Submitted:
20 August 2024
Posted:
20 August 2024
You are already at the latest version
Abstract

Keywords:
1. Introduction
2. Photosynthesis and Global Balances of Matter and Energy
2.1. Energy Inputs in Perspective
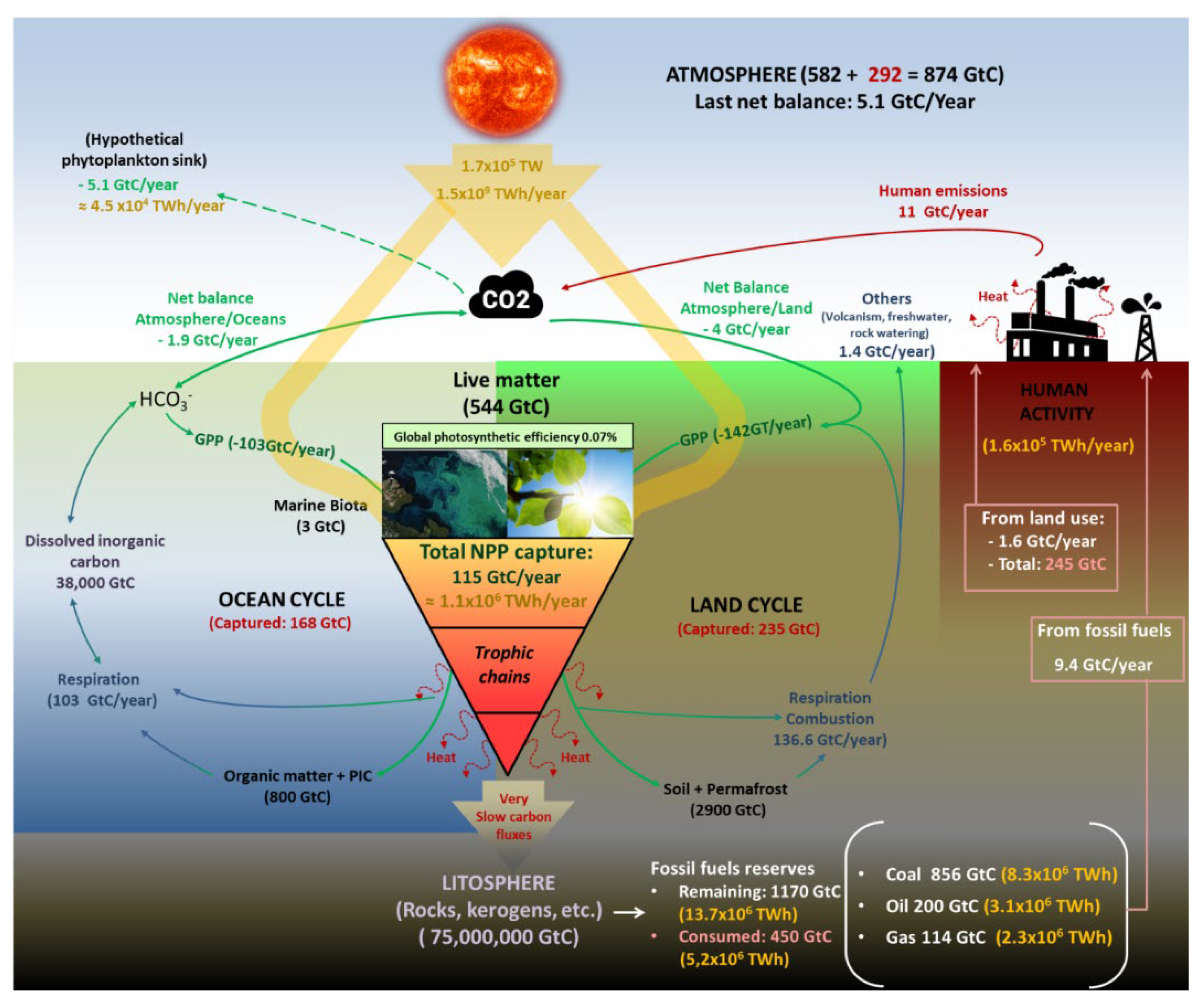
2.2. Carbon Cycle and Civilization
2.3. Photosynthetic Efficiency
3. Phytoplankton Culture
3.1. A Brief History
3.2. Photobioreactors (PBRs)
3.2.1. Open Ponds/Artificial Lakes
3.2.2. Closed PBRs
3.2.3. Indoor/Outdoor PBRs
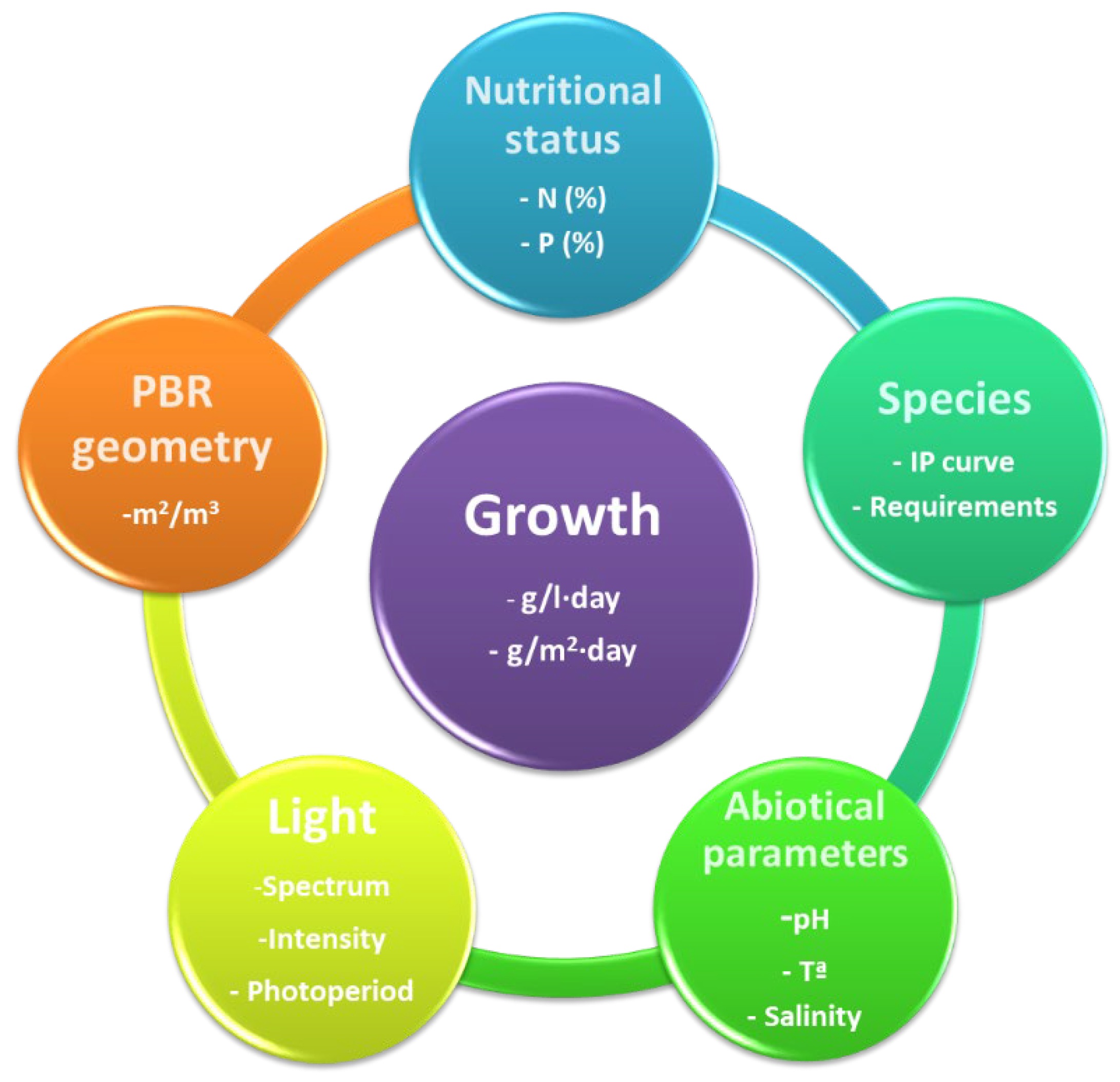
3.3. Key Parameters for the Growth
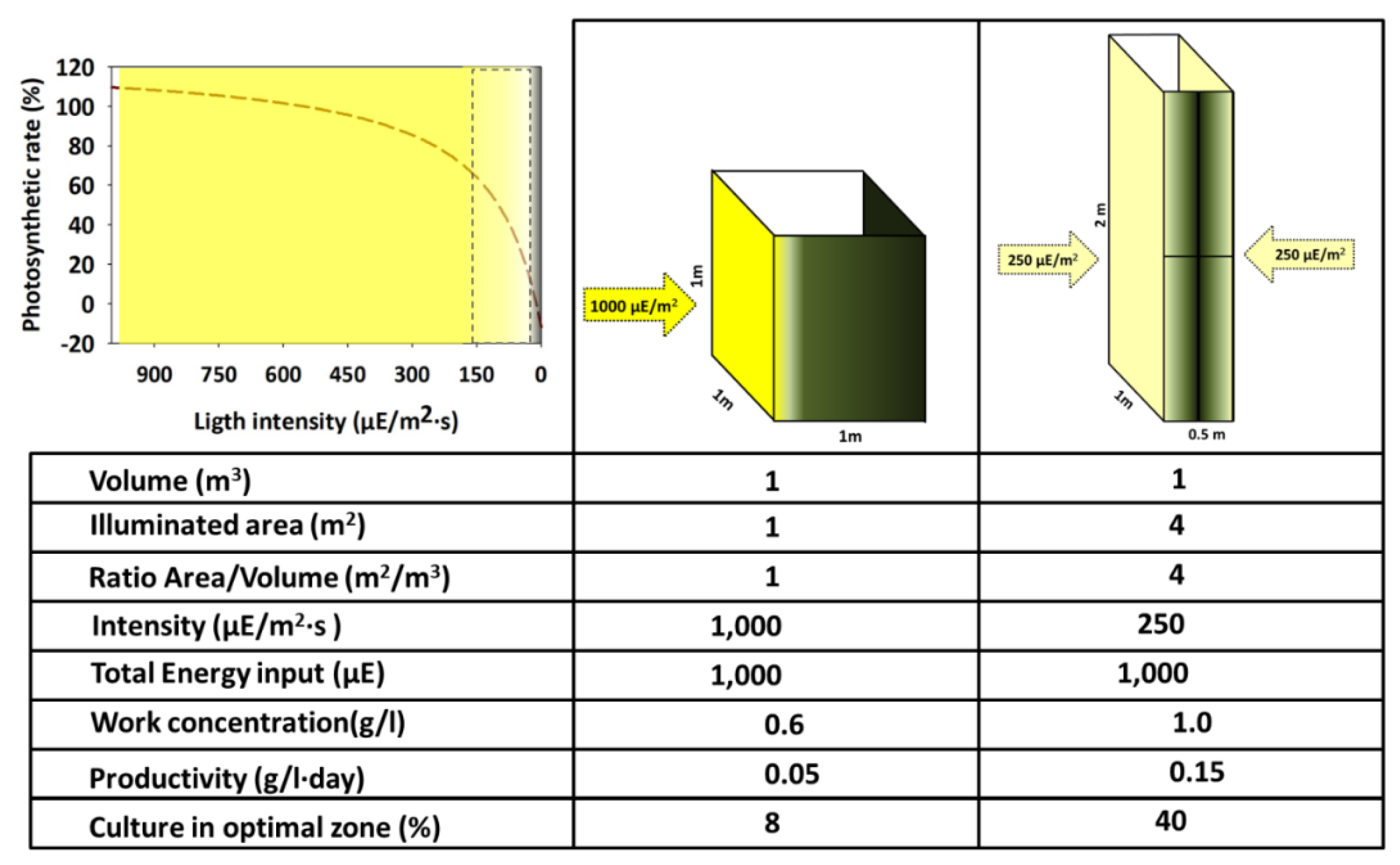
3.3.1. Light
3.3.2. Other Abiotic Parameters
- a)
- Temperature:
- b)
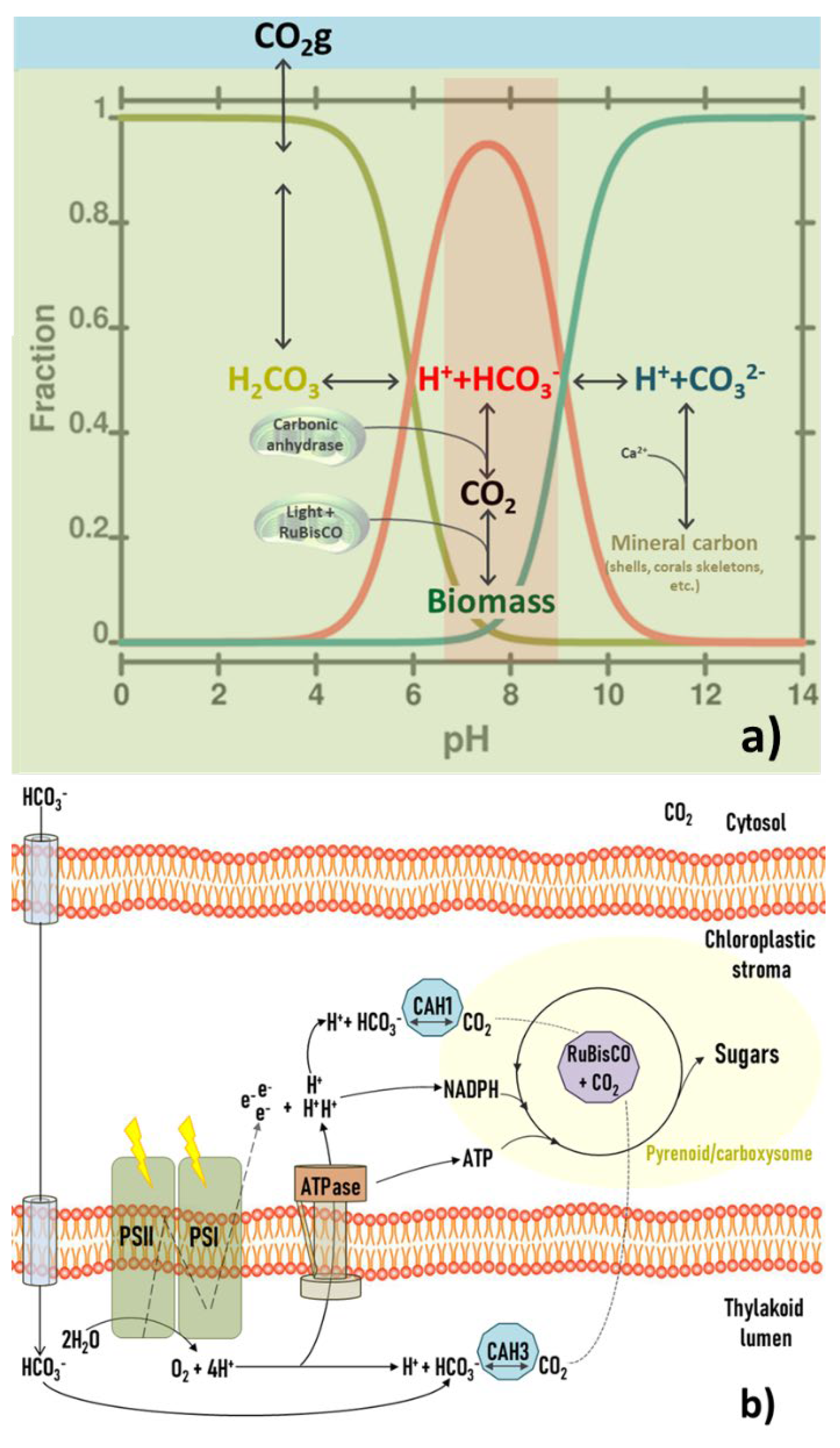
- Medium pH
- -
- In the absence of any gas exchange, protons are gradually consumed as they are incorporated into new organic compounds through the Calvin Cycle, increasing pH. This increase can also cause the precipitation of carbonates, negatively impacting photosynthesis since e pH values are outside the optimal range and a lack of precursors accumulates
- -
- When the culture medium is aerated with air (0.04% CO2), the pH value will reach an equilibrium that mainly depends on the water alkalinity. The maximum pH value under these conditions is usually around 8.3, beyond which carbonate starts to precipitate. When the photosynthetic demand for CO2 is less than the atmospheric CO2 solubilization rate, the pH stays stable and functions as a buffer system. However, if the CO2 demand exceeds the atmospheric delivery rate due to the increment in cellular concentration and/or light intensity, the initial scenario of proton depletion will be gradually reached.
- -
- In some culture systems, the medium is aerated with enriched-CO2 air. The final pH value, after reaching equilibrium, goes down linearly concerning the concentration of this gas in the air. At a CO2 concentration between 0.5% and 2.0%, the pH remains strongly buffered between 7.0 and 8.5 (depending on the alkalinity of the medium), which is suitable for the growth of most species. At the photosynthetic level, this strategy ensures an ample supply of CO2, even in high cell concentrations or light intensities. Hence, CO2 is never depleted, so the pH remains constantly buffered, and the photosynthesis rate is never limited.
- c)
- Major ions and trace metals:
3.3.3. Nutritional Status
3.3.4. Culture Mixing
- a)
- Through mechanical devices such as paddle wheels [64, 65] or hydraulic pumps [66], the origin currents and flows rise up the biomass.
- b)
- Through pneumatic devices that allow bubbling with air or CO2-enriched air generating a current toward the atmosphere and airlift circulation [67].
- c)
- Through mixed systems that use both strategies [32].
3.4. Estimations of Growth
3.5. Potential Numbers of Production
4. Mitigating Fossil Energy Dependence by Enhancing Photosynthesis
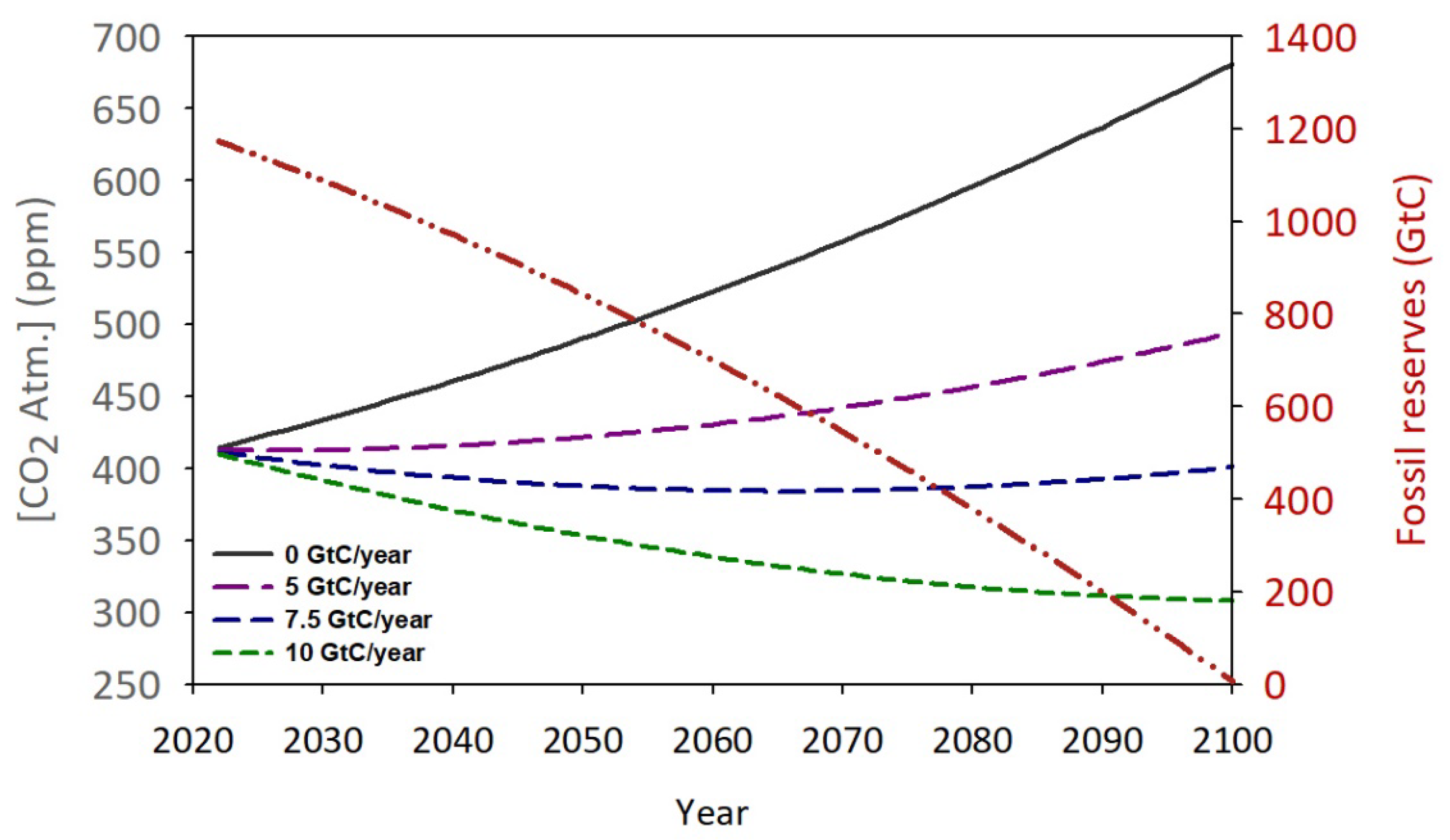
4.1. Fossil Fuel Reserves and Potential Evolution
4.2. Biological Capture of CO2
4.3. A Matter about Figures and Politics
4.4. Technical Challenges
4.4.1. Photobioreactor Features
4.4.2. Water Use
4.4.3. Species and Growth Medium
4.4.4. Mixing System
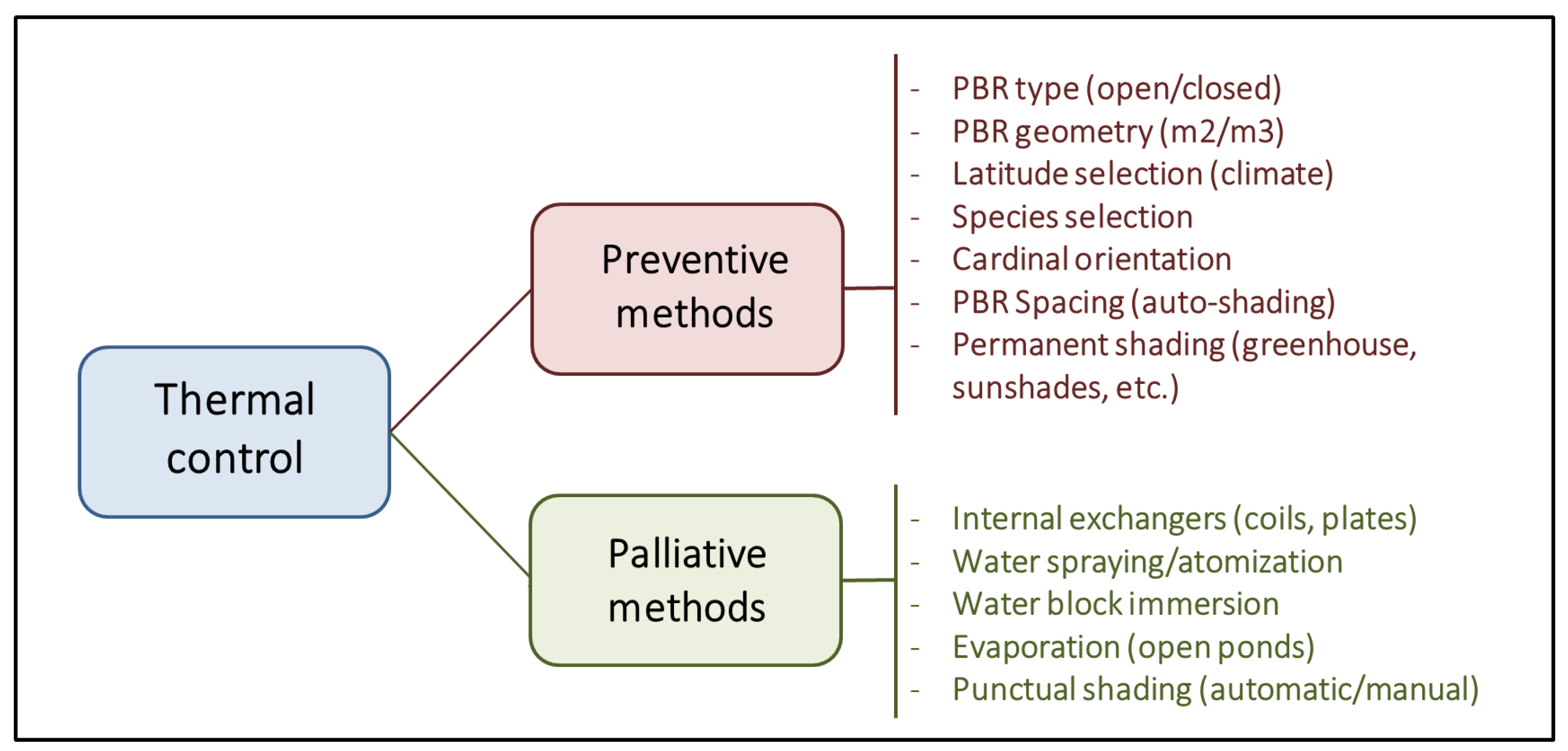
4.4.5. Temperature Control
4.4.6. Sources of Nutrients
4.4.7. Biomass Management
5. Discussion
- a)
- Implementing a specialized scientific-technical sector to design an propose suitable regions for establishing these CO2 sinks would be necessary. This sector should standardise the protocol for defining a region as suitable within a specific legal framework
- b)
- Promoting measures to establish a profitable sector from the culture and harvest of phytoplankton and leading to producing an attractive and valuable product for the market.
- c)
- Promoting measures to develop a profitable industry for processing the large amount of biomass produced, which can generate added value and demand for the product in such a way that transcends to other sectors, such as energy, human or animal food, or stored as fixed carbon
- d)
- Creation of international organizations and laws to manage the use of fixed carbon to prevent its new release into the atmosphere.
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ntergovernmental Panel on Climate Change (IPCC). (2023). Summary for Policymakers. In Climate Change 2021 – The Physical Science Basis: Working Group I Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change 2023, pp. 3–32. frontmatter, Cambridge: Cambridge University Press. [CrossRef]
- Head, M.J.; Steffen, W.; Fagerlind, D.; Waters, C.N.; Poirier, C.; Syvitski, J.; Zalasiewicz, J.A.; Barnosky, A.D.; Cearreta, A.; Jeandel, C.; Leinfelder, R.; McNeill, J.; Rose, N.L.; Summerhayes, C.; Wagreich, M.; Zinke, J. The Great Acceleration is real and provides a quantitative basis for the proposed Anthropocene Series/Epoch. Episodes 2022, 45, 45,359–376. [Google Scholar] [CrossRef]
- Nag, B.; Makaranga, A.; Kareya, M.S.; Nesamma, A.A.; Jutur, P.P. Photosynthetic Cell Factories, a New Paradigm for Carbon Dioxide (CO₂) Valorization. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Inamuddin, Altalhi, T., Eds.; Elsevier, 2023; pp. 463–480. [CrossRef]
- Rapf, R.; Vaida, V. Sunlight as an Energetic Driver in the Synthesis of Molecules Necessary for Life. Physical Chemistry Chemical Physics 2016, 18, 20067–20084. [Google Scholar] [CrossRef] [PubMed]
- Ruban, A. Evolution under the sun: optimizing light harvesting in photosynthesis. Journal of Experimental Botany 2015, 66, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.P.; Wood, A.P. The Chemolithotrophic Prokaryotes. In, E. Rosenberg, E.F. DeLong, S. Lory, E. Stackebrandt, & F. Thompson (Eds.).The Prokaryotes 2013, pp. 753–793. Springer, Berlin, Heidelberg. [CrossRef]
- Hammarström, L. Overview: capturing the sun for energy production. Ambio, 2012, 41, 103–1007. [Google Scholar] [CrossRef]
- Stocker, B.D.; Yu, Z.; Massa, C.; Joos, F. Holocene peatland and ice-core data constraints on the timing and magnitude of CO2 emissions from past land use. Proceedings of the National Academy of Sciences of the United States of America 2017, 114, 1492–1497. [Google Scholar] [CrossRef] [PubMed]
- Moriarty, P.; Honnery, D. Global bioenergy: Problems and prospects. International Journal of Global Energy Issues 2007, 27, 231–249. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). (2023). Global Carbon and Other Biogeochemical Cycles and Feedbacks. In Climate Change 2021 – The Physical Science Basis: Working Group I Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change 2023, pp. 673–816. chapter, Cambridge: Cambridge University Press. [CrossRef]
- Friedlingstein, P. Global carbon budget 2022. Earth System Science Data 2022, 14, 4811–4900. [Google Scholar] [CrossRef]
- Quetin, G.R.; Famiglietti, C.A.; Dadap, N.C.; Bloom, A.A.; Bowman, K.W.; Diffenbaugh, N.S.; Liu, J.; Trugman, A.T.; Konings, A.G. Attributing Past Carbon Fluxes to CO₂ and Climate Change: Respiration Response to CO₂ Fertilization Shifts Regional Distribution of the Carbon Sink. Global Biogeochemical Cycles 2023, 37, e2022GB007478. [Google Scholar] [CrossRef]
- Barber, J. Photosynthetic energy conversion: natural and artificial. Chemical Society Reviews 2009, 38, 185–196. [Google Scholar] [CrossRef]
- BP. Statistical Review of World Energy 2022. 71th ed. https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2022-full-report.pdf. (accessed on January of 2023).
- Chatterjee, A.; Gierach, M.; Sutton, A.; Feely, R.; Crisp, D.; Eldering, A.; Gunson, M.; O'Dell, C.; Stephens, B.; Schimel, D. (2017). Influence of El Niño on atmospheric CO2 over the tropical Pacific Ocean: Findings from NASA’s OCO-2 mission. Science 2017, 358, pp–eaam5776. [Google Scholar] [CrossRef] [PubMed]
- Khatiwala, S.; Primeau, F.; Hall, T. Reconstruction of the history of anthropogenic CO2 concentrations in the ocean. Nature 2009, 462, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Dupont, S.; Pörtner, H.O. A snapshot of ocean acidification research. Marine Biology 2013, 160, 2282–2282. [Google Scholar] [CrossRef]
- Ballantyne, A.P.; Liu, Z.; Anderegg, W.R.; Yu, Z.; Stoy, P.; Poulter, B.; Vanderwall, J.; Watts, J.; Kelsey, K.; Neff, J. Reconciling carbon-cycle processes from ecosystem to global scales. Frontiers in Ecology and the Environment 2021, 19, 57–65. [Google Scholar] [CrossRef]
- Hall, D.O.; Rao, K.K. Photosynthesis, 6th ed.; Cambridge University Press 1999, 214 pp.
- Davis, S.; Lebauer, D.; Long, S. Light to liquid fuel: Theoretical and realized energy conversion efficiency of plants using Crassulacean Acid Metabolism (CAM) in arid conditions. Journal of Experimental Botany 2014, 65, pp.eru163. [Google Scholar] [CrossRef]
- Edwards, E.J. Evolutionary trajectories, accessibility, and other metaphors: the case of C4 and CAM photosynthesis. New Phytologist 2019, 223, 1742–1755. [Google Scholar] [CrossRef]
- Zhu, X.G.; Long, S.P.; Ort, D.R. What is the maximum efficiency with which photosynthesis can convert solar energy into biomass? Current Opinion Biotechnology 2008, 19, 153–159. [Google Scholar] [CrossRef]
- Vecchi, V.; Barera, S.; Bassi, R.; Dall'Osto, L. Potential and Challenges of Improving Photosynthesis in Algae. Plants 2020, 9, 67. [Google Scholar] [CrossRef]
- Treves, H.; Lucius, S.; Feil, R.; Stitt, M.; Hagemann, M.; Arrivault, S. Operation of Carbon-Concentrating Mechanisms in Cyanobacteria and Algae requires altered poising of the Calvin-Benson cycle. bioRxiv 2022, 2022.08.23.504937. [CrossRef]
- Singh, U.B.; Ahluwalia, A. Microalgae: A promising tool for carbon sequestration. Mitigation and Adaptation Strategies for Global Change 2013, 18, 73–95. [Google Scholar] [CrossRef]
- Paul, S.; Bera, S.; Dasgupta, R.; Mondal, S.; Roy, S. Review on the Recent Structural Advances in Open and Closed Systems for Carbon Capture through Algae. Energy Nexus 2021, 4, 100032. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Biology of microalgae. Microalgae in Health and Disease Prevention. Elsevier 2018, pp. 23–72. [CrossRef]
- Ciferri, O. Spirulina, the edible microorganism. Microbiology Reviews 1983, 47(4), 551–578. [Google Scholar] [CrossRef] [PubMed]
- Dangeard, P. Sur une algue bleue alimentaire pour l'homme: Arthrospira platensis (Nordstedt) Gomont. Actes Soc. Linn. Boreaux Extr. Procés-verbaux 1940, 91, 39–41. [Google Scholar]
- Geohegan, M. Unicellular Algae as a source of food. Nature 1951, 168, 426–427. [Google Scholar] [CrossRef] [PubMed]
- Borowitzka, M.A. High-value products from microalgae—their development and commercialisation. Journal of Applied Phycology 2013, 25, 743–756. [Google Scholar] [CrossRef]
- Huang, Q.; Jiang, F.; Wang, L.; Yang, C. Design of Photobioreactors for Mass Cultivation of Photosynthetic Organisms. Engineering 2017, 3, 318–329. [Google Scholar] [CrossRef]
- Wang, B.; Lan, C.Q.; Horsman, M. Closed photobioreactors for production of microalgal biomasses. Biotechnology advances 2012, 30, 904–912. [Google Scholar] [CrossRef]
- Tredici, M.R.; Chini Zittelli, G.; Rodolfi, L. Photobioreactors. In M. C. Flickinger (Ed.), Encyclopedia of industrial biotechnology. Bioprocess, Bioseparation, and Cell Technology 2009, pp. 1–15. [CrossRef]
- Oswald, W.J.; Golueke, C.G. Biological transformation of solar energy. Advances in Applied Microbiology 1969, 2, 223–262. [Google Scholar] [CrossRef]
- Brenner, A.; Abeliovich, A. Water purification: Algae in wastewater oxidation ponds. In A. Richmond and Q. Hu (Eds.), Handbook of Microalgal Culture, 2nd ed., 2013, pp. 595–601. [CrossRef]
- Slegers, P.M.; Wijffels, R.H.; van Straten, G.; van Boxtel, A.J.B. Design Scenarios for Flat Panel Photobioreactors. Applied Energy 2011, 88, 3342–53. [Google Scholar] [CrossRef]
- Fernández, I.; Acién, F.G.; Berenguel, M.; Guzmán, J.L. First principles model of a tubular photobioreactor for microalgal production. Industrial & Engineering Chemistry Research 2014, 53, 11121–11136. [Google Scholar] [CrossRef]
- Torzillo, G.; Chini Zittelli, G. Tubular Photobioreactors. In A. Prokop, R. Bajpai & M. Zappi (Eds.), Algal Biorefineries 2015, pp. 105–116. Springer, Cham. [CrossRef]
- Nogueira, N.; Nascimento, F.; Cunha, C.; Cordeiro, N. Nannochloropsis gaditana grown outdoors in annular photobioreactors: Operation strategies. Algal Research 2020, 101913. [Google Scholar] [CrossRef]
- Ugwu, U.; Aoyagi, H.; Uchiyama, H. Photobioreactors for Mass Cultivation of Algae. Bioresource Technology 2008, 99, 4021–4028. [Google Scholar] [CrossRef]
- Masojídek, J.; Ranglová, K; Lakatos, G. E.; Silva Benavides, A.M.; Torzillo, G. Variables Governing Photosynthesis and Growth in Microalgae Mass Cultures. Processes 2021, 9, 820. [Google Scholar] [CrossRef]
- Carvalho, A.P.; Silva, S.O.; Baptista, J.M.; Malcata, F.X. Light requirements in microalgal photobioreactors: an overview of biophotonic aspects. Applied Microbiology and Biotechnology 2011, 89, 1275–1288. [Google Scholar] [CrossRef]
- Parlevliet, D.; Moheimani, N. Efficient conversion of solar energy to biomass and electricity. Aquatic Biosystems 2014, 10, 4. [Google Scholar] [CrossRef]
- Michaelis, L.; Menten, M.L.; Johnson, K.A.; Goody, R.S. The original Michaelis constant: translation of the 1913 Michaelis-Menten paper. Biochemistry 2011, 50, 8264–9. [Google Scholar] [PubMed]
- Straka, L.; Rittmann, B.E. (2019). Growth kinetics and mathematical modeling of Synechocystis sp. PCC 6803 under flashing light. Biotechnology and Bioengineering 2019, 116, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Mairet, F.; Bayen, T. The promise of dawn: Microalgae photoacclimation as an optimal control problem of resource allocation. Journal of Theoretical Biology 2021, 515, 110597. [Google Scholar] [CrossRef]
- Ananthi, V.; Kathirvel, B.; Pugazhendhi, A.; Arun, A. Impact of abiotic factors on biodiesel production by microalgae. Fuel 2021, 284, 118962. [Google Scholar] [CrossRef]
- Barten, R.; Djohan, Y.; Evers, W.; Wijffels, R.; Barbosa, M. Towards industrial production of microalgae without temperature control: The effect of diel temperature fluctuations on microalgal physiology. Journal of Biotechnology 2021, 336, 56–63. [Google Scholar] [CrossRef]
- Uyar, B.; Kapucu, N. Passive temperature control of an outdoor photobioreactor by phase change materials. Journal of Chemical, Technology and Biotechnology 2015, 90, 915–920. [Google Scholar] [CrossRef]
- Gao, K. Approaches and involved principles to control pH/pCO2 stability in algal cultures. Journal of Applied Phycology 2021, 33, 3497–3505. [Google Scholar] [CrossRef]
- Fox, J.M.; Zimba, P.V. Minerals and trace elements in microalgae. In I. A. Levine & J. Fleurence (Eds.), Microalgae in health and disease prevention 2018, pp. 177–193. Academic Press. [CrossRef]
- Carvalho, J.C.; Bittencourt Sydney, E.; Assú Tessari, L.F.; Soccol, C.R. (2019). Chapter 2 - Culture media for mass production of microalgae. In: Ashok Pandey, J.-S., Chang, C.R., Lee, D.-J., Chisti, Y. (eds.) Biofuels from Algae (2nd ed.) 2019. Elsevier, pp.33-50. [CrossRef]
- Yaakob, M.A.; Mohamed, R.M.S.R.; Al-Gheethi, A.; Aswathnarayana Gokare, R.; Ambati, R.R. Influence of Nitrogen and Phosphorus on Microalgal Growth, Biomass, Lipid, and Fatty Acid Production: An Overview. Cells 2021, 10, 393. [Google Scholar] [CrossRef]
- Ben-Amotz, A.; Avron, M. On the factors, which determine massive beta-carotene accumulation in the halotolerant alga Dunaliella bardawil. Plant Physiology 1983, 72, 593–597. [Google Scholar] [CrossRef]
- Boussiba, S.; Vonshak, A. Astaxanthin accumulation in the green alga Haematococcus pluvialis. Plant Cell Physiology 1991, 32, 1077–1082. [Google Scholar] [CrossRef]
- Drira, M.; Elleuch, J.; Ben Hlima, H.; Hentati, F.; Gardarin, C.; Rihouey, C.; Le Cerf, D.; Michaud, P.; Abdelkafi, S.; Fendri, I. Optimization of Exopolysaccharides Production by Porphyridium sordidum and Their Potential to Induce Defense Responses in Arabidopsis thaliana against Fusarium oxysporum. Biomolecules 2021, 11, 282. [Google Scholar] [CrossRef] [PubMed]
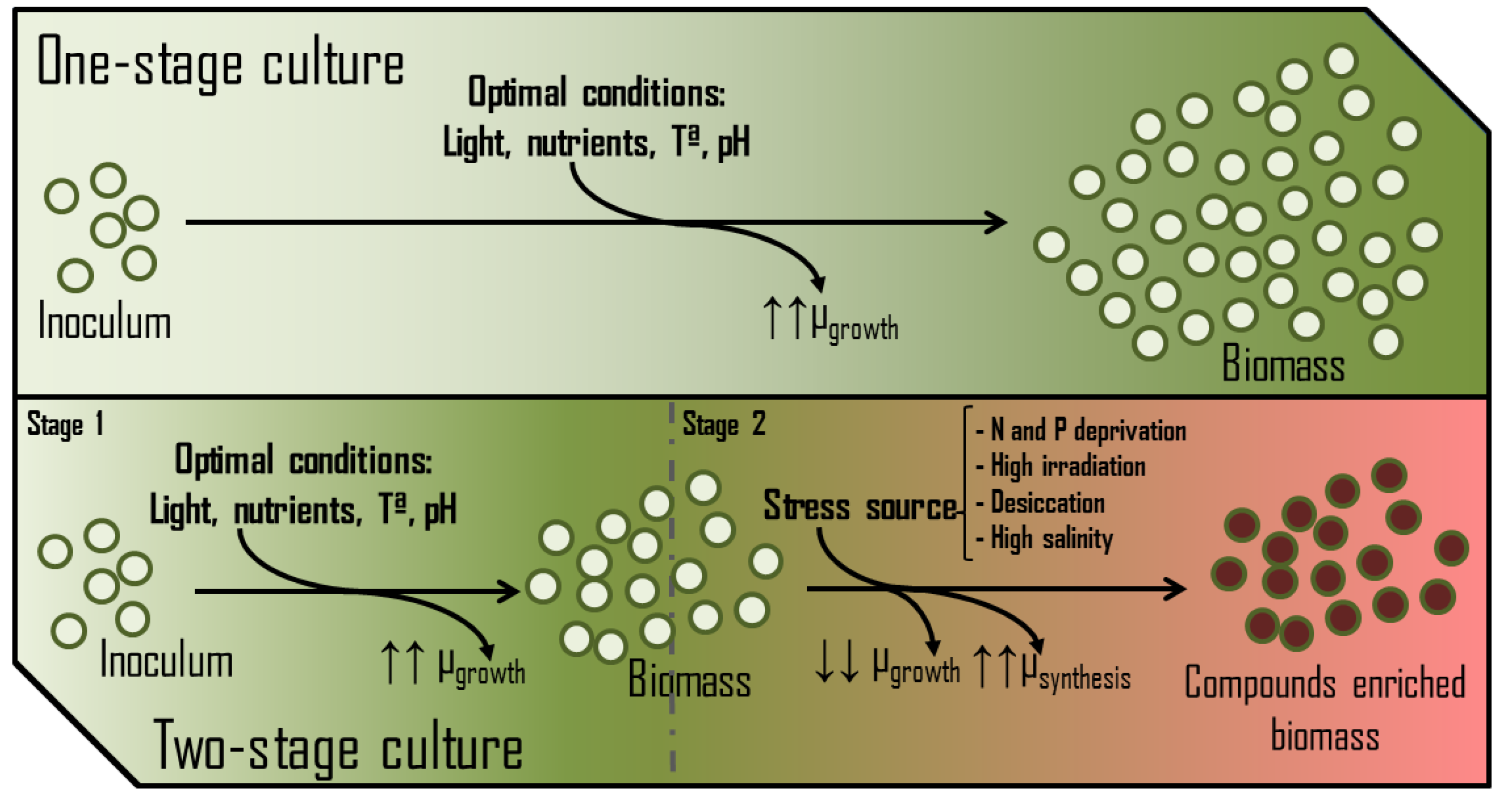
- Liyanaarachchi, V.; Premaratne, M.; Ariyadasa, T.; Nimarshana, V.; Malik, A. Two-stage cultivation of microalgae for production of high-value compounds and biofuels: A review. Algal Research 2021, 57, 102353. [Google Scholar] [CrossRef]
- Hegemann, P. Vision in microalgae. Planta 1997, 203, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Walsby, A.E. Gas vesicles. Microbiological Review 1994, 58, 94–144. [Google Scholar] [CrossRef]
- St Laurent, L.; Garrett, C. The Role of Internal Tides in Mixing the Deep Ocean. Journal of Physical Oceanography 2022, 32, 2882–2899. [Google Scholar] [CrossRef]
- Chanquia, S.N.; Vernet, G.; Kara, S. Photobioreactors for cultivation and synthesis: Specifications, challenges, and perspectives. Engineering in Life Sciences 2022, 22, 712–724. [Google Scholar] [CrossRef]
- Souza Kirnev, P.C.; Vandenberghe LP de, S.; Soccol, C.R.; Carvalho JC de Mixing Agitation in Photobioreactors In, H. Thatoi, S. Mohapatra, & S. K. Das (Eds.), Innovations in Fermentation and Phytopharmaceutical Technologies 2022, pp. 13–35. Elsevier. [CrossRef]
- Rogers, J.; Rosenberg, J.; Guzman, B.; Oh, V.; Mimbela, L.-E.; Ghassemi, A.; Betenbaugh, M.; Oyler, G. A critical analysis of paddlewheel-driven raceway ponds for algal biofuel production at commercial scales. Algal Research 2013, 4, 76–88. [Google Scholar] [CrossRef]
- Hreiz, R.; Sialve, B.; Morchain, J.; Escudié, R.; Steyer, J.-P.; Guiraud, P. Experimental and numerical investigation of hydrodynamics in raceway reactors used for algaculture. Chemical Engineering Journal 2014, 250, 230–239. [Google Scholar] [CrossRef]
- Fan, F.; Fei, Z.; Wan, M.; Huang, J.; Wang, W.; Bai, W.; He, M.; Li, Y. The optimization of centrifugal pump driving horizontal tubular photobioreactor for enhancing astaxanthin production using heterotrophic Haematococcus pluvialis. Journal of Biotechnology 2021, 341, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Mirón, A.; García Camacho, F.; Contreras Gómez, A.; Grima, E.M.; Chisti, Y. Bubble-column and airlift photobioreactors for algal culture. AIChE Journal 2000, 46, 1872–1887. [Google Scholar] [CrossRef]
- Wang, C.; Lan, C.Q. Effects of shear stress on microalgae - A review. Biotechnology advances 2018, 36, 986–1002. [Google Scholar] [CrossRef]
- Hoffman, J.; Pate, R.; Drennen, T.; Quinn, J. Techno-economic assessment of open microalgae production systems. Algal Research 2017, 23, 51–57. [Google Scholar] [CrossRef]
- Schagerl, M.; Siedler, R.; Konopáčová, E.; Ali, S.S. Estimating Biomass and Vitality of Microalgae for Monitoring Cultures: A Roadmap for Reliable Measurements. Cells 2022, 11, 2455. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.; Nam, K.; Seo, Y.H.; Kim, J.Y.; Lee, S.; Lee, Y.G.; Yang, J.W. Study of Optical Configurations for Multiple Enhancement of Microalgal Biomass Production. Scientific Reports 2019, 9, 1723. [Google Scholar] [CrossRef]
- Illman, A.M.; Scragg, A.H.; Shales, S.W. Increase in Chlorella strains calorific values when grown in low nitrogen medium. Enzyme and Microbial Technology 2000, 27, 631–635. [Google Scholar] [CrossRef]
- Borowitzka, A.M.; Moheimani, R.N. Algae for Biofuels and Energy. Developments in Applied Phycology 2013, 5. Preface, Springer Dordrecht Heidelberg New York London. [CrossRef]
- De Vree, J.H.; Bosma, R.; Janssen, M.; Barbosa, M.J.; Wijffels, R.H.; Lamers, P.P. Comparison of four outdoor pilot-scale photobioreactors. Biotechnology for Biofuels 2015, 8, 215. [Google Scholar] [CrossRef]
- BP. Statistical Review of World Energy 2021. 70th ed. https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2021-full-report.pdf (accessed on January of 2023).
- Ritchie, H.; Roser, M.; Rosado, P. Energy. Our World in Data 2022. Retrieved from https://ourworldindata.org/energy (accessed on January of 2023).
- Princiotta, F.T. The Climate Mitigation Challenge-Where Do We Stand? Journal of Air & Waste Management Association 2021, 71, 1234–1250. [Google Scholar] [CrossRef]
- Clémençon, R. The Two Sides of the Paris Climate Agreement: Dismal Failure or Historic Breakthrough? The Journal of Environment & Development 2016, 25, 3–24. [Google Scholar] [CrossRef]
- Sayre, R. Microalgae: The Potential for Carbon Capture. BioScience 2010, 60, 722–727. [Google Scholar] [CrossRef]
- Prasad, R.; Gupta, S.K.; Shabnam, N.; Oliveira, C.Y.B.; Nema, A.K.; Ansari, F.A.; Bux, F. Role of Microalgae in Global CO2 Sequestration: Physiological Mechanism, Recent Development, Challenges, and Future Prospective. Sustainability 2021, 13, 13061. [Google Scholar] [CrossRef]
- Derakhshandeh, M.; Atici, T.; Tezcan Un, U. Evaluation of Wild-Type Microalgae Species Biomass as Carbon Dioxide Sink and Renewable Energy Resource. Waste Biomass Valorization 2021, 12, 105–121. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Land use in agriculture by the numbers 2020. Retrieved 2022. https://www.fao.org/sustainability/news/detail/en/c/1274219/ (accessed on March of 2023).
- Ritchie, H.; Roser, M.; Rosado, P. China: CO2 country profile. Our World in Data 2020. Retrieved from https://ourworldindata.org/co2/country/china?country=~CHN (accessed on March of 2023).
- Ebhodaghe, S.O.; Imanah, O.E.; Ndibe, H. Biofuels from microalgae biomass: A review of conversion processes and procedures. Arabian Journal of Chemistry 2022, 15, 103591. [Google Scholar] [CrossRef]
- Singh, G.; Patidar, S.K. Microalgae harvesting techniques: A review. Journal of Environmental Management 2018, 217, 499–508. [Google Scholar] [CrossRef]
- Carmichael, W.; Drapeau, C.; Anderson, D. Harvesting of Aphanizomenon flos-aquae Ralfs ex Born. & Flah. Var. flos-aquae (Cyanobacteria) from Klamath Lake for human dietary use. Journal of Applied Phycology 2000, 12, 585–595. [Google Scholar] [CrossRef]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.; Verspagen, J.; Visser, P. Cyanobacterial blooms. Nature Reviews Microbiology 2018, 16, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Olofsson, M.; Suikkanen, S.; Kobos, J.; Wasmund, N.; Karlson, B. Basin-specific changes in filamentous cyanobacteria community composition across four decades in the Baltic Sea. Harmful Algae 2020, 91, 101685. [Google Scholar] [CrossRef] [PubMed]
- Olofsson, M.; Klawonn, I.; Karlson, B. Nitrogen fixation estimates for the Baltic Sea indicate high rates for the previously overlooked Bothnian Sea. Ambio 2021, 50, 203–214. [Google Scholar] [CrossRef]
- Falcón, L.I.; Cipriano, F.; Chistoserdov, A.Y.; Carpenter, E.J. Diversity of diazotrophic unicellular cyanobacteria in the tropical North Atlantic Ocean. Applied and Environmental Microbiology 2002, 68, 5760–5764. [Google Scholar] [CrossRef]
- Pruvost, J.; Goetz, V.; Artu, A.; Das, P.; Aljabri, H. Thermal modeling and optimization of microalgal biomass production in the harsh desert conditions of State of Qatar. Algal Research 2019, 38, 101381. [Google Scholar] [CrossRef]
- Schmitt, R. Salinity and the Global Water Cycle. Oceanography 2008, 21, 12–19. [Google Scholar] [CrossRef]
- De Luca, R.; Bezzo, F.; Béchet, Q.; Bernard, O. Meteorological Data-Based Optimal Control Strategy for Microalgae Cultivation in Open Pond Systems. Complexity 2019, 4363895. [Google Scholar] [CrossRef]
- Tian, H.; Bian, Z.; Shi, H.; Qin, X.; Pan, N.; Lu, C.; Pan, S.; Tubiello, F.N.; Chang, J.; Conchedda, G.; Liu, J.; Mueller, N.; Nishina, K.; Xu, R.; Yang, J.; You, L. Zhang, B. History of anthropogenic Nitrogen inputs (HaNi) to the terrestrial biosphere: A 5 arcmin resolution annual dataset from 1860 to 2019. Earth System Science Data 2022, 14, 4551. [Google Scholar] [CrossRef]
- Mahata, C.; Mishra, S.; Dhar, S.; Ray, S.; Mohanty, K.; Das, D. Utilization of dark fermentation effluent for algal cultivation in a modified airlift photobioreactor for biomass and biocrude production. Journal of Environmental Management 2022, 330, 11712. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Mella-Herrera, R.A.; Golden, J.W. Cyanobacterial heterocysts. Cold Spring Harbor Perspectives in Biology 2010, 2, a000315. Available online: https://cshperspectives.cshlp.org/content/2/4/a000315. [CrossRef] [PubMed]
- Qadir, M.; Drechsel, P.; Jiménez Cisneros, B.; Kim, Y.; Pramanik, A.; Mehta, P.; Olaniyan, O. Global and regional potential of wastewater as a water, nutrient and energy source. Natural Resource Forum 2020, 44, 40–51. [Google Scholar] [CrossRef]
- Wang, L.; Qin, T.; Zhao, J.; Zhang, Y.; Wu, Z.; Cui, X.; Zhou, G.; Li, C.; Guo, L.; Jiang, G. Exploring the nitrogen reservoir of biodegradable household garbage and its potential in replacing synthetic nitrogen fertilizers in China. PeerJ 2022, 10, e12621. [Google Scholar] [CrossRef]
- Udayan, A.; Sirohi, R.; Sreekumar, N.; Sang, B.-I.; Sim, S.J. Mass Cultivation and Harvesting of Microalgal Biomass: Current Trends and Future Perspectives. Bioresource Technology 2022, 344, 126406. [Google Scholar] [CrossRef]
- Hachicha, R.; Elleuch, F.; Ben Hlima, H.; Dubessay, P.; de Baynast, H.; Delattre, C.; Pierre, G.; Hachicha, R.; Abdelkafi, S.; Michaud, P.; Fendri, I. Biomolecules from Microalgae and Cyanobacteria: Applications and Market Survey. Applied Sciences 2022, 12, 1924. [Google Scholar] [CrossRef]
- Onyeaka, H.; Miri, T.; Kechrist, O.; Hart, A.; Anumudu, C.; Al-sharify, Z.T. Minimizing carbon footprint via microalgae as a biological capture. 100007. Energy Nexus 2021, 4, 100032. [Google Scholar] [CrossRef]
- Han, Y.; Hoekman, S.; Cui, Z.; Jena, U.; Das, P. Hydrothermal liquefaction of marine microalgae biomass using co-solvents. Algal Research 2019, 38, 101421. [Google Scholar] [CrossRef]
- Aliyu, A.; Lee, J.; Harvey, A. Microalgae for biofuels: A review of thermochemical conversion processes and associated opportunities and challenges. Bioresource Technology Reports 2021, 15, 103591. [Google Scholar] [CrossRef]
- Ganesh Saratale, R.; Kumar, G.; Banu, R.; Xia, A.; Periyasamy, S.; Saratale, G.D. A critical review on anaerobic digestion of microalgae and macroalgae and co-digestion of biomass for enhanced methane generation. Bioresource Technology 2018, 262, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Romero, A.; Martin, M.; Boyer, A.; Bolzoni, R.; Matricon, L.; Sassi, J.F.; Steyer, J.P.; Delrue, F. Microalgae adaptation as a strategy to recycle the aqueous phase from hydrothermal liquefaction. Bioresource Technology 2023, 371, 128631. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, H.; Rosado, P.; Roser, M. Meat and Dairy Production. Our World in Data 2017. https://ourworldindata.org/meat-production (accessed on March of 2023).
- Mottet, A.; De Haan, C.; Falcucci, A.; Tempio, G.; Opio, C.; Gerber, P.J. Livestock: On our plates or eating at our table? A new analysis of the feed/food debate. Global Food Security 2017, 14. [Google Scholar] [CrossRef]
- Kusmayadi, A.; Leong, Y.K.; Yen, H.W.; Huang, C.Y.; Chang, J.S. Microalgae as sustainable food and feed sources for animals and humans – Biotechnological and environmental aspects. Chemosphere 2021, 271, 129800. [Google Scholar] [CrossRef]
- Madeira, M.; Cardoso, C.; Lopes, P.; Coelho, D.; Afonso, C.; Bandarra, N.; Prates, J. Microalgae as feed ingredients for livestock production and meat quality: A review. Livestock Science 2017, 205, 47–59. [Google Scholar] [CrossRef]
- Valente, L.M.P.; Cabrita, A.R.J.; Maia, M.R.G.; Valente, I.M.; Engrola, S.; Fonseca, A.J.M.; Ribeiro, D.M.; Lordelo, M.; Martins, C.F.; Cunha, L.F.; Almeida, A.M.; Freire, J.P.B.; Galanakis, C.M. Microalgae as feed ingredients for livestock production and aquaculture. In Microalgae 2021, pp. 239–312. Academic Press. [CrossRef]
- Saadaoui, I.; Rasheed, R.; Aguilar, A.; Cherif, M.; Al Jabri, H.; Sayadi, S.; Manning, S.R. Microalgal-based feed: promising alternative feedstocks for livestock and poultry production. Journal of Animal Science and Biotechnology 2021, 12, 76. [Google Scholar] [CrossRef]
- Ahmad, A.; Hassan, S.W.; Banat, F. An overview of microalgae biomass as a sustainable aquaculture feed ingredient: food security and circular economy. Bioengineered 2022, 13, 9521–9547. [Google Scholar] [CrossRef]
- Henchion, M.; Hayes, M.; Mullen, A.M.; Fenelon, M.; Tiwari, B. Future protein supply and demand: Strategies and factors influencing a sustainable equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Bele, V.; Rajagopal, R.; Goyette, B. Closed -loop bioeconomy opportunities through the integration of microalgae cultivation with anaerobic digestion: A critical review. Bioresource Technology Reports 2023, 21, 101336. [Google Scholar] [CrossRef]
- Rajagopal, R.; Mousavi, S.E.; Goyette, B.; Adhikary, S. Coupling of Microalgae Cultivation with Anaerobic Digestion of Poultry Wastes: Toward Sustainable Value Added Bioproducts. Bioengineering 2021, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Leena, M.C.; Hausrath, E.M.; Ming, D.W.; Adcock, C.T.; Raymond, J.; Remias, D.; Ruemmele, W.P. Investigating the Growth of Algae Under Low Atmospheric Pressures for Potential Food and Oxygen Production on Mars. Frontiers in Microbiology 2021, 12. [Google Scholar] [CrossRef]

| Country | Emissions (Mt CO2/year) | Total Surface (x106 Km2) | Proportional culture surface (x103 Km2) | % of Total surface |
|---|---|---|---|---|
| China | 10,065 | 9.60 | 494.9 | 5.2 |
| USA | 5,416 | 9.83 | 266.3 | 2.7 |
| India | 2,654 | 3.29 | 130.5 | 4.0 |
| Rusia | 1,711 | 17.10 | 84.1 | 0.5 |
| Japan | 1,162 | 0.38 | 57.1 | 15.1 |
| Germany | 759 | 0.36 | 37.3 | 10.4 |
| Iran | 720 | 1.65 | 35.4 | 2.1 |
| South Korea | 659 | 0.10 | 32.4 | 32.4 |
| Saudi Arabia | 621 | 2.15 | 30.5 | 1.4 |
| Indonesia | 615 | 1.90 | 30.2 | 1.6 |
| Canada | 568 | 9.98 | 27.9 | 0.3 |
| Mexico | 477 | 1.97 | 23.5 | 1.2 |
| South Africa | 468 | 1.22 | 23.0 | 1.9 |
| Brazil | 457 | 8.52 | 22.5 | 0.3 |
| Turkey | 428 | 0.78 | 21.0 | 2.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





