1. Introduction
Human T-cell lymphotropic virus type 1 (HTLV-1)-associated myelopathy/tropical spastic paraparesis (HAM/TSP) is a chronic progressive myelopathy associated with HTLV-1 infection [
1]. It is estimated that over 10 million people are infected with HTLV-1 in the world, where in 2-10% of these, it manifest in clinical disease such as adult-onset T-cell leukemia and HAM/TSP [
2,
3,
4]. HTLV-1 can spread from exchange of bodily fluids such as during sexual intercourse, from mother to child or through blood transfusions from an infected donor [
4,
5]. HAM/TSP is endemic in several parts of the world such as middle east, South America, Japan, and in the indigenous population in the USA and Australia [
2,
6].
CNS damage in HAM/TSP is believed to be mediated by the recruitment, activation, and expansion of HTLV-1-infected CD4+ T-cells and HTLV-1-specific CD8+ cytotoxic T lymphocytes (CTLs) in the intrathecal compartment [
7,
8,
9]. These infiltrates release proinflammatory cytokines, including interferon gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α), and promote the secretion of chemokines from resident cells [
10]. This creates a self-perpetuating focus of inflammation within the CNS compartment that culminates in bystander damage of neuronal tissue [
4,
11,
12]. Inflammatory changes have been reported in both the spinal cord and brain of participants with HAM/TSP. By histopathology, Aye et al. (2000) [
13] reported that patients with active-chronic inflammation in the spinal cord also had perivascular inflammatory infiltrates in the brain.
Radiologically, spinal cord atrophy and brain lesion formation has long been understood to be a feature of HAM/TSP [
14,
15,
16,
17,
18,
19]. Some in vivo studies have reported that the frequency of cerebral white matter hyperintensities (WMH) is greater in HAM/TSP patients than healthy controls, but others have reported no difference between patient and control groups [
17,
20,
21]. To date, therefore, no consensus has been reached regarding the prevalence of brain abnormalities in HAM/TSP nor their relation to spinal cord degradation or clinical disability [
20,
21,
22,
23]. We have previously demonstrated that spinal cord atrophy can be estimated in vivo with a semi-automated tool that measures spinal cord cross sectional area (SCCSA) in the cervical and thoracolumbar spine from magnetic resonance imaging (MRI) [
15,
16,
18,
24]. Participants diagnosed with HAM/TSP had significant spinal cord atrophy that began in the thoracic region and progressed to the cervical cord. The severity of thoracic cord atrophy was positively correlated with HTLV-1 proviral load, CD8+ T-cell frequency in the CSF, and clinical disability scores [
15]. By histopathology, spinal cord thinning in HAM/TSP is associated with demyelination and loss of axons, which predominate in the lateral columns [
4].
Sometimes multiple sclerosis (MS), a neuroinflammatory disease affecting the brain and spinal cord, can present as a progressive myelopathy having similar clinical features as HAM/TSP. MS affects more than 2.8 million people worldwide with unclear etiology [
25] and studying HAM/TSP in concert with MS can help characterize shared immunopathogenic mechanisms and identify therapies that can be translated from one disease to the other. MRI of the brain and cervical spinal cord is useful for both diagnosing and monitoring disease progression and treatment response [
26,
27]. Recent studies have also demonstrated that primary and secondary progressive forms of multiple sclerosis (PPMS and SPMS, together called progressive MS or P-MS), but not relapsing remitting (RRMS), are associated with c-spine atrophy, particularly at the C4-5 vertebral body level corresponding to the cervical enlargement [
15,
24]. In addition to spinal cord atrophy, the MS brain is mainly characterized by WMH called lesions as well as significant atrophy of both normal appearing gray and white matter [
28,
29]. Since patients with HAM/TSP are often initially misdiagnosed as having progressive form of multiple sclerosis (P-MS), especially in areas of the world in which HAM/TSP is not endemic, developing tools may provide clues for differentials with other inflammatory myelopathies are important for patient management.
To evaluate the prevalence and role of WMH and atrophy in the CNS of participants with HAM/TSP in comparison with control subjects with no neurological symptoms (HVs), we retrospectively measured cervical and thoracolumbar SCCSA and brain tissue volumes using routinely acquired MR images. SCCSA was averaged for the regions corresponding to vertebral body levels C2-3, C4-5, and T4-9, and brain tissue was segmented into gray matter (GM), white matter (WM), cerebrospinal fluid (CSF), and WMH. Spinal cord and brain tissue volumes were then correlated to various measures of clinical disability. Group averages for HAM/TSP were also compared to participants clinically diagnosed with MS and principal component analysis was used to evaluate which radiological variables were relevant to the differentiation of these neurological conditions.
2. Materials and Methods
2.1. Study Design and Participants
This was a retrospective study that involved HAM/TSP, RRMS, progressive MS (P-MS), and HVs. Natural history studies in HAM/TSP and MS were approved by the institutional review board at the NIH (NCT numbers: NCT00034723 and NCT00001248) and participants included after informed consent. Participants underwent neurological examinations and clinical disability was assessed using expanded disability status scale (EDSS), Scripps neurologic rating scale (SNRS), time to complete a 25-foot walk (T25FW), and the time to complete 9-hole peg test (9-HPT). HAM/TSP participants were also rated by Instituto de Pesquisas de Cananeia (IPEC). MS and were categorized into RRMS, SPMS, and PPMS phenotypes and, participants with SPMS and PPMS were considered together as one group termed progressive MS (P-MS).
2.2. MR Imaging and Analysis
All participants underwent 3T MRI of the brain and spinal cord (Siemens Healthcare GmbH, Erlangen, Germany) with a 20-channel or a 32-channel head coil and 24-channel spine matrix coil. To measure spinal cord thinning, T1-weighted scans of the cervical and thoracic spinal cord (3D gradient recalled echo sequence, repetition time = 8 ms, echo time = 3 ms, flip angle = 18 degrees, 1-mm isotropic resolution, total acquisition time of about 3 min 30 sec each for C- and T- spine) were obtained for each subject. Cervical and thoracic images were stitched together using table position information on the DICOM headers and scripts written in house and spinal cord cross sectional area (SCCSA) was measured as previously described [
15,
18,
24]. Briefly, the user manually selected the region of the spinal cord corresponding to vertebral body levels C1 and T10 using scripts written in Matlab (MathWorks Inc., Natick, MA). Axial images perpendicular to the selected cord edge were then automatically reformatted at each point and cross-sectional area was calculated. Analysis was checked by both manual and automated quality assurance steps and SCCSA was plotted against normalized distance from C1 to T10. For statistical comparisons, SCCSA for each subject was also averaged over the regions corresponding to vertebral levels C2-3, C4-5, and T4-9. These three regions were chosen as they are not affected by inter-subject anatomical differences and have unique pathological and clinical implications; C2-3 comprises predominantly white matter tracts, C4-5 corresponds to the cervical enlargement and has higher gray matter content than the C2-3 region, and T4-9 houses white matter tracts for lower extremity innervation and has been shown to be the first region to degrade in HAM/TSP [
15]. Due to either lack of scan availability or poor scan quality, SCCSA data was not acquired for 8 healthy controls, 3 HAM/TSP participants, 4 RRMS participants, and 3 P-MS participants.
To evaluate brain tissue volumes, the machine learning program Classification Using DErivative Based Features (C-DEF) [
30,
31] was used to perform automated brain segmentations from MR images. T1-weighted (T1-MP2RAGE, IR-TFL sequence, TR/TE/TI1/TI2 = 5000/2.9/700/2500 ms, FA=4,5 deg, 176 slices, 1 mm isotropic resolution, acquisition time=8 min 20 sec) and fluid attenuated inversion recovery (FLAIR, 3D IR-TSE sequence, TR/TE/TI = 4800/352/1800 ms, 176 slices, 1 mm isotropic resolution, acquisition time=5 min 22 sec) contrasts were obtained for each subject and analyzed by C-DEF algorithm as previously described [
30]. In brief, models were trained on participants with variable lesion severity, two separate models were created for scans acquired with 20-channel and 32-channel head coils. Analysis was conducted using voxel data from MP2RAGE sequence (including the two inversion images and denoised T1-weighted image) and FLAIR images, and brain tissue was segmented into GM, WM, CSF, and white matter hyperintensities (lesions). All segmentations were quality checked by S.V.O., a trained neurologist with 6 years of experience in MS imaging. Segmentations that failed the quality assurance (13/113 scans), which was typically due to the misidentification of large lesions as GM, were manually corrected using 3DSlicer. Manual segmentations were performed by E.H.S. and quality checked by S.V.O. All tissue volumes were normalized to intracranial volume to give brain fractions and adjusted for age. All brain scans analyzed were acquired within one day of the subject’s spine scans.
2.3. Statistical Methods
Box-Cox transformation was applied to the outcome variables with non-normal distribution: the natural logarithm for lesion fraction and PBMC-PVL, inverse transformation for T25FW, and square transformation for 9-HPT. Test avg. Shapiro-Wilk test was used to test the normality of the (Studentized) residuals. For each of the 11 outcome variables, analysis of covariance (ANCOVA) was applied to examine the group-wise differences using Tukey’s method to adjust for multiple comparisons. Sex and age were considered as covariate variables, for which variables with p > 0.1 were dropped from the model. Pearson simple and partial correlation analyses were conducted to examine the association between the 4 brain variables and the 3 spine variables with age as a covariate (sex had no effect on any outcome variables) for each of the 4 clinical groups separately. These correlation analyses were also applied to examine the association between the 4 clinical variables and the 3 spine variables. Finally, principal component analysis was performed using three spine variables (C4-5, C2-3, T4-9) and three lesion variables (log transformed number of brain lesions, log transformed lesion fraction, log transformed median lesion volume of each participant), where the number of lesions and lesion-fraction were pre-adjusted for age (the other 4 variables were not associated with age). Varimax rotation was applied to derive orthogonal (uncorrelated) components, which were used to evaluate the group-wise difference by ANOVA and Tukey’s method. SAS version 9.4 was used for all analyses, and a p-value < 0.05 was considered to be statistically significant.
3. Results
3.1. Subsection
In total, 113 individuals were included in the study comprising 24 HVs, 43 HAM/TSP, 26 RRMS, and 20 P-MS. Demographic and clinical information for the cohort is summarized in
Table 1.
3.1. Spinal Cord Atrophy
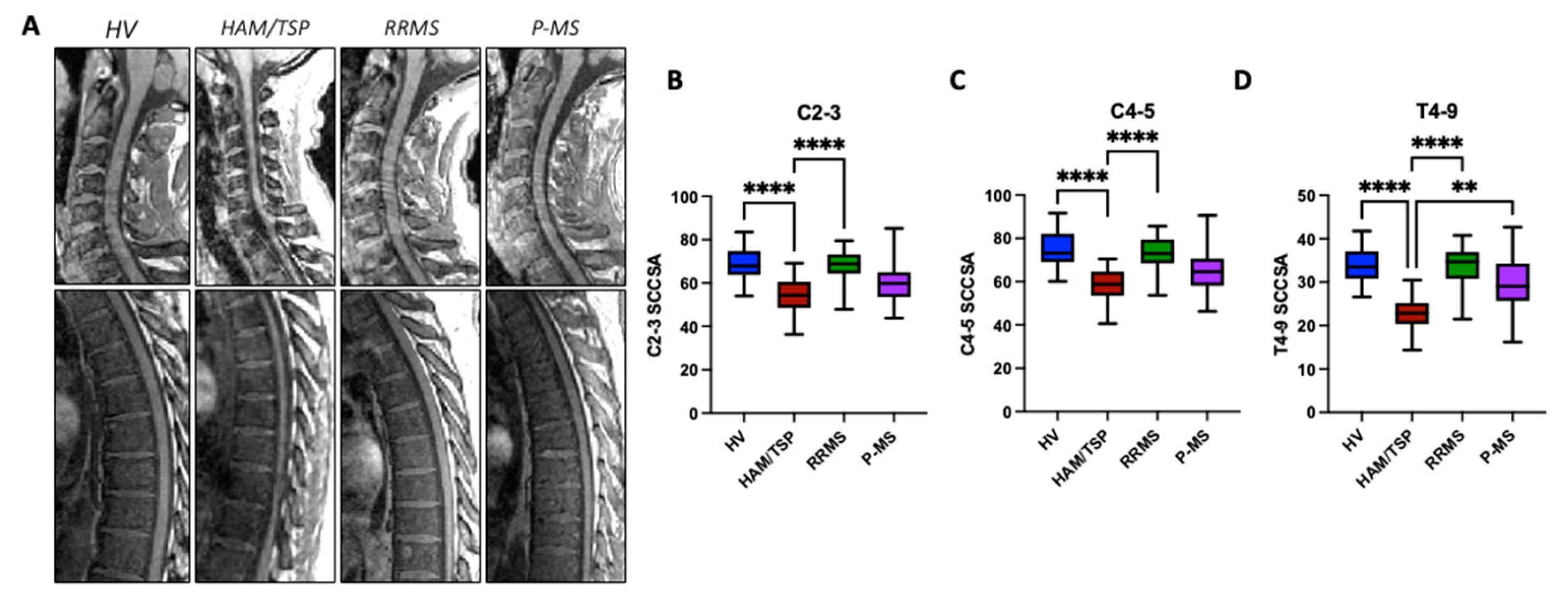
As shown in the representative mid-sagittal MR images of the cervical and thoracolumbar spine for one subject from each group, there is visible spinal cord thinning in HAM/TSP (
Figure 1A). To quantitatively compare the degree of spinal cord thinning across groups, average SCCSA was calculated for three representative regions of the cord corresponding to vertebral body levels C2-3, C4-5, and T4-9 (
Figure 1B-D). HAM/TSP participants had significantly lower average SCCSA at C2-3 (54.0±8 mm2), C4-5 (57.8±8 mm2), and T4-9 (22.7±4 mm2) compared to HV (C2-3: 69.4±8 mm2, C4-5: 75.1±9 mm2, T4-9: 34.1±4 mm2; p < 0.0001) and RRMS (67.6±8 mm2, 72.7±9 mm2, 33.4±5 mm2; p < 0.0001) averages (
Figure 1B-D). There were no statistically significant differences in average SCCSA for RRMS or P-MS (C2-3: 60.7±11 mm2; C4-5: 66±13 mm2, T4-9: 29.7±7 mm2; p>0.05) compared to HVs, but a trend towards lower area at C2-3 was observed in P-MS (p = 0.062). There were no significant correlations between SCCSA at any region and age or sex (data not shown).
3.2. Brain Lesion Volume and Atrophy
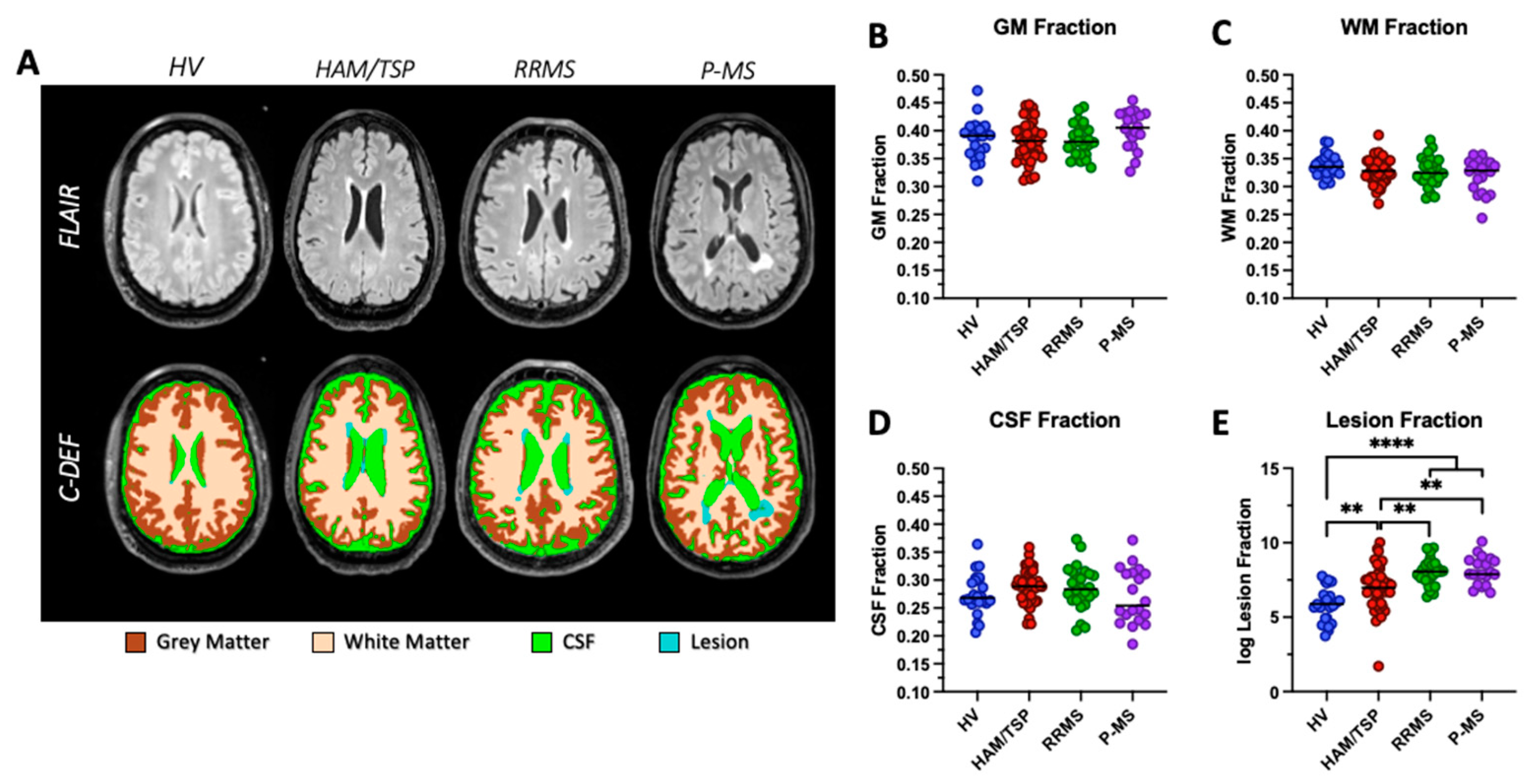
Brain segmentations were completed on all 113 individuals. FLAIR images and C-DEF segmentation masks from a representative participant in each diagnosis group is shown in
Figure 2A. Tissue was segmented into GM (brown), WM (beige), CSF (green), and lesions (blue) and normalized to total intracranial volume to obtain brain fractions. All brain tissue fractions were significantly correlated with age (GM fraction, p < 0.0001; WM fraction, p = 0.0008; CSF fraction, p < 0.0001; lesion fraction, p < 0.0001), so age-adjusted brain fractions were used for downstream statistical analyses. No statistically significant differences in age-adjusted GM fraction, WM fraction, or CSF fraction were observed between HAM/TSP and other groups in our cohort (
Figure 2 B-D). A significant increase in average age-adjusted lesion fraction was observed in HAM/TSP (0.004±0.008), RRMS (0.004±0.004), and P-MS (0.0087±0.014) compared to HVs (0.0005±0.0007; all p < 0.002,
Figure 2E). Average age-adjusted lesion fraction in HAM/TSP was significantly lower than both RRMS and P-MS averages (p = 0.0067, p = 0.0014, respectively,
Figure 2E).
3.3. Correlation of Radiological and Clinical Measures
On average, participants with HAM/TSP and P-MS had more severe clinical disability than participants with RRMS as indicated by significant increases in median EDSS (6.5, 6, and 1.75, respectively; p < 0.0001) and significant decreases in median SNRS (69, 65, and 93, respectively; p < 0.0001,
Table 1). Pearson partial correlation coefficients for the relationship between SCCSA or brain lesion fraction and all disability measures are summarized in
Table 2.
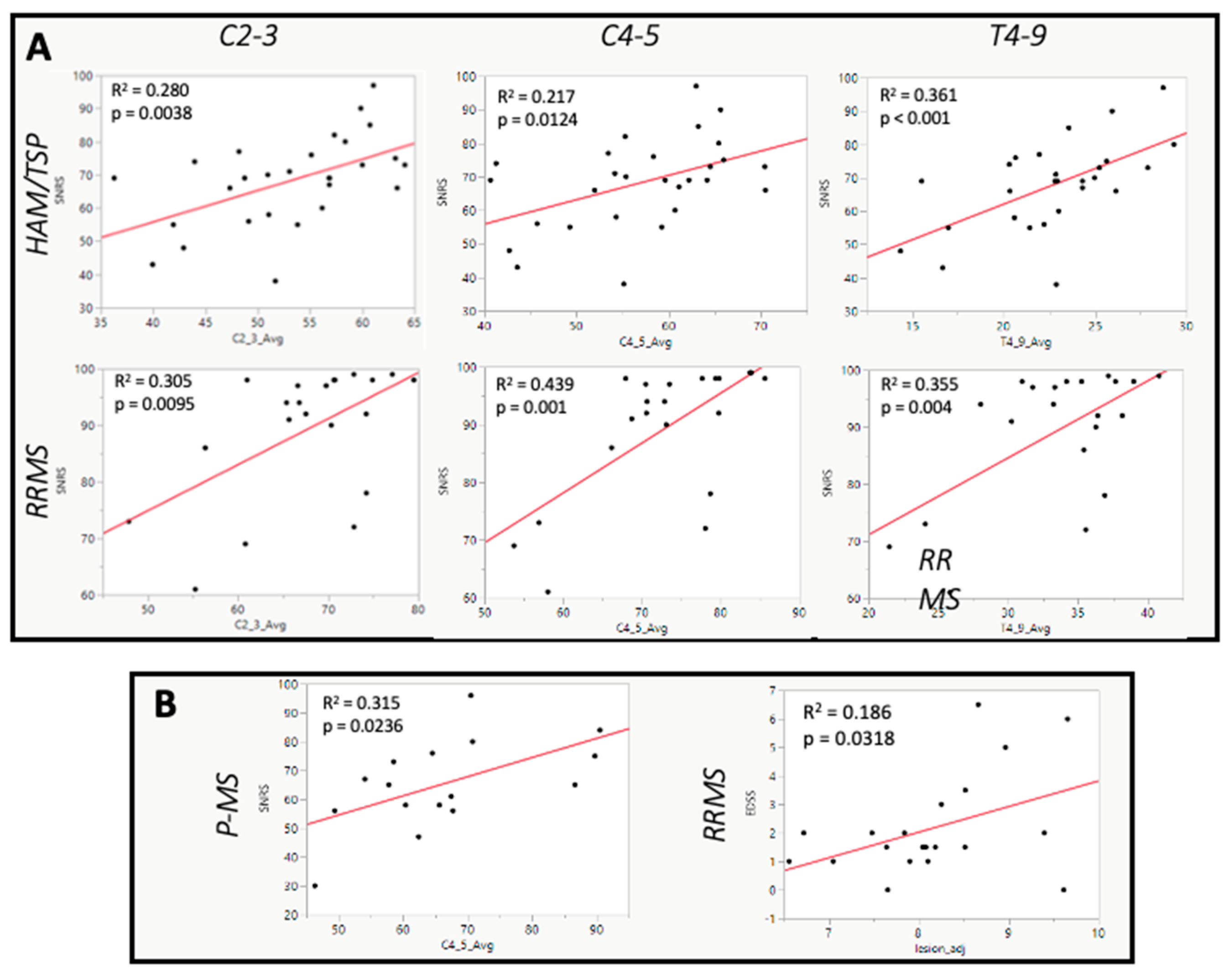
In both HAM/TSP and RRMS, SCCSA at all regions of the cord was significantly positively correlated with SNRS score (
Table 2,
Figure 3A), indicating that participants with thinner spinal cords had worsened clinical disability. In HAM/TSP, the strongest correlation was observed at the level of T4-9 (r = 0.60, p < 0.001,
Table 2,
Figure 3A). This region was also significantly negatively correlated with EDSS (r = -0.40, p = 0.0331) and T25FW (r = -0.55, p = 0.0031). The cervical enlargement was the only level of the cord to have a significant correlation between cross-sectional area and SNRS in P-MS (r = 0.56, p = 0.0221,
Figure 3B). Importantly, brain lesion fraction was not correlated with any disability rating scale in HAM/TSP (
Table 2). By contrast, in RRMS, higher brain lesion fraction was significantly correlated with increasing EDSS (r = 0.47, p = 0.0318), T25FW (r = 0.56, p = 0.0031) and 9-HPT (r = 0.48, p = 0.0234,
Table 2 and
Figure 3B).
3.4. Principal Component Results
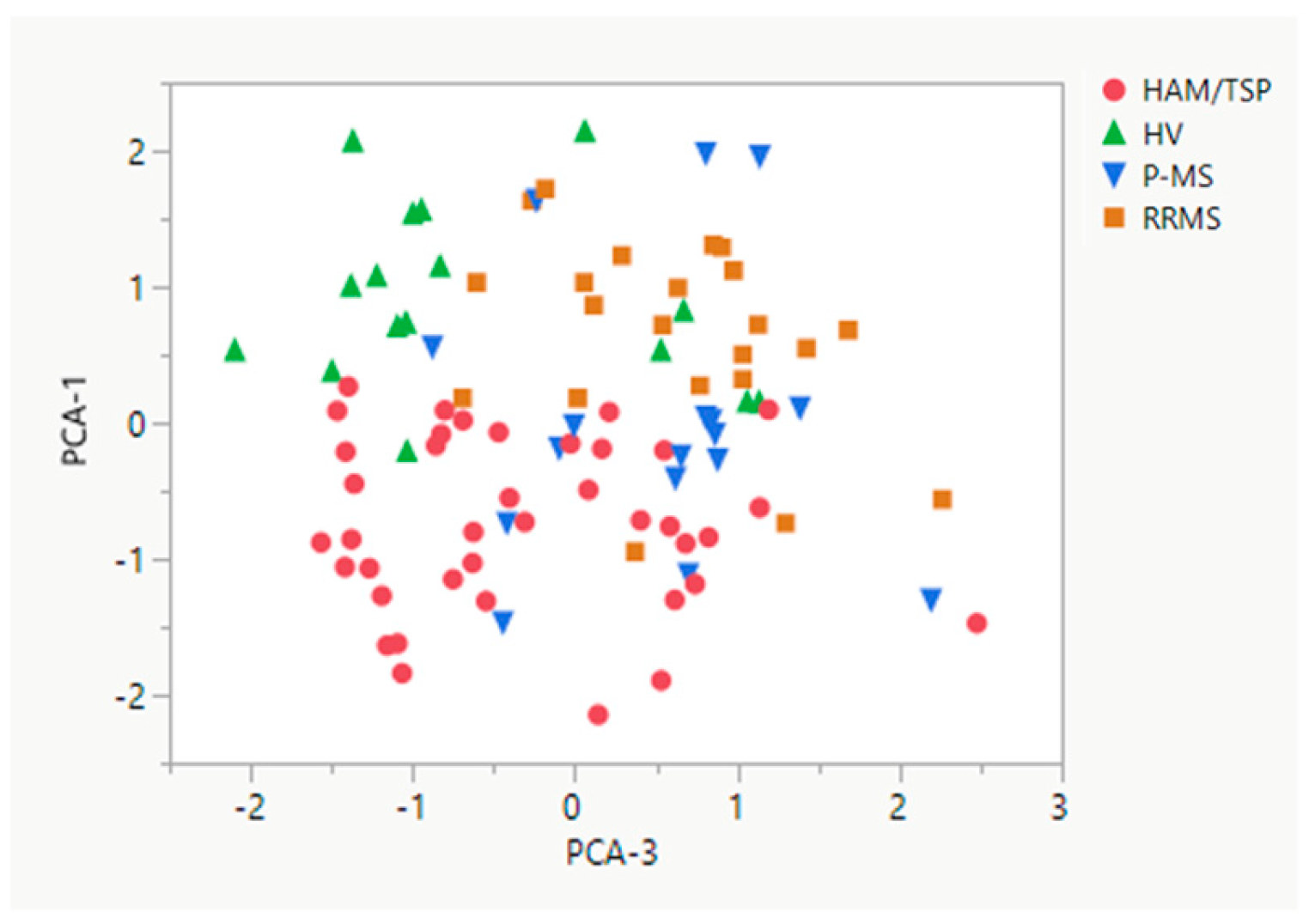
As shown in
Figure 4, principal component analysis demonstrated that radiological variables were clustered in three principal components. The first component contained all three regional SCCSA measures while the second and third components contained information pertaining to brain lesion load (lesion fraction and number of radiological hyperintensities in component two; median volume of radiological hyperintensities in component three). Component one, SCCSA measures, was sufficient to differentiate HAM/TSP from all other groups (HV, p < 0.0001; RRMS, p < 0.0001; and P-MS, p = 0.0031,
Figure 4). Component three, volume of radiological hyperintensities in the brain, was also able to separate HAM/TSP from RRMS and P-MS (p < 0.001 and p < 0.01, respectively), but not from HVs (p = 0.633).
4. Discussion
The radiological hallmark of HAM/TSP is spinal cord thinning, but conflicting evidence has been reported regarding the prevalence of brain abnormalities in the disease. Using routinely acquired MR images, we retrospectively measured spinal cord thinning and brain lesion volume and atrophy in HAM/TSP, compared to control participants to evaluate the degree of damage to each compartment and its correlation with clinical disability measures. In addition, these metrics were also compared to a limited cohort of participants clinically diagnosed with MS to look for patterns of radiological changes in various progressive myelopathies.
We have previously shown that participants with HAM/TSP had thinner spinal cords than healthy participants and participants with RRMS [
15,
16,
18,
24]. More severe thinning in the thoracic cord was correlated with both worsened clinical disability and elevated immune markers [
15]. In agreement with our previous reports, here we demonstrate significantly lower average SCCSA at the vertebral body levels of C2-3, C4-5, and T4-9 in participants with HAM/TSP compared to healthy volunteers and RRMS (
Figure 1B-D).
Based on our previous spine analysis, it was of interest to evaluate if there were radiological abnormalities in the brain for HAM/TSP participants. We used C-DEF, a machine-learning program that has been previously validated in MS and people living with human immunodeficiency virus (HIV), to perform automated brain segmentations and tissue volume measurements from MR images [
30,
31,
32]. Brain tissue was segmented into GM, WM, CSF, and lesions, normalized to total intracranial volume, and adjusted for age. Significantly, we found that participants with HAM/TSP had an increase in age-adjusted brain lesion fraction compared to healthy controls, though it was lower than both RRMS and P-MS averages (
Figure 2E). There was no evidence of GM, WM, or whole brain atrophy in HAM/TSP compared to control participants. This finding is consistent with previous radiological and histopathological studies that have reported the occurrence of brain lesions, but not atrophy, in a subset of participants with HAM/TSP [
21,
22,
23,
33]. Kalil et al. (2021) [
21]evaluated the occurrence of brain white matter hyperintensities by MRI in 22 participants with HAM/TSP compared to healthy controls and asymptomatic carriers of HTLV-1. They reported that lesions were more frequent in HAM/TSP participants than asymptomatic carriers and occurred preferentially in periventricular white matter. Another study also reported a higher frequency of white matter lesions by MRI in a cohort of 28 HAM/TSP participants, but the observation that lesions predominated in older participants suggested that they may reflect age-related degenerative processes rather than disease-specific pathology [
23]. These initial studies, however, relied on low sample sizes and a binary assessment of lesions as present or absent. We extended these early findings by evaluating a larger HAM/TSP cohort, measuring lesion volume using a novel machine learning algorithm to reflect lesion burden more completely, and adjusting all brain measures for age to control for age-related degenerative processes. After controlling for these confounding variables, we still found a significant increase in lesion burden in our HAM/TSP cohort compared to control subjects.
Evidence of inflammatory changes in the brain of HAM/TSP participants has also been found by histopathological analyses [
13,
22]. Aye et al. (2000) [
13] reported that HAM/TSP participants with active-chronic spinal cord lesions had perivascular inflammatory infiltrates in the brain, but those with inactive-chronic spinal cord lesions did not. Brain parenchymal infiltration was minimal and primarily comprised CD8+ T-cells, which also predominated in the spinal cord. Other studies reported that brain lesions on MRI were associated with demyelination, astrocytic gliosis, and hyaline thickening of small vessels, but not inflammatory cell infiltration [
22]. These studies suggested that inflammation is likely not restricted to the spinal cord in HAM/TSP, but that inflammatory changes in the brain may not be as severe or diffuse as those in the cord.
To explore the relationship between damage to each anatomical region and clinical disability, we evaluated the correlation between spinal cord thinning or brain lesion fraction and score on various clinical disability scales. All participants were rated by EDSS, SNRS, T25FW, and the 9-HPT and HAM/TSP participants were also evaluated by IPEC. In HAM/TSP, we observed a significant correlation between spinal cord thinning in every region and worsened clinical disability (
Table 2). For most clinical variables examined, the strongest correlation was observed in the thoracic cord. This finding is consistent with previous literature, which reported that the thoracic cord is the first to degrade in HAM/TSP and is the region with the most prominent immune cell infiltration, inflammation, demyelination, and thinning [
13,
14,
15]. Importantly, there were no statistically significant correlations between age-adjusted brain lesion fraction and any clinical disability measure in HAM/TSP. Collectively, these results suggest that although HAM/TSP participants may develop brain lesions at a greater rate than healthy ageing populations, brain lesions are less likely related to the clinical disability of HAM/TSP.
Finally, we compared the number of radiological hyperintensities and their average volume in each of our groups. Using principal component analysis, we demonstrated that spinal cord thinning was sufficient to differentiate participants with HAM/TSP from all other groups. Interestingly, we also found that the volume of radiological hyperintensities separated HAM/TSP participants from participants with both RRMS and P-MS. This suggests that in the absence of complete spinal cord imaging and volume measures, the evaluation of brain white matter hyperintensities may aid in the differentiation of HAM/TSP from progressive forms of MS. This is clinically significant as MS, particularly the primary progressive phenotype, is included in the differential diagnosis of HAM/TSP. In our experience, many of our HAM/TSP participants have once carried a diagnosis of progressive MS. Current methods to differentiate these myelopathies include serological and polymerase chain reaction (PCR) testing of blood and CSF for the presence of HTLV-1, which are not routinely done in a clinical setting. Expanding our repertoire of tools that can help differentiate these conditions, therefore, may improve the speed and accuracy of diagnosis. Since there are no known treatments that promote remyelination or CNS repair, early and accurate diagnosis is critical to ensure that participants with chronic progressive myelopathies begin disease modifying therapies as early as possible in the disease course to help prevent irreversible neurological damage.
The present study has several limitations. The major limitation of this study is the low sample size in the MS groups. The low sample size may result in increased variability, especially when adjusting for age. Indeed, the age of participants in the HV and HAM and P-MS was different and could have contributed to some of the negative results seen in the MS groups. Future studies will focus on including more subjects in each group that have brain and spinal cord scans done concurrently. Secondly, it is well known that the clinical rating scales included here are skewed towards rating motor symptoms [
34]. We did not include any rating of cognitive function, therefore we cannot comment on any relationship that may exist between the radiological variables measured here and cognitive impairment, which has been reported in a subset of HAM/TSP participants [
21,
35]. As asymptomatic carriers of HTLV-1 are rarely seen in our clinics, we were unable to evaluate if the elevated brain lesion fraction reported here is related to the pathogenesis of HAM/TSP or more broadly to infection with HTLV-1.
5. Conclusions
We have shown that the cohort of HAM/TSP participants had both spinal cord atrophy and elevated age-adjusted brain lesion fraction compared to healthy volunteers. Average brain lesion fraction in HAM/TSP was significantly lower than both RRMS and progressive MS averages. In HAM/TSP, we found strong correlations between spinal cord thinning at all regions and worsened clinical disability, with the strongest correlations being observed in the thoracic cord. Importantly, we did not observe any correlation between brain lesion fraction and any measure of clinical disability in HAM/TSP. Using principal component analysis, we demonstrated that spinal cord thinning can differentiate HAM/TSP from all other groups and median volume of radiological hyperintensities can differentiate HAM/TSP from both RRMS and P-MS. These findings suggest that HAM/TSP participants may develop brain lesions at a greater rate than can be attributed to age-related degenerative processes, but these brain lesions may not be related to clinical disability in this patient population. The present work reaffirms the importance of measuring spinal cord thinning as an indicator of disease progression and severity in HAM/TSP and suggest that in the absence of complete cervical and thoracolumbar spine data, radiological hyperintensities in the brain may be a useful disease marker.
Author Contributions
Conceptualization, GN and SJ; methodology, , and GN; software, GN; validation, EHS, SVO, WF, JO, ICMC, DSR, GN, and SJ; formal analysis, WHS, SVO, TW, MS, YM, MG, SA, GN; investigation, EHS; resources, DSR, JO, ICMC, and SJ; data curation, EHS, SVO, TW, YM, and SA; writing—original draft preparation, EHS, SVO, TW, GN, and SJ; writing—review and editing, EHS, SVO, TW, ICMC, DSR, GN, and SJ; visualization, EHS and GN; supervision, GN and SJ; project administration, GN and SJ; funding acquisition, DSR and SJ. All authors have read and agreed to the published version of the manuscript
Funding
This research was supported by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke, National Institutes of Health.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of the National Institutes of Health (NCT numbers: NCT00034723 and NCT00001248).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data and software used in the study can be shared upon request, according to the data sharing policy of the National Institute of Health’s and as per the specific study protocol.
Acknowledgments
We thank the study participants for their time and effort. We also thank the staff of the NINDS Neuroimmunology Clinic, NIH Clinical Center support staff, and the staff of the Functional MRI core facility for care of, and collection of clinical and radiological data from the study participants.
Conflicts of Interest
The authors declare no conflicts of interest pertaining to the topic of this study.
References
- Araujo, A.Q. and M.T. Silva, The HTLV-1 neurological complex. Lancet Neurol. 2006, 5, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Gessain, A. and O. Cassar, Epidemiological Aspects and World Distribution of HTLV-1 Infection. Front. Microbiol. 2012, 3, 388. [Google Scholar] [CrossRef] [PubMed]
- Nosaka, K. , et al., Epidemiological and clinical features of adult T-cell leukemia-lymphoma in Japan, 2010-2011: A nationwide survey. Cancer Sci. 2017, 108, 2478–2486. [Google Scholar] [CrossRef] [PubMed]
- Yamano, Y. and T. Sato, Clinical pathophysiology of human T-lymphotropic virus-type 1-associated myelopathy/tropical spastic paraparesis. Front. Microbiol. 2012, 3, 389. [Google Scholar] [CrossRef]
- Boostani, R. , et al., Human T-lymphotropic virus type I and breastfeeding; systematic review and meta-analysis of the literature. Iran. J. Neurol. 2018, 17, 174–179. [Google Scholar]
- Einsiedel, L.J. , et al., The prevalence and clinical associations of HTLV-1 infection in a remote Indigenous community. Med. J. Aust. 2016, 205, 305–309. [Google Scholar] [CrossRef]
- Jacobson, S. , et al., Circulating CD8+ cytotoxic T lymphocytes specific for HTLV-I pX in patients with HTLV-I associated neurological disease. Nature 1990, 348, 245–248. [Google Scholar] [CrossRef]
- Kubota, R. , et al., Demonstration of human T lymphotropic virus type I (HTLV-I) tax-specific CD8+ lymphocytes directly in peripheral blood of HTLV-I-associated myelopathy/tropical spastic paraparesis patients by intracellular cytokine detection. J. Immunol. 1998, 161, 482–488. [Google Scholar] [CrossRef]
- Nagai, M. , et al. , Increased HTLV-I proviral load and preferential expansion of HTLV-I Tax-specific CD8+ T cells in cerebrospinal fluid from patients with HAM/TSP. Ann Neurol 2001, 50, 807–812. [Google Scholar]
- Ando, H. , et al., Positive feedback loop via astrocytes causes chronic inflammation in virus-associated myelopathy. Brain 2013, 136, 2876–2887. [Google Scholar] [CrossRef]
- Levin, M.C. , et al., Immunologic analysis of a spinal cord-biopsy specimen from a patient with human T-cell lymphotropic virus type I-associated neurologic disease. N. Engl. J. Med. 1997, 336, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, E. , et al., Visualization of HTLV-1-specific cytotoxic T lymphocytes in the spinal cords of patients with HTLV-1-associated myelopathy/tropical spastic paraparesis. J. Neuropathol. Exp. Neurol. 2015, 74, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Aye, M.M. , et al., Histopathological analysis of four autopsy cases of HTLV-I-associated myelopathy/tropical spastic paraparesis: inflammatory changes occur simultaneously in the entire central nervous system. Acta Neuropathol. 2000, 100, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Akizuki, S. , et al., An autopsy case of human T-lymphotropic virus type I-associated myelopathy. Hum. Pathol. 1988, 19, 988–990. [Google Scholar] [CrossRef]
- Azodi, S. , et al., Imaging spinal cord atrophy in progressive myelopathies: HTLV-I-associated neurological disease (HAM/TSP) and multiple sclerosis (MS). Ann. Neurol. 2017, 82, 719–728. [Google Scholar] [CrossRef]
- Evangelou, I.E., R. Massoud, and S. Jacobson, HTLV-I-associated myelopathy/tropical spastic paraparesis: semiautomatic quantification of spinal cord atrophy from 3-dimensional MR images. J. Neuroimaging 2014, 24, 74–78. [Google Scholar] [CrossRef]
- Godoy, A.J. , et al., Characterization of cerebral white matter lesions of HTLV-I-associated myelopathy/tropical spastic paraparesis in comparison with multiple sclerosis and collagen-vasculitis: a semiquantitative MRI study. J. Neurol. Sci. 1995, 133, 102–111. [Google Scholar] [CrossRef]
- Liu, W. , et al., In vivo imaging of spinal cord atrophy in neuroinflammatory diseases. Ann. Neurol. 2014, 76, 370–378. [Google Scholar] [CrossRef]
- Taniguchi, A. , et al., Spinal cord anteroposterior atrophy in HAM/TSP: Magnetic resonance imaging and neuropathological analyses. J. Neurol. Sci. 2017, 381, 135–140. [Google Scholar] [CrossRef]
- Griffith, C. , et al., Brain volume measurements in patients with human T-cell lymphotropic virus-1-associated tropical spastic paraparesis. J. Neurovirol. 2006, 12, 349–355. [Google Scholar] [CrossRef]
- Kalil, R.S. , et al., Association between high proviral load, cognitive impairment, and white matter brain lesions in HTLV-1-infected individuals. J. Neurovirol. 2021, 27, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Ogata, A. , et al., MRI-pathological correlate of brain lesions in a necropsy case of HTLV-I associated myelopathy. J. Neurol. Neurosurg. Psychiatry 1993, 56, 194–196. [Google Scholar] [CrossRef] [PubMed]
- Puccioni-Sohler, M. , et al., HAM/TSP: association between white matter lesions on magnetic resonance imaging, clinical and cerebrospinal fluid findings. Arq. Neuropsiquiatr. 2012, 70, 246–251. [Google Scholar] [CrossRef]
- Mina, Y. , et al., Cervical and thoracic cord atrophy in multiple sclerosis phenotypes: Quantification and correlation with clinical disability. Neuroimage Clin. 2021, 30, 102680. [Google Scholar] [CrossRef] [PubMed]
- Walton, C. , et al., Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult. Scler. 2020, 26, 1816–1821. [Google Scholar] [CrossRef]
- Wattjes, M.P. , et al., 2021 MAGNIMS-CMSC-NAIMS consensus recommendations on the use of MRI in patients with multiple sclerosis. Lancet Neurol. 2021, 20, 653–670. [Google Scholar] [CrossRef]
- Thompson, A.J. , et al., Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Losseff, N.A. , et al., Progressive cerebral atrophy in multiple sclerosis. A serial MRI study. Brain 1996, 119, 2009–2019. [Google Scholar] [CrossRef]
- Rocca, M.A. , et al., Brain MRI atrophy quantification in MS: From methods to clinical application. Neurology 2017, 88, 403–413. [Google Scholar] [CrossRef]
- Selvaganesan, K. , et al., Robust, atlas-free, automatic segmentation of brain MRI in health and disease. Heliyon 2019, 5, e01226. [Google Scholar] [CrossRef]
- Dieckhaus, H. , et al., Logistic Regression-Based Model Is More Efficient Than U-Net Model for Reliable Whole Brain Magnetic Resonance Imaging Segmentation. Top. Magn. Reson. Imaging 2022, 31, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Chien, A. , et al., White and Gray Matter Changes are Associated With Neurocognitive Decline in HIV Infection. Ann. Neurol. 2024, 95, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Takatani, M. , et al., Clinical and laboratory features of HTLV-I asymptomatic carriers and patients with HTLV-I-associated myelopathy/tropical spastic paraparesis from the Brazilian Amazon. Rev. Inst. Med. Trop. 2017, 59, e5. [Google Scholar] [CrossRef] [PubMed]
- Nortvedt, M.W. , et al., Quality of life in multiple sclerosis: measuring the disease effects more broadly. Neurology 1999, 53, 1098–1103. [Google Scholar] [CrossRef]
- Kamrani, M. , et al., Cognitive deficits in HTLV-1 patients. J. Neurovirol. 2023, 29, 416–424. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).