Submitted:
20 August 2024
Posted:
21 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Collection and obtaining of essential oil
2.2. GC-MS analysis
2.3. GC-FID analysis
2.4. Antibacterial activity
2.4.1. Determination of minimum inhibitory concentration (MIC)
2.4.2. Antibiotic modulation tests
2.4.3. Determination of the MIC reduction factor
2.4.4. Statistical analysis
3. Results and discussion
3.1. Composition of C. grewioides essential oil
3.2. Antibacterial activity
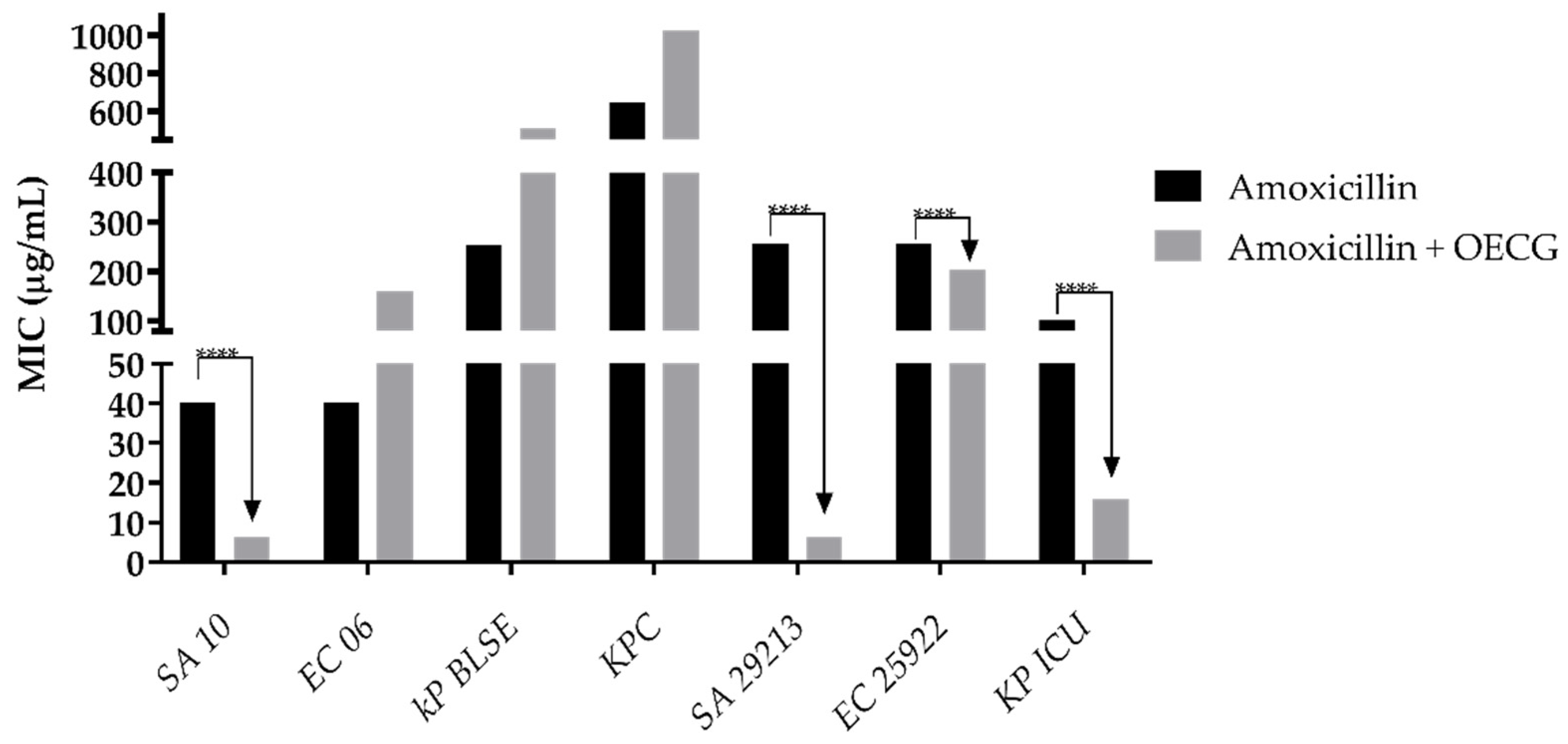
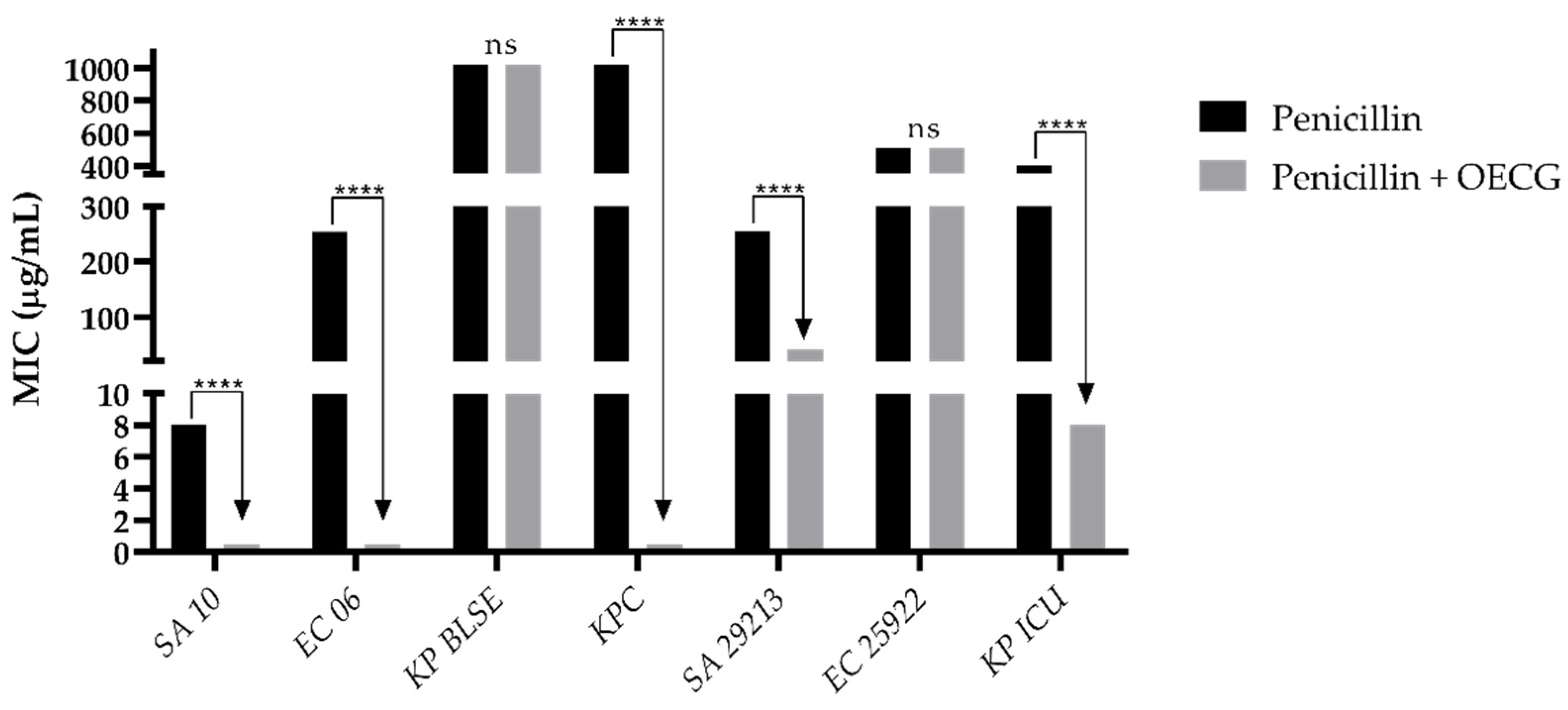
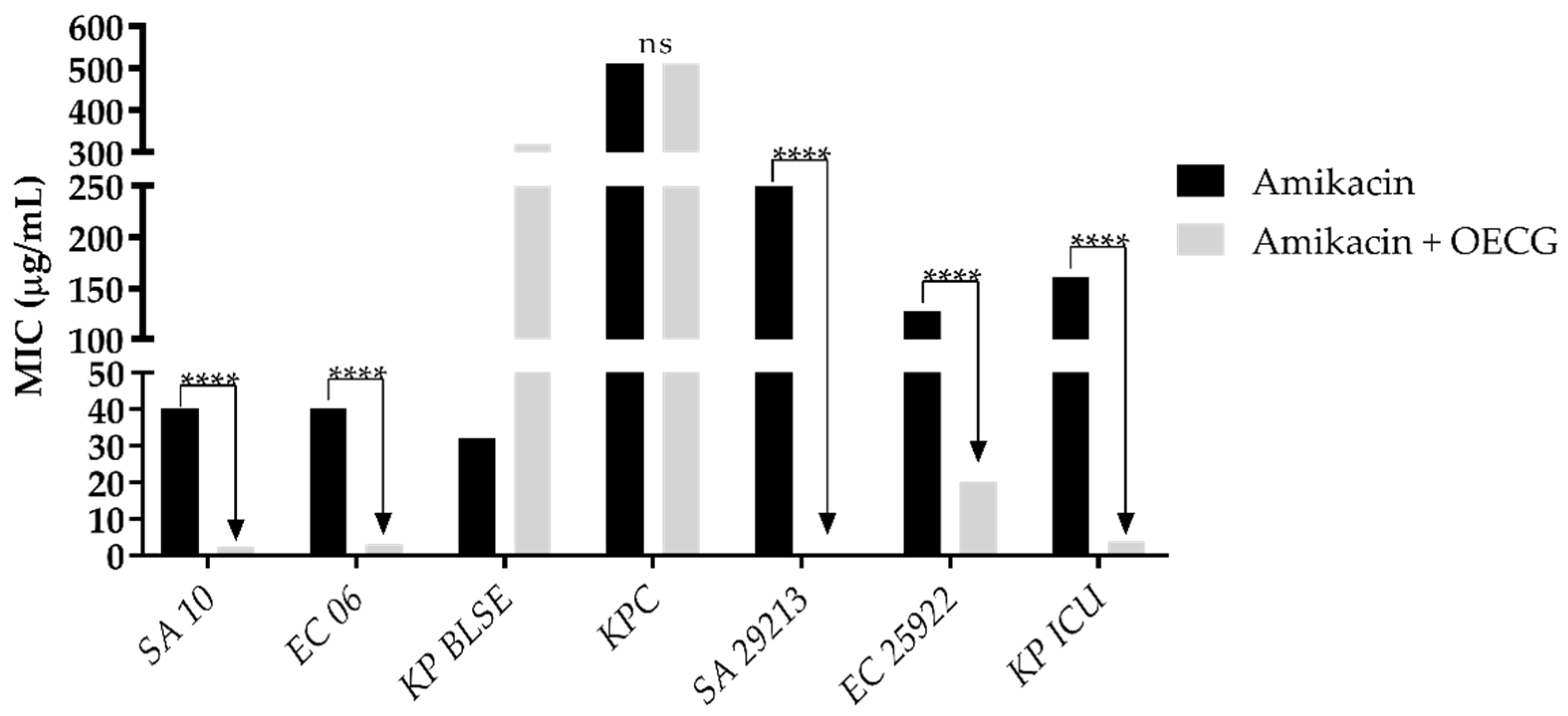
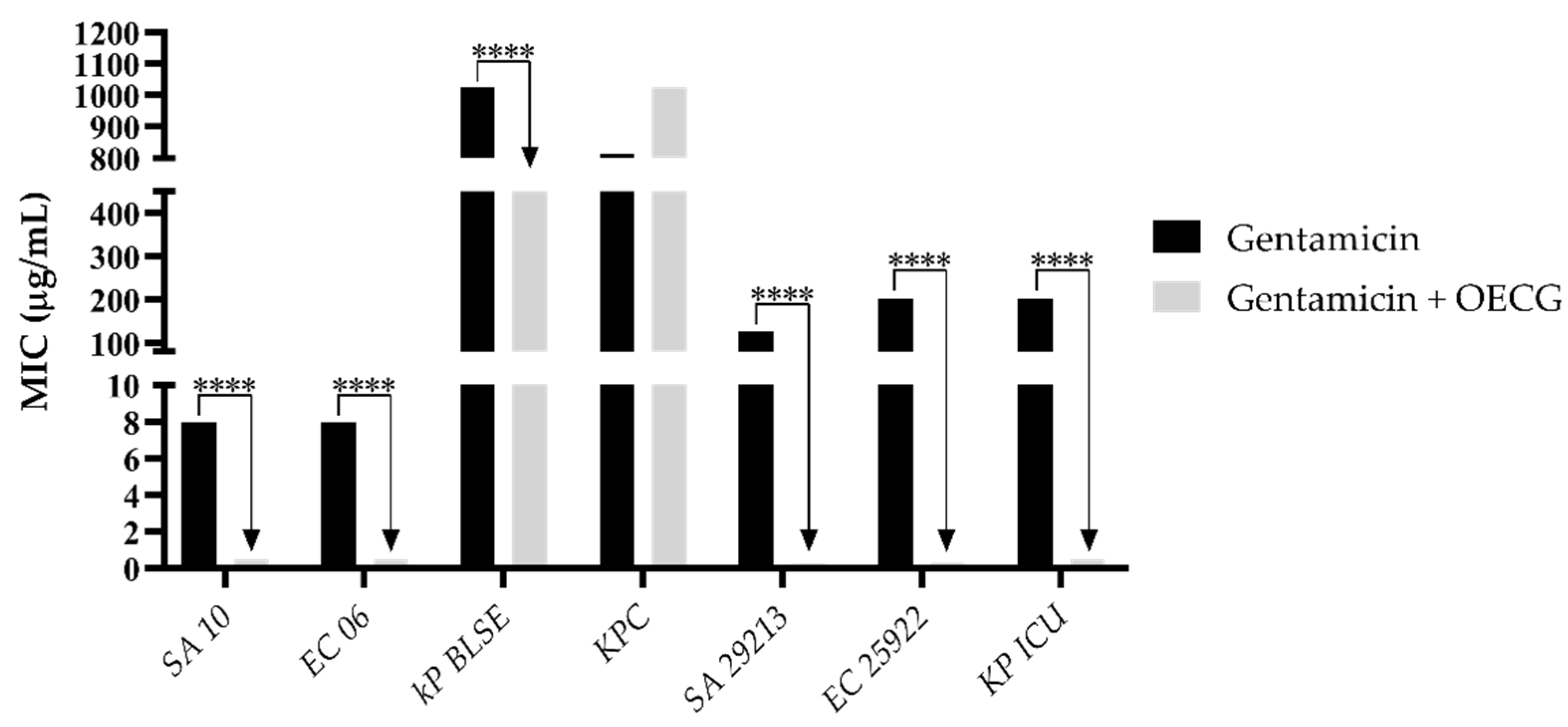
3.3. Modulating activity
4. Conclusions
Author Contributions
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costa, A. R., Lima Silva, J., Lima, K. R. R., Rocha, M. I., Barros, L. M., da Costa, J. G. M., ... & Coutinho, H. D. M. Rhaphiodon echinus (Nees & Mart.) Schauer: Chemical, toxicological activity and increased antibiotic activity of antifungal drug activity and antibacterial. Microb. Pathog., v. 107, p. 280-286, 2017. [CrossRef]
- Silva, Líllian Oliveira Pereira; Nogueira, Joseli Maria da Rocha. Resistência bacteriana: potencial de plantas medicinais como alternativa para antimicrobianos. Rev. bras. anal. clin, p. 21-27, 2021.
- <named-content content-type="background:white">Marisco, G. Marisco, G., & Rocha, R. Estudos etnobotânicos em comunidades indígenas no Brasil. 2016. https://www.arca.fiocruz.br/handle/icict/19250.
- Souza, R. K. D. , da Silva, M. A. P., de Menezes, I. R. A., Ribeiro, D. A., Bezerra, L. R., & de Almeida Souza, M. M. Ethnopharmacology of medicinal plants of carrasco, Nordeste Brazil. Journal of Ethnopharmacology, v. 157, p. 99-104, 2014.
- Oliveira, S. D. D. S. , De Oliveira E Silva, A. M., Blank, A. F., Nogueira, P. C. D. L., Nizio, D. A. D. C., Almeida-Pereira, C. S., Pereira, R.O, Menezes-Sá, T. S. A., Santana, M. H. S. & Arrigoni-Blank, M. D. F. Radical scavenging activity of the essential oils from Croton grewioides Baill accessions and the major compounds eugenol, methyl eugenol and methyl chavicol. J. Essent. Oil Res. v. 33, n. 1, p. 94-103, 2021. [CrossRef]
- Lima, F. C. , Sousa, D. F., Ferreira, J. M., Lima Jr, R. C., Tomé, A. R., Cardoso, J. H. L., Queiroz, M. G. R. & Campos, A. R. Croton zehntneri Essential Oil Prevents Acetaminophen-Induced Acute Hepatotoxicity in Mice. v. 2, n. 4, 2008.
- Sousa, A. J. , Oliveira, G. L., Fonseca, L., Rocha, M. S., Rai, M., Santos, F. E., & Lima, S. G. D. Antioxidant properties of Croton zehntneri Pax et Hoffm. Essential oil and its inclusion complex with β-cyclodextrin prepared by spray drying. Journal of the Brazilian Chemical Society, v. 33, p. 1244-1253, 2022.
- Andrade, T. C. , Lima, S. G., Freitas, R. M., Rocha, M. S., Islam, T., SILVA, T. G., & Militao, G. C.Isolation, characterization and evaluation of antimicrobial and cytotoxic activity of estragole, obtained from the essential oil of Croton zehntneri (euphorbiaceae). An. Acad. Bras. Ciênc. v. 87, p. 173-182, 2015. [CrossRef]
- Coelho-de-Souza, A. N. , Lahlou, S., Barreto, J. E., Yum, M. E., Oliveira, A. C., Oliveira, H. D., Celedônioa, N.R., Feitosaa, R.G.F., Duarte, G.P., Santosa, C.F., De Albuquerque A.A.C. & Leal-Cardoso, J. H. Essential oil of Croton zehntneri and its major constituent anethole display gastroprotective effect by increasing the surface mucous layer. Fundamental & clinical pharmacology, v. 27, n. 3, p. 288-298, 2013. [CrossRef]
- Cabral, P. H. B. , de Morais Campos, R., Fonteles, M. C., Santos, C. F., Cardoso, J. H. L., & do Nascimento, N. R. F. Effects of the essential oil of Croton zehntneri and its major components, anethole and estragole, on the rat corpora cavernosa. Life sci, v. 112, n. 1-2, p. 74-81. [CrossRef]
- Sá, N. A. R. , Bruno, J. B., Guerreiro, D. D., Cadenas, J., Alves, B. G., Cibin, F. W. S., Leal-Cardoso, J.H., Gastal, E.L. & Figueiredo, J. R. Anethole reduces oxidative stress and improves in vitro survival and activation of primordial follicles. Braz J Med Biol Res. v. 51, 2018. [CrossRef]
- Cavalcanti, J. M. , Leal-Cardoso, J. H., Diniz, L. R. L., Portella, V. G., Costa, C. O., Linard, Alves, C.K., Rocha, M.V.A.P., Lima, C.C., Cecatto, V.M.& Coelho-de-Souza, A. N. The essential oil of Croton zehntneri and trans-anethole improves cutaneous wound healing. J. Ethnopharmacol, v. 144, n. 2, p. 240-247, 2012. [CrossRef]
- Adams, R.P. , 2001. Identification of Essential Oil Components by Gas Chromatography/ Quadrupole Mass Spectroscopy. Allured Pub, Corp, Illinois.
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard-. Ninth Edition. CLSI document Clinical and Laboratory Standards Institute, 2012.
- Coutinho, H. D. , Costa, J. G., Lima, E. O., Falcão-Silva, V. S., & Siqueira-Júnior, J. P. Enhancement of the antibiotic activity against a multiresistant Escherichia coli by Mentha arvensis L. and chlorpromazine. Chemotherapy, v. 54, n. 4, p. 328-330, 2008.
- Santos, H. S. , Furtado, E. F., Bertini, L. M., Bandeira, P. N., Albuquerque, M. R., Menezes, J. E., Trevisan, M. T. S. & Lemos, T. L. Chemical composition and cholinesterase inhibition of essential oils of three chemotypes from Croton zehntneri. Rev. Latinoam. Quím, v. 38, n. 1, p. 45-51, 2010. [CrossRef]
- Silva, C. G. , Zago, H. B., Júnior, H. J., da Camara, C. A., de Oliveira, J. V., Barros, R., Schwartz, M. O. E. & Lucena, M. F. Composition and insecticidal activity of the essential oil of Croton grewioides Baill. against Mexican bean weevil (Zabrotes subfasciatus Boheman). J. Essent. Oil Res, v. 20, n. 2, p. 179-182. 2008. [CrossRef]
- Ribeiro, S. M. , Bonilla, O. H., & Lucena, E. M. P. Influência da sazonalidade e do ciclo circadiano no rendimento e composição química dos óleos essenciais de Croton spp. da Caatinga. Iheringia, Sér. Bot. v. 73, n. 1, p. 31-38, 2018. [CrossRef]
- Morais, Lilia Aparecida Salgado. Influência dos fatores abióticos na composição química dos óleos essenciais. Hortic Bras, v. 27, n. 2, p. S4050-4063, 2009. http://www.alice.cnptia.embrapa.br/alice/handle/doc/577686.
- Costa, J. G. , Rodrigues, F. F., Angélico, E. C., Pereira, C. K., Souza, E. O. D., Caldas, G. F.R., Silva, M. R., Santos, N. K. A., Mota, M.L. & Santos, P. F. D. Composição química e avaliação da atividade antibacteriana e toxicidade do óleo essencial de Croton zehntneri (variedade estragol). Rev. Bras. Farmacogn, v. 18, p. 583-586, 2008. [CrossRef]
- Siqueira, R. J. B. , Leal-Cardoso, J. H., Couture, R., & Lahlou, S. Role of capsaicin-sensitive sensory nerves in mediation of the cardiovascular effects of the essential oil of Croton zehntneri leaves in anaesthetized rats. Clin. Exp. Pharmacol. Physiol. v. 33, n. 3, p. 238-247, 2006. [CrossRef]
- Leite, G. D. O. , Penha, A. R. S., Costa, J. G. M. D., & Campos, A. R. Anxiogenic like effect of Croton zehntneri Pax et Hoffm leaves essential oil in mice. J. Essent. Oil-Bear. Plants, v. 12, n. 5, p. 546-550, 2009. [CrossRef]
- Taveira, F. S. N. , De Lima, W. N., Andrade, E. H. A., & Maia, J. G. S. Seasonal essential oil variation of Aniba canelilla. Biochem. Syst. Ecol. v. 31, n. 1, p. 69-75, 2003. [CrossRef]
- Oliveira, A. L. D. , Soares, M. M., Santos, T. C. D., & Santos, A. Mecanismos de resistência bacteriana a antibióticos na infecção urinária. UNINGÁ Rev. v. 20, n. 3, 2014.
- Rodrigues, Fabíola FG; Costa, JGM; Coutinho, HDM. Synergy effects of the antibiotics gentamicin and the essential oil of Croton zehntneri. Phytomedicine, v. 16, n. 11, p. 1052-1055, 2009. [CrossRef]
- Scherer, C. B. , Botoni, L. S., & Costa-Val, A. P. Mecanismos de ação de antimicrobianos e resistência bacteriana. Veterinária, v. 4, n. 13, p. 12-20, 2016.
- Arruda, C. J. M. , de Almeida Siqueira, V. F., de Souza, F. J. M., das Neves Silva, J. L., Ferreira, K., & Faro, A. Revisão bibliográfica de antibióticos beta-lactâmicos. Revista Saúde em Foco, 2019. [Google Scholar]
- Cornaglia, G. , Giamarellou, H., & Rossolini, G. M. Metallo-β-lactamases: a last frontier for β-lactams? Lancet Infect. Dis, v. 11, n. 5, p. 381-393, 2011. [CrossRef]
- Queenan, A. M. , & Bush, K. Carbapenemases: the versatile β-lactamases. Clin Microbiol Rev, v. 20, n. 3, p. 440-458, 2007. [CrossRef]
- Nogueira, H. S. , de Oliveira Xavier, A. R. E., de Sousa Xavier, M. A., Carvalho, A. A., Monção, G. A., & Barreto, N. A. P. Antibacterianos: principais classes, mecanismos de ação e resistência. Unimontes Cient. v. 18, n. 2, p. 96-108, 2016.
- Monge, K. M. M. (2013). Carbapenémicos: tipos y mecanismos de resistência bacterianos. Rev. méd. Costa Rica Centroam. 70(608), 599-605.
- Medeiros, V. M. , do Nascimento, Y. M., Souto, A. L., Madeiro, S. A. L., de Oliveira Costa, V. C., Silva, S. M. P., Silva, V. S. F., Agra, M. F., Siqueira-Júnior J. P. & Tavares, J. F. Chemical composition and modulation of bacterial drug resistance of the essential oil from leaves of Croton grewioides. Microb. Pathog, v. 111, p. 468-471, 2017. [CrossRef]
- Coutinho, H. D. M. , Matias, E. F. F., Santos, K. K. A., Tintino, S. R., Souza, C. E. S., Guedes, G. M. M., Santos F.A.D, Costa J.G.M., Falcão-Silva V.S. & Siqueira-Júnior, J. P. Enhancement of the norfloxacin antibiotic activity by gaseous contact with the essential oil of Croton zehntneri. JYP, v. 2, n. 4, p. 362-364, 2010. [CrossRef]
- Nicolson, K. , Evans, G., & O'Toole, P. W. Potentiation of methicillin activity against methicillin-resistant Staphylococcus aureus by diterpenes. FEMS Microbiol. Lett. v. 179, n. 2, p. 233-239, 1999. [CrossRef]
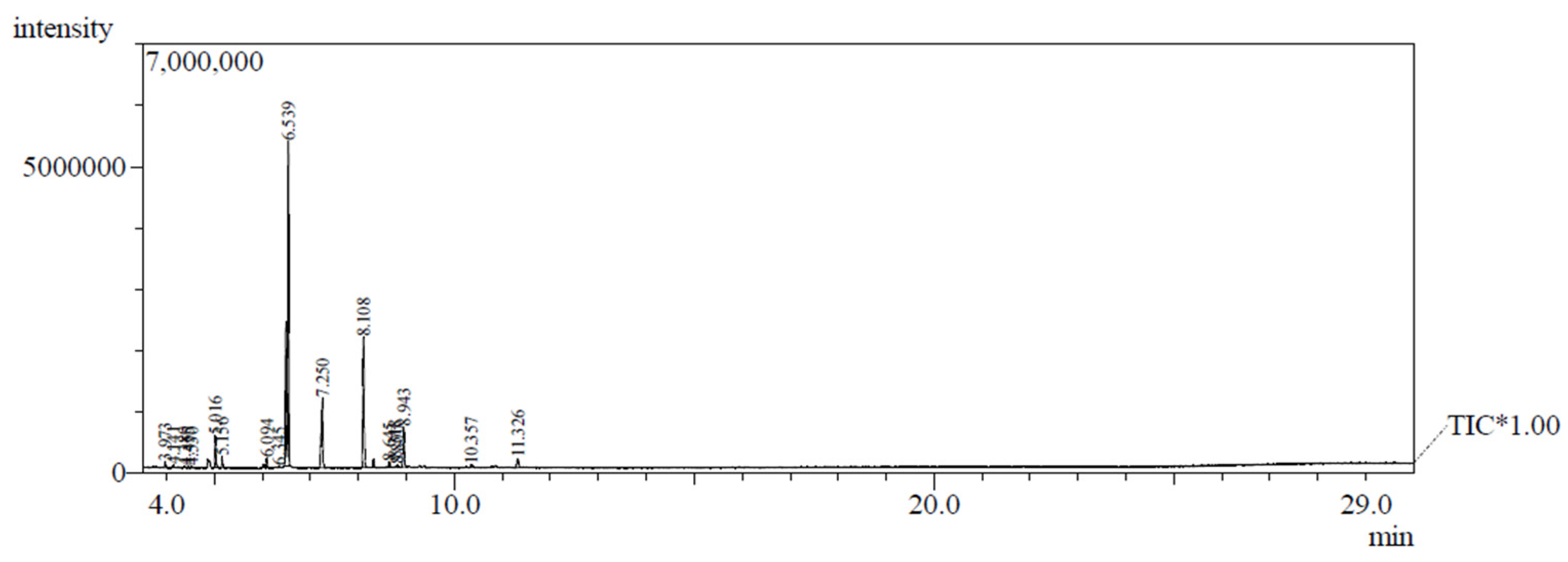
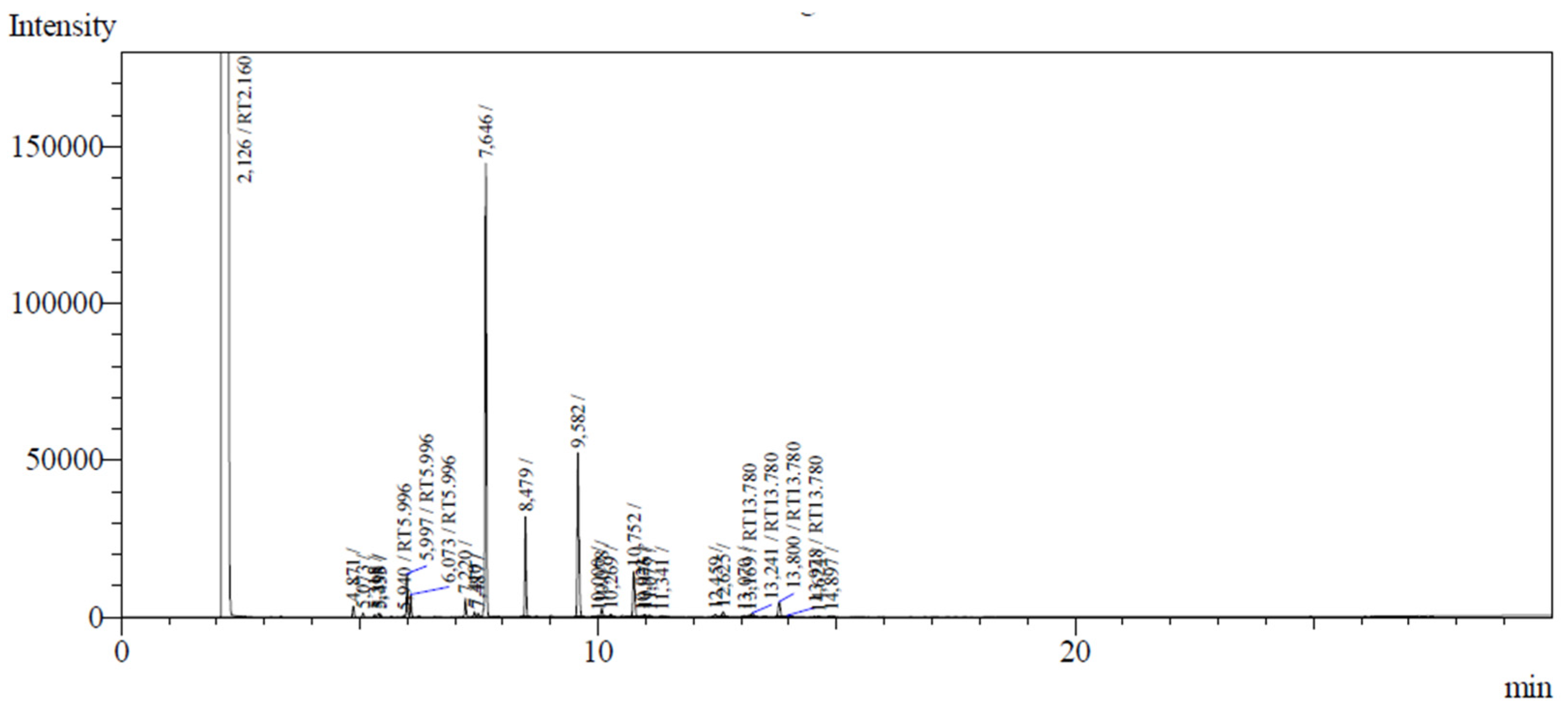
| Compounds | (%) | RT (min) | IR1 | IR2 |
| estragole | 50.34 | 6.53 | 1.246 | 1.226 |
| methyl eugenol | 19.39 | 8.10 | 1.339 | 1.359 |
| anisole | 10.99 | 7.25 | 1.283 | 1.264 |
| methyl-trans-isoeugenol | 6.09 | 8.94 | 1.403 | 1.422 |
| 1,8-cineole | 4.2 | 5.01 | 1.156 | 1.164 |
| α-selinene | 1.97 | 11.32 | 1.628 | 1.648 |
| β-elemene | 1.37 | 8.31 | 1.355 | 1.375 |
| β-ocimene | 1.09 | 5.15 | 1.165 | 1.145 |
| camphor | 1.08 | 6.09 | 1.223 | 1.243 |
| α-pinene | 0.82 | 3.97 | 1.070 | 1.050 |
| trans-caryphyllene | 0.81 | 8.64 | 1.380 | 1.400 |
| caryphyllene oxide | 0.42 | 10.35 | 1.553 | 1.572 |
| camphene | 0.36 | 4.14 | 1.090 | 1.097 |
| β-myrcene | 0.29 | 4.53 | 1.122 | 1.142 |
| 2-β-pinene | 0.25 | 4.45 | 1.116 | 1.119 |
| isoledene | 0.25 | 8.80 | 1.391 | 1.373 |
| isoborneol | 0.17 | 6.34 | 1.236 | 1.255 |
| sabinense | 0.11 | 4.38 | 1.111 | 1.123 |
| Total | 100 % |
| Organisms | MIC (µg/mL) | |
| Multidrug-resistant strains | SA 10 | ≥1024 |
| EC 06 | ≥1024 | |
| KPC | 512 | |
| KP BLSE | 256 | |
| Standard strains | EC 25922 | ≥1024 |
| SA 29213 | ≥1024 | |
| KP ICU | 512 |
| Substance | Organisms | MIC of antibiotics (µg/mL) | |||
| Amoxicillin | Penicillin | Gentamicin | Amikacin | ||
| Control | SA 10 | 40 | 8 | 8 | 40 |
| EC 06 | 40 | 256 | 8 | 40 | |
| KP BLSE | 256 | 1024 | 1024 | 32 | |
| KPC | 645 | 1024 | 812 | 512 | |
| KP ICU | 101 | 406 | 203 | 161 | |
| SA 29213 | 256 | 256 | 128 | 256 | |
| EC 25922 | 256 | 512 | 203 | 128 | |
| OECG test | SA 10 | 6 | 0.5 | 0.5 | 2 |
| EC 06 | 161 | 0.5 | 0.5 | 3 | |
| KP BLSE | 256 | 1024 | 256 | 161 | |
| KPC | 1024 | 0.5 | 1024 | 512 | |
| KP ICU | 16 | 8 | 0.5 | 4 | |
| SA 29213 | 6 | 40 | 0.25 | 0.30 | |
| EC 25922 | 203 | 512 | 0.30 | 20 | |
| FRC | SA 10 | 85 % | 93.75 % | 93.75 % | 95 % |
| EC 06 | - 302.5 % | 99.80 % | 93.75 % | 92.5 % | |
| KP BLSE | 0 % | 0 % | 75 % | - 403.12 % | |
| KPC | - 58.76 % | 99.95 % | - 26.10 % | 0 % | |
| KP ICU | 84.16 % | 98.02 % | 99.75 % | 97.51 % | |
| SA 29213 | 97.65 % | 84.37 % | 99.80 % | 99.88 % | |
| EC 25922 | 20.7 % | 0 % | 99.85 % | 84.37 % | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





