Submitted:
19 August 2024
Posted:
22 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Powders
2.3. Methods of Analysis
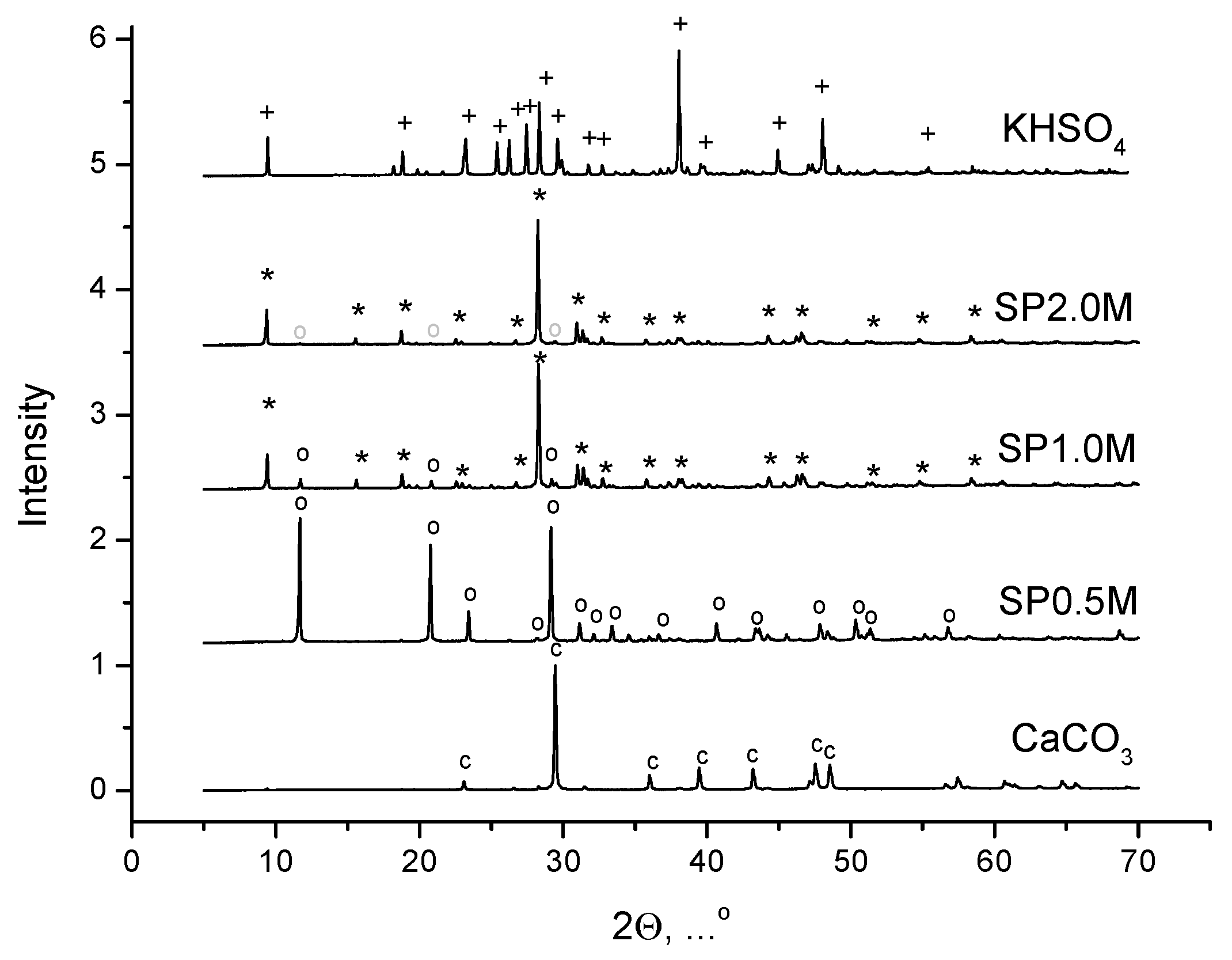
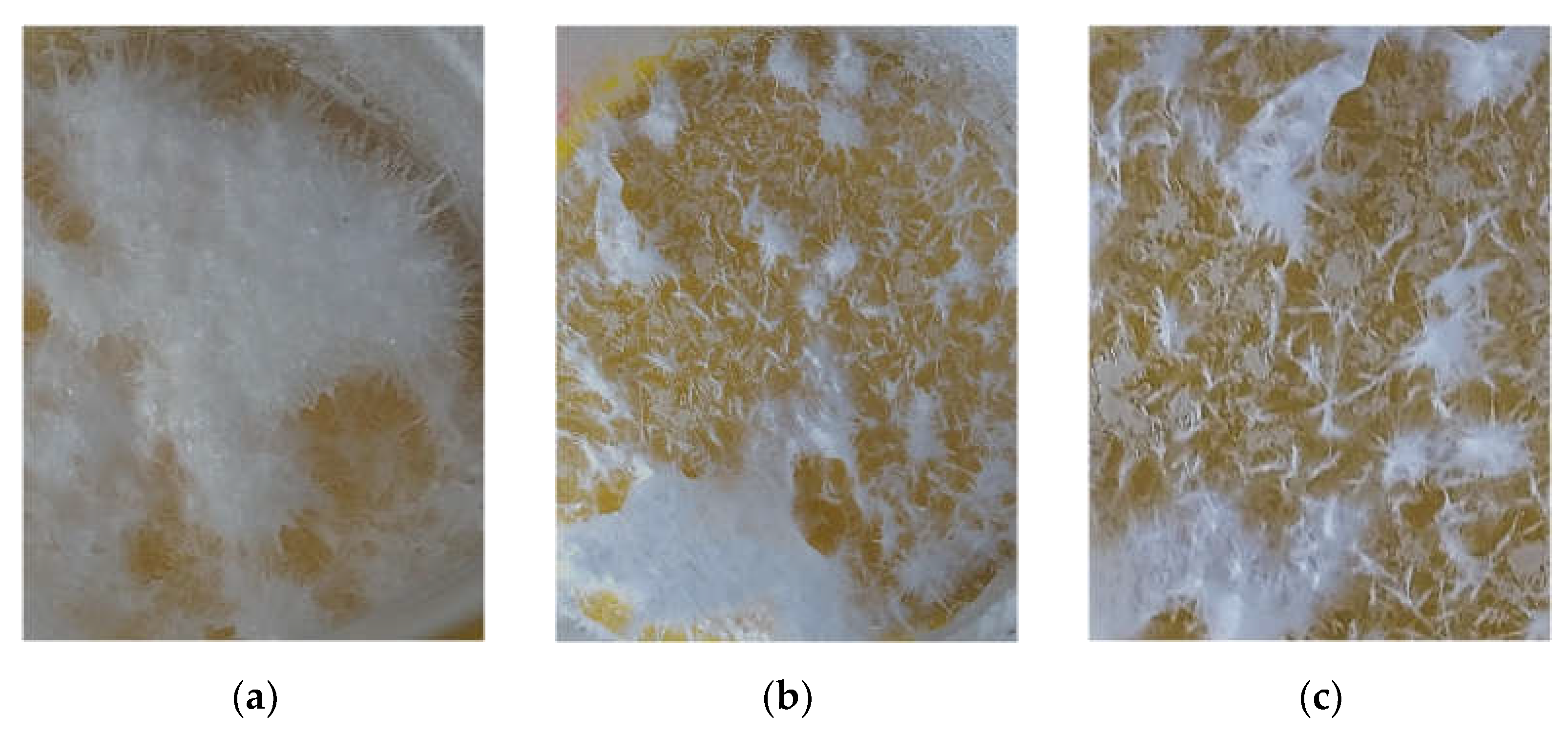
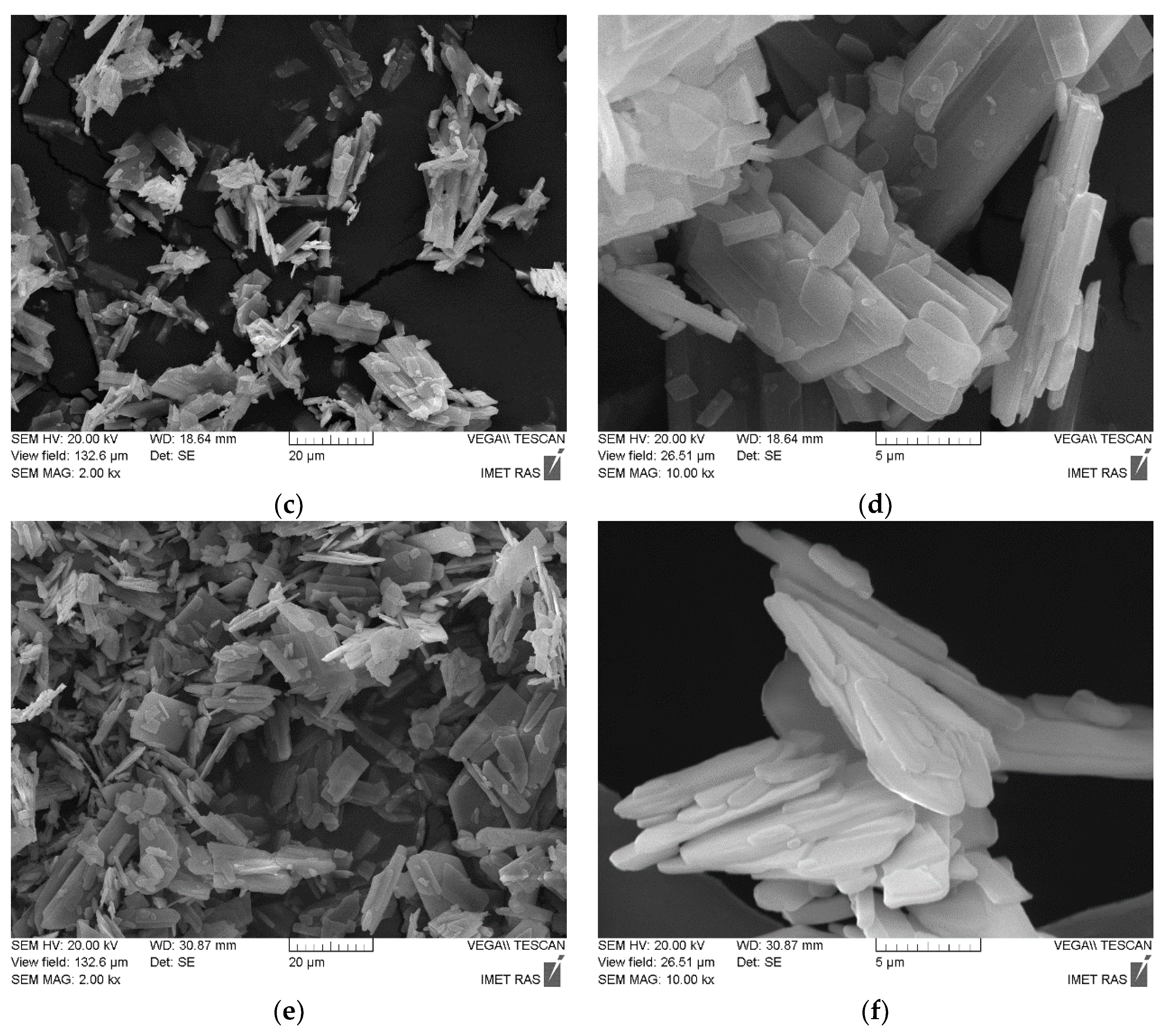
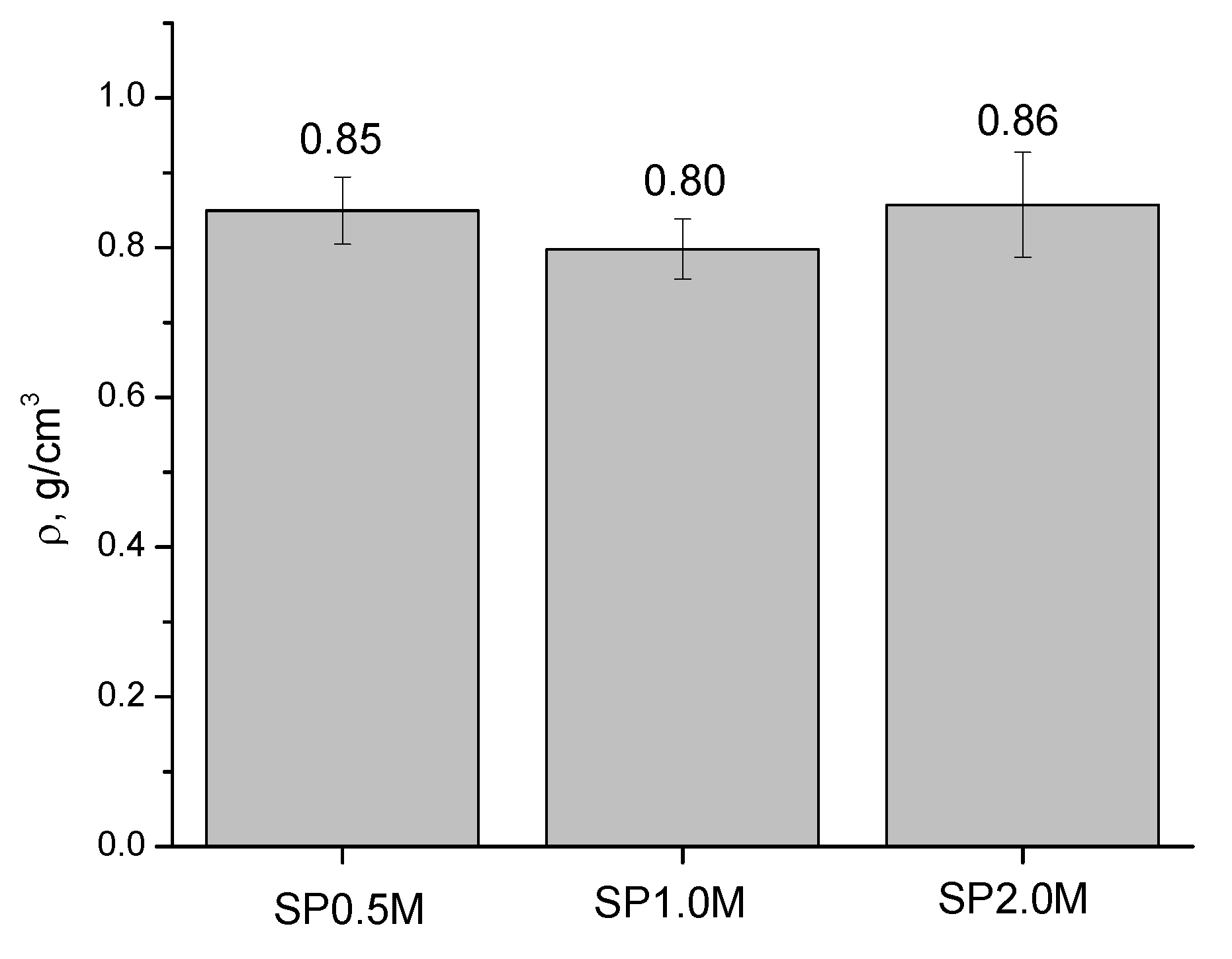
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Damidot, D., & Glasser, F. P. Thermodynamic investigation of the CaO-Al2O3-CaSO4-K2O-H2O system at 25°C. Cem. Concr. Res., 1993, 23(5), 1195-1204. [CrossRef]
- Timakov, I. S., Grebenev, V. V., Komornikov, V. A., Zainullin, O. B., Makarova, I. P., Selezneva, E. V., & Kuzmin, I. I. Production of Complex Hydrosulphates in the K3H(SO4)2–Rb3H(SO4)2 Series: II. Phase Equilibria in the K2SO4–Rb2SO4–H2SO4–H2O System. Crystallogr. Rep. 2022, 67(3), 455-463. [CrossRef]
- Eriksen, K. M., Fehrmann, R., Hatem, G. et al. Conductivity, NMR, Thermal Measurements, and Phase Diagram of the K2S2O7−KHSO4 System. The Journal of Physical Chemistry. 1996, 100(25), 10771-10778. [CrossRef]
- Komornikov, V.A., Timakov, I.S., Makarova, I.P., Selezneva, E.V., Grebenev, V.V., & Zainullin, O.B. (2020, December). The Study of Phase Equilibria and Superprotonic Crystals in the (NH4)2SO4 - K2SO4 - H2SO4 - H2O system. J. Phys. Conf. Ser. 2020 1686 (1), 012048). IOP Publishing. [CrossRef]
- Freyer, D., Voigt, W. Crystallization and phase stability of CaSO4 and CaSO4–based salts //Monatshefte für Chemie/Chemical Monthly. 2003, 134(5), 693-719. [CrossRef]
- Safronova, T.V.; Belokozenko, M.A.; Yahyoev, S.O.; Shatalova, T.B.; Kazakova, G.K.; Peranidze, K.K.; Toshev, O.U.; Khasanova, S.S. Ceramics Based on CaSO4⋅2H2O Powder Synthesized from Ca(NO3)2 and (NH4)2SO4. Inorg. Mater. 2021, 57, 867–873. [CrossRef]
- Zhou, J.; Yuan, F.; Peng, S.; Xie, H.; Wu, P.; Feng, P.; Gao, C.; Yang, Y.; Guo, W.; Lai, D.; et al. Tunable Degradation Rate and Favorable Bioactivity of Porous Calcium Sulfate Scaffolds by Introducing Nano-Hydroxyapatite. Appl. Sci. 2016, 6, 411. [CrossRef]
- Mananda, A.B., Harada, H., Halem, H.I.A., Mitoma, Y., & Keiko, F. Study of potassium recovery from biomass ash using tartaric acid and syngenite method. J. Mater. Scie. Chem. Eng. 2021, 9(5), 39-52. [CrossRef]
- Otani, T., Ando, H., Goshima, T., Mizuta, K., & Nii, S. Enhanced recovery of potassium from sugarcane molasses for fertilizer. Sugar Tech, 2023, 25(4), 820-826. [CrossRef]
- Neto, J. D. S. A., Angeles, G., & Kirchheim, A. P. Effects of sulfates on the hydration of Portland cement–A review. Constr. Build. Mater. 2021, 279, 122428. [CrossRef]
- Arceo, H.B., Glasser, F.P. Fluxing reactions of sulphate and carbonates in cement clinkering. I. Systems CaSO4-K2SO4 and K2SO4-CaCO3. Cem. Concr. Res. 1990, 20(6), 862-868. [CrossRef]
- Schiefer, C., & Plank, J. Mechanochemical syngenite as hydration accelerator for anhydrite-based self-levelling floor screeds. Constr. Build. Mater., 2021, 308, 124982. [CrossRef]
- Pandey, A., Sonkawade, R.G., Sahare, P.D. Thermoluminescence and photoluminescence characteristics of nanocrystalline K2Ca2(SO4)3:Eu. J. Phys. D. 2002, 35(21), 2744. [CrossRef]
- Safronova, T.V.; Akhmedov, M.M.; Shatalova, T.B.; Tikhonova, S.A.; Kazakova, G.K. Ceramics in the K2O–CaO–SO3–P2O5 System. Russ. J. Inorg. Chem. 2021, 66, 1057–1066. [CrossRef]
- Kuznetsov, A.I.; Safronova, T.V.; Shatalova, T.B.; Filippov, Y.Y.; Vaymugin, L.A.; Vlasenko, V.S.; Likhanov, M.S. Materials in the CaO-K2O-SO3-H2O System Based on Powder Mixtures including Calciolangbeinite K2Ca2(SO4)3 and Calcium Sulfate Anhydrite CaSO4. Ceramics 2023, 6, 1434-1448. [CrossRef]
- García-Florentino, C., Gomez-Nubla, L., Huidobro, J., Torre-Fdez, I., Ruíz-Galende, P., Aramendia, J., ... & Madariaga, J.M. Interrelationships in the gypsum–syngenite–görgeyite system and their possible formation on mars. Astrobiology, 2021, 21(3), 332-344. [CrossRef]
- Huidobro, J., Aramendia, J., Arana, G., Hausrath, E. M., & Madariaga, J. M. The effect of low temperature on the Raman spectra of calcium-rich sulfates on Mars. Annals of Glaciology. 2023, 64(91), 57-62. [CrossRef]
- Ju, E., Shi, E., Xin, Y., Cao, H., Liu, C., Liu, P., ... & Ling, Z. Laboratory synthesis, spectroscopic characteristics, and conversion relationships of five calcium sulfate double salts relevant to Mars. Icarus. 2023. 401, 115610. [CrossRef]
- Jentzsch, P.V., Bolanz, R.M., Ciobotă, V., Kampe, B., Rösch, P., Majzlan, J., & Popp, J. Raman spectroscopic study of calcium mixed salts of atmospheric importance. Vib. Spectrosc. 2012, 61, 206-213. [CrossRef]
- Jurišová, J. et al., Phase diagram of the system CaSO4-K2SO4-KNO3-Ca(NO3)2-H2O. Acta Chim. Slov. 2014, 7 (1), 20—24. [CrossRef]
- Goncharik, I.I., Shauchuk, V.V., Kudzina, O.A., & Mozheyko, F.F. Preparation of potassium sulfate in a reaction of potassium chloride and calcium sulfate. Proceedings of the National Academy of Sciences of Belarus, Chemical Series. 2017, (3), 98-103. (In Russian) https://vestichem.belnauka.by/jour/article/view/276.
- Schiefer, C., & Plank, J. Solventless Mechanochemical Synthesis of Phase Pure Syngenite. Chemistry-Methods, 2021, 1(1), 78-84. [CrossRef]
- Smillie, S.; Moulin, E.; Macphee, D.E.; Glasser, F.P. Freshness of cement: Conditions for syngenite CaK2(SO4)2 H2O formation. Adv. Cem. Res. 1993, 19, 93–96. [CrossRef]
- ICDD (2010). PDF-4+ 2010 (Database), edited by Dr. Soorya Kabekkodu, International Centre for Diffraction Data. Newtown Square. PA. USA. http://www.icdd.com/products/pdf2.htm.
- Matović, V., Erić, S., Kremenović, A., Colomban, P., Srećković-Batoćanin, D., & Matović, N. The origin of syngenite in black crusts on the limestone monument King's Gate (Belgrade Fortress, Serbia)–the role of agriculture fertiliser. Journal of Cultural Heritage, 2012, 13(2), 175-186. [CrossRef]
- Sanytsky, M., Kropyvnytska, T., & Shyiko, O. Effect of Potassium Sulfate on the Portland Cement Pastes Setting Behavior. Chem. Chem. Technol. 2023, 17(1), 170-178. [CrossRef]
- Wieczorek-Ciurowa, K. Topochemistry of thermal dehydration of syngenite K2Ca(SO4)2·H2O. Thermochimica acta, 1985, 92, 485-487. [CrossRef]
- Dankiewicz, J., & Wieczorek-Ciurowa, K. Kinetic study of the thermal dehydration of syngenite K2Ca(SO4)2H2O under isothermal conditions. J. Therm. Anal. Calorim. 1978, 13(3), 543-552. [CrossRef]
- Rowe, J.J., Morey, G.W., & Hansen, I.D. The binary system K2SO4-CaSO4. J. Inorg. Nucl. Chem. 1965. 27(1), 53-58. [CrossRef]
- Kloprogge, J.T., Ding, Z., Martens, W.N., Schuiling, R.D., Duong, L.V., & Frost, R.L. Thermal decomposition of syngenite, K2Ca(SO4)2·H2O. Thermochimica Acta, 2004. 417(1), 143-155. [CrossRef]
- Pekov I.V., Zelenski M.E., Zubkova N.V. et al. Calciolangbeinite, K2Ca2(SO4)3, a new mineral from the Tolbachik volcano, Kamchatka, Russia. Mineralogical Magazine. 2012, 76(3), 673-682. [CrossRef]


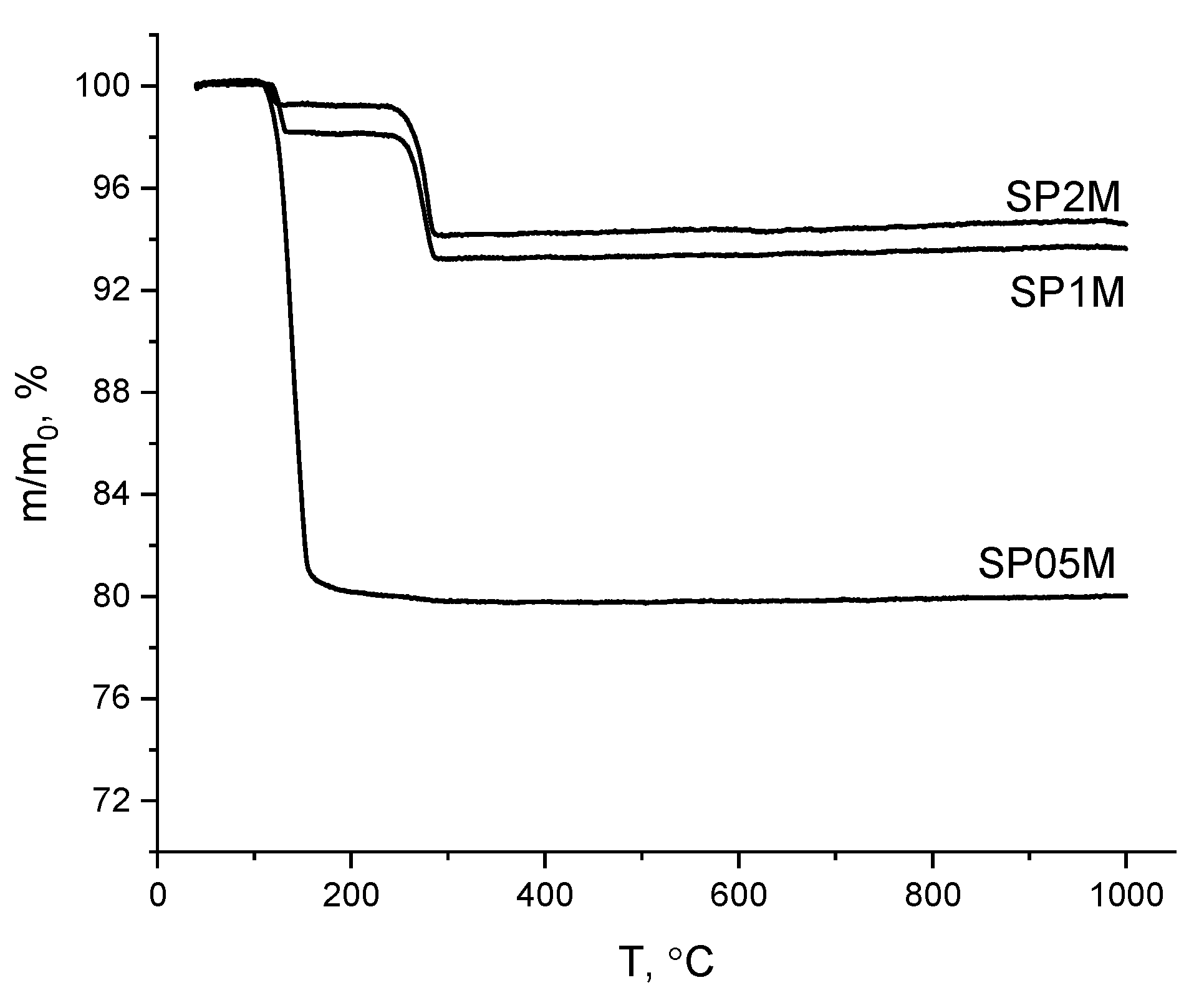
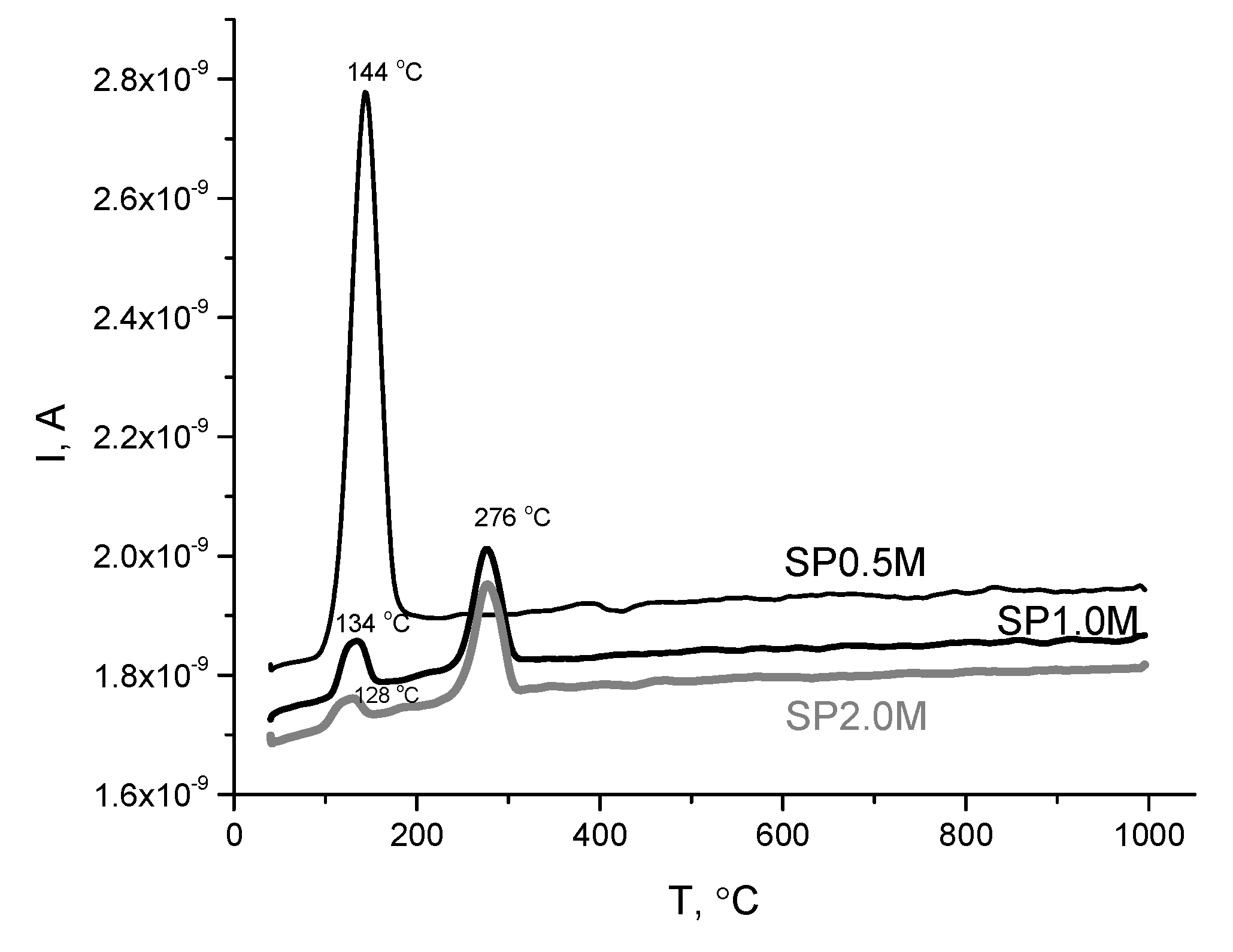
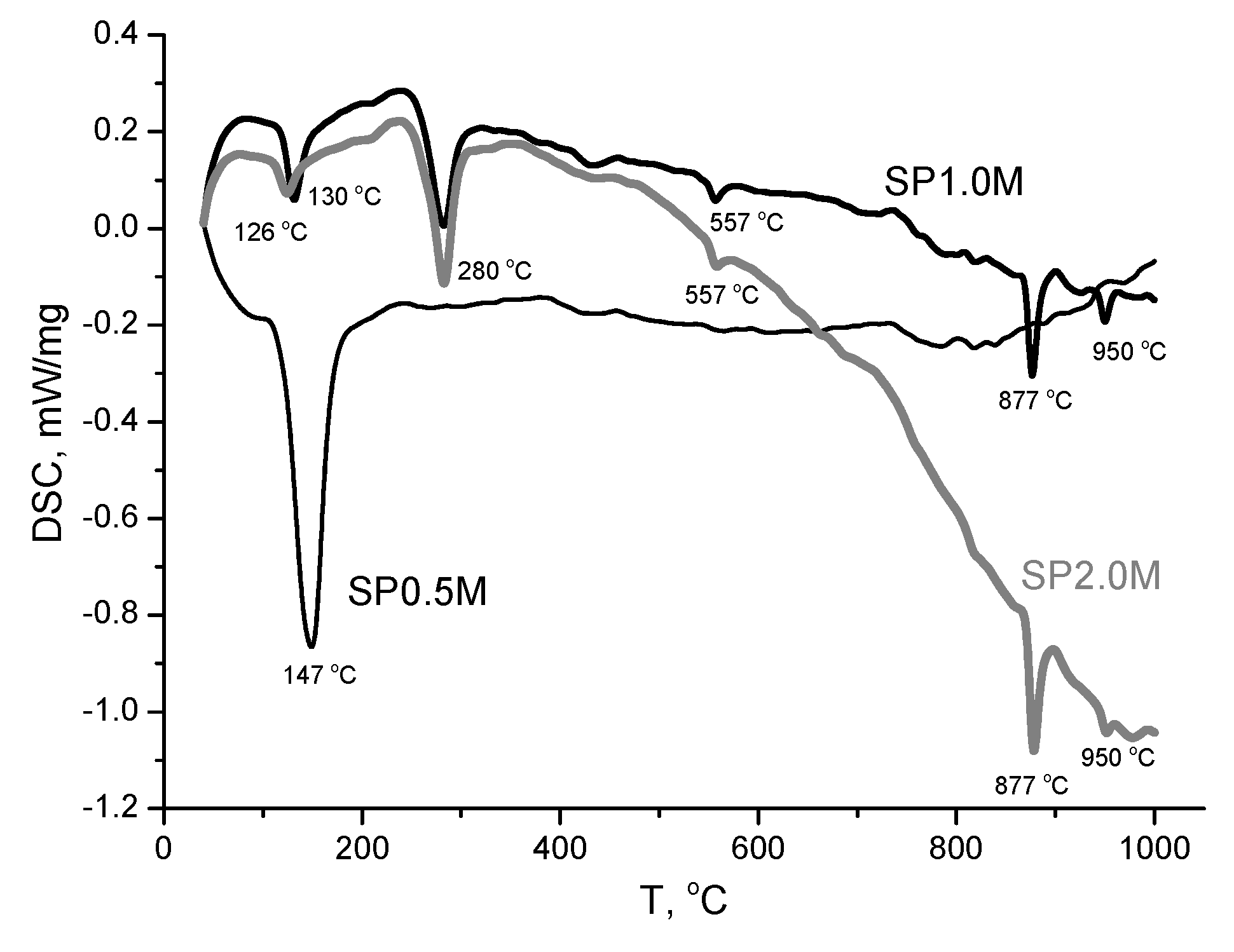
| Labeling1 | Concentration of the KHSO4 solution, mol/l | Volume of the solution, ml | The amount of substances by reaction, mol | Mass of reagents, g | Expected mass of K2Ca(SO4)2·H2O, g | ||
|---|---|---|---|---|---|---|---|
| KHSO4 | CaCO3 | KHSO4 | CaCO3 | ||||
| SP0.5M | 0.5 | 400 | 0.2 | 0.1 | 27.2 | 10.0 | 32.8 |
| SP1.0M | 1 | 400 | 0.4 | 0.2 | 54.4 | 20.0 | 65.6 |
| SP2.0M | 2 | 400 | 0.8 | 0.4 | 108.8 | 40.0 | 131.2 |
| Labeling | Expected mass of K2Ca(SO4)2·H2O, g | Mass of synthesized powder, g | Phase composition of synthesized powder1, mass % | |
|---|---|---|---|---|
| K2Ca(SO4)2·H2O (#96-900-8129)1 |
CaSO4·2H2O (#96-901-7314)1 |
|||
| SP0.5M | 32.8 | 15.5 | 0 | 100 |
| SP1.0M | 65.6 | 58.2 | 92.9 | 7.1 |
| SP2.0M | 131.2 | 114.3 | 98.7 | 1.3 |
| Labeling | Mass of extracted product, g | Phase composition of extracted products1, mass % | |
|---|---|---|---|
| K2Ca(SO4)2·H2O (#96-900-8129)1 |
K2SO4 (#96-900-7570)1 |
||
| Ex-SP0.5M | 17.21 | 62.2 | 37.8 |
| Ex-SP1.0M | 5.8 | 49.1 | 50.9 |
| Ex-SP2.0M | 5.6 | 83.3 | 16.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





