1. Introduction
Cotton, recognized as one of the pivotal agricultural commodities globally, is distinguished by the cellulose-rich fibers produced by its seed coat cells, which serve as the primary natural fiber utilized in the textile industry [
1]. Concurrently, the cotyledons of cottonseeds are abundant in oils and proteins, rendering them not only suitable for the extraction of cottonseed oil but also as a raw material for producing protein-rich livestock feed [
2]. Consequently, both the cotton fibers and the seeds are indispensable resources for societal development. Presently, cotton research predominantly focuses on enhancing fiber yield and quality, with comparatively less emphasis on seed quality. Traditional cotton breeding research has primarily aimed at increasing lint yield, which has inadvertently led to a trend of diminishing seed size [
3]. Smaller seeds, due to their inadequate nutritional content, exhibit reduced viability, thereby constraining further improvements in fiber quality [
4]. In the pursuit of cultivating high-quality cotton varieties, superior cotton seeds emerge as a critical factor that cannot be overlooked [
5]. However, the molecular mechanisms and regulatory networks governing cotton seed size remain inadequately explored, thereby limiting the understanding and application of cotton seed quality improvement.
Seeds, serving as the reproductive entities of most plants, have long been acknowledged as a critically adaptive characteristic in terms of their size [
6]. In angiosperms, the developmental trajectory of seeds commences with the process of double fertilization, which subsequently gives rise to a diploid embryo and a triploid endosperm [
7].The embryo and endosperm are encased within the seed coat, with the coordinated growth and regulation of these components ultimately determining the seed’s size [
8]. The developmental process of seeds is also a manifestation of genetic influence, as evidenced by numerous genes that have been validated in the model crop
Arabidopsis to regulate seed organ development [
9,
10]. Examples include genes associated with endosperm size, such as
HAIKU1 (
IKU1) and
IKU2 [
11,
12,
13]; genes influencing ovule integument cell growth, like
TRANSPARENT TESTA GLABRA2 (
TTG2) [
14]and
APETALA2 (
AP2) [
15]; and genes affecting embryo development, such as the simultaneous knockout of
ARABIDOPSIS HISTIDINE KINASE2 (
AHK2),
AHK3, and
AHK4, which leads to the formation of large embryos within seeds [
16].Previous research has elucidated several regulatory mechanisms involved in seed development, including the IKU (HAIKU) pathway, the ubiquitin-proteasome pathway, G protein signaling pathways (guanosine triphosphate), mitogen-activated protein kinase (MAPK) signaling pathways, plant hormones, and transcriptional regulators [
17]. These pathways and factors interact with one another, collectively orchestrating the developmental processes of seeds, thereby impacting seed size and quality.
During the developmental process of cotton seeds, the concomitant growth and development of fibers and ovules are distinctive features that markedly differentiate cotton seed development from that of other seeds. The outer integument of cotton seeds is adorned with numerous vascular bundles, which are responsible for transporting assimilates produced by photosynthesis to the ovules, while the inner epidermis of the ovule is nourished by filial tissues [
18,
19,
20]. The formation of the cotton embryo commences with the zygote’s emergence during double fertilization, followed by the central cell’s development into endosperm tissue. The key events in cotton embryogenesis unfold sequentially across three overlapping phases: morphogenesis, maturation, and desiccation [
21]. The morphogenesis phase initiates with the formation of the fertilized egg and persists until 25 days post-anthesis (DPA), at which point the embryo attains its full length [
22]. Embryo development begins with the asymmetric division of the fertilized egg, yielding a small apical cell and a large basal cell, both exhibiting polarity. The apical cell evolves into the embryo, whereas the basal cell forms the suspensor, which serves as a conduit for nutrient supply to the developing embryo [
23,
24]. Embryo development progresses through globular, heart, and torpedo stages, with its size predominantly contingent upon the actual size of the egg cell. The maturation phase spans from 20 to 45 DPA, characterized by rapid accumulation of oils and proteins in the cotyledons. The final phase, embryonic desiccation, commences around 35 DPA, featuring the embryo’s preparation for entering a dry, quiescent state [
25]. Cotton seed embryos require substantial carbohydrates to fuel their development. Upon maturation, the dry weight of cotton fibers constitutes 40% to 50% of the total seed dry weight (inclusive of seeds and fibers), indicating a competitive relationship between cotton seed embryos and fibers for assimilates transported from photosynthesis [
26]. This competitive dynamic significantly impacts the development and ultimate yield of cotton seeds.
To date, genetic research pertaining to the regulation of cotton seed size has been relatively sparse, with only a handful of genes such as
DA1 [
27],
GW2 [
28],
GRDP1 [
29], and
SAP [
30] identified as possessing the potential to influence the size of cotton seeds. To further elucidate the molecular regulatory mechanisms governing seed size in cotton and to explore the critical genes affecting cotton seed yield and quality, this study selected two cotton materials with significant phenotypic differences, namely N10 and N12. Through phenotypic analysis under multiple environmental conditions, these two materials exhibited notable differences in seed size. In this study, we compared the transcriptome data of N10 and N12 to unearth potential candidate genes affecting seed size. With in-depth analysis, we identified a candidate gene named
GhUXS5, which showed significant differential expression between the two materials, suggesting it may play a role in regulating seed size in cotton. The findings of this study provide valuable insights for the identification of candidate genes and understanding of the molecular mechanisms that regulate cotton seed size.
2. Results
2.1. Obtaining and Phenotypic Analysis of Cotton Seed Size Materials
Based on the data pertaining to the size traits of cotton seeds collected from four locations (Alaer and Shihezi in Xinjiang, Anyang in Henan, and Xingtai in Hebei) during 2017-2018, two lines, designated as N10 and N12, were identified. These materials exhibited significant differences in several cotton seed traits, including the weight of a hundred seeds weight (seed index), seed area, seed perimeter, seed length, seed width, and seed diameter (
Table 1 and
Table 2). Given the presence of a hull around the cotton seeds, additional measurements were taken on the traits of the cotton kernels to further verify the seed characteristics. A correlation analysis showed an extremely high degree of correlation among the various size traits of the seeds, indicating that the presence of a hull does not interfere with the study of cotton seed size traits (
Figure S1).
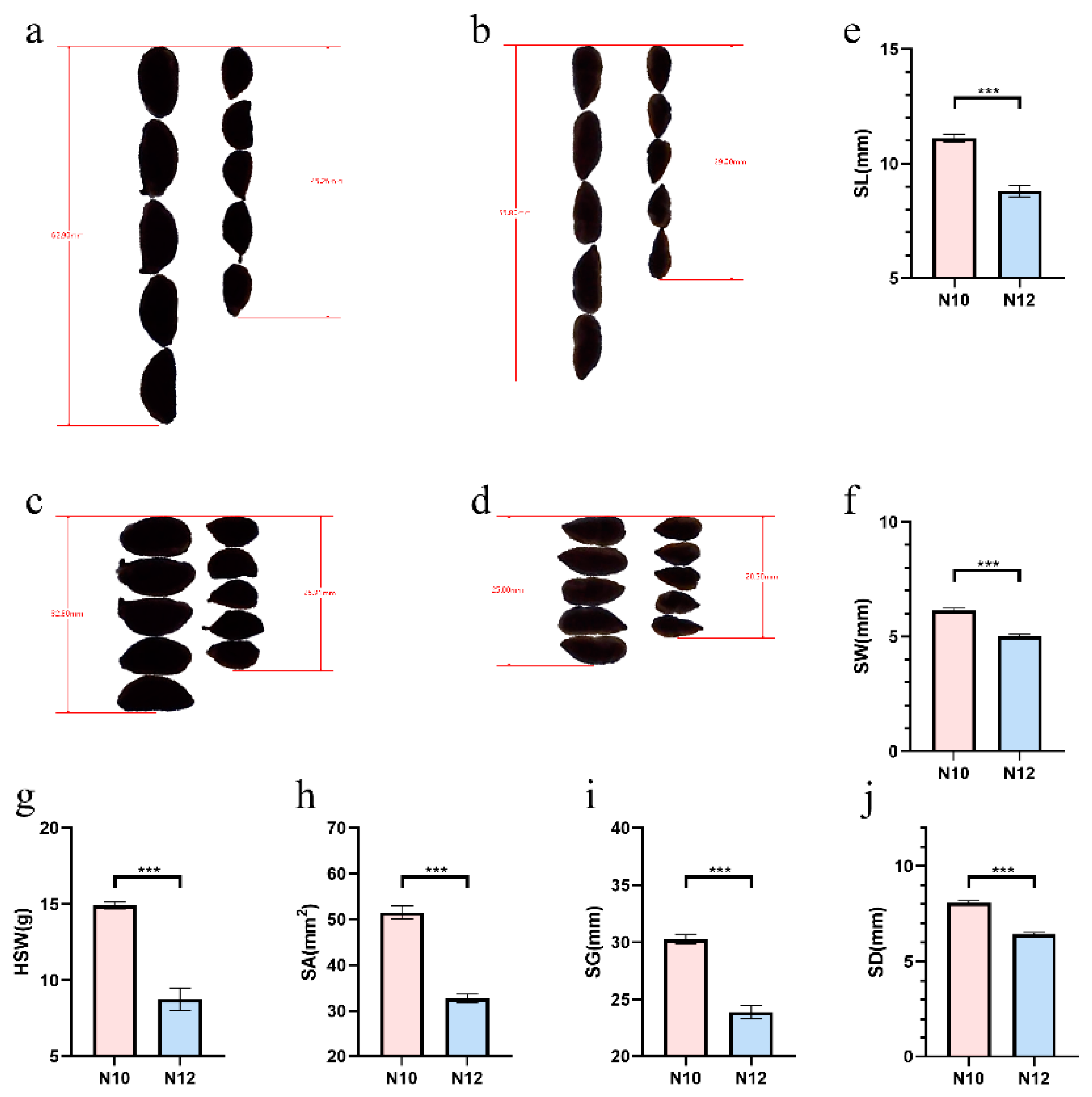
Following multiple generations of self-pollination, the two cotton lines, N10 and N12, maintained their significant phenotypic differences in seed size (
Figure 1a-d). Specific data revealed that the hundred-seed weight (seed index) of N10 was only 58.8% of that of N12. In terms of morphological parameters such as seed area, perimeter, length, width, and diameter, the values for N10 were 63.4%, 79%, 79.2%, 81.7%, and 79.5% of those for N12, respectively (
Figure 1e-j). These data clearly illustrate the significant differences in seed size phenotypes between N10 and N12, providing ideal genetic materials for studying the genetic regulation of cotton seed size.
2.2. Transcriptome Assembly and Sample Clustering
To understand the changes at the transcription level, RNA-seq of seeds in different developmental stages (5, 20, 30, and 35 DPA) were carried out. A total of 496.37 million reads were generated in this study (
Table S1). From each sample, 148.48Gb were obtained on average. The percentage for Q20 and Q30 was above 95%, and 86%, respectively. These findings demonstrated that the quality of RNA-Seq was suitable for further investigation.
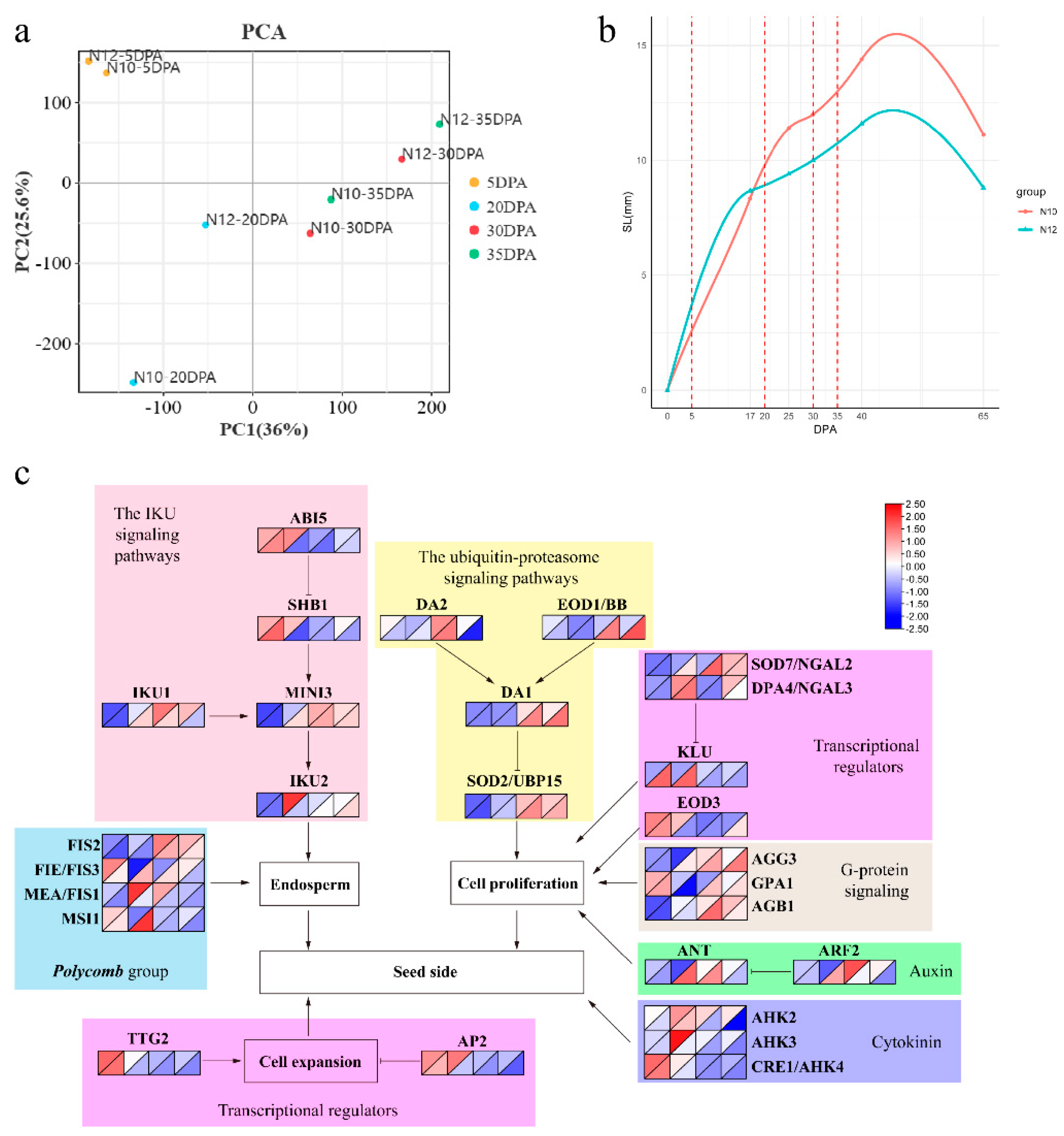
The results of principal component analysis (PCA) indicated that the two lines ed together during the same period (
Figure 2a). However, at 20 DPA, the two lines exhibited a more distant clustering relationship, suggesting that 20 DPA may be a critical period influencing seed size traits.
2.3. Cottonseed Growth Curve and Gene Expression Network
In vitro culture experiments were conducted on ovules harvested from N10 and N12 on the day of flowering. The lengths of cotton seeds at various developmental stages were measured and growth curves constructed (
Figure 2b). The results revealed a significant divergence in growth rates between N10 and N12 at 20 DPA. Subsequent PCA pinpointed 20 DPA as a critical juncture where seed size differences between the two genotypes become pronounced. This discovery offers vital insights into the growth dynamics and genetic regulation of cotton seed size development.
We then further compared a gene expression network specific to cotton seed size by amalgamating RNA-seq data with recognized pathways known to modulate seed size in
Arabidopsis (
Figure 2c). In the context of the IKU signaling pathway, the expression of
ABI5,
SHB1, and
IKU2 was robust during the initial stage, particularly at the 20 DPA phase, where the expression magnitude in N10 was notably superior to that in N12. The expression of
IKU1 and
MINI3 escalated at the later stages, particularly noticeable at 30 DPA. In the ubiquitin-proteasome signaling pathway,
DA1,
DA2,
EOD1/BB, and
SOD2/UBP15 exhibited a high expression at the 30-35 DPA phase, with a more pronounced expression level in N12. Similarly, within the G-protein signaling pathway, expression levels of
AGG3,
GPA1, and
AGB1 in N12 were also higher than in N10 at the 30-35 DPA phase. Within the transcriptional regulators pathway,
DPA4/NGAL3,
KLU,
EOD3,
TTG2, and
AP2 manifested higher expression in the incipient stage of ovule development, where KLU showcased higher expression in N12, while
EOD3 and
AP2 exhibited higher expression in N10.
SOD7/NGAL3 featured a more significant expression level at the 30-35 DPA stage, its expression level in N12 was markedly superior to that in N10. In the context of the hormone signaling pathway,
ANT and
ARF2, relevant to auxin, demonstrated higher expression levels at the 20-30 DPA phase, while AHKs associated with cytokinins manifested higher expression in the initial stage, with their expression levels in N10 surpassing those in N12.
2.4. Transcriptome Differences between N10 and N12 during Seed Development
The variations in gene expression were examined with the comparison of the four different seed developmental stages, using thresholds of more than log2 (fold change) ≥2 and adjusted p-value less than 0.05. The highest number of differentially expressed genes (DEGs) was observed at the 20 DPA stage, totaling 15,646 (
Figure S2a). This was followed by 35 DPA (5,567 DEGs), and 30 DPA (5,561 DEGs). The lowest number of DEGs was noted at 5 DPA, with merely 375 DEGs. At the 20 DPA and 30 DPA stages, the number of up-regulated genes surpassed that of down-regulated genes, whereas the opposite was observed at the 5 DPA and 35 DPA stages (
Figure S2b).
2.5. Temporal Pattern Clusters of N10 and N12
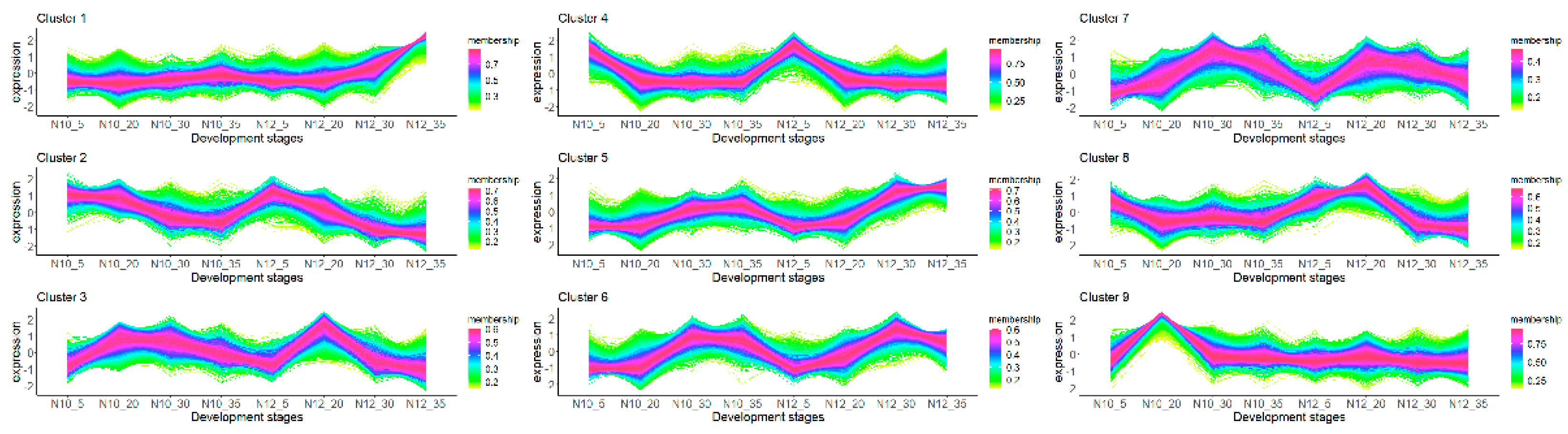
We clustered the gene expression profiles for all developmental stages. A total of nine distinct clusters of temporal patterns were observed (
Figure 3), each indicative of different expression kinetics and suggesting unique regulatory mechanisms. Notably, Clusters 4, 5, and 6 displayed relatively consistent expression patterns in both the N10 and N12 lines regardless of time points. This indicates that these genes have relatively consistent functions and play similar roles in both lines. In contrast, Clusters 3, 7, and 8 exhibited higher expression levels in N12 compared to N10 at the 20 days post-anthesis (DPA) stage, while the opposite trend was observed for Clusters 2 and 9. Furthermore, Cluster 1 demonstrated high expression in N12 at 35 DPA. This suggests that these genes may play distinct roles in the formation of cottonseed morphology.
2.6. Establishment of Weighed Gene Co-Expression Network Analysis (WGCNA)
Seed maturation is an intricate process of biological activity, orchestrated by a vast array of functional gene networks. These networks may coalesce into a variety of gene regulatory modules (GRMs), which collaborate in a concerted effort throughout the entire maturation sequence. The WGCNA [
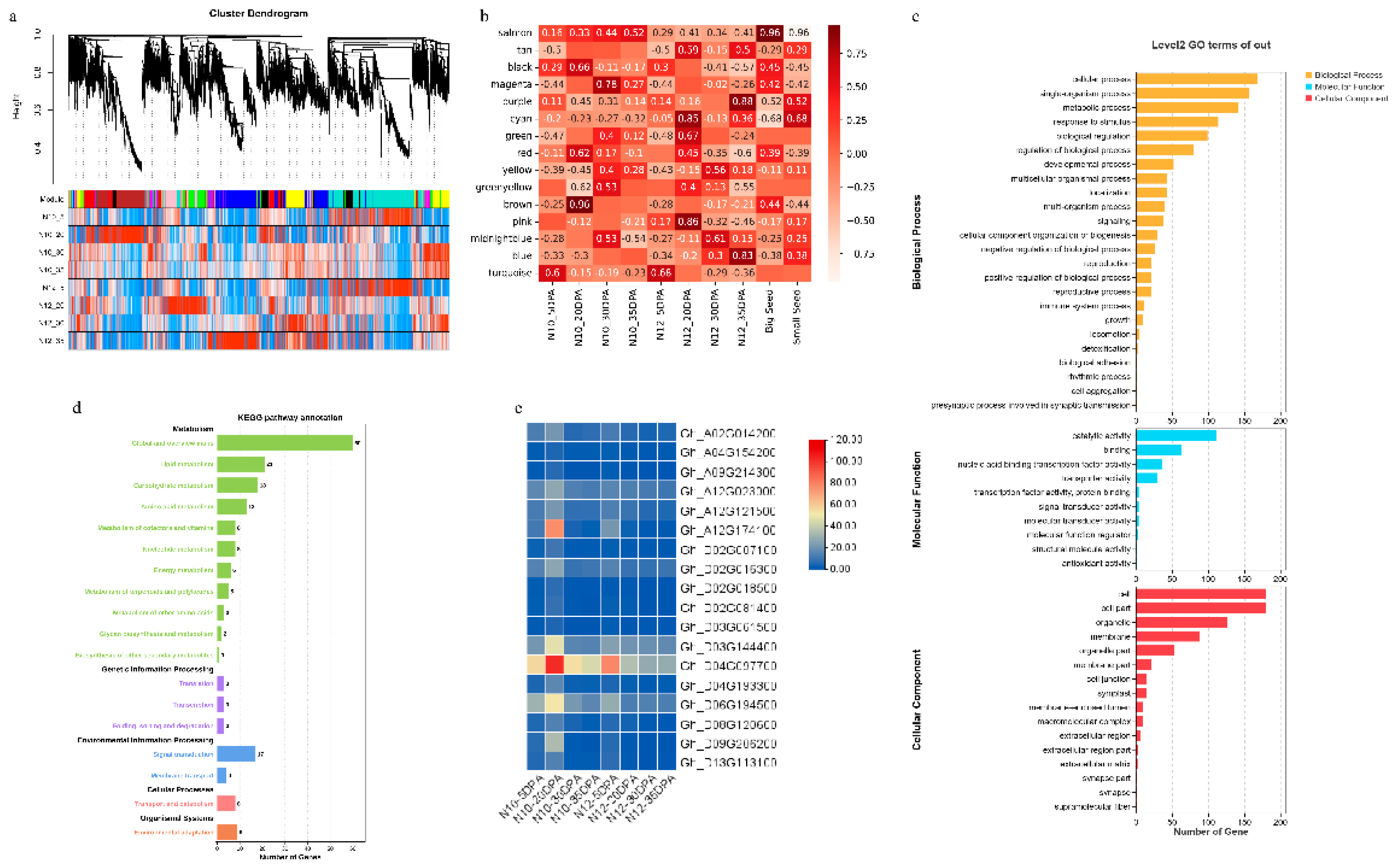
31] unveiled a spectrum of 15 GRMs (
Figure 4a-b), each mirroring the intricate tapestry of biological processes inherent in seed maturation. Within this array of modules, the turquoise module stood out as the most expansive, boasting a repertoire of 8,618 genes, in stark contrast to the midnightblue module, which was identified as the most diminutive, comprising a mere 37 genes. These insights not only cast light on the heterogeneity of gene regulation intricacies during seed maturation but also furnish an invaluable compendium for the further exploration into the genetic orchestration underlying seed development.
To elucidate the roles of various gene regulatory modules (GRMs) in seed development, we embarked on an analysis to discern the correlation between GRMs and phenotypic expressions at four distinct temporal junctures, focusing on large and small seed phenotypes. Our investigation revealed a multifaceted pattern of association between GRMs and phenotypic manifestations. Notably, the Turquoise GRM demonstrated a pronounced correlation with the phenotype at 5 DPA; whereas the magenta, yellow, greenyellow, and midnightblue GRMs were predominantly linked with the phenotype at 30 DPA. The salmon, purple, and blue GRMs were found to be associated with the phenotype at 35 DPA. The Black and brown GRMs exhibited a significant correlation with the large seed phenotype N10 at 20 DPA, while the cyan, green, tan, and pink GRMs were distinctly correlated with the small seed phenotype N12 at the same developmental stage. The Red GRM was observed to correlate with both phenotypes at 20 DPA. This intricate web of correlations underscores the complexity inherent in the processes of seed formation, development, and maturation, implicating a diverse array of biological pathways including substance accumulation and hormone regulation. This complexity gives rise to the emergence of distinct modules at varying developmental milestones, highlighting the intricate interplay between genetic regulation and phenotypic expression in seed development.
2.7. Cottonseed Size Candidate Gene Screening
To gain insight into the genetic determinants of cotton seed size traits, our study concentrated on the pivotal 20 DPA stage, marked by the most pronounced growth rate disparities and differential gene expressions between the N10 and N12 lines. Through meticulous gene expression profiling, we observed that Cluster 9 displayed the most significant expression trend divergences between the two lines at this crucial stage. Further, employing WGCNA, we pinpointed a black GRM tightly linked with N10 at 20 DPA and significantly associated with variation in cotton seed size, thus deeming it the most pivotal GRM influencing this trait. Subsequent to these analyses, we identified 413 candidate genes for further scrutiny.
To explore the roles and regulatory mechanisms of these candidate genes during fiber development, we conducted comprehensive gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses. The GO enrichment analysis sorted the candidate genes into three main categories: biological processes, cellular components, and molecular functions (
Figure 4c). Within the biological processes, these genes predominantly enriched categories related to cellular processes, metabolism, and hormonal activity. Concerning molecular functions, the genes showed a significant enrichment in terms related to catalytic activity and binding, highlighting substantial genetic variations in these processes between the two materials. Our KEGG enrichment analysis indicated significant enrichment of these genes across 18 pathways (
Figure 4d), predominantly within metabolic pathways, suggesting that the principal differences between N10 and N12 may reside in metabolic activities. Among these, a notable cluster of genes was enriched in global and overview maps, lipid metabolism, carbohydrate metabolism, and amino acid metabolism. Particularly within the carbohydrate metabolism pathway—crucial for seed size development—18 genes were enriched.
Among these, we focused on
Gh_D03G144400, annotated as
GhUXS5, which encodes for UDP-glucuronate decarboxylase 5 (
Figure 4e). This enzyme plays a vital role in the biosynthesis of the core tetrasaccharide integral to glycosaminoglycan biosynthesis. Notably,
GhUXS5 was markedly upregulated in N10 at 20 DPA, with diminished expression at other stages, positing it as a key candidate gene potentially pivotal in influencing cotton seed size.
2.8. GhUXS5 Is a Candidate Gene That Affects Seed Size Function
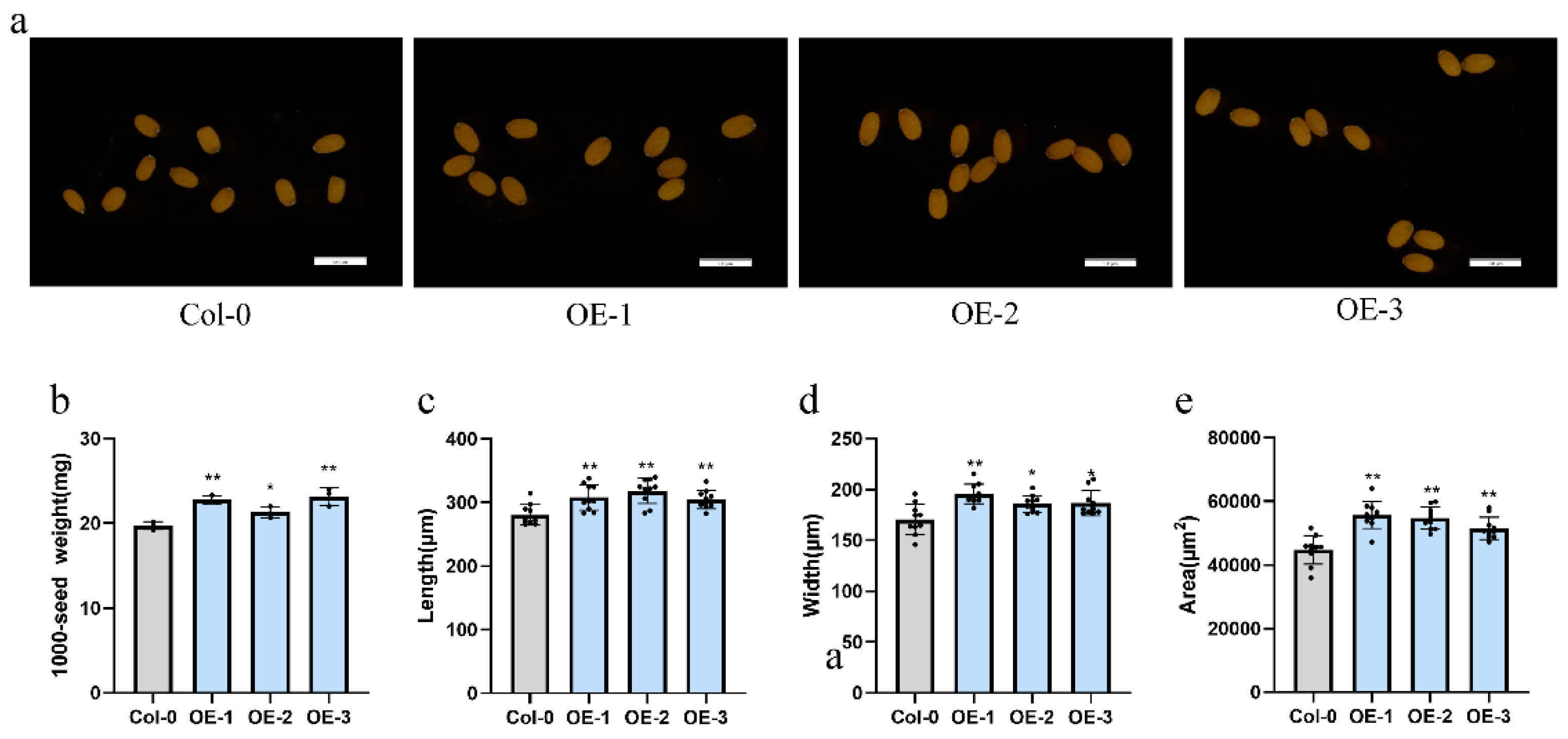
To probe the functional role of
GhUXS5 in seed development, we employed the GV3101 strain of Agrobacterium tumefaciens to generate overexpression (OE) lines of
GhUXS5 in
Arabidopsis thaliana via the floral dip method. Subsequently, we selected three distinct
Arabidopsis GhUXS5-OE lines for phenotypic assessment (
Figure 5a). Comparative analysis between the
GhUXS5-OE and the wild type (WT) revealed that the seeds from the OE lines exhibited a significant increase in weight (
Figure 5b). Additionally, morphological differences were observed in the seeds of the OE lines compared to the wild type, with notable increases in length, width, and overall area (
Figure 5d-f). Collectively, these findings suggest that the expression of
GhUXS5 may enhance seed development, indicating its potential as a genetic resource for seed size improvement.
3. Discussion
Cotton holds an indispensable position in global agricultural production; it is not only foundational to the textile industry but also an essential component of the edible oil sector. The fibers produced from the outer growth of the cotton ovule are the raw materials for textiles, while the oil and protein contained within the seeds provide key raw materials for the food and feed industries. Accordingly, both cotton fiber and seed are pivotal to societal advancement. However, in comparison to major field crops such as wheat, rice, and maize — where research into the regulatory pathways of seed size is well-developed and mature — studies concerning the regulatory pathways of cotton seed are relatively scarce. Despite the significant contributions of cotton seeds to national food security and the economy, research in this area remains far from exhaustive. In this investigation, we selected two cotton materials with notable differences in seed size, N10 and N12, to probe deeply into the regulatory mechanisms of seed size. Given the complex structure of cotton seeds, comprising both the hull and the kernel, our study meticulously measured both parts separately, including parameters such as hundred-seed weight, grain length, grain width, area, perimeter, and diameter. Correlation analyses affirmed a high degree of association between the cotton seed and the kernel, suggesting that the influence of the cotton hull on seed size is relatively minor. This discovery implies that the impact of the cotton hull can be reliably discounted when analyzing seed size. Subsequent transcriptomic analyses were conducted at different developmental stages (ovules at 5, 20, 30, and 35 days post-anthesis), and ultimately the GhUXS5 gene was identified as a significant candidate gene affecting seed size.
The embryogenesis of cotton can be delineated into three overlapping stages: the morphogenesis period (0-25 days post-anthesis, DPA), the maturation period (20-45DPA), and the desiccation period (post-35DPA). To investigate the variations in gene expression throughout these stages, and particularly their influence on the regulation of seed size, this study pinpointed several critical junctures for transcriptomic analysis, including 5DPA marking morphogenesis, 20DPA initiating maturation, 30DPA transitioning to desiccation, and 35DPA commencing the dry-down phase. This intermittent sampling strategy was devised to focus on analyzing the patterns of gene expression at each pivotal phase of cotton embryo development. Employing principal component analysis (PCA) and differential expression analysis, we discerned significant disparities in the gene expression profiles of materials N10 and N12 at 20DPA. Subsequent ex vitro culture experiments confirmed that 20DPA represents a critical period for notable differences in seed size between the two materials. This discovery indicates that the variance in gene expression at the onset of the maturation period may have a decisive impact on the formation of cotton seed size.
Given the current limited understanding of the pathways governing cotton seed development, this study endeavored to construct a gene expression network for cotton seed development by drawing upon the expression networks of seed size development in Arabidopsis thaliana. However, the findings revealed that despite our attempts to infer the regulatory network of cotton seed development through homologous genes, we were unable to directly identify key genes affecting seed size. Moreover, the majority of gene expression trends in the cotton seed transcriptome were opposite to those observed in Arabidopsis, a phenomenon that may reflect the genetic complexity of cotton as a tetraploid plant. To overcome this challenge, we employed differential expression analysis, clusters of temporal patterns, and weighted gene co-expression network analysis (WGCNA) to screen 413 candidate genes that potentially influence cotton seed size from the transcriptome data. Through further Gene Ontology (GO) analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis, we identified the GhUXS5 gene located in the carbohydrate metabolism pathway as a key candidate gene affecting cotton seed size. The discovery of GhUXS5 provides a new perspective on understanding the molecular mechanisms of seed size regulation and may offer a new target for cotton breeding. Given the crucial role of carbohydrate metabolism pathways in plant growth and development, the function of GhUXS5 in this pathway may have significant implications for the development and maturation processes of cotton seeds.
In dicot plants, xylose serves as an important component of cell wall hemicellulose and pectin polysaccharides [
32]. The biosynthesis of UDP-xylose occurs through a two-step reaction process. Firstly, UDP-glucose is converted to UDP-glucuronic acid (UDP-GlcA) in the presence of UDP-glucose dehydrogenase (UGD). Subsequently, the enzyme UDP-glucuronic acid decarboxylase (UXS) catalyzes the irreversible decarboxylation of UDP-GlcA to generate UDP-xylose [
33]. UXS plays a crucial role in the interconversion of nucleotide sugar, and its activity has been observed in higher plants. The cloning of the UXS gene was first accomplished in the fungus Cryptococcus neoformans [
34]. In tobacco, multiple isoforms of UXS have been isolated, and their expression has been identified in tissues associated with secondary cell wall development. Moreover, downregulation of certain genes through antisense expression is associated with reduced levels of xylan in these tissues [
35]. In Arabidopsis, six isoforms of UXS protein have been isolated, of which three, namely UXS3, UXS5, and UXS6, have been found to encode enzymes localized in the cytoplasm. Significant reduction in secondary cell wall thickening is observed when these UXS isoforms are downregulated or mutated [
36,
37]. Additionally, UXS3 has been demonstrated to affect the accumulation of indole-3-acetic acid (IAA) in rice [
38]. Therefore, GhUXS5 may influence cotton seed size by affecting cell wall changes and the content of IAA. Furthermore, our laboratory has discovered through other studies that this gene also impacts the elongation of cotton fibers [
39], indicating its multifunctionality and broad applicability.
4. Materials and Methods
4.1. Plant Materials and Sampling
Two distinct cotton lines, N10 (characterized by large seeds) and N12 (distinguished by small seeds), were sourced from a reconstructed population of recombinant inbred lines (RILs). These lines were subjected to cultivation across eight diverse environments, encompassing locations such as Alaer and Shihezi in Xinjiang, Anyang in Henan, and Xingtai in Hebei, during the years of 2017 and 2018, with an additional test conducted in Anyang, Henan, in 2023. Upon the maturation of the cotton bolls, manual harvesting ensued, followed by the extraction of seeds with lint through the processes of ginning and delinting utilizing concentrated sulfuric acid. From each line, a selection of one hundred seeds was meticulously delinted. The Wanshen SC-G automatic seed tester was then employed to accurately assess six attributes of seed and kernel size for both the N10 and N12 lines under varying environmental conditions and capture photos. The primary seed traits evaluated included hundred seed weight (HSW, also called seed index in cotton, g), seed length (SL, mm), seed width (SW, mm), seed area (SA, mm2), seed perimeter (SG, mm), and seed diameter (SD, mm). The kernel traits primarily encompassed hundred kernel weight (HKW, g), kernel length (KL, mm), kernel width (KW, mm), kernel area (KA, mm2), kernel perimeter (KG, mm), and kernel diameter (KD, mm). To conduct a comparative analysis of the cotton seed size traits between N10 and N12, a Student’s t-test was implemented.
4.2. RNA Extraction, Library Construction and RNA-Seq Analysis
In 2023, the two lines were grown field plots using a paired experiment with three replications in the experimental farm, Anyang, Henan, in 2023. RNA was extracted from ovules at 5, 20, 30, and 35 days post-anthesis (DPA) after flowers at 0 DPA were tagged for RNA sequencing (RNA-seq). The ovules were meticulously transported into a grinding vessel and swiftly submerged in liquid nitrogen. This step guaranteed consistent low-temperature processing through the consistent addition of more liquid nitrogen.
Subsequent to the collection, ovules were carefully excised using a pestle and tweezers, ensuring the integrity of each specimen. Each ovule fragment was then isolated and thoroughly triturated into a fine powder using a pestle. Following this, approximately 100 mg of the powdered sample was carefully apportioned into pre-chilled 2.0 ml centrifuge tubes, which were then promptly preserved in liquid nitrogen, awaiting subsequent analysis.
Subsequently, around 100 mg of the fiber powder sample was loaded into a pre-cooled 2.0 ml centrifuge tube, which was then placed in liquid nitrogen for storage and further processing. Total RNA was isolated utilizing the FastPure Plant Total RNA Isolation kit, specifically designed for samples abundant in polysaccharides and polyphenolics, provided by Nanjing. The isolation procedure was meticulously followed as per the guidelines provided by the kit’s producer. The integrity of RNA was evaluated through visual examination of the fractionated 18S and 28S rRNA bands, which were separated via electrophoresis on a 1.5% agarose gel, applied to the isolated total RNAs. Subsequently, the concentration of RNA was quantified using the Nanodrop 2000 spectrophotometric device (produced by Thermo Fisher Scientific, located in Waltham, MA, USA) by assessing the optical density ratio at 260 nm and 280 nm (OD260/OD280). Altogether, eight libraries of cDNA (comprising two distinct genotypes across four stages of development) were assembled and subsequently sequenced on the Illumina NovaSeq 6000 platform (provided by Illumina, located in San Diego, CA, USA) to analyze the transcriptomic profile.
After transcriptome sequencing, the raw data was filtered by BMKCloud (BMK, Qingdao, China). During the process of filtering the sequences, any sequences containing adapters, reads that possessed over 5% bases denoted as ‘N’, and reads where more than 20% were of low quality (quality score ≤ 15) were excluded from the subsequent analysis. The filtered sequences were aligned to the G. hirsutum TM-1 reference genome [
40], using the Hisat2 alignment tool with standard parameters [
41]. The aligned files were treated by samtools, and transcription abundance of the whole genes was quantitatively estimated by StringTie (FPKM was used) [
42]. We have submitted all the sequenced raw datasets to the NCBI short read archives (SRA; accession number PRJNA1120209).
4.3. Principal Component Analysis, Ovule In Vitro Culture and Growth Curves
A Principal Component Analysis (PCA) was implemented on the transcriptome data compiled from two samples spanning four distinct stages, utilizing the OmicShare tool (
https://www.omicshare.com/tools). In the proceeding steps, ovules harvested at 0 days post-anthesis (DPA) were subjected to disinfection via a 0.1% mercury(II) chloride solution for a duration of 5 minutes. The boll shell was then carefully dissected using a surgical knife, followed by the meticulous extraction of the ovules using sterilized tweezers and their placement in a detached culture medium. Subsequently, these ovules were incubated in the absence of light at a temperature of 30°C, enabling the observation of their growth over time. In addition, the dimensions of the cotton seeds were accurately measured, and the data were graphically represented using R software, thus providing a visually intuitive depiction of the developmental trajectory.
4.4. Gene Expression Network
Drawing from Li’s (2019) [
17] research on the molecular network that regulates plant seed size, we utilized Blast to screen for cotton genes that showed the highest homology with the genes controlling seed size in Arabidopsis. Subsequently, based on the transcriptome data, we selected the cotton genes exhibiting the highest expression levels. Ultimately, we constructed a gene expression network comprising six pathways that regulate seed size, incorporating a total of 27 genes.
4.5. Differential Expression Analysis
In order to analyze differentially expressed genes (DEGs), we employed DESeq2 and calculated Padj values to adjust the threshold of P values. We defined genes with |log2FoldChange| ≥ 2 and Padj ≤ 0.01 as differentially expressed genes. Based on the transcriptional abundance of genes between the two varieties, we defined genes with higher expression levels in the large-seed variety during ovule development as up-regulated genes, and those with lower expression levels in the large-seed variety as down-regulated genes.
4.6. Clusters of Temporal Patterns
Genes exhibiting an FPKM value exceeding 5 at a particular timepoint were classified as expressed genes. Utilizing this criterion, a total of 38,947 expressed genes were identified. The fuzzy c-means algorithm [
43] was employed to cluster gene expression profiles across all developmental stages. Drawing upon the transcriptional abundance of the two materials under investigation, an analysis was performed using the TCseq package within the R programming language. The culmination of this analysis yielded nine distinct clusters, each representing a unique transcriptomic model.
4.7. WGCNA Pipeline
Gene co-expression networks were established through the execution of Weighted Gene Correlation Network Analysis (WGCNA) using the pertinent R software package [
31]. Genes with a maximum value among eight samples smaller than five were excluded. The remaining genes were selected for the construction of the weighted gene co-expression network. A soft-thresholding criterion was chosen based on an R2 value surpassing 0.85. The parameter for merging modules, referred to as mergeCutHeight, was set at a level of 0.35 to facilitate module classification. To evaluate the association between modules and phenotypes, the Pearson correlation coefficient between the module’s eigengene and the phenotype was calculated using the statistics package in Python. For the construction of gene regulatory networks (GRNs), Pearson correlations between the expression levels of the target gene and other candidate genes were computed. The GRNs were documented in the form of tab-separated tables. In this study, functional enrichment analysis was conducted using GO and KEGG enrichment analysis via cottonFGD (
https://cottonfgd.net/).
4.8. GhUXS5 Overexpression Arabidopsis Phenotypic Identification
Seeds reaped from the T3 generation of Arabidopsis, comprising both overexpressing specimens and those of wild-type, were utilized for the quantification of the thousand seed weight, with each instance encompassing the enumeration of 1000 seeds, executed thrice for repetition. Subsequently, the dimensions of the seeds were captured photographically via a stereomicroscope. Ten seeds from an identical field of view were selected, and their respective lengths, widths, and surface areas were measured utilizing ImageJ software.
5. Conclusions
In this study, transcriptomic analysis of two lines, N10 and N12, during cotton embryo development was conducted to systematically screen 413 genes as candidate genes influencing seed size, ultimately identifying GhUXS5 as a key candidate gene. Through GO and KEGG analysis, the functional role of GhUXS5 in the carbohydrate metabolism pathway was further revealed, which plays a crucial role in regulating cotton seed size. Additionally, preliminary gene functional validation was performed to further confirm the importance of GhUXS5 in cotton embryo development. The results of this study hold significant scientific value in deepening our understanding of the molecular mechanisms underlying cotton seed growth and development. By uncovering key genes and associated pathways, we can gain a better understanding of the regulatory mechanisms of cotton seed size. This is of great significance for breeders, as they can utilize these findings to design and manipulate the desired seed size traits, thereby enhancing the economic value and adaptability of cotton crops.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Figure S1: Correlation of various traits of cottonseed and kernel in cotton materials N10 and N12; Figure S2: RNA-Seq analysis was used to study differentially expressed genes (DEGs) during the developmental stages of cottonseeds. (a) Venn diagram of DEGs across comparisons at different time periods. (b) Vertical bar charts showing the number of upregulated and downregulated DEGs (based on fold changes between different periods) (DEGs number with FDR-adjusted p-value ≤ 0.05); Table S1: Summary of RNA_seq data quality in 25 libraries.
Author Contributions
Bing Jia: analysed and summed all the data, drew the figures and wrote the manuscript. Pan Feng and Jikun Song: participated in sample preparation. Bingbing Zhang, Caoyi Zhou and Yajie Wang: participated in data collection, and analysis. Man Wu and Jinfa Zhang: advised the experiment and revised the manuscript. Quanjia Chen and guided the experiment and participated in the discussion of the manuscript. Jiwen Yu: directed the experiments and revised the manuscript. All authors read and approved the final manuscript.
Funding
The authors thank supports by The National Key Research and Development Program of China (2022YFD1200300 & 2023YFD2301201), the STI 2030-Major Projects 2023ZD04038, the Scientific Research Project of Anyang city of China (2023C01NY003), the Scientific Research Project of Henan Province of China (222102110209), the Agricultural Improved Seed Joint Project of Henan Province of China (2022010301).
Data Availability Statement
Data will be made available on request. All data and materials supporting our findings are included in the Materials and Methods section. Details are provided in the attached files. All the transcriptome raw data we sequenced was deposited in the NCBI short read archives (SRA; accession number PRJNA1120209).
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Chen, Y.; Liu, Y.; Chen, Y.; Zhang, Y.; Zan, X. Design and preparation of polysulfide flexible polymers based on cottonseed oil and its derivatives. Polymers 2020, 12, 1858. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y. L. Recent advances in understanding cotton fibre and seed development. Seed Sci. Res. 2005, 15, 269–280. [Google Scholar] [CrossRef]
- Dowd, M. K.; Pelitire, S. M.; Delhom, C. D. Seed-Fiber Ratio, Seed Index, and Seed Tissue and Compositional Properties of Current Cotton Cultivars. J. Cotton Sci. 2018, 22, 60–74. [Google Scholar] [CrossRef]
- Zhao, J.; Bai, W. Q.; Zeng, Q. W.; Song, S. Q.; Zhang, M.; Li, X. B.; Hou, L.; Xiao, Y. H.; Luo, M.; Li, D. M.; Luo, X. Y.; Pei, Y. Moderately enhancing cytokinin level by down-regulation of GhCKX expression in cotton concurrently increases fiber and seed yield. Mol. Breed. 2015, 35, 11. [Google Scholar] [CrossRef]
- Atique-ur-Rehman; Kamran, M.; Afzal, I. Production and processin of quality cotton seed. In Cotton Production and Uses; 2020; pp. 547–570. [Google Scholar]
- Silvertown, J. The paradox of seed size and adaptation. Trends in ecology & evolution 1989, 4, 24–26. [Google Scholar]
- Chaudhury, A. M.; Koltunow, A.; Payne, T.; Luo, M.; Tucker, M. R.; Dennis, E.; Peacock, W. J. Control of early seed development. Annual Review of Cell and Developmental Biology 2001, 17, 677–699. [Google Scholar] [CrossRef]
- Figueiredo, D. D.; Köhler, C. Signalling events regulating seed coat development. Biochem. Soc. Trans. 2014, 42, 358–363. [Google Scholar] [CrossRef]
- Cai, G. Q.; Yang, Q. Y.; Yang, Q.; Zhao, Z. X.; Chen, H.; Wu, J.; Fan, C. C.; Zhou, Y. M. Identification of candidate genes of QTLs for seed weight in Brassica napus through comparative mapping among Arabidopsis and Brassica species. BMC Genetics 2012, 13. [Google Scholar] [CrossRef]
- Kesavan, M.; Song, J. T.; Seo, H. S. Seed size: a priority trait in cereal crops. Physiol. Plant. 2013, 147, 113–120. [Google Scholar] [CrossRef]
- Sun, X. D.; Shantharaj, D.; Kang, X. J.; Ni, M. Transcriptional and hormonal signaling control of Arabidopsis seed development. Curr. Opin. Plant Biol. 2010, 13, 611–620. [Google Scholar] [CrossRef]
- Luo, M.; Dennis, E. S.; Berger, F.; Peacock, W. J.; Chaudhury, A. MINISEED3 (MINI3), a WRKY family gene, and HAIKU2 (IKU2), a leucine-rich repeat (LRR) KINASE gene, are regulators of seed size in Arabidopsis. Proc. Natl. Acad. Sci. U. S. A. 2005, 102, 17531–17536. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, X. J.; Kang, X. J.; Zhao, X. Y.; Zhang, X. S.; Ni, M. SHORT HYPOCOTYL UNDER BLUE1 Associates with MINISEED3 and HAIKU2 Promoters in Vivo to Regulate Arabidopsis Seed Development. Plant Cell 2009, 21, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C. S.; Kolevski, B.; Smyth, D. R. TRANSPARENT TESTA GLABRA2, a trichome and seed coat development gene of Arabidopsis, encodes a WRKY transcription factor. Plant Cell 2002, 14, 1359–1375. [Google Scholar] [CrossRef]
- Jofuku, K. D.; Omidyar, P. K.; Gee, Z.; Okamuro, J. K. Control of seed mass and seed yield by the floral homeotic gene APETALA2. Proc. Natl. Acad. Sci. U. S. A. 2005, 102, 3117–3122. [Google Scholar] [CrossRef] [PubMed]
- Riefler, M.; Novak, O.; Strnad, M.; Schmulling, T. Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development, and cytokinin metabolism. Plant Cell 2006, 18, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Xu, R.; Li, Y. H. Molecular Networks of Seed Size Control in Plants. In Annual Review of Plant Biology, Vol 70; Merchant, S. S., Ed.; Annual Reviews: Palo Alto, 2019; Volume 70, pp. 435–463. [Google Scholar]
- Ryser, U.; Schorderet, M.; Jauch, U.; Meier, H. Ultrastructure of the “fringe-layer”, the innermost epidermis of cotton seed coats. Protoplasma 1988, 147, 81–90. [Google Scholar] [CrossRef]
- Ruan, Y. L.; Chourey, P. S.; Delmer, D. P.; PerezGrau, L. The differential expression of sucrose synthase in relation to diverse patterns of carbon partitioning in developing cotton seed. Plant Physiol. 1997, 115, 375–385. [Google Scholar] [CrossRef]
- Pugh, D. A.; Offler, C. E.; Talbot, M. J.; Ruan, Y. L. Evidence for the Role of Transfer Cells in the Evolutionary Increase in Seed and Fiber Biomass Yield in Cotton. Mol. Plant. 2010, 3, 1075–1086. [Google Scholar] [CrossRef]
- West, M. A. L.; Harada, J. J. Embryogenesis in Higher Plants: An Overview. The Plant cell 1993, 5, 1361–1369. [Google Scholar] [CrossRef]
- Reeves, R. G.; Beasley, J. O. The development of the cotton embryo. J. Agric. Res. 1935, 51, 935–944. [Google Scholar]
- Pollock, E. G.; Jensen, W. A. Cell development during early embryogenesis in Capsella and Gossypium. American Journal of Botany 1964, 51, 915–21. [Google Scholar] [CrossRef]
- Yeung, E. C.; Meinke, D. W. Embryogenesis in Angiosperms: Development of the Suspensor. The Plant cell 1993, 5, 1371–1381. [Google Scholar] [CrossRef] [PubMed]
- Turley, R. B.; Chapman, K. D. Ontogeny of cotton seeds: gametogenesis, embryogenesis, germination, and seedling growth; Springer: New York, 2010; pp. 332–341. [Google Scholar]
- Lee, J. J.; Woodward, A. W.; Chen, Z. J. Gene expression changes and early events in cotton fibre development. Ann. Bot. 2007, 100, 1391–1401. [Google Scholar] [CrossRef]
- Yang, S. X.; Huang, L.; Song, J. K.; Liu, L. S.; Bian, Y. Y.; Jia, B.; Wu, L. Y.; Xin, Y.; Wu, M.; Zhang, J. F.; Yu, J. W.; Zang, X. S. Genome-Wide Analysis of DA1-Like Genes in Gossypium and Functional Characterization of GhDA1-1A Controlling Seed Size. Front. Plant Sci. 2021, 12, 11. [Google Scholar] [CrossRef]
- Huang, L.; Yang, S. X.; Wu, L. Y.; Xin, Y.; Song, J. K.; Wang, L.; Pei, W. F.; Wu, M.; Yu, J. W.; Ma, X. Y.; Hu, S. L. Genome-Wide Analysis of the GW2-Like Genes in Gossypium and Functional Characterization of the Seed Size Effect of GhGW2-2D. Front. Plant Sci. 2022, 13, 10. [Google Scholar] [CrossRef]
- Zhao, T.; Wu, H. Y.; Wang, X. T.; Zhao, Y. Y.; Wang, L. Y.; Pan, J. Y.; Mei, H.; Han, J.; Wang, S. Y.; Lu, K. N.; Li, M. L.; Gao, M. T.; Cao, Z. Y.; Zhang, H. L.; Wan, K.; Li, J.; Fang, L.; Zhang, T. Z.; Guan, X. Y. Integration of eQTL and machine learning to dissect causal genes with pleiotropic effects in genetic regulation networks of seed cotton yield. Cell Reports 2023, 42, 21. [Google Scholar] [CrossRef] [PubMed]
- Liu, X. Y.; Hou, J.; Chen, L.; Li, Q. Q.; Fang, X. M.; Wang, J. X.; Hao, Y. S.; Yang, P.; Wang, W. W.; Zhang, D. S.; Liu, D. X.; Guo, K.; Teng, Z. H.; Liu, D. J.; Zhang, Z. S. Natural variation of GhSI7 increases seed index in cotton. Theor. Appl. Genet. 2022, 135, 3661–3672. [Google Scholar] [CrossRef]
- Futschik, M. E.; Carlisle, B. Noise-robust soft clustering of gene expression time-course data. Journal of bioinformatics and computational biology 2005, 3, 965–88. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 2008, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Ebert, B.; Rautengarten, C.; Guo, X. Y.; Xiong, G. Y.; Stonebloom, S.; Smith-Moritz, A. M.; Herter, T.; Chan, L. J. G.; Adams, P. D.; Petzold, C. J.; Pauly, M.; Willats, W. G. T.; Heazlewood, J. L.; Scheller, H. V. Identification and Characterization of a Golgi-Localized UDP-Xylose Transporter Family from Arabidopsis. Plant Cell 2015, 27, 1218–1227. [Google Scholar] [CrossRef]
- Zhang, D. M.; Pan, Y. X.; Zhang, Y.; Li, Z. K.; Wu, L. Q.; Liu, H. W.; Zhang, G. Y.; Wang, X. F.; Ma, Z. Y. Antisense expression of Gossypium hirsutum UDP-glucuronate decarboxylase in Arabidopsis leads to changes in cell wall components. Genet. Mol. Res. 2016, 15, 12. [Google Scholar] [CrossRef]
- Bar-Peled, M.; Griffith, C. L.; Doering, T. L. Functional cloning and characterization of a UDP-glucuronic acid decarboxylase:: The pathogenic fungus Cryptococcus neoformans elucidates UDP-xylose synthesis. Proc. Natl. Acad. Sci. U. S. A. 2001, 98, 12003–12008. [Google Scholar] [CrossRef] [PubMed]
- Bindschedler, L. V.; Tuerck, J.; Maunders, M.; Ruel, K.; Petit-Conil, M.; Danoun, S.; Boudet, A. M.; Joseleau, J. P.; Bolwell, G. P. Modification of hemicellulose content by antisense down-regulation of UDP-glucuronate decarboxylase in tobacco and its consequences for cellulose extractability. Phytochemistry 2007, 68, 2635–2648. [Google Scholar] [CrossRef] [PubMed]
- Kuang, B. Q.; Zhao, X. H.; Zhou, C.; Zeng, W.; Ren, J. L.; Ebert, B.; Beahan, C. T.; Deng, X. M.; Zeng, Q. Y.; Zhou, G. K.; Doblin, M. S.; Heazlewood, J. L.; Bacic, A.; Chen, X. Y.; Wu, A. M. Role of UDP-Glucuronic Acid Decarboxylase in Xylan Biosynthesis in Arabidopsis. Mol. Plant. 2016, 9, 1119–1131. [Google Scholar] [CrossRef]
- Zhong, R. Q.; Teng, Q. C.; Haghighat, M.; Yuan, Y. X.; Furey, S. T.; Dasher, R. L.; Ye, Z. H. Cytosol-Localized UDP-Xylose Synthases Provide the Major Source of UDP-Xylose for the Biosynthesis of Xylan and Xyloglucan. Plant Cell Physiol. 2017, 58, 156–174. [Google Scholar] [CrossRef] [PubMed]
- Ruan, N.; Dang, Z. J.; Wang, M. H.; Cao, L. Y.; Wang, Y.; Liu, S. T.; Tang, Y. J.; Huang, Y. W.; Zhang, Q.; Xu, Q.; Chen, W. F.; Li, F. C. FRAGILE CULM 18 encodes a UDP-glucuronic acid decarboxylase required for xylan biosynthesis and plant growth in rice. J. Exp. Bot. 2022, 73, 2320–2335. [Google Scholar] [CrossRef]
- Song, J.; Jia, B.; Feng, P.; Xi, H.; Zhao, W.; Xi, H.; Dong, Y.; Pei, W.; Ma, J.; Zhang, B.; Wang, L.; Wu, M.; Zhang, J.; Yu, J. Transcriptome analysis reveals potential of down-regulated genes in cotton fiber improvement. Industrial Crops and Products 2024, 217. [Google Scholar] [CrossRef]
- Yang, Z. E.; Ge, X. Y.; Yang, Z. R.; Qin, W. Q.; Sun, G. F.; Wang, Z.; Li, Z.; Liu, J.; Wu, J.; Wang, Y.; Lu, L. L.; Wang, P.; Mo, H. J.; Zhang, X. Y.; Li, F. G. Extensive intraspecific gene order and gene structural variations in upland cotton cultivars. Nat. Commun. 2019, 10, 13. [Google Scholar] [CrossRef]
- Kim, D.; Landmead, B.; Salzberg, S. L. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–U121. [Google Scholar] [CrossRef]
- Trapnell, C.; Pachter, L.; Salzberg, S. L. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 2009, 25, 1105–1111. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).