
[178] |
| SLA | PEGDMA | BMSCs | Gel patch to damaged cardiac tissue | Placement of the MSC-laden, microchanneled gel patch improved in the ejection fraction, fractional shortening, and stroke volume. | [168] |
| Pneumatic-extrusion | Fibrinogen, gelatin, aprotinin, HA | hESCs-derived EVCs | Cardiac tissues | Spheroids derived from hESC-derived endothelial and EVCs offer greater potential for engineering complex vascular structures compared to single cells. | [179] |
| Micro-continuous optical printing | GelMA, HAGM | hiPSC-CMs | Cardiac micro-tissue (for drug testing) | The micro-tissue exhibited a well-organized sarcomere structure and a marked upregulation in the expression of maturity markers. | [180] |
| Pneumatic 3D printing | Fibrinogen, gelatin, aprotinin, glycerol, HA | NRCMs | Functional and contractile cardiac tissue constructs | They produced an organised structure with physiological and biomechanical characteristics similar to native heart tissue. | [181] |
| 3D printing technology | Biomaterials | Cellular Therapies | Application | Outcomes | Reference |
|---|---|---|---|---|---|
| SLM | Ti6Al4V, Matrigel | BMSCs | Mandibular bone defect reconstruction | Scaffold-Matrigel-BMSCs with enhanced bioactivity and mechanical properties for bone repair. | [192] |
| 3D bioprinting | Alginate, β-TCP | MG-63 fibroblasts | Bone defects | 10 % alginate/β-TCP improved cells proliferation and alkaline phosphatase activity | [193] |
| GelMA, Sr-CSH | BMSCs | Critical-size bone defects | GelMA-Sr–CSH scaffolds indicated complete regeneration of critical-size bone defects. | [195] | |
| Alginate, PLGA | BMP-2, hUCMSCs | Bone tissue engineering | hUCMSCs printing with Alginate/ BMP-2 loaded into PLGA improved osteogenesis of the printed cells as demonstrated by higher rates of ALP activity, calcium deposition, expression of genes associated with osteogenesis, and mineralization when compared with controls. | [196] | |
| Vat photopolymerization | HA multi-substituted with Mg2+, Sr2+, and Zn2+ ions | n/a | Cancellous bone defects | It is a suitable technique for manufacturing highly complex trabecular structures for bone regeneration. | [194] |
| Extrusion 3D Bioprinting | PCL, ACP | n/a | Bone defects | It presented suitable properties, such as compressive strength, pore size, rigidity and repeatability (related to the interconnectivity between the pores). | [50] |
| GelMA | DPSCs | Bone regeneration | DPSCs in GelMA bioprinted presented better osteogenic differentiation potential | [197] | |
| PCL, HAp, PEGDA | DPSCs | Bone regeneration | PCL/HANp/PEGDA revealed hydrophilic properties, suitable mechanical performance and significantly higher cell viability than the other groups. | [188] | |
| DLP-based bioprinting | GelMA, dextran emulsion | BMSCs | Bone regeneration | It promoted the proliferation, migration, dissemination and differentiation of encapsulated BMSCs. | [198] |
| Laser Powder Bed Fusion | Ti6Al4V, Ta | r-BMSCs | Orthopedic clinical applications | It is mechanically compatible, favourable to the adhesion, proliferation and differentiation of r-BMSC in osteoblasts. | [191] |
| PCL, HAp | BMSCs | Human mandibular trabecular bone regeneration | The biodegradable structures (with DO and RD elementary unit cell geometry) demonstrated their suitability as supports for bone regeneration. | [199] | |
| Binder-jetting | Fe-Mn-Ca, Mg | n/a | Cranio-maxillofacial bone defects | The Fe-Mn and Fe-Mn-1Ca constructs confirmed higher degradation from the addition of Ca to the 3D printed constructs and good cytocompatibility. | [200] |
| Laser Directed Energy Deposition | Commercially pure Ti | n/a | Dental restoration | Commercially pure grade 4 titanium produced by Laser Directed Energy Deposition has a higher mechanical response than other techniques, which can be attributed to the modification of the microstructure inherent in the process. | [201] |
| Ti-Nb, Ti-Zr-Nb | n/a | Orthopedic and dental applications | Ti-35Zr-25Nb presented a lower modulus of elasticity, higher hardness, good corrosion resistance and in vitro biocompatibility. | [202] | |
| Sheet lamination | AISI 302 | n/a | Bone regeneration | The scaffold prototype was designed and fabricated with the parameters selected through experimental tests and using the mathematical model. | [203] |
| Laser-assisted bioprinting | BioRoot RCS® | SCAPs, hUVECs | Bone regeneration | The application of laser-assisted bioprinting techniques with this ink failed to provide complete bone repair, whether the SCAPs were printed in direct proximity or not. | [204] |
| 3D printing technology | Biomaterials | Cellular Therapies | Application | Outcomes | Reference |
|---|---|---|---|---|---|
| DLP bioprinting | GelMA | dECM, hiHep cells | Hepatic functional restoration | It was found that the addition of hepatic dECM to the biotints improved printing capacity and cell viability. Moreover, hiHep cells spread more and showed better functions in the liver microtissue. | [223] |
| 3D bioprinting | HA, alginate, gelatin | dECM, PMHs | Functional in vitro liver tissue models | The inclusion of dECM enhanced the hepatic function of hepatocyte spheroids. | [224] |
| Alginate | hiPSCs, hESCs | Mini-liver tissue structures | Using this approach, researchers have been successfully bioprinting hPSCs whilst preserving their pluripotency or directing their differentiation towards specific cell types. | [225] | |
| NovoGel 2.0 | iPSC-derived hepatocyte | Functional in vitro liver tissue models | It demonstrated a method for rapidly fabricating multicellular 3D liver constructs in a multi-well format, displaying critical liver functions such as albumin production, cholesterol biosynthesis, fibrinogen and transferrin production and inducible CYP 1A2 and CYP 3A4 activities. | [210] | |
| Alginate | HepaRG cells | Hepatorganoids: Liver tissue model | The transplantation of 3DP-HOs significantly improved the survival of mice (with liver failure) | [218] | |
| Gelatin, Alginate | Primary HCC cell lines | In vitro models for patient-specific drug screening for HCC | The generated models preserved the key characteristics of the original HCCs, including consistent biomarker expression, stable genetic alterations, and maintained expression profiles. | [219] | |
| PCL | dECM, BMMSCs, HepG2 | 3D cell printing-based liver tissue engineering | The liver dECM bioink proved to have excellent printing capacity without significant cell death during the process. It also improved stem cell differentiation and HepG2 cell function. | [226] | |
| Alginate | HepG2 | Reconstruction of liver tissues or organs | The cells proliferated well in the scaffold and the expression of liver-specific genes increased. | [221] | |
| GelMA, alginate, CNC | NIH/3T3 fibroblasts, hepG2 | Bicellular liver lobule-mimetic structures | NIH/3T3 cells proliferated within the rigid 135ACG matrix and aligned along the 135ACG/GelMA interface due to durotaxis, whereas HepG2 cells exclusively formed spheroids within the GelMA matrix. High albumin production was noted in the 3D two-cell co-culture of hepG2 and NIH/3T3, indicating that the improvement in liver cell function can be contributed to soluble chemical factors. | [222] | |
| Pneumatic extrusion | Gelatin | HUH7 | Model hepatocyte system | It has been proved that the scaffold geometry, using well-defined gelatine constructs, modulates hepatocyte function. | [220] |
| PCL, collagen | PrHCs, hUVECs, hLFs | Liver tissue engineering | The 3D cell printed construct comprising a capillary-like network enhanced the protein secretion and metabolism of PrHCs. It demonstrated a great potential for functional liver tissue regeneration. | [227] | |
| Alginate, gelatin | hECM, human HepaRG liver cells | 3D liver model for infection and transduction studies | It was demonstrated that supplementing an alginate/gelatin bioink with hECM enhanced cell viability and hepatic metabolic activity in a 3D-printed humanized liver model. | [228] | |
| Extrusion bioprinting | Alginate | HepG2/C3A, EA.hy926 | Hepatic lobules within a highly vascularized construct | The bioprinting of multiscaled hepatic lobules within a highly vascularized construct was successfully produced, presenting higher albumin secretion, urea production, albumin, MRP2, and CD31 protein levels, when compared to other groups, and cytochrome P450 enzyme activity | [229] |
| HA, Collagen I | L × 2, aHSC | 3D bioprinted liver model | The formulations seemed to facilitate cell viability, in line with the biomatrices used. The printed bioinks containing primary human hepatocytes were monitored over 2 weeks, showing that they maintain urea and albumin production and responded adequately to acetaminophen. | [230] | |
| Alginate, CNCs | Fibroblasts, Human hepatoma cells | Liver-mimetic structures | This bioink showed excellent shear-thinning property, extrudability, and shape fidelity after the deposition. The bioprinting caused minimal cell damage. | [231] |
| 3D printing technology | Biomaterials | Cellular Therapies | Application | Outcomes | Reference |
|---|---|---|---|---|---|
| 3D bioprinting | Collagen, SS, GAM | hDFs, hU-VECs, MUVECs, BALB/3T3 fibroblast | Vascularized Skin substitute | Collagen-2SS-GAM presented a high capacity of blood vessel formation, graft-host integration, and wound repair in vivo. | [242] |
| Extrusion-based 3D printer and electrospinning | Dex-VEGF, Gel-Kr | n/a | Construct to accelerate wound healing | The BL-VEGF structure is presented as an ideal sample for accelerating the healing of full thickness skin wounds. | [243] |
| PCL, AMX, alginate, gelatin | rhEGF | Repair defective skin tissue and wound healing | The PCL-AMX@SG-rhEGF scaffold presented great drug release and antibacterial characteristics. In vitro and in vivo studies suggested that the structure could promote cell adhesion and proliferation and the healing of skin wounds, presenting biocompatibility. | [241] | |
| DLP-based 3D printing | GelMA/HA, NB/LAP | hSFs, hUVECs | Functional living skin | Bioink demonstrated rapid gelation, adjustable mechanical properties, good biocompatibility and adhesion to tissues. The in vivo study demonstrated that the living skin had an immediate defence response and was more effective in promoting dermal regeneration, including the formation of skin appendages, in large animals models. | [244] |
| SF, PEG4A | NIH 3T3 fibroblas, keratinocytes | Artificial skin model | SF-polyethylene glycol hydrogels presented higher cell proliferation, and the thickest keratin layer was produced with SF-PEG4A hydrogels when compared to PEG4A alone. | [245] | |
| Extrusion-based bioprinting (in situ) | Alginate, gelatin, PRP | DFs, ESCs | Wound repair | The addition of PRP enhanced the cellular behaviour of the cells, regulated vascular endothelial cell tube formation and macrophage polarisation in a paracrine manner. In in-situ bioprinting, the addition of PRP accelerated high-quality wound closure, modulated inflammation and started angiogenesis when compared to alginate-gelatine bioink. | [246] |
| Extrusion-based bioprinting | GelMA | ADM, HaCaTs, hUVECs, fibroblasts | Functional skin model | The in vivo results showed that the functional skin model stimulated wound healing and re-epithelialisation, promoted the secretion of dermal extracellular matrix and angiogenesis, and enhanced the quality of wound healing. | [240] |
| PU, gelatin | Fibroblasts, keratinocytes, EPCs | Skin tissue engineering. | Curvilinear-bioprinted hydrogel showed superior structural integrity over planar-bioprinted hydrogel. In the treatment of large and irregular rat skin wounds, the curvilinear-bioprinted tri-cell-laden hydrogel achieved complete repair within 28 days. | [247] | |
| Alginate, honey | 3T3 fibroblast | Skin tissue engineering | The incorporation of honey resulted in bioprinted scaffolds with enhanced cell proliferation, without any visible decrease in printability. | [248] | |
| SF, gelatin | hDFs, hKCs | Full-thickness skin constructs | A silk and gelatine bioink have been printed that can be used to recapitulate a series of biological and design parameters comparable to human skin. | [239] | |
| 3D Bioprinting | PecMA | hNDFs | Biomimetic skin constructs | The hydrogels produced showed to be highly versatile, allowing fine-tuning of the rheological and viscoelastic properties. Furthermore, they provided a suitable microenvironment that supports the deposition of endogenous ECM, rich in collagen and fibronectin, by entrapped hNDFs. | [249] |
| Rat tail type I collagen | hDFs, hKCs, hECs, hPCs, | Multilayered vascularized human skin grafts | In vitro, hKCs replicate and mature to become a multi-layered barrier, whilst hECs and hPCs self-assemble into interconnected microvascular networks. In vivo, the presence of hPCs in the printed dermis increased the invasion of the graft by the host's microvessels and the creation of an epidermal network. | [250] | |
| PSCs, alginate, gelMA, gelatin | hUVECS, hUCMSCs | Skin tissue grafts | The incorporation of PSCs revealed enhanced cell proliferation and higher expression of genes related to angiogenesis in vitro. In vivo experiments showed an improved wound healing effect, characterized by an increase in angiogenesis and collagen deposition. | [233] | |
| Pneumatic-assisted extrusion freeforming | Alginate, gelatin | hSFs | Bioactive dermal substitute scaffold | The three-phase crosslinking technique can produce dermal substitute supports with physicochemical and biological properties suitable to be used in skin tissue engineering. | [251] |
| Inkjet printing | PLGA, prednisolone | n/a | Personalized dermal patches for treatment of skin diseases | The first steps have been made towards the concept of manufacturing personalized patches by inkjet printing that can be made for poorly soluble pharmaceuticals. The model drug prednisolone was successfully processed and printed in the format of a nanosuspension. The PLGA nanoparticles and the patches produced demonstrated a prolonged release of the pharmaceutical. | [252] |
| 3D printing technology | Biomaterials | Cellular Therapies | Application | Outcomes | Reference |
|---|---|---|---|---|---|
| Extrusion-based 3D Bioprinting | SilMA, Pectin, PecMA, SF | NSPCs | Neural tissue engineering applications or in vitro brain models | The SilMA/pectin biotints showed adjustable mechanical properties, biocompatibility and a highly propitious environment for neural induction. | [259] |
| PU | NSCs | Neural tissue engineering | NSCs proliferated and differentiated favourably in PU2 bioprinted hydrogels. In the in vivo model of neural injury in the zebrafish embryo, the injection of hydrogels loaded with NSCs promoted the repair of the damaged central nervous system. Additionally, the function of adult zebrafish with traumatic brain injury was recovered after the injection of constructs with NSCs. | [262] | |
| Gelatin, alginate, fibrinogen, nanofibrillate cellulose, matrigel, HA | NPCs, astrocytes | Study the connection between human neuronal networks, model pathological processes and provide a platform for drug testing. | Imprinted neuronal progenitors differentiate into neurons and form functional neuronal circuits within and among tissue layers with specificity within weeks. | [263] | |
| Laser Assisted Bioprinting | PLGA | NE-4C | Artificial neural tissue constructs | The comparative study demonstrated that the topological clue plays a relevant role in the development of clusters on the support, but the bioprinted laminin dots appeared to regulate the strength of the bond between them, paving the way for controlling the functional morphology of artificial neural tissue constructions. | [264] |
| 3D Bioprinting | PU | FoxD3 plasmids, phDFs | Personalized drug screening or neuroregeneration. | The phDFs printed with FoxD3 in the thermo-responsive PU hydrogel demonstrated that they could be reprogrammed and differentiated into a neural tissue-like structure at 14 days after induction. | [265] |
| Collagen, fibrin gel | VEGF, C17.2 | Neural tissue regeneration | The bioprinting of fibrin gel incorporating VEGF supported the sustained release of growth factors on the collagen support. | [266] | |
| 3D printing technology based on low-temperature extrusion | Collagen, chitosan | IFN-γ, NSC-derived exosomes | Neurological recovery after traumatic brain injury | The 3D printed collagen/chitosan structure showed good biological and mechanical properties, providing a suitable microenvironment for the differentiation of NSCs and the secretion of exosomes. Furthermore, this scaffold was involved in multiple pathological processes after traumatic brain injury in rats, significantly enhancing neurodeficiency. | [267] |
| Electrospinning | PCL, OMT, gelatin, PEGDA, β-cyclodextrin | Spinal cord extracellular matrix | Treatment of spinal cord injuries | The manufactured scaffolds led to the differentiation of NSCs into neurons and inhibited their differentiation into astrocytes. These scaffolds created a suitable microenvironment for spinal cord tissue regeneration in vivo and guided the directional growth of axons. Furthermore, transplantation of the scaffolds with OMT enhanced the motor function of rats with spinal cord injuries. | [260] |
| DLP printing | GelMA, chitosan, PEDOT | n/a | Neural tissue repair | The GelMA/Chitosan-PEDOT hydrogel showed good and stable electrical conductivity, enhanced mechanical strength and noticeable biocompatibility. In vivo tests showed that the hydrogels promoted nerve regeneration and helped muscle recovery in the repair of sciatic nerve defects in rats. | [268] |
| Microextrusion-based 3D bioprinting | GelMA, PEGDA, PEDOT, CSMA | NSCs | Nerve injury repair | The 3D bioprinted electroconductive hydrogel showed biocompatibility, suitable mechanical strength and good conductivity, thus promoting the adhesion, growth and proliferation of NSCs. | [269] |
| Additive-lathe 3D bioprinting | GelMA, PEGDA | BMSCs | Peripheral nerve injury repair | An integrated two-layer nerve conduit was obtained, in which the BSMCs inside the printed nerve conduits demonstrated very good cell viability and extended morphology. In vitro culture of PC12 cells revealed that the growth of neurons is significantly enhanced in nerve conduits embedded with BMSCs. | [270] |
| SLA | GelMA, PEGDA | Pc-12 | Multi-physical model for cell-laden nerve scaffolds | This model can predict in advance, in in vitro culture experiments, which are the main areas of cell growth in the neural scaffold and can thus increase the oxygen concentration in these areas in order to further increase the concentration of cells. | [261] |
| Electrohydrodynamic jet | PCL, rGO | PC-12 | Peripheral nerve injury repair | The addition of rGO to scaffolds results in softer materials that enhance neural differentiation. In vitro studies with PC12 cells show increased cell proliferation and improved support for neural differentiation in PCL/rGO scaffolds compared to PCL-only scaffolds, as confirmed by RT-PCR and immunocytochemistry analyses. | [271] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).