1. Introduction
Autism spectrum disorders (ASD), evident from early childhood, are neurodevelopmental conditions characterized by deficits in communication, socialization, and cognitive abilities. Common features include restricted interests and repetitive behaviors, complicating relationships [
1]. Globally, ASD diagnoses in children are increasing, with the U.S. showing a significant rise to 1 in 36 children [
2]. ASD is four times more prevalent in boys than girls [
2], potentially due to male fetuses' greater vulnerability to prenatal stressors [
3,
4,
5]. Causes may involve defective genes, chromosomal abnormalities, medical conditions, viruses, prenatal stressors, and environmental factors affecting brain development and central nervous system function [
1,
2,
3,
4,
5,
6]. Early diagnosis and intervention (birth to 36 months) significantly enhance a child's development [
7]. Diagnosis relies on clinical criteria from the International Classification of Diseases (ICD-10) and the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) [
8,
9]. Symptom severity varies from mild to severe, affecting the level of support needed for social communication and repetitive behaviors [
10].
Families of children with ASD may exhibit characteristics such as advanced parental age [
11,
12,
13,
14], low socioeconomic status [
5,
15], psychiatric history [
16], other neurodevelopmental disorders in siblings [
17], infertility history [
3], maternal exposure to pesticides, toxic chemicals [
18,
19], and loud noises during pregnancy [
3]. Additionally, maternal smoking, alcohol consumption [
20], obstetric complications [
3,
12], hyperemesis, epilepsy, hypertension, polycystic ovary syndrome [
21], fish oil supplementation during pregnancy [
22], birth order [
11,
13], and family size significantly influence autistic children [
23]. Preeclampsia, eclampsia [
24], iron deficiency [
25], and immune stress from maternal inflammation and third-trimester infections like rubella, cytomegalovirus, or Toxoplasma gondii are also identified as ASD risk factors [
1,
6,
21].
Pre-pregnancy overweight, particularly with rapid weight gain during pregnancy, is identified as a risk factor for gestational diabetes [
12,
13]. This condition is linked to neurodevelopmental disorders in offspring [
26].
Research indicates that ASD is frequently linked to multiple gestation [
13], maternal medical interventions during labor, such as oxytocin exposure with epidural analgesia [
27], preterm delivery [
12,
28], and various pregnancy and delivery complications [
21], including cesarean section [
12], abnormal fetal presentation [
13], fetal distress [
13], postpartum hemorrhage due to uterine atony, and prolonged labor [
13]. Newborn studies highlight issues such as low Apgar scores [
13], oxytocin-induced labor, low birth weight [
12,
13,
14,
21], birth asphyxia [
21], infections [
21], epilepsy [
21], and neonatal complications [
13,
21].
Adaptive functioning impairment's level dictates the severity of intellectual disability, making both IQ and adaptive functioning crucial in assessing ASD [
28]. Researchers explored how intrauterine toxicity and labor complications relate to ASD symptoms, IQ, and adaptive functioning [
28].
This study aims to elucidate the role of prenatal factors in the likelihood of an ASD diagnosis and their impact on the subsequent functionality of ASD. Few reports have addressed prenatal influences on the severity of clinical presentations in children with ASD, particularly regarding their adaptive and intellectual functioning, especially in Greece [
29]. Factors such as very low birth weight (< 1500g), premature delivery, and low Apgar scores may directly affect adaptive functioning, while gestational diabetes and advanced parental age were not highlighted [
14,
29]. Hadjkacem et al. [
30] found no significant association between clinical severity and prenatal factors in children with ASD. Conversely, another study indicated that preeclampsia and gestational diabetes are associated with greater overall severity, especially in stereotypical behavior and socio-communicative impairments in children with ASD [
31].
Our research aims to enhance existing literature and address research gaps by identifying and analyzing correlations between various study variables. It seeks to provide prenatal data on ASD in Greece to support evidence-based planning and resource development nationally and regionally. Specifically, the objectives are to estimate ASD prevalence in Greece and identify regional disparities in children aged 4 to 7. The primary goal is to investigate prenatal risk factors associated with the emergence of ASD in children, along with autism functioning.
The prevalence of ASD has significantly risen over the past 20 years [
32], posing serious social challenges due to population growth and escalating rehabilitation costs. Our findings are likely to support the theory that ASD is multifactorial, with certain risk factors demonstrating statistical significance. This research was conducted to examine whether different prenatal factors, obtained from maternal self-questionnaires, are correlated with increased severity in the clinical presentation of ASD, as well as with intellectual and adaptive functioning, in a group of children who were diagnosed with ASD and underwent comprehensive clinical evaluations.
2. Materials and Methods
The present research employed a quantitative, descriptive, and exploratory methodology. The study’s sample consisted of mothers residing in various regions of Greece, whose children were diagnosed with ASD. Only mothers participated in the study, as they served as the primary caregivers for their children and had extensive knowledge of their pregnancy, delivery, and postpartum experiences.
A structured questionnaire was developed to gather data, incorporating closed-ended and open-ended questions. The questionnaire provided anonymized data on the number of autism cases, gender, year of birth, and year of first diagnosis. Mothers of children with ASD completed a questionnaire containing demographic, clinical, and other data, including a Demographic Characteristics Questionnaire, which provided information about their personal medical history, pregnancy, delivery, and postpartum experiences. This research project was carried out by selecting mothers with children diagnosed with ASD, aged 4 - 7 years, from specialized schools, day centers, and Disabled and Special Needs Kids Clubs and Activities, across the nation in Greece, born between 2017 and 2020. A telephone call was made, and an email was sent to the representatives of the aforementioned institutions (the school director, the scientific manager of the day center, or the president of the disabled association) in 340 Greek centers, to explain the study's objectives and goals and to facilitate further communication. The research team contacted each center at least twice during the questionnaire collection period to ensure and minimize errors and biases in the collection process. These centers provide support and counseling to children and their families, intending to promote inclusive education and ensure the optimal integration of every child into the appropriate educational setting. Participants who did not possess adequate proficiency in the Greek language were not included in the study.
The study was retrospective and cross-sectional in design, aimed at examining various aspects of the maternal perinatal period. The researchers aimed to achieve accurate and scientifically reliable outcomes by concentrating on pivotal aspects, including the creation of an appropriate questionnaire and employing a proportionate sampling design for the Greek population. Specifically, for the study's application within the Greek population, the functional status of the children was assessed using three distinct categories of functionality (high, moderate, and low). The data collection phase extended from April 2024 to June 2024.
This research was undertaken upon obtaining approval from the Ethics Committee of the University of West Attica, located in Athens (Reference Number: 29346/08-04-2024), and from the respective specialized schools, day centers, and Disabled and Special Needs Kids Clubs and Activities spanning the nation of Greece. Only members of the research team had access to the data, which were utilized solely for scientific purposes in alignment with the study's objectives. Prior to signing the consent form, participants were informed both verbally and in writing about the study's aim, confidentiality, anonymity, voluntary participation, and the option to withdraw at any time. The protection of the participants' personal data was ensured through the anonymous completion of the questionnaires.
Before disseminating the questionnaires, a robust coding process was implemented to safeguard the anonymity of all individuals involved. An exclusive identifier code was allotted to each participant, which served to replace any personal information on the questionnaire with the assigned code. This code was employed to track the responses without revealing the identity of the respondents. The coding system was designed to ensure that only the principal researcher and the supervisor of the study had access to the key that linked the codes to specific individuals, and this key was stored securely in an encrypted digital format. Additionally, all data collected were entered and analyzed anonymously, with findings reported in aggregate form to further prevent the identification of any participant. These measures were crucial to maintaining the trust of the participants and the integrity of the research process. During the course of the study, participants were provided with either face-to-face or telephone support to assist them in completing the questionnaires, should they encounter any difficulties. The team's cohesion and cooperation facilitated the resolution of any consultation or persuasion difficulties with the groups.
The questionnaire referred to children diagnosed with "Autism spectrum disorders" according to DSM-5 or "Autism" as defined by ICD-10. A child can only be classified as "ASD" based on a clinical diagnosis issued by a public sector child neurologist, child psychiatrist, or developmental pediatrician. The survey questionnaires were disseminated to various agencies in Attica and Thessaly through postal mail, while the distribution to other regions of Greece was conducted within a three-month timeframe at the expense of the research team. The primary objective was to safeguard the personal data of the individuals included in the sample. In furtherance of this objective, each participant was notified of the absolute confidentiality of their personal information, and the researcher team assured them that the data would be utilized exclusively for academic purposes. After completing the distribution process, the leading researcher collected the completed questionnaires via mail and prepaid courier service. With the understanding that they concurred with the aforementioned, participants signed the consent form, and the process continued. The participants in the study provided their informed consent to participate voluntarily and confidentially, as indicated by their completion of the questionnaire. This consent was obtained following a thorough explanation of the study's purpose, nature, and data handling procedures. A total of 517 mothers responded to the invitation between April and June 2024.
Descriptive statistics were calculated to summarize the characteristics of the study sample. This included calculating means, standard deviations (SDs), medians, and ranges for continuous variables such as age, weight, height, gestational age, length of labor, and birth weight. For categorical variables, frequencies and percentages were determined to describe the distribution of family structure, education level, family income, geographic residence, functional level of children with ASD, gender distribution, birth order, and family history of developmental or psychiatric disorders. To compare the characteristics of children with high versus low/ moderate functionality in ASD, inferential statistical tests, including Independent Sample t-tests and Chi-Square Tests were performed.
Furthermore, a binomial logistic regression model was utilized to identify predictors of functionality in children with ASD. In this analysis, we included only the significant variables derived from inferential statistical tests to further account for their potential influence on the functionality outcomes. Odds ratios (OR) and 95% confidence intervals (CI) were reported. The model's fit was evaluated using the Akaike Information Criterion (AIC) and pseudo-R² values (Cox & Snell’s R² and Nagelkerke’s R²). Multicollinearity was assessed using Variance Inflation Factor (VIF) and tolerance. Tolerance values below 0.1 (equivalent to VIF above 10) indicate serious multicollinearity. The model's predictive accuracy, sensitivity, specificity, and area under the curve (AUC) were also assessed to ensure its reliability and discriminatory ability. All analyses were performed in Jamovi (version 2.5.5) and the visualizations were created using R (version 4.4.1). Statistical significance was set at p ≤ 0.05.
3. Results
3.1. Descriptive Statistics of Continuous Variables
Table S1 presents the descriptive statistics for a range of continuous variables pertinent to the study. The mean age of mothers in the sample was 40.2 years (SD = 5.42), with a median age of 40 years, and ranging from 21 to 63 years. Fathers were, on average, 43.3 years old (SD = 6.19), with a median age of 43 years, and ages ranging from 24 to 70 years. The mean age of children diagnosed with ASD was 6.89 years (SD = 3.94), with a median of 6 years and an age range of 1 to 28 years. The average age at ASD diagnosis was 3.47 years (SD = 2.29), with a median age of 3 years, ranging from 0 to 24 years.
Pre-pregnancy weight of mothers had a mean of 67.2 kg (SD = 33.4), a median of 63 kg, and ranged from 34 to 761 kg. At the end of pregnancy, the mean weight was 80.4 kg (SD = 29.1), with a median of 78 kg, and ranged from 49 to 651 kg. The mean height at the end of pregnancy was 164 cm (SD = 16.1), with a median of 165 cm, and a range from 1.52 to 188 cm. Fathers' age at conception averaged 36.0 years (SD = 5.61), with a median of 36 years and ranged from 20 to 65 years. Gestational age at birth had a mean of 37.9 weeks (SD = 2.74), with a median of 38 weeks and ranged from 9 to 42 weeks. The mean duration of labor was 4.88 hours (SD = 6.14), with a median of 2 hours and ranged from 0 to 48 hours. The mean birth weight of children was 3089 grams (SD = 744), with a median of 3150 grams and a range of 2.90 to 4850 grams.
3.2. Descriptive Statistics of Sociodemographic Variables
Table S2 summarizes the sociodemographic characteristics of the sample. Most families consisted of two parents living together (90.1%), with a smaller proportion of single-parent families (4.6%). Regarding educational attainment, 43.5% of mothers had tertiary education, followed by 28.4% holding a master's degree. Fathers' education levels showed 47.6% had secondary education, while 35.0% had tertiary education. Annual family income predominantly fell within the €20001-40000 range (42.1%), followed by €10001-20000 (38.1%). The majority of families resided in Attica (36.6%) and Central Macedonia (13.4%), with other regions having smaller representations.
3.3. Descriptive Statistics of ASD-Related Variables
As detailed in
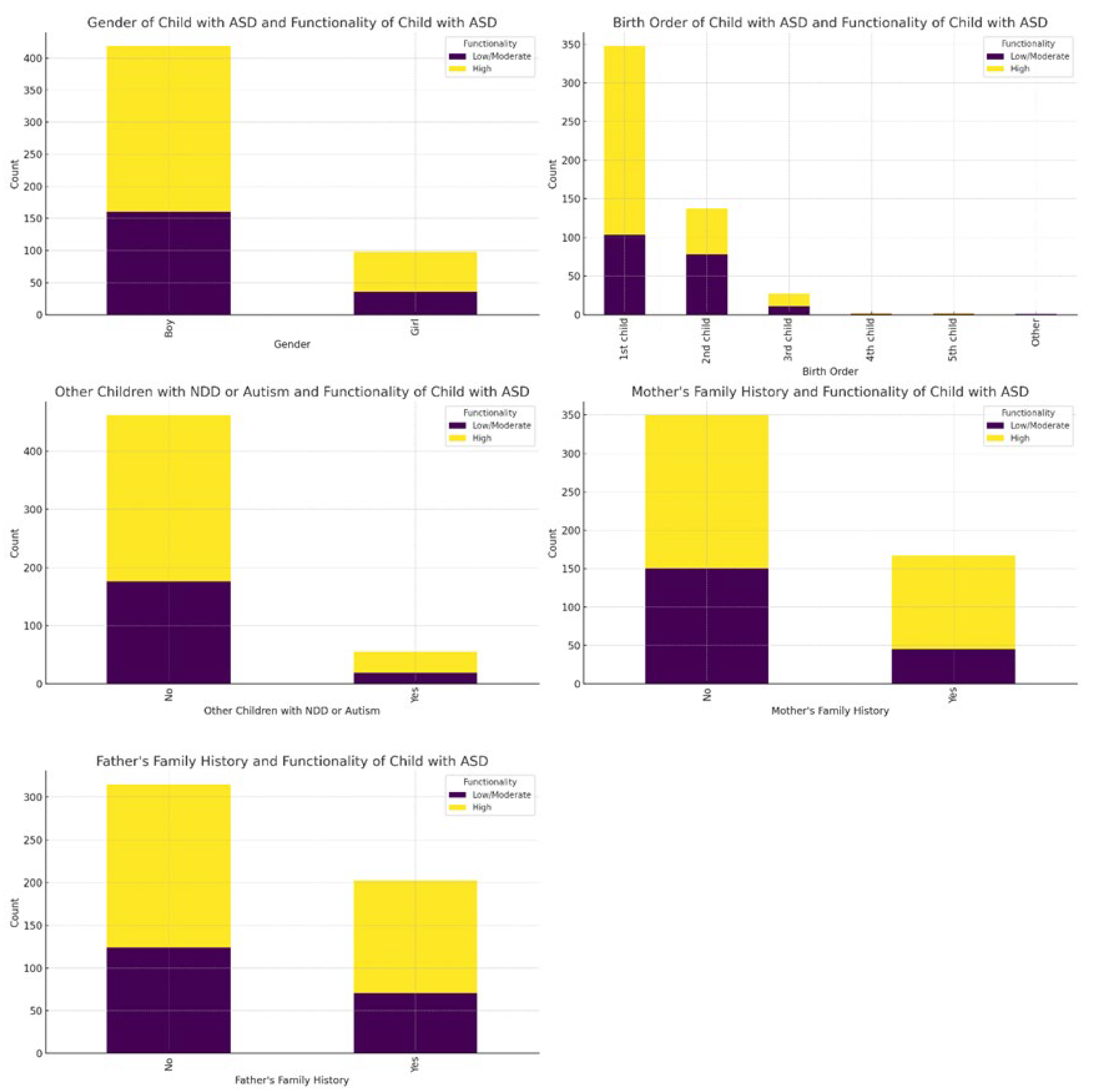
Table S3, the functionality levels of children with ASD were categorized as high (62.3%) and low/moderate (37.7%). A significant majority of the children with ASD were boys (81.0%), with girls comprising 19.0%. Most of these children were the first-born (67.3%), followed by second-born children (26.5%). Only 10.6% of families reported having other children with neurodevelopmental difficulties. Family history showed that 32.3% of mothers and 39.1% of fathers had a history of autism, developmental disorders, epileptic seizures, or depression/anxiety disorders.
3.4. Descriptive Statistics of Peri- and Postnatal Variables
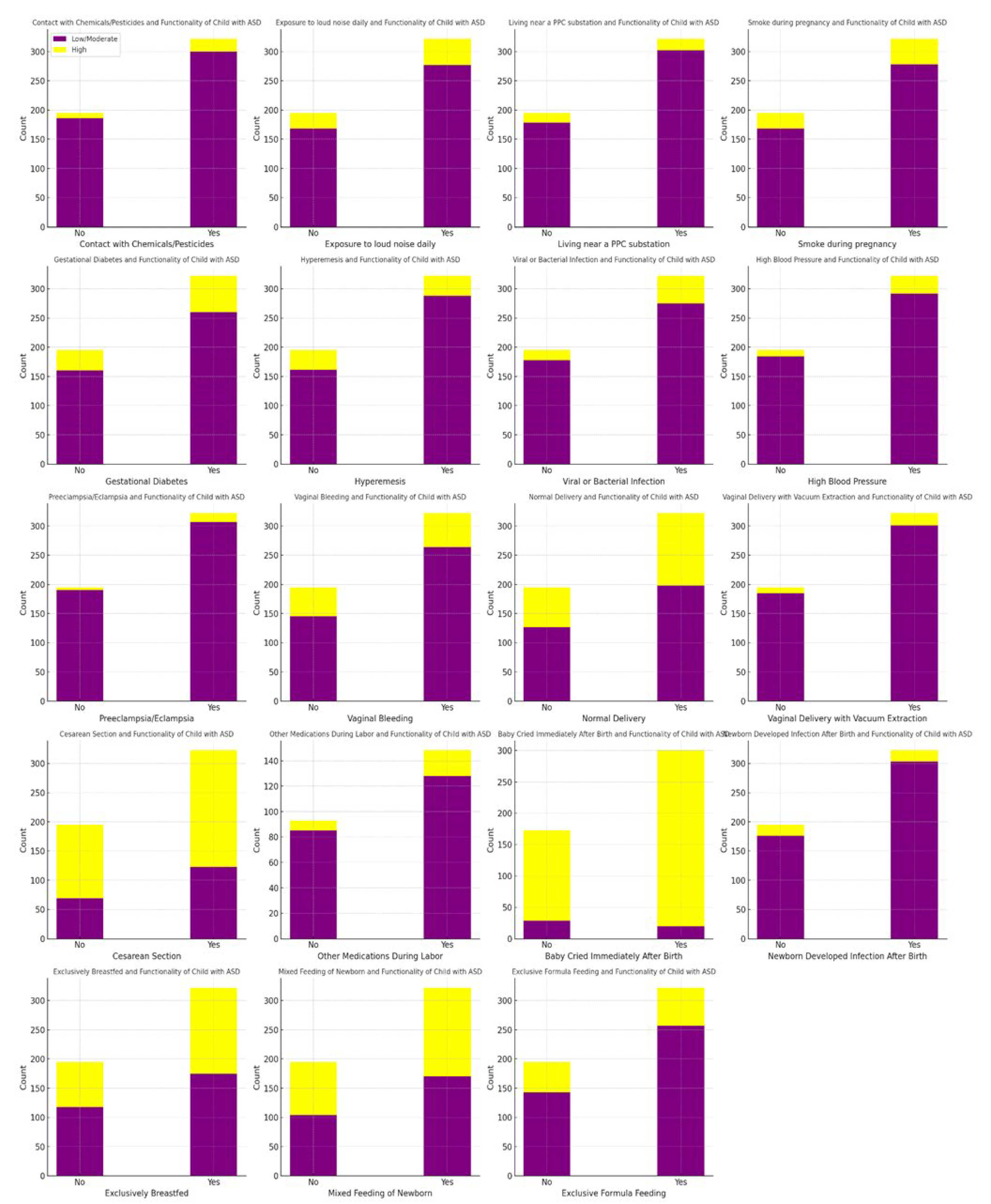
Table S4 provides an overview of peri- and postnatal variables. Exposure to chemicals during pregnancy was reported by 6.0% of mothers, while 19.0% smoked during pregnancy. Gestational diabetes was present in 18.8% of cases, and 13.2% of mothers experienced hyperemesis. Regarding delivery, 62.9% of births were via cesarean section, while 37.1% were normal deliveries. Exclusive breastfeeding was reported for 43.3% of newborns, whereas 47.0% received mixed feeding (breastfeeding and formula). Additionally, 22.6% of newborns were exclusively formula-fed.
3.5. Comparison of Continuous Variables by Functionality of Child with ASD
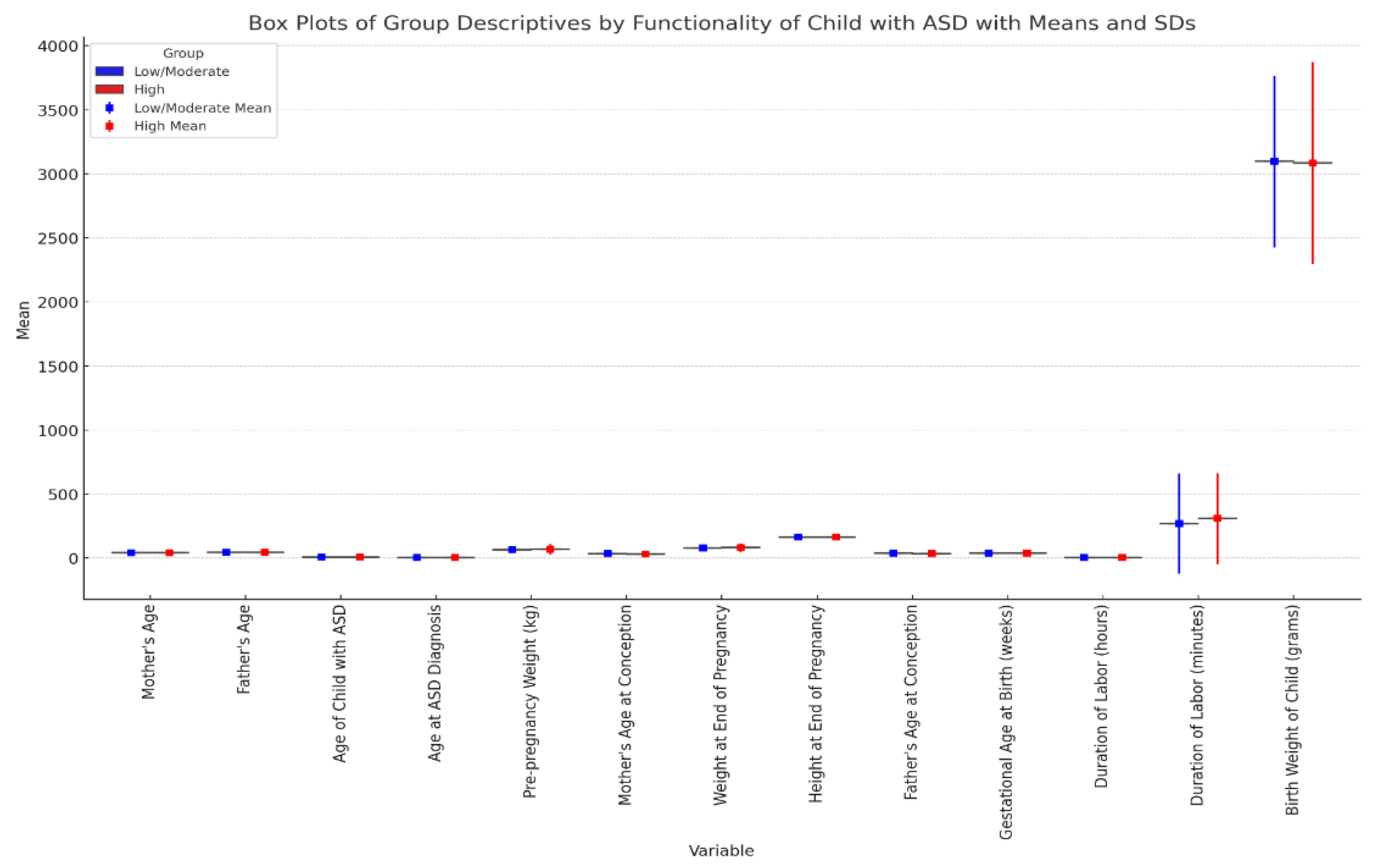
Tables S5 and S6 provide the results of independent sample t-tests and group statistics comparing various continuous variables based on the functionality of children with ASD (categorized as Low/Moderate and High). There was a significant difference in the age of mothers between the two groups (t(515) = 2.214, p = 0.027). Mothers of children with low/moderate functionality were older (M = 40.86, SD = 5.38) compared to mothers of children with high functionality (M = 39.78, SD = 5.41) (
Figure 1). A highly significant difference was found in the age at ASD diagnosis (t(515) = -5.431, p < 0.001). Children with high functionality were diagnosed later (M = 3.89, SD = 2.59) compared to those with low/moderate functionality (M = 2.78, SD = 1.46) (
Figure 1). There was also a significant difference in the age of mothers at conception between the two groups (t(515) = 2.443, p = 0.015). Mothers of children with low/moderate functionality were older at conception (M = 33.63, SD = 5.05) compared to mothers of children with high functionality (M = 32.53, SD = 4.86) (
Figure 1).
Other continuous variables, such as the father's age, age of the child with ASD, pre-pregnancy weight, weight at the end of pregnancy, height at the end of pregnancy, father's age at conception, gestational age at birth, duration of labour, and birth weight of the child, did not show significant differences between the two groups (p > 0.05).
3.6. Comparison of Sociodemographic Categorical Variables by Functionality of Child with ASD
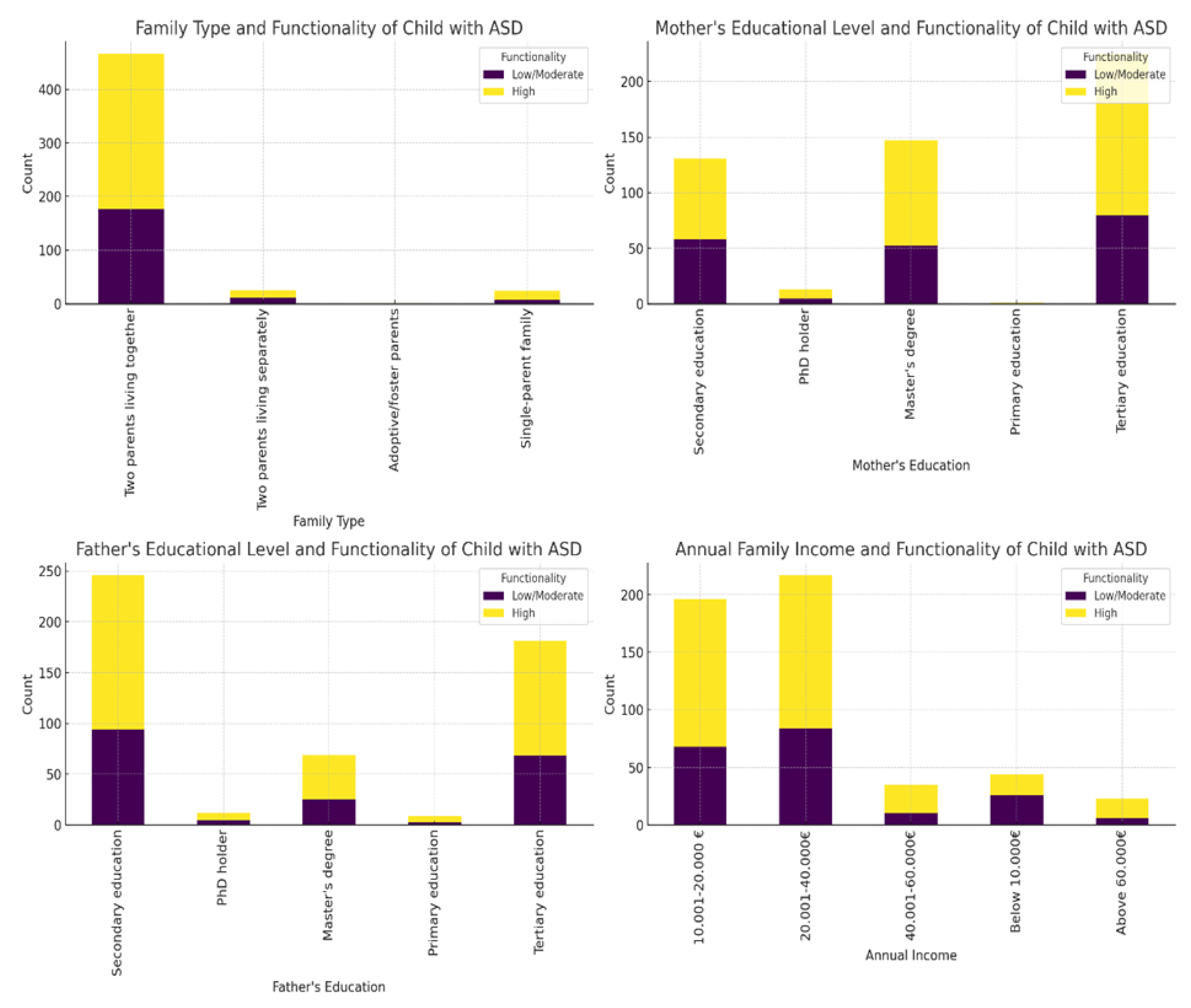
The analysis of sociodemographic categorical variables (
Table S2) using the Chi-square test showed that there were significant differences only in annual family income between the groups (χ² = 12, df = 4, p = 0.017). Families of children with low/moderate functionality were more likely to fall into lower income brackets compared to families of children with high functionality, as depicted in
Figure 2. The other sociodemographic categorical variables (family type, mother's educational level, and father's educational level) did not show significant results, as their p-values were greater than 0.05 (
Figure 2). This was also the case for the current family residence (
Figure S1).
3.7. Comparison of ASD-Related Categorical Variables by Functionality of Child with ASD
The analysis of ASD-related categorical variables (
Table S3) using Chi-square test showed that there were significant differences in the birth order of the child and their functionality with ASD (χ2 = 33.3, df = 5, p < 0.001) as well as in the mother's family history with autism, developmental disorders, epileptic seizures, depression, or anxiety disorder (χ2 = 12.2, df = 1, p < 0.001). Specifically, the first-born children and second-born children had a higher proportion of high functionality compared to later-born children (3rd child, 4th child, etc.), and a higher proportion of children with a high level of functionality had a mother's family history of these conditions compared to children with a low/moderate level of functionality (
Figure 3).
3.8. Comparison of Peri- and Postnatal Categorical Variables by Functionality of Child with ASD
The analysis of peri- and postnatal categorical variables (
Table S4) using the Chi-square test showed significant differences in several factors associated with the functionality of children with ASD. These factors include hyperemesis (severe vomiting) (χ² = 5.3, df = 1, p = 0.03), viral or bacterial infection (χ² = 3.87, df = 1, p = 0.05), vaginal bleeding (χ² = 4.28, df = 1, p = 0.04), vaginal bleeding during the pregnancy of the child with ASD (χ² = 12.6, df = 1, p = 0.006), and whether the baby cried immediately after birth (χ² = 15, df = 1, p = 0.001). Specifically, severe vomiting during pregnancy, viral or bacterial infections during pregnancy, vaginal bleeding during pregnancy, vaginal bleeding during the pregnancy of child with ASD, and the baby crying immediately after birth are associated with lower functionality in children with ASD (
Figure 4).
3.9. Multivariate Analysis of Factors Related to the Functionality of Child with ASD
The binomial logistic regression model (
Table 1) revealed significant predictors of functionality in children with ASD, including maternal age (OR = 1.06, 95% CI [1.00, 1.12]), age at diagnosis (OR = 0.65, 95% CI [0.55, 0.77]), family income (Below 10,000€ vs. 10,001-20,000€, OR = 2.58, 95% CI [1.21, 5.50]), birth order (2nd child vs. 1st child, OR = 2.71, 95% CI [1.70, 4.29]), mother's family history with autism, developmental disorders, epileptic seizures, depression, or anxiety disorder (OR = 0.58, 95% CI [0.36, 0.91]), viral or bacterial infection during pregnancy (OR = 0.49, 95% CI [0.25, 0.97]), vaginal bleeding during pregnancy (1st Trimester vs. No Bleeding, OR = 6.43, 95% CI [1.55, 26.77]; 3rd Trimester vs. No Bleeding, OR = 13.02, 95% CI [2.15, 78.93]), and whether the baby cried immediately after birth (OR = 0.39, 95% CI [0.19, 0.81]).
The model demonstrated a good fit, with several measures indicating its adequacy. The deviance of the model was 554, and the AIC was 598. The pseudo-R² values, including Cox & Snell’s R² (0.220) and Nagelkerke’s R² (0.300), suggested a reasonable amount of variance explained by the model. The overall model test yielded a χ² value of 128 with 21 degrees of freedom, which was statistically significant (p < 0.001), indicating that the model significantly predicted the outcome variable (
Table 1).
Based on the collinearity statistics, there is no severe multicollinearity in the model, and the predictors are sufficiently independent of each other to produce stable regression coefficients (
Table S7). The predictive measures of the model indicated an overall accuracy of 72.6%, with a specificity of 85.7%, a sensitivity of 51.0%, and an area under the curve (AUC) of 0.79. These results suggest that the model had a good discriminatory ability between high and low/moderate functionality in children with ASD (
Figures S2 and S3).
4. Discussion
The occurrence of ASD has been linked to a range of prenatal, perinatal, and neonatal factors. An increasing number of studies have identified several perinatal and postnatal factors that may be causally associated with ASD, such as fetal distress [
13], multiple pregnancy [
13], preterm delivery [
12,
28], meager birth weight [
12,
13,
14,
21], and low Apgar score [
13].
The present study examines the various factors impacting the functionality of children with ASD through the use of statistical methods and extensive data. The study offers comprehensive descriptive statistics for both continuous and categorical variables. The primary focus of this research is on demographic and physical health variables such as the age of parents at the time of conception, gestational age, and birth weight of the child. For instance, the study highlights the broad range of pre-pregnancy weights among mothers, drawing attention to the diverse physical conditions that existed before pregnancy. With regard to categorical variables, the sociodemographic data indicates that the majority of children come from dual-parent households, and the distribution across different educational levels and incomes suggests a diverse sample.
This research examining high-functioning children with those possessing low or moderate functionality in ASD disclosed multiple noteworthy findings. The key predictors identified through the binomial logistic regression model were Maternal Age, Age at Diagnosis, Family Income, Birth Order, and Mother's family history with autism, developmental disorders, epileptic seizures, depression, or anxiety disorder.
A relationship was revealed between increased parental age and reduced cognitive functionality in the offspring. For each additional year in the mother's age, the probability of the child displaying low or moderate cognitive abilities rises by 6%. While this effect is statistically significant, the increase is relatively small. This finding implies that older maternal age and age at conception may pose potential risks. Prior research [
33] has shown that older parental age at the time of birth is correlated with both the severity and incidence of ASD in their progeny. Still, a substantial disparity was discovered in the age of diagnosis, where children with greater functionality were diagnosed at a later age than those with lower functional capacity. For every additional year's postponement in the diagnosis of ASD, the likelihood of the child having low or moderate functionality diminishes by 35%. This suggests that an earlier diagnosis is associated with higher functionality in children with ASD and may be related to more pronounced ASD symptoms, as the functioning of children may be enhanced with earlier interventions [
34,
35].
The binomial logistic regression analysis identified several variables that impact functionality, such as vaginal bleeding, infections, socioeconomic factors, and birth order. Vaginal bleeding during pregnancy, especially in the first and third trimester, emerged as a strong predictor of reduced functionality. Mothers who experienced vaginal bleeding in the first trimester were found to have 543% higher odds of having children with low/ moderate functionality. Similarly, mothers who experienced vaginal bleeding in the third trimester were found to have 1202% higher odds of having children with low/ moderate functionality. Pregnancy complications, whether they occur early or late in gestation, have been consistently linked to reduced functional outcomes. Notably, other surveys have also reported significantly higher rates of bleeding during pregnancy among mothers of autistic children [
36], particularly during the mid-trimester [
37].
The relationship between prenatal infections caused by viruses or bacteria and cognitive outcomes in offspring is complex and may exhibit paradoxical results. Despite this complexity, our research has shown a substantial decrease in the probability of reduced functionality in children born to mothers who encountered these infections during their pregnancy. Specifically, these children showed a 51% decrease in the probability of having low/ moderate functionality. The somewhat counterintuitive results observed in this study may be related to the influence of immune responses or other biological processes on cognitive development. A similar study found no connection between maternal inflammation and ASD [
38]. However, evidence suggests that infections reported during pregnancy are associated with neurodevelopment disorders, particularly ASD [
39]. Our study unveils that the relationship between prenatal infections and cognitive outcomes is multifaceted and intricate, encompassing a range of potential biological factors. While further research is required to fully understand these complex interactions, our results highlight the significance of considering the possible long-term consequences of prenatal infections on offspring cognitive development.
The relationship between family income and functional outcomes in children is of significant interest. A lower family income has been associated with diminished functional outcomes, which may suggest inequities in healthcare access or the availability of early intervention services. According to our data, families with annual incomes of less than €10,000 had a 158% higher likelihood of having children with low/moderate functionality compared to those with incomes ranging from €10,001 to €20,000. This finding highlights the connection between socioeconomic status and adverse functionality outcomes, as families with higher incomes may have greater access to specialized mental health services and more extensive social support. Moreover, studies have confirmed a positive relationship between socioeconomic disparities and the prevalence of children with ASD [
40].
The status of being a second-born child correlated with an increased probability of exhibiting lower functionality when compared to first-borns, suggesting potential dilution effects of resources or parental attention. Specifically, second-born children demonstrated a 171% heightened likelihood of manifesting low/ moderate functionality relative to their first-born counterparts. This data underscores the significant impact of birth order, where subsequent children are more prone to experiencing lower levels of functionality. A comparable study also revealed that the size of the family and the increasing birth order in children with ASD were correlated with heightened functional and cognitive limitations [
41].
Neonates who manifested immediate crying following birth demonstrated a 61% reduction in the likelihood of possessing low/ moderate functionality in the present study. This observation indicates that prompt vocalization post-delivery, a marker of neonatal vigor and health, correlates with more favorable functional outcomes in later life. Research findings reinforce the potential of crying as a biomarker of an infant’s physical and emotional health status [
42]. Yet, studies employing observation of vocalization behaviors post-delivery have been very limited, reducing the external generalization of the findings.
Furthermore, it is noteworthy that individuals with a family history of autism or related developmental disorders exhibit a 42% lower likelihood of experiencing low to moderate functionality, implying that inherited or familial elements may provide a protective influence. Nevertheless, this protective effect is not uniformly supported across the literature. A contrasting study [
43] suggests that parental psychiatric disorders, particularly those affecting mothers, may increase the risk of ASD in their children. This risk can be attributed to epigenetic mechanisms that may occur during fetal development, indicating that maternal psychiatric conditions could have a deleterious impact on the neurodevelopment of the offspring [
44].
These seemingly contradictory findings underline the complexity of genetic and environmental interactions in the development and manifestation of ASD. While some familial factors may confer protection and contribute to better functionality, others, particularly related to maternal mental health, may increase the risk of developing ASD through mechanisms that alter gene expression during critical periods of prenatal development. This dual perspective calls for a nuanced understanding of the multifactorial nature of ASD, recognizing that both protective and risk factors can coexist within the familial and genetic context [
45,
46,
47].
Upon evaluating the study's strengths, it can be asserted that the comprehensive sample size of 517 children aged between 4 and 7 years offers substantial information on maternal history, pregnancy, childbirth, and neonatal data. Furthermore, the participating centers' services are accessible to children and families from both public and private schools, ensuring a diverse sample without the presence of institutional barriers. Additionally, the study's design considers socioeconomic status, effectively addressing potential geographical disparities and variations. The regression model demonstrated adequate predictive power, with reasonable sensitivity and specificity. The area under the curve (AUC) was robust, suggesting the model's good discriminatory ability between high and low/ moderate functionality levels.
The study has several limitations that should be taken into account. Firstly, it is possible that not all cases of ASD were captured, particularly those who did not seek treatment at the study centers. This may include individuals who are milder or have higher functioning levels. Secondly, the quality of the data collected was dependent on the availability and accessibility of family records, as well as the level of awareness and participation of the centers in the study.
Lastly, it should be noted that the study did not independently verify the ASD diagnoses, but rather relied on records provided by the participating centers.
The results of the study indicate the importance of developing targeted services focused on early intervention, rehabilitation, social care, and integration. It is recommended that policymakers prioritize early diagnosis and accessible early identification and intervention services. Our findings underscore the complex interplay of genetic, environmental, and socioeconomic factors in determining the outcomes of ASD.
These results have significant implications for developing effective early screening and intervention strategies. They emphasize the necessity of considering a wide range of influences to improve the functional outcomes of children with ASD. The detailed statistical analysis conducted in this study offers important insights into the predictors of ASD functionality, providing avenues for targeted interventions and policy adjustments.
Systematic screening of toddlers and increasing awareness among parents, guardians, and healthcare professionals are crucial. Standardizing case-recording procedures and creating an ASD register at the national and regional levels would facilitate systematic monitoring and evaluation of resources and services.
5. Conclusions
This research constitutes a comprehensive investigation of ASD in Greece, intending to evaluate the prevalence of ASD at both the national and regional levels, utilizing the functional capacity of children as a basis for assessment. The present study establishes one of the limited contemporary studies that furnishes data essential for estimating the disorder's burden and informing the formulation and implementation of regional and national service development plans. The study employs statistical analyses of an extensive dataset, which includes various demographic and physiological variables, to explore the factors that influence the functionality of children with ASD. The dataset encompasses predictors such as maternal age, the timing of ASD diagnosis, family income, and birth order.
Several factors, including advanced maternal age, delayed ASD diagnosis, lower family income, and higher birth order, were linked to reduced functionality in children with ASD. The study highlighted significant associations between older maternal age, delayed diagnosis, and poorer functional outcomes, highlighting the need for early intervention. Additionally, children from lower-income families and those with higher birth order in larger families exhibited significantly lower functionality, possibly due to diluted parental resources and socioeconomic disparities. The research also identified complex interactions between prenatal health concerns and child development, with prenatal issues like vaginal bleeding and infections potentially having paradoxical effects through immune responses. Interestingly, a family history of neurological or psychiatric conditions appeared to protect functionality in children with ASD. The regression model employed demonstrated strong predictive power, enhancing the accuracy of distinguishing functionality levels in children with ASD.
The present study sheds light on the significant role that prenatal and early life factors play in determining the functionality of individuals with ASD, thus providing evidence-based information that is essential for estimating the global burden of the disorder and guiding the development of national and regional services. The results of this research underscore the importance of early diagnosis and intervention, systematic screening, and addressing disparities in service provision to improve outcomes for individuals with ASD.
Supplementary Materials
The following supporting information can be downloaded at: Preprints.org, Table S1. Descriptive Statistics of Continuous Variables, Table S2. Descriptive Statistics of Sociodemographic Categorical Variables, Table S3. Descriptive Statistics of ASD-Related Categorical Variables, Table S4. Descriptive Statistics of Peri- and Postnatal Categorical Variables, Table S5. Comparison of Continuous Variables by Functionality of Child with ASD, Table S6. Group Descriptives of Continuous Variables by Functionality of Child with ASD, Table S7. Collinearity Statistics of Binominal Logistic Regression, Figure S1. The visualization of the Chi-square test results for the Current Family Residence, Figure S2. Cut-Off Plot of the Binominal Logistic Regression Prediction Model, Figure S3. ROC Curve of the Binominal Logistic Regression Prediction Model
Author Contributions
Conceptualization, Ai.S and An.S; methodology, An.S; software, E.D; validation, An.S; D.A; D.M. and E.D; formal analysis, E.D; investigation, Ai.S; resources, An.S; data curation, Ai.S; P.D; CRA.; writing—original draft preparation, Ai.S and An.S; writing—review and editing, An.S and E.D; visualization, An.S and A.L; supervision, An.S; project administration, D.M; D.A and An.S; funding acquisition, An.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the University of West Attica (protocol code 29346 / 08-04-2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study, including written informed consent from all participants for publication of this manuscript.
Data Availability Statement
Due to the sensitive nature of the data, it is not publicly available.
Acknowledgments
We extend our heartfelt gratitude to the participant mothers whose collaboration and openness were vital to the success of our study. Through the disclosure of sensitive and detailed personal information about their lives and the health of their children, valuable insights into the prevalence of ASD and regional discrepancies have been gained, significantly enhancing our comprehension of the factors that impact the development and functioning of children with ASD. We are deeply thankful to the personnel of the various facilities that supported our research. Their commitment and assistance were essential in facilitating the structured data collection process, providing support, and ensuring the smooth conduct of this study across different regions of Greece.
During the preparation of this work, the AI tool Chat GPT was used to improve the readability and language of the manuscript, and subsequently, the authors revised and edited the content produced by the AI tool as necessary, taking full responsibility for the ultimate content of the present manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hyman, S.L.; Levy S.E.; Myers, S.M. Identification, Evaluation, and Management of Children With Autism Spectrum Disorder. Pediatrics 2020, 145. [CrossRef]
- Maenner, M.J.; Warren, Z.; Robinson Williams, A.; Amoakohene, E.; Bakian, A.V.; Bilder, D.A.; Durkin, M.S.; Fitzgerald, R.T.; Furnier, S.M.; Hughes, M.M.; Ladd-Acosta, C.M.; McArthur, D.; Pas, E.T.; Salinas, A.; Vehorn, A.; Williams, S.; Esler, A.; Grzybowski, A.; Hall-Lande, J.; Nguyen, R. H N; Pierce, K.; Zahorodny, W.; Hudson, A.; Hallas, L.; Mancilla, K.C.; Patrick, M.; Shenouda, J.; Sidwell, K.; DiRienzo, M.; Gutierrez, J.; Spivey, M.H.; Lopez, M.; Pettygrove, S.; Schwenk, Y.D.; Washington, A.; Shaw, K.A. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2020. MMWR Surveill Summ 2023, 72, 1–14. [CrossRef]
- Kinney, D.K.; Munir, K.M.; Crowley, D.J.; Miller, A.M. Prenatal stress and risk for autism. Neuroscience and Biobehavioral Reviews 2008, 32, 1519–1532. [CrossRef]
- Kinney, D.K.; Miller, A.M.; Crowley, D.J.; Huang, E.; Gerber, E. Autism Prevalence Following Prenatal Exposure to Hurricanes and Tropical Storms in Louisiana. Journal Autism Developmental Disorders 2008, 38, 481–488. [CrossRef]
- Manzari, N.; Matvienko-Sikar, K.; Baldoni, F.; W O'Keeffe, G.; Khashan, A.S. Prenatal maternal stress and risk of neurodevelopmental disorders in the offspring: A systematic review and meta-analysis. Social Psychiatry and Psychiatric Epidemiology 2019, 54, 1299–1309. [CrossRef]
- Jiang, H.Y.; Xu, L.L.; Shao, L.; Xia, R.M.; Yu, Z.H.; Ling, Z.X.; Yang, F.; Deng, M.; Ruan, B. Maternal infection during pregnancy and risk of autism spectrum disorders: A systematic review and meta-analysis. Brain, Behavior, and Immunity 2016, 58, 165–172. [CrossRef]
- Vivanti, G.; Prior, M.; Williams, K.; Dissanayake, C. Predictors of Outcomes in Autism Early Intervention: Why Don’t We Know More? Front. Pediatr 2014, 2, 58. [CrossRef]
- American Psychological Association (APA). Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th ed.; American Psychiatric Publishing: Arlington, VA, USA, 2013; pp. 50-59.
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems, 10th revision, 5th ed.; World Health Organization: Geneva, Switzerland, 2016; pp. 336.
- Autism Speaks. Autism diagnosis criteria: DSM-5. Available online: https://www.autismspeaks.org/autism-diagnosis-criteria-dsm5 (accessed on 8 April 2024).
- Durkin, M.S.; Maenner, M.J.; Newschaffer, C.J.; Lee, L.C.; Cunniff, C.M.; Daniels, J.L.; Kirby, R.S.; Leavitt, L.; Miller, L.; Zahorodny, W.; Schieve, L.A. Advanced Parental Age and the Risk of Autism Spectrum Disorder. American Journal of Epidemiology 2008, 168, 1268–1276. [CrossRef]
- Wang, C.; Geng, H.; Liu, W.; Zhang, G. Prenatal, perinatal, and postnatal factors associated with autism: A meta-analysis. Medicine (Baltimore) 2017, 96, e6696. [CrossRef]
- Gardener, H.; Spiegelman, D.; Buka, S.L. Perinatal and neonatal risk factors for autism: A comprehensive meta-analysis. Pediatrics 2011, 128, 344-355. [CrossRef]
- Itzchak, E.B.; Lahat, E.; Zachor, D.A. Advanced parental ages and low birth weight in autism spectrum disorders--rates and effect on functioning. Res Dev Disabil 2011, 32, 1776-1781. [CrossRef]
- Patrick, M.E.; Hughes, M.M.; Ali, A.; Shaw, K.A.; Maenner, M.J. Social vulnerability and prevalence of Autism Spectrum Disorder, Metropolitan Atlanta Developmental Disabilities Surveillance Program (MADDSP). Annals of Epidemiology 2023, 83, 47-53.e1. [CrossRef]
- Sipsock, D.; Tokadjian, H.; Righi, G.; Morrow, E.M.; Sheinkopf, S.J.; Rhode Island Consortium for Autism Research and Treatment (RI-CART). Autism severity aggregates with family psychiatric history in a community-based autism sample. Autism Res 2021, 14, 2524-2532. [CrossRef]
- Garrido, D.; Carballo, G.; Garcia-Retamero, R. Siblings of children with autism spectrum disorders: Social support and family quality of life. Quality of Life Research 2020, 29, 1193–1202. [CrossRef]
- Xu, Y.; Yang, X.; Chen, D.; Xu, Y.; Lan, L.; Zhao, S.; Liu, Q.; Snijders, A.M.; Xia, Y. Maternal exposure to pesticides and autism or attention-deficit/hyperactivity disorders in offspring: A meta-analysis. Chemosphere 2023, 313, 137459. [CrossRef]
- Volk, H.E.; Ames, J.L.; Chen, A.; Fallin, M.D.; Hertz-Picciotto, I.; Halladay, A.; Hirtz, D.; Lavin, A.; Ritz, B.; Zoeller, T.; Swanson, M. Considering Toxic Chemicals in the Etiology of Autism. Pediatrics 2022, 149, e2021053012. [CrossRef]
- Pop-Jordanova, N.; Demerdzieva, A. How Alcohol Damages Brain Development in Children. Prilozi (Makedon Akad Nauk Umet Odd Med Nauki) 2022, 43, 29-42. [CrossRef]
- Hisle-Gorman, E.; Susi, A.; Stokes, T.; Gorman, G.; Erdie-Lalena, C.; Nylund, C.M. Prenatal, perinatal, and neonatal risk factors of autism spectrum disorder. Pediatric Research. 2018, 84, 190-198. [CrossRef]
- Rodrigues, E.L.; Figueiredo, P.S.; Marcelino, G.; de Cássia Avellaneda Guimarães, R.; Pott, A.; Santana, L.F.; Hiane, P.A.; Aragão do Nascimento, V.; Bogo, D.; de Cássia Freitas, K. Maternal Intake of Polyunsaturated Fatty Acids in Autism Spectrum Etiology and Its Relation to the Gut Microbiota: What Do We Know? Nutrients 2023, 15, 1551. [CrossRef]
- Mohammadi, M.; Zarafshan, H. Family function, Parenting Style and Broader Autism Phenotype as Predicting Factors of Psychological Adjustment in Typically Developing Siblings of Children with Autism Spectrum Disorders. Iran J Psychiatry 2014, 9, 55-63.
- Zhen Lim, T.X.; Pickering, T.A.; Lee, R.H.; Hauptman, I.; Wilson, M.L. Hypertensive disorders of pregnancy and occurrence of ADHD, ASD, and epilepsy in the child: A meta-analysis. Pregnancy Hypertens 2023, 33, 22-29. [CrossRef]
- McWilliams, S.; Singh, I.; Leung, W.; Stockler, S.; Ipsiroglu, O.S. Iron deficiency and common neurodevelopmental disorders-A scoping review. PLoS ONE 2022, 17, e0273819. [CrossRef]
- Carpita, B.; Muti, D.; Dell’Osso, L. Oxidative Stress, Maternal Diabetes, and Autism Spectrum Disorders. Oxidative Medicine and Cellular Longevity 2018, 2018, 3717215. [CrossRef]
- Qiu, C.; Carter, S.A.; Lin, J.C.; Shi, J.M.; Chow, T.; Desai, V.N.; Nguyen, V.T.; Spitzer, J.; Feldman, R.K.; Xiang, A.H. Association of Labor Epidural Analgesia, Oxytocin Exposure, and Risk of Autism Spectrum Disorders in Children. JAMA Netw Open 2023, 6, e2324630. [CrossRef]
- Perrone-McGovern, K.; Simon-Dack, S.; Niccolai, L. Prenatal and Perinatal Factors Related to Autism, IQ, and Adaptive Functioning. J Genet Psychol 2015, 176, 1-10. [CrossRef]
- Traver, S.; Geoffray, M.M.; Mazières, L.; Geneviève, D.; Michelon, C.; Picot, M.C.; Baghdadli, A. Association between prenatal and perinatal factors and the severity of clinical presentation of children with ASD: Report from the ELENA COHORT. J Psychiatr Res 2021, 137, 634-642. [CrossRef]
- Hadjkacem, I.; Ayadi, H.; Turki, M.; Yaich, S.; Khemekhem, K.; Walha, A.; Cherif, L.; Moalla, Y.; Ghribi, F. Prenatal, perinatal and postnatal factors associated with autism spectrum disorder. J Pediatr (Rio J) 2016, 92, 595-601. [CrossRef]
- Chien, Y.L.; Chou, M.C.; Chou, W.J.; Wu, Y.Y.; Tsai, W.C.; Chiu, Y.N.; Gau, S.S.F. Prenatal and perinatal risk factors and the clinical implications on autism spectrum disorder. Autism 2019, 23, 783-791. [CrossRef]
- Russell, G.; Stapley, S.; Newlove-Delgado, T.; Salmon, A.; White, R.; Warren, F.; Pearson, A.; Ford, T. Time trends in autism diagnosis over 20 years: A UK population-based cohort study. Journal of Child Psychology and Psychiatry 2022, 63, 674–682. [CrossRef]
- Kavanaugh, B.C.; Gabert; T.; Rhode Island Consortium for Autism Research and Treatment (RI-CART); Jones, R.N.; Sheinkopf, S.J.; Morrow, E.M. Parental age and autism severity in the Rhode Island Consortium for Autism Research and Treatment (RI-CART) study. Autism Res 2022, 15, 86-92. [CrossRef]
- Campbell, M.; Schopler, E.; Cueva, J.E.; Hallin, A. Treatment of autistic disorder. J Am Acad Child Adolesc Psychiatry 1996, 35, 134-143. [CrossRef]
- Mandell, D.S.; Novak, M.M.; Zubritsky, C.D. Factors Associated With Age of Diagnosis Among Children With Autism Spectrum Disorders. Pediatrics 2005, 116, 1480–1486. [CrossRef]
- Mason-Brothers, A.; Ritvo, E.R.; Guze, B.; Mo, A.; Freeman, B.J.; Funderburk, S J.; Schroth, P C. Pre-, peri-, and postnatal factors in 181 autistic patients from single and multiple incidence families. J Am Acad Child Adolesc Psychiatry 1987, 26, 39-42. [CrossRef]
- Torrey, E.F.; Hersh, S.P.; McCabe, K.D. Early childhood psychosis and bleeding during pregnancy. A prospective study of gravid women and their offspring. J Autism Child Schizophr 1975, 5, 287-297. [CrossRef]
- Zerbo, O.; Qian, Y.; Yoshida, C.; Grether, J.K.; Van de Water, J.; Croen, L.A. Maternal Infection during Pregnancy and Autism Spectrum Disorders. J Autism Dev Disord 2015, 45, 4015–4025. [CrossRef]
- Shuid, A.N.; Jayusman, P.A.; Shuid, N.; Ismail, J.; Kamal Nor, N.; Mohamed, I.N. Association between Viral Infections and Risk of Autistic Disorder: An Overview. Int J Environ Res Public Health 2021, 18, 2817. [CrossRef]
- Yu, T.; Lien, Y.J.; Liang, F.W.; Kuo, P.L. Parental Socioeconomic Status and Autism Spectrum Disorder in Offspring: A Population-Based Cohort Study in Taiwan. Am J Epidemiol 2021, 190, 807-816. [CrossRef]
- Alvares, G.A.; Licari, M.K.; Stevenson, P.G.; Bebbington, K.; Cooper, M.N.; Glasson, E.J.; Tan, D.W.; Uljarević, M.; Varcin, K.J.; Wray, J.; J O Whitehouse, A. Investigating associations between birth order and autism diagnostic phenotypes. J Child Psychol Psychiatry 2021, 62, 961-970. [CrossRef]
- Laguna, A.; Pusil, S.; Acero-Pousa, I.; Zegarra-Valdivia, J.A.; Paltrinieri, A.L.; Bazán, A.; Piras, P.; Palomares I Perera, C.; Garcia-Algar, O.; Orlandi S. How can cry acoustics associate newborns' distress levels with neurophysiological and behavioral signals? Front Neurosci 2023, 17, 1266873. [CrossRef]
- Chien, Y.L.; Wu, C.S.; Chang, Y.C.; Cheong, M.L.; Yao, T.C.; Tsai, H.J. Associations between parental psychiatric disorders and autism spectrum disorder in the offspring. Autism Res 2022, 15, 2409-2419. [CrossRef]
- Banik, A.; Kandilya, D.; Ramya, S.; Stünkel, W.; Chong, Y.S.; Dheen, S.T. Maternal Factors that Induce Epigenetic Changes Contribute to Neurological Disorders in Offspring. Genes (Basel) 2017, 8, 150. [CrossRef]
- Almandil, N.B.; Alkuroud, D.N.; AbdulAzeez, S.; AlSulaiman, A.; Elaissari, A.; Borgio, J.F. Environmental and Genetic Factors in Autism Spectrum Disorders: Special Emphasis on Data from Arabian Studies. Int J Environ Res Public Health 2019, 16, 658. [CrossRef]
- Tordjman, S.; Somogyi, E.; Coulon, N.; Kermarrec, S.; Cohen, D.; Bronsard, G.; Bonnot, O.; Weismann-Arcache, C.; Botbol, M.; Lauth, B.; Ginchat, V.; Roubertoux, P.; Barburoth, M.; Kovess, V.; Geoffray, M.M.; Xavier, J. Gene × Environment interactions in autism spectrum disorders: Role of epigenetic mechanisms. Front Psychiatry 2014, 5, 53. [CrossRef]
- Hon Kei Yip, B.; Bai, D.; Mahjani, B.; Klei, L.; Pawitan, Y.; Hultman, C.M.; Grice, D.E.; Roeder, K.; Buxbaum, J.D.; Devlin, B.; Reichenberg, A.; Sandin, S. Heritable Variation, With Little or No Maternal Effect, Accounts for Recurrence Risk to Autism Spectrum Disorder in Sweden. Biol Psychiatry 2018, 83, 589-597. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).