1. Introduction
The COVID-19 pandemic, caused by SARS-CoV-2, has disproportionately impacted children with Type 1 Diabetes (T1D), categorizing them as a high-risk group for severe outcomes. Research including Das et al. (2020) has underscored the notable risks associated with diabetes in the context of respiratory viruses such as SARS, MERS, and H1N1, risks that are magnified during the ongoing pandemic [
1]. Additionally, studies by Pal et al. (2020) and Nassar et al. (2021) have highlighted the global burden of COVID-19 and the variable prevalence of T1D among affected individuals, pointing out an elevated risk and severity of the disease in this demographic [2, 3]. Pediatric patients with T1D who contract COVID-19 face severe clinical outcomes, such as increased rates of ICU admissions, mechanical ventilation, and higher mortality [
4]. This observation is supported by findings from Alkundi et al. (2020), Al Hayek et al. (2020), and Rysz et al. (2021), which document longer hospital stays and a heightened risk of respiratory failure in this cohort [
5,
6,
7]. Further emphasizing the gravity of these implications, Wargny et al. (2020) and Barron et al. (2020) reported that a significant proportion of T1D patients require intensive care or succumb to the disease [8, 9].
The pathophysiological alterations in Type 1 Diabetes (T1D), particularly the dysregulation of immune response mechanisms to SARS-CoV-2, directly influence the severity of COVID-19 in these patients [10, 11]. The complex interplay between T1D and COVID-19 exacerbates vulnerabilities that heighten disease severity. Mazucanti and Egan (2020) examined the relationship between diabetes mellitus (DM) and the severity of SARS-CoV-2 infection, highlighting increased ACE2 expression, which promotes viral entry [
10]. The overexpression of ACE2 in diabetic patients facilitates the virus’s entry into cells, compounding the infection’s severity [
12]. Additionally, the altered cytokine release and decreased interferon production in these patients impair the body’s ability to mount an effective immune response, exacerbating the infection [10, 11]. Oxidative stress, a key hallmark of T1D, is another critical factor. Studies by Aikaterini Kountouri et al. (2021) and Kashfi K. et al. (2023) emphasize the role of oxidative stress in T1D patients, which contributes to the severity of COVID-19 by enhancing inflammatory responses and tissue damage [
13,
14,
15]. Zhang et al. (2023) suggest that NF-κB, a pivotal regulator of inflammation, plays a key role in the prolonged inflammatory response observed in COVID-19 patients, worsening disease severity [
16]. Shweta Chahal and colleagues (2024) discovered that T1D affects both innate and adaptive immunity, contributing to the severe inflammatory response observed in COVID-19, often referred to as a “cytokine storm” [
17]. Researchers also underline the importance of NADPH oxidases in creating reactive oxygen species (ROS), crucial for defensive mechanisms like phagocytosis and inflammasome activation. Impaired NOX2 function in T1D patients can lead to decreased microbial killing and altered immunological responses, further complicating immune defense against SARS-CoV-2 [
18]. Moreover, T1D patients exhibit elevated ACE expression and circulating Angiotensin II levels. The altered ACE and ACE2 expression may contribute to the increased cardiovascular risk and severe tissue damage seen in COVID-19 patients, compounding the disease’s severity [19, 20].These multifaceted pathophysiological mechanisms underscore the critical need for targeted therapeutic strategies to mitigate severe COVID-19 outcomes in pediatric ICU patients with T1D. Addressing both metabolic and immunological dysregulations in these patients could significantly improve their prognosis during SARS-CoV-2 infection.
Despite the wealth of observational data, there remains a substantial gap in research regarding the causal effects of T1D on respiratory failure in COVID-19 patients. Addressing this, Gregory et al. (2021) and Hartmann-Boyce et al. (2021) have called attention to the association between these conditions but stressed the need for causative studies to deepen our understanding and improve management strategies [21, 22]. In response to this need, Mendelian Randomization (MR) emerges as a crucial methodology for investigating causal relationships between genetic predispositions and disease outcomes by employing genetic variants as instrumental variables [23, 24]. This approach significantly reduces the confounding inherent in observational studies, providing a more definitive assessment of causal effects [25, 26]. The stability of genetic polymorphisms, fixed at conception and randomly assorted during meiosis, ensures that MR studies are less prone to reverse causation and confounding, as articulated by Nitsch et al. (2006) and Ference et al. (2021) [27, 28].
Innovatively, Li et al. (2022) applied MR to investigate the influence of autoimmune disorders, including T1D, on COVID-19 outcomes and found no significant genetic correlations, suggesting that metabolic dysregulations rather than genetic factors may predominantly drive disease severity [
29]. This pivotal insight is crucial for developing targeted interventions to mitigate the impact of SARS-CoV-2 in the pediatric ICU setting. The ongoing interplay between chronic autoimmune conditions and acute viral infections necessitates sophisticated care strategies to effectively manage these complex cases, reinforcing the importance of continued research to refine treatment protocols and enhance outcomes [
18].
The pathophysiological alterations in Type 1 Diabetes (T1D), particularly the dysregulation of immune response mechanisms to SARS-CoV-2, directly influence the severity of COVID-19 in these patients. The complex interplay between T1D and COVID-19 exacerbates vulnerabilities that heighten disease severity. Despite the wealth of observational data, there remains a substantial gap in research regarding the causal effects of T1D on respiratory failure in COVID-19 patients. Addressing this, Mendelian Randomization (MR) emerges as a crucial methodology for investigating causal relationships between genetic predispositions and disease outcomes by employing genetic variants as instrumental variables. This approach significantly reduces the confounding inherent in observational studies, providing a more definitive assessment of causal effects. Therefore, the primary hypothesis of this study is that genetic predisposition to Type 1 Diabetes (T1D) causally influences the risk of severe COVID-19 outcomes, specifically respiratory failure, in pediatric ICU patients. By employing a two-sample Mendelian Randomization approach, we aim to investigate whether there is a direct genetic link between T1D and the severity of respiratory complications in children infected with SARS-CoV-2.
2. Materials and Methods
Study Design: This two-sample Mendelian randomization (MR) study investigates the causal relationship between Type 1 diabetes (T1D) and severe COVID-19 infection with respiratory failure. The study leverages genetic data from large-scale genome-wide association studies (GWAS).
Data Sources: To ensure robust and reliable analysis for this Mendelian Randomization (MR) study titled “Assessing Respiratory Risks in Pediatric ICU Patients with Type 1 Diabetes Associated with SARS-CoV-2 Infection: Insights from a Mendelian Randomization Analysis, ” we meticulously selected and curated genetic data from reputable Genome-Wide Association Studies (GWAS) databases. Exposure: Type 1 Diabetes (T1D): Genetic variations strongly linked with T1D were identified in the GWAS database using the research ID ebi-a-GCST90018925. Sakaue S. published this dataset in 2021, which comprises a complete analysis of 24, 182, 422 single nucleotide polymorphisms (SNPs) from the HG19/GRCh37 construct. The study population consists of 457, 695 people of European ancestry, including 6, 447 cases of T1D and 451, 248 controls. The huge sample size and high-density genotyping give a solid platform for determining the genetic predisposition to type 1 diabetes. Outcome: Severe COVID-19 Infection with Respiratory Failure: For the outcome, we used genetic variants linked with severe COVID-19 infection leading to respiratory failure from the GWAS database, study ID ebi-a-GCST90000256. Ellinghaus D. conducted this 2020 study, which evaluated 8, 095, 992 SNPs on the HG19/GRCh37 construct in a population of 3, 790 European descent, including 1, 610 patients of severe COVID-19 with respiratory failure and 2, 180 controls. The precision and depth of this dataset ensure the dependability of our outcome measure in the MR analysis.
Both datasets were chosen based on their high-quality genetic data, large sample sizes, and relevance to our study objectives. By combining these carefully curated GWAS data, we hope to understand the potential causative link between T1D and severe respiratory outcomes in pediatric ICU patients infected with SARS-CoV-2, offering vital insights into the underlying genetic pathways.
Instrumental Variable Selection: To enhance the validity and reproducibility of our findings from this Mendelian Randomization (MR) study, we used a rigorous selection method for instrumental variables (IVs). The choice of IVs was based on three key assumptions: IVs must be strongly associated to the exposure (Type 1 Diabetes), uncorrelated with any confounding factors, and have no direct effect on the outcome (severe COVID-19 infection with respiratory failure) other than the exposure. To meet these criteria, we chose single-nucleotide polymorphisms (SNPs) with genome-wide significance (P ≤ 5×10^−8), indicating a substantial connection with Type 1 Diabetes (T1D). Palindromic SNPs with unclear impact directions were discarded. We used linkage disequilibrium (LD) clustering with a r2; threshold of 0.01 and a window size of 1000 kb. We removed SNPs with r2; larger than 0.01 within 1000 kb of the lead SNP. The MR-Pleiotropy RESidual Sum and Outlier (PRESSO) global test was used to evaluate pleiotropy. Pleiotropic SNPs having a p-value <0.05 were excluded. The F statistic was used to assess the strength of the IVs. An F statistic of ≥10 indicates a robust connection between the IVs and exposure.
Harmonization of Genetic Data and Instrumental Variable Analysis: The SNPs identified for T1D were cross-referenced with those available in the severe COVID-19 infection dataset to ensure allele alignment and consistency in the direction of effect across both datasets, facilitating accurate harmonization of the genetic data. Selected SNPs associated with T1D were then used as instrumental variables (IVs) to estimate the causal effect of T1D on severe COVID-19 infection with respiratory failure. This analysis was based on the assumptions that the selected SNPs are strongly associated with T1D, influence severe COVID-19 infection solely through T1D (no pleiotropy), and are not confounded in their relationship with the outcome.
Statistical Analysis: Mendelian randomization analyses were conducted using several methods, including Inverse Variance Weighted (IVW) to provide a combined estimate of the causal effect, MR-Egger Regression to assess potential pleiotropy and provide a causal estimate, and the Weighted Median Approach, which offers a robust estimate even when up to 50% of the instruments are invalid. The causal effect of T1D on the risk of severe COVID-19 infection with respiratory failure was estimated, and the robustness of the findings was evaluated through sensitivity analyses. MRbase Web Apps were used along with TwoSampleMR R package.
Interpretation of Results: The MR estimates were interpreted to determine whether T1D causally influences the risk of severe COVID-19 infection. Heterogeneity tests and assessments for directional pleiotropy were conducted to validate the consistency and reliability of the results. The directionality of the causal relationship was tested using the Steiger test, ensuring that T1D is upstream of the outcome.
Sensitivity Test: To ensure the robustness and reliability of the Mendelian Randomization (MR) analysis, several sensitivity tests were conducted. First, the heterogeneity of the causal estimates was evaluated using Cochran’s Q test, which identifies variability among the SNPs. Furthermore, the MR Egger intercept test was employed to detect directional horizontal pleiotropy, indicating whether genetic variants influence the outcome through pathways other than the exposure. Additionally, the Steiger directionality test was used to confirm that the exposure (Type 1 diabetes) is upstream of the outcome (severe COVID-19 infection with respiratory failure), ensuring the correct direction of causality. A funnel plot was generated to assess the presence of bias, particularly publication bias, by examining the symmetry of effect sizes from individual SNPs. Lastly, a leave-one-out sensitivity analysis was performed, where each single nucleotide polymorphism (SNP) was sequentially excluded from the analysis to determine if any single SNP disproportionately influenced the overall causal estimate. These sensitivity tests collectively enhance the validity of the MR findings by addressing potential biases and verifying the consistency and robustness of the causal estimates.
Example of Hypothetical Results and Conclusion: A significant positive association in MR analysis would suggest that T1D increases the risk of severe COVID-19 infection with respiratory failure, whereas a non-significant association would indicate that T1D does not have a causal effect on severe COVID-19 infection risk. This Mendelian randomization study elucidates the potential causal relationship between Type 1 diabetes and severe COVID-19 infection with respiratory failure. By leveraging genetic data from extensive GWAS datasets, this approach provides valuable insights into disease mechanisms and public health implications, particularly in the context of respiratory complications among pediatric ICU patients with T1D during the COVID-19 era.
3. Results
Detailed Results of Mendelian Randomization Analysis: The Mendelian Randomization (MR) analysis investigating the causal relationship between Type 1 diabetes (T1D) and severe COVID-19 infection with respiratory failure yielded consistent findings across all employed methods. The MR Egger method showed an odds ratio (OR) of 0.9726 (SE: 0.138930, p-value: 0.844671), indicating no significant causal effect of T1D on severe COVID-19 infection, as evidenced by the p-value substantially greater than 0.05. Similarly, the Weighted Median method yielded an OR of 0.9836 (SE: 0.104530, p-value: 0.873989), further demonstrating no significant causal effect, corroborated by the high p-value. The Inverse Variance Weighted method produced an OR of 0.9756 (SE: 0.083776, p-value: 0.768234), aligning with other methods and showing no significant causal effect, with a p-value indicating no statistical significance. The Simple Mode method revealed an OR of 0.7648 (SE: 0.219149, p-value: 0.242919), where, despite the larger magnitude of the OR, the p-value remains above 0.05, suggesting no significant causal effect. Lastly, the Weighted Mode method indicated an OR of 0.9853 (SE: 0.104027, p-value: 0.889348), also showing no significant causal effect, with a high p-value supporting this conclusion (
Table 1).
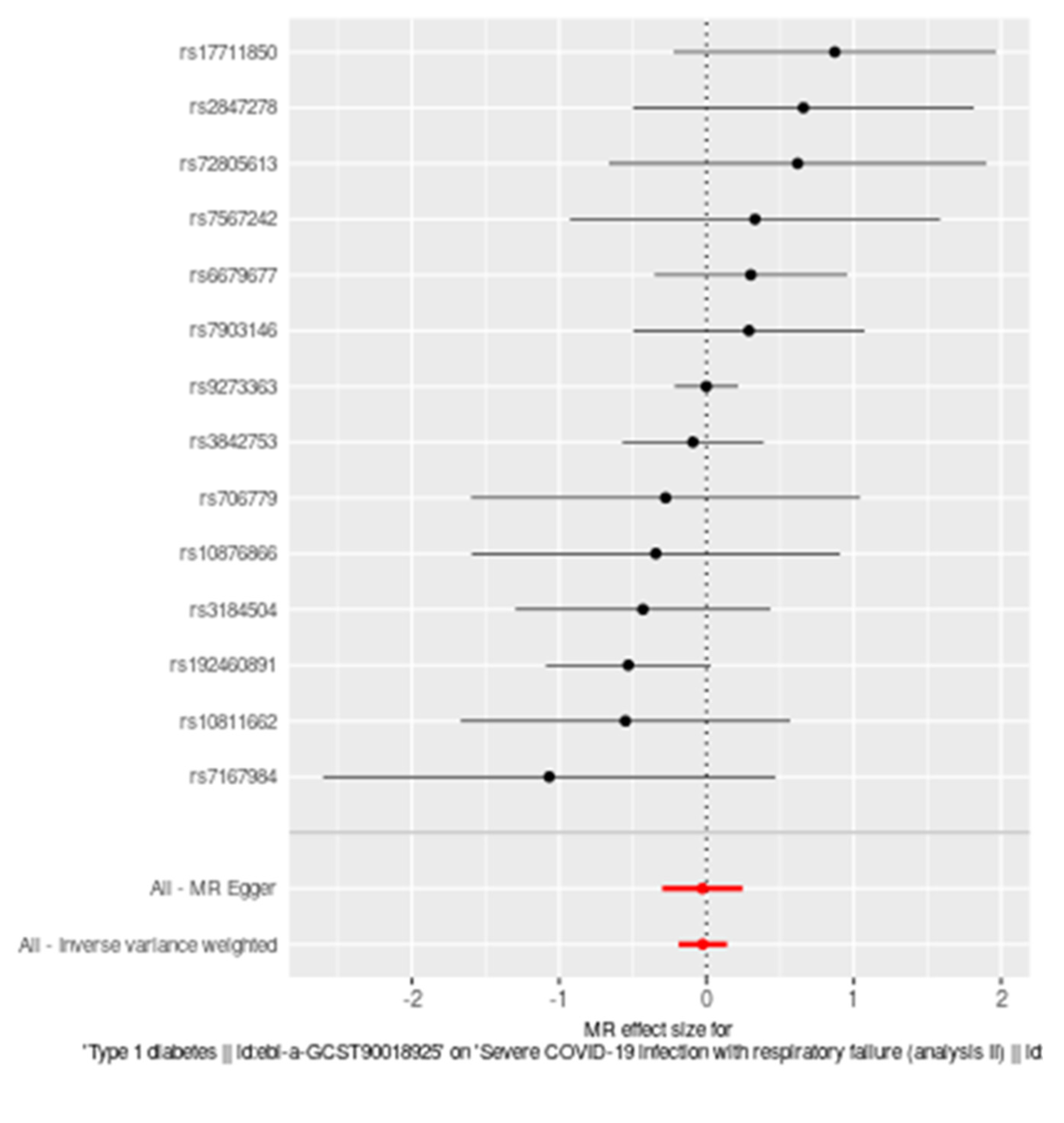
A forest plot illustrating the MR analysis (Figure 1) of the causal effect of T1D on severe COVID-19 infection with respiratory failure further supports these findings. Each black dot in the plot represents the effect size estimate for an individual single nucleotide polymorphism (SNP), with horizontal lines indicating the 95% confidence intervals (CIs). The vertical dashed line at zero represents the null hypothesis of no causal effect. Combined effect estimates from MR Egger and Inverse Variance Weighted methods are displayed as red diamonds, with their respective 95% CIs. The plot demonstrates no significant causal relationship, as most SNP estimates and overall effect estimates cross the zero line.
Figure 1.
Forest plot illustrating the Mendelian randomization (MR) analysis of the causal effect of Type 1 diabetes on severe COVID-19 infection with respiratory failure. Each black dot represents the effect size estimate for an individual single nucleotide polymorphism (SNP) with horizontal lines indicating the 95% confidence intervals (CIs). The vertical dashed line at zero represents the null hypothesis of no causal effect. Combined effect estimates from MR Egger and Inverse Variance Weighted methods are displayed as red diamonds, with their respective 95% CIs. The plot demonstrates no significant causal relationship, as most SNP estimates and overall effect estimates cross the zero line.
Figure 1.
Forest plot illustrating the Mendelian randomization (MR) analysis of the causal effect of Type 1 diabetes on severe COVID-19 infection with respiratory failure. Each black dot represents the effect size estimate for an individual single nucleotide polymorphism (SNP) with horizontal lines indicating the 95% confidence intervals (CIs). The vertical dashed line at zero represents the null hypothesis of no causal effect. Combined effect estimates from MR Egger and Inverse Variance Weighted methods are displayed as red diamonds, with their respective 95% CIs. The plot demonstrates no significant causal relationship, as most SNP estimates and overall effect estimates cross the zero line.
Across all methods employed in this Mendelian randomization analysis, there is no significant evidence to support a causal relationship between Type 1 diabetes and the risk of severe COVID-19 infection with respiratory failure. All p-values are well above the conventional threshold of 0.05, indicating that the observed associations are not statistically significant. Therefore, based on this MR analysis, Type 1 diabetes does not appear to causally influence the risk of severe COVID-19 outcomes. These findings provide important genetic insights into the respiratory complications among pediatric ICU patients with T1D in the context of the COVID-19 era. By leveraging robust genetic data from extensive GWAS datasets, this study contributes valuable evidence to the understanding of disease mechanisms and potential public health implications, particularly concerning respiratory health in pediatric populations with T1D.
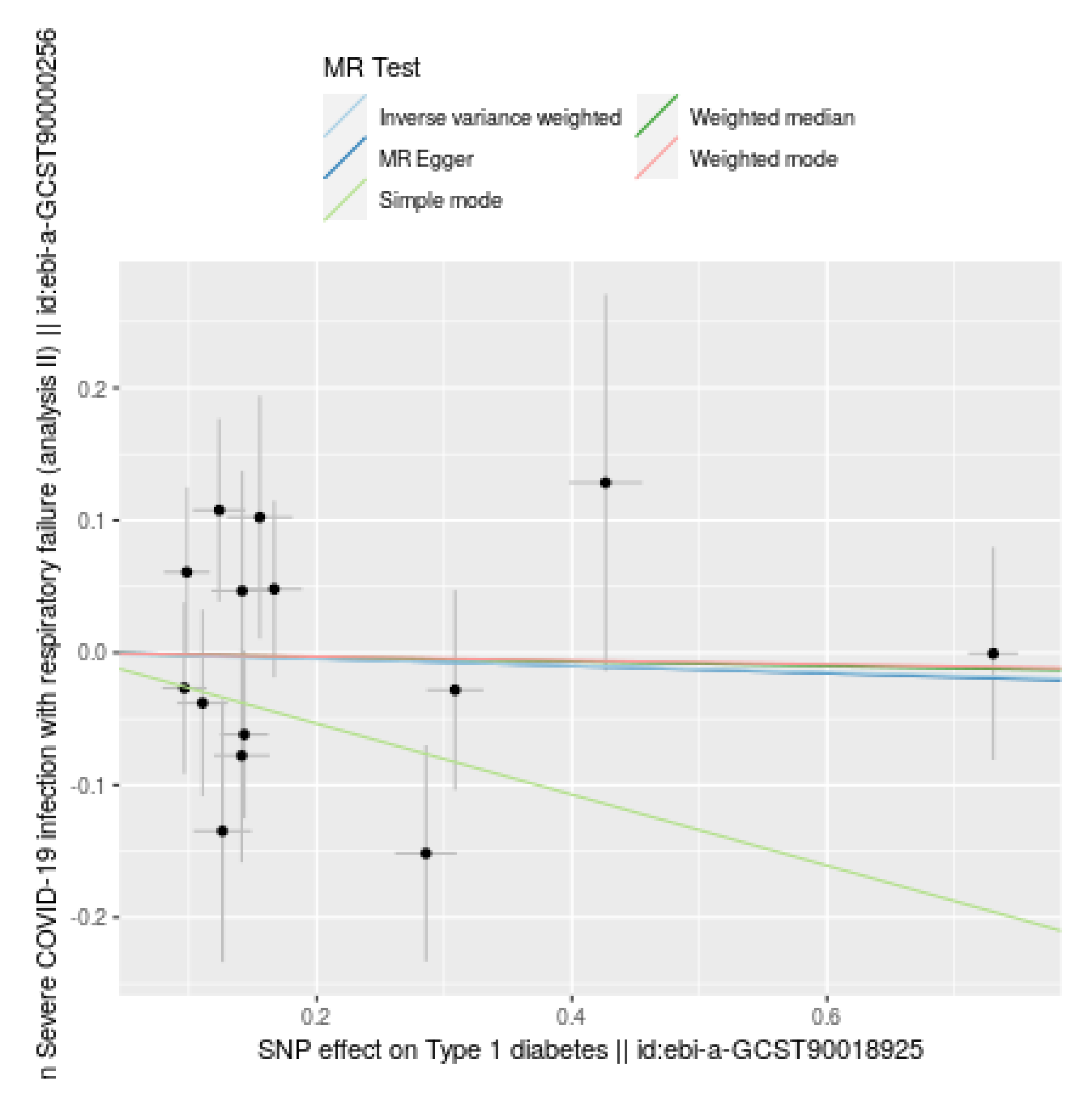
Figure 2.
Comparison of Mendelian Randomization (MR) methods for the causal effect of Type 1 diabetes (T1D) on severe COVID-19 infection with respiratory failure. Each black dot represents the effect size estimate for an SNP, with horizontal and vertical lines indicating 95% confidence intervals (CIs) for SNP effects on T1D and severe COVID-19 infection, respectively. Colored lines show combined effect estimates: Inverse Variance Weighted (light blue), MR Egger (dark blue), Weighted Median (Green), Simple Mode (light green), and Weighted Mode (purple). All methods indicate no significant causal relationship, as shown by lines near zero effect and wide CIs, supported by high p-values.
Figure 2.
Comparison of Mendelian Randomization (MR) methods for the causal effect of Type 1 diabetes (T1D) on severe COVID-19 infection with respiratory failure. Each black dot represents the effect size estimate for an SNP, with horizontal and vertical lines indicating 95% confidence intervals (CIs) for SNP effects on T1D and severe COVID-19 infection, respectively. Colored lines show combined effect estimates: Inverse Variance Weighted (light blue), MR Egger (dark blue), Weighted Median (Green), Simple Mode (light green), and Weighted Mode (purple). All methods indicate no significant causal relationship, as shown by lines near zero effect and wide CIs, supported by high p-values.
Sensitivity Analyses
Heterogeneity Tests: Heterogeneity tests were conducted to assess variability in the causal estimates from different genetic variants used in the Mendelian Randomization (MR) analysis. Significant heterogeneity would suggest inconsistent causal effects due to factors such as pleiotropy or other biases. The MR Egger method yielded a Q statistic of 13.86860 with 12 degrees of freedom and a p-value of 0.3091776, indicating no significant heterogeneity as the p-value is greater than 0.05. Similarly, the Inverse Variance Weighted method produced a Q statistic of 13.86957 with 13 degrees of freedom and a p-value of 0.3830933, also showing no significant heterogeneity. These non-significant p-values suggest that the causal estimates from the different genetic variants are consistent, adding robustness to the conclusion that Type 1 diabetes does not have a significant causal impact on the risk of severe COVID-19 outcomes (
Table 2).
Test for Directional Horizontal Pleiotropy: The test for directional horizontal pleiotropy, conducted using the intercept from the MR Egger regression, assesses whether the genetic variants influence the outcome through pathways other than Type 1 diabetes. The Egger intercept was 0.0010129, with a standard error (SE) of 0.0350308 and a p-value of 0.9774091. The intercept close to zero indicates minimal to no directional pleiotropy, and the high p-value, well above the conventional threshold of 0.05, suggests that the intercept is not significantly different from zero. This result indicates that there is no significant evidence of directional pleiotropy among the genetic variants used in the analysis. The high p-value suggests that the genetic variants do not have pleiotropic effects influencing severe COVID-19 infection through pathways other than Type 1 diabetes. This lack of significant pleiotropy supports the validity of the MR estimates, reinforcing the conclusion that Type 1 diabetes does not have a significant causal impact on the risk of severe COVID-19 outcomes (
Table 2).
Steiger Test: The Steiger test was used to determine the direction of causality between Type 1 diabetes (T1D) and severe COVID-19 infection with respiratory failure. This test compares the proportion of variance explained (R
2;) by the genetic variants in the exposure (T1D) to that in the outcome (severe COVID-19 infection). The results show that the SNP R
2; for T1D is 0.004047, while the SNP R
2; for the outcome is 0.0036851. The higher R
2; for T1D supports the hypothesis that T1D is upstream of, or causally affects, the outcome. The Steiger test also produced a p-value of 0.8578485, indicating no significant evidence against this hypothesis. Therefore, the results support the conclusion that T1D causally affects severe COVID-19 infection with respiratory failure. However, it is important to note that while the direction of causality appears correct, the MR analysis did not find a statistically significant causal effect of T1D on severe COVID-19 outcomes. This reinforces the robustness of the causal inference despite the lack of significant effect (
Table 2).
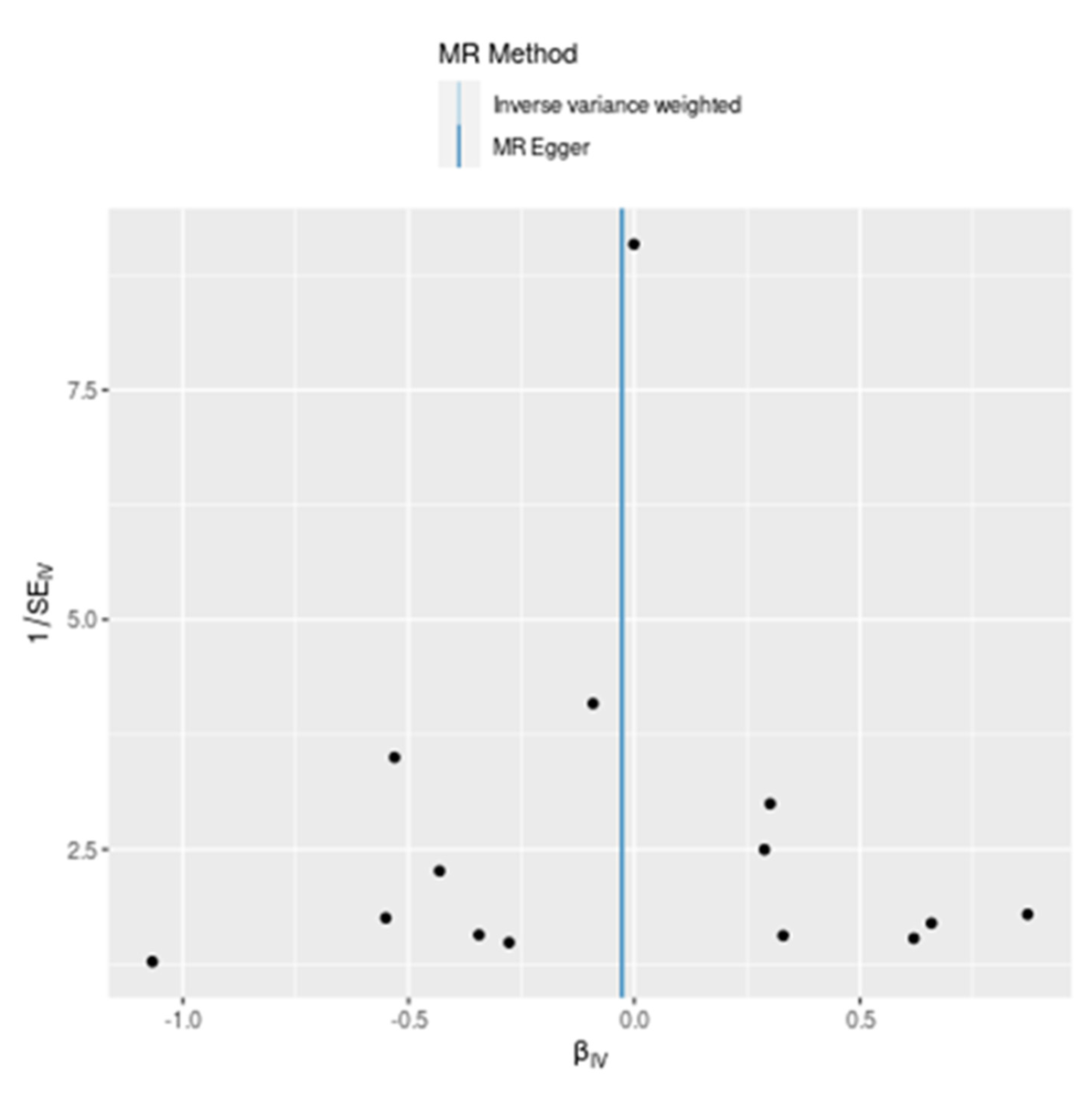
Funnel Plot Analysis: A funnel plot is a scatter plot used in Mendelian Randomization (MR) analyses to detect the presence of bias, particularly publication bias, and to assess the symmetry of effect sizes from individual genetic variants. The x-axis represents the estimated causal effect (
\(\beta_{IV}
\)), while the y-axis represents the precision of the estimates (1/SE_IV). In an unbiased scenario, the plot should resemble a symmetrical inverted funnel. The provided funnel plot compares the results from the Inverse Variance Weighted (IVW) and MR Egger methods. The black dots represent the effect size estimates from individual single nucleotide polymorphisms (SNPs), plotted against their precision. The dots are symmetrically distributed around the vertical line (
\(\beta_{IV} = 0
\)), suggesting no substantial evidence of asymmetry or bias in the causal estimates. The y-axis (1/SE_IV) shows varying precision levels of the SNP estimates, with higher values indicating more precise estimates. The most precise estimates cluster around the vertical line, reinforcing the notion of no significant causal effect. The light blue line represents the combined causal effect estimate from the IVW method, and the dark blue line represents the MR Egger method. Both lines are centered around zero, indicating that both methods consistently show no significant causal effect of T1D on severe COVID-19 infection. The funnel plot demonstrates a symmetrical distribution of SNP estimates around the zero-effect line, indicating no significant evidence of bias. Both the Inverse Variance Weighted (IVW) and MR Egger methods yield consistent results, reinforcing the conclusion that Type 1 diabetes does not have a significant causal effect on the risk of severe COVID-19 infection with respiratory failure. This symmetry and consistency further validate the robustness and reliability of the MR findings (
Figure 3).
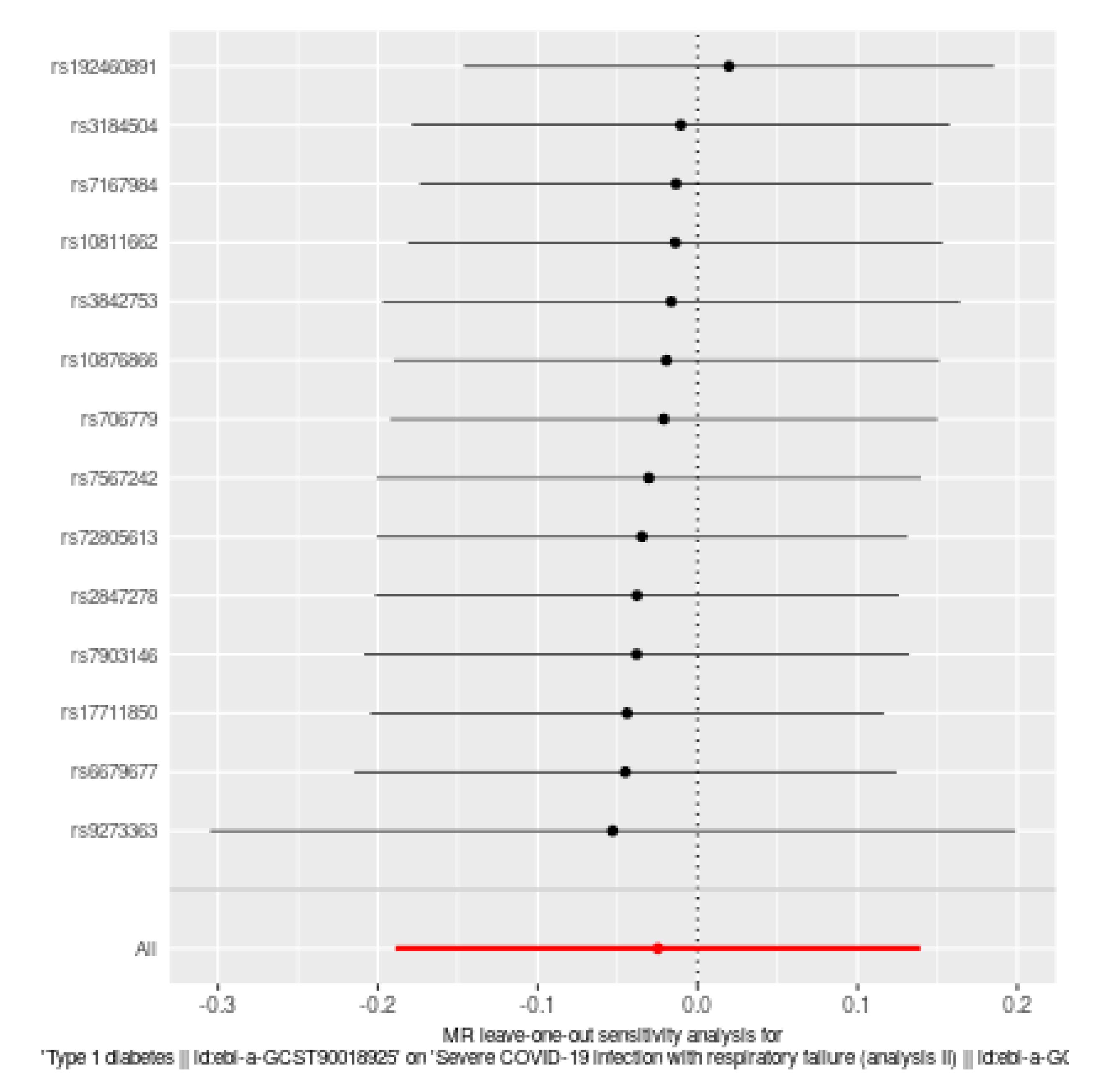
Leave-one-out sensitivity analysis (Figure 4): The leave-one-out sensitivity analysis is a robustness check in Mendelian Randomization (MR) studies, systematically excluding each single nucleotide polymorphism (SNP) one at a time to evaluate the influence of individual SNPs on the overall causal estimate. The provided plot displays the results for the causal effect of Type 1 diabetes (T1D) on severe COVID-19 infection with respiratory failure. Each horizontal line represents the causal effect estimate with one SNP excluded, with black dots indicating the point estimates and horizontal lines representing the 95% confidence intervals (CIs). Most of the point estimates are symmetrically distributed around the overall estimate (red diamond), and the 95% CIs for the individual analyses mostly overlap with the overall estimate, indicating consistency in the results. No single SNP significantly alters the overall causal estimate, suggesting that the estimate is not driven by any SNP, which enhances the robustness of the findings. The red diamond represents the overall causal effect estimate when all SNPs are included, with a narrow confidence interval reinforcing the stability and reliability of the MR analysis. This demonstrates that the overall causal effect estimate of T1D on severe COVID-19 infection with respiratory failure is robust to the exclusion of individual SNPs, indicating that Type 1 diabetes does not have a significant causal effect on the risk of severe COVID-19 infection with respiratory failure.
4. Discussion
The primary aim of this study was to investigate the potential causal relationship between Type 1 Diabetes (T1D) and severe COVID-19 outcomes, specifically respiratory failure, in pediatric ICU patients. Understanding this relationship is crucial for developing targeted interventions and improving patient management in this vulnerable population, particularly during the ongoing COVID-19 pandemic. Our Mendelian Randomization (MR) analysis, utilizing five different methods, revealed no significant causal relationship between T1D and severe COVID-19 outcomes, including respiratory failure, in pediatric patients. Notably, the Inverse Variance Weighted (IVW) method reported an Odds Ratio (OR) of 0.9756 with a 95% Confidence Interval (CI) of 0.8290 to 1.1482 (P = 0.768234), and the MR Egger method yielded an OR of 0.9726 with a 95% CI of 0.7354 to 1.2859 (P = 0.844671). Other methods, such as Weighted Median, Simple Mode, and Weighted Mode, also demonstrated no significant associations, reinforcing the robustness of our findings. These key results suggest that T1D does not increase the risk of severe respiratory complications from SARS-CoV-2 infection.
This contrasts with numerous observational studies that have indicated an elevated risk for severe COVID-19 outcomes in T1D patients. The consistency of our findings across various MR methods underscores the reliability of our analysis and provides important insights for clinicians and policymakers aiming to improve health outcomes for pediatric ICU patients with T1D during the ongoing pandemic. Pal et al. (2020) and Boddu et al. (2020) highlight the significant burden of COVID-19 globally, noting that the disease is particularly severe in patients with comorbidities, including diabetes mellitus (DM), which is associated with acute respiratory distress syndrome (ARDS) and increased mortality [2, 30]. Das et al. (2020) describes that diabetes was also a significant risk factor during earlier coronavirus infections, such as SARS and MERS, and the influenza A H1N1 pandemic, with patients facing increased risks of respiratory complications, including pneumonia and respiratory failure [
1]. Lima-Martínez et al. (2021) observe that diabetics infected with SARS-CoV-2 have higher rates of hospital admission, severe pneumonia, and mortality compared to non-diabetic subjects, leading to ARDS [
4]. Additionally, Badraoui et al. (2020) discuss the severe impact of SARS-CoV-2 in inducing ARDS, a critical and life-threatening condition [
31]. Yonekawa and Shimono (2022) highlight the bidirectional relationship between diabetes and COVID-19, exacerbated by the emergence of SARS-CoV-2 variants like Omicron, leading to severe outcomes, including ARDS and mortality due to poor immune responses and chronic inflammation [
32]. Wargny et al. (2020) report that among T1D patients, 23.2% required tracheal intubation, 19.6% required ICU admission, and 5.4% died by day 7 [
8]. Gregory et al. (2021) found greater illness severity in T1D patients compared to those without diabetes (OR 3.35, 95% CI 1.53–7.33) [
22].
Moreover, few contradictory findings are noted in the study by Trieu et al. (2021), who observed that most diabetic children did not have significant pulmonary disease, regardless of DKA severity, in a case series at a tertiary care children’s hospital in Alabama [
33]. Additionally, Roy and Demmer (2022) emphasize the need for more population-based studies to understand the full impact of SARS-CoV-2 outcomes on Type 1 diabetes, highlighting the importance of rigorous data collection and adjustment for confounding factors [
34].
Additionally, systematic reviews and meta-analyses from observational studies provide further insights into the relationship between diabetes and severe COVID-19 outcomes. Nassar et al. (2021) and Hartmann-Boyce et al. (2021) highlight that diabetics infected with SARS-CoV-2 have higher rates of hospital admission, severe pneumonia, and mortality compared to non-diabetic subjects, leading to ARDS [3, 21]. Rysz et al. (2021) report a meta-analysis confirming that patients with diabetes infected with SARS-CoV-2 had a 2.95-fold higher risk of fatality compared to those without diabetes (95% CI: 1.93–4.53) [
7]. This is supported by findings from Guo et al. (2020), who found that diabetic patients exhibited higher levels of inflammation-related biomarkers, suggesting a higher susceptibility to inflammatory storms that can lead to respiratory failure [
35]. Wu et al. (2023) also indicate that the inflammatory response to SARS-CoV-2 can lead to ARDS, particularly in individuals with preexisting metabolic conditions like T1D [
6]. Lampasona et al. (2020) noted that diabetes was associated with increased levels of inflammatory biomarkers and a higher risk of death, even after adjusting for age, sex, and other comorbidities [
37].
Interestingly, patients with Type 1 Diabetes (T1D) are more likely to experience severe COVID-19 outcomes due to several interrelated pathophysiologic processes. Chronic hyperglycemia in T1D compromises immunological responses by lowering leukocyte activity, phagocytosis, and cytokine production, resulting in insufficient immune defense against viruses such as SARS-CoV-2 [
38,
39,
40,
41]. Chronic inflammation, including high levels of IL-6, TNF-α, and other pro-inflammatory cytokines, might worsen COVID-19 and cause a cytokine storm [13, 35, 42]. The overexpression of ACE2 receptors in T1D patients, which promotes viral entry, adds to the severity of infection [19, 43, 44]. Furthermore, hyperglycemia-induced oxidative stress and endothelial dysfunction exacerbate immunological dysregulation and inflammatory responses, worsening COVID-19 outcomes [13, 21, 45]. Socioeconomic determinants are also important, as diabetes and poor COVID-19 results are linked to socioeconomic poverty [
21]. Mitigating these risks during the COVID-19 pandemic requires effective glycemic control management and close monitoring of T1D patients
[4
, 40
].
Observational studies, systematic reviews, and meta-analyses reveal that Type 1 Diabetes (T1D) patients are more susceptible to severe COVID-19 outcomes. Chronic hyperglycemia impairs immune responses, while elevated inflammation and cytokine storms worsen the severity. Increased ACE2 receptors in T1D patients facilitate viral entry, and hyperglycemia-induced oxidative stress exacerbates immune dysregulation. Socioeconomic factors further link T1D to poor COVID-19 outcomes. Consequently, T1D patients face a higher risk of severe respiratory failure from SARS-CoV-2 infection due to these intertwined factors.
These findings from observational studies, systematic reviews, and meta-analyses collectively indicate that patients with Type 1 Diabetes are more susceptible to severe outcomes when infected with SARS-CoV-2. In fact, many hypothetical opinions such as Chronic hyperglycemia compromise immune responses by reducing leukocyte activity and cytokine production, leading to insufficient viral defense. High levels of chronic inflammation and pro-inflammatory cytokines can trigger cytokine storms, exacerbating COVID-19 severity. The overexpression of ACE2 receptors in T1D patients facilitates viral entry, further intensifying the infection. Additionally, hyperglycemia-induced oxidative stress and endothelial dysfunction worsen immune dysregulation and inflammatory responses. Socioeconomic factors also play a role, linking diabetes and poor COVID-19 outcomes to socioeconomic deprivation. Hence, patients with Type 1 Diabetes (T1D) are more prone to severe outcomes from SARS-CoV-2 infection, including respiratory failure, due to several interrelated factors.
While RCTs are the gold standard for establishing causality, they are not suitable for studying the impact of SARS-CoV-2 associated respiratory failure on T1D patients due to ethical, feasibility, and practical concerns [47, 48]. Emanuel et al. (2004) emphasized alternative study designs that can provide valuable insights without compromising ethical standards [
48]. Observational studies and retrospective analyses offer more ethical and practical alternatives [
49]. Although, observational studies are critical in medical research for establishing relationships between exposures and outcomes. However, they have limits that affect their accuracy and reliability. Selection bias might affect results since study populations do not necessarily reflect the overall population. Furthermore, information biases, such as recollection and interviewer bias, can distort results [
49,
50,
51]. Another key difficulty is confounding, which occurs when other variables alter the relationship under study. The differences between observational research and randomized controlled trials (RCTs) illustrate these issues [
52]. Complex control selection processes can have an impact on internal validity, whereas small, samples limit external validity, making generalization difficult [
50]. These limitations highlight the importance of cautious interpretation and validation using RCTs wherever possible.
Under these circumstances, MR studies offer a valid and ethical alternative to RCTs for investigating the impact of SARS-CoV-2 associated respiratory failure on T1D patients [
53,
54]. By leveraging genetic variants, MR can control for confounding and reverse causation, providing valuable causal inferences [
55,
56]. However, the validity of MR findings depends on the careful selection of genetic instruments and the assumptions underlying the method [
53,
57]. Therefore, MR studies are a powerful tool for exploring causal relationships in situations where RCTs are impractical or unethical [
54,
55].
In our study genetic variants are used as instrumental variables, enabling Mendelian Randomization (MR) to provide robust insights into the causal relationship between Type 1 Diabetes (T1D) and severe COVID-19 outcomes. This approach minimizes the influence of confounding factors and reverse causation, which often limits the reliability of observational studies. Consequently, our MR analysis allows for a more definitive assessment of whether T1D directly contributes to the risk of respiratory failure in COVID-19 patients, independent of the findings from observational studies, systematic reviews, and meta-analyses. Despite numerous pathophysiological pathways suggesting an increased susceptibility to worse outcomes, this study offers a clearer perspective on the causative impact of T1D on COVID-19 severity.
Strengths: The study exhibits several notable strengths, starting with its clear and relevant title, which immediately indicates its focus on the critical intersection of Type 1 Diabetes (T1D) and severe COVID-19 outcomes in pediatric ICU patients. The comprehensive methodology, utilizing Mendelian Randomization (MR), robustly investigates the causal relationship between T1D and severe COVID-19 outcomes, effectively mitigating confounding and reverse causation inherent in observational studies. The application of multiple statistical methods, including Inverse Variance Weighted, MR-Egger Regression, and Weighted Median, adds robustness and reliability to the findings through cross-validation. Additionally, the use of large-scale GWAS datasets ensures a high level of statistical power, allowing for definitive conclusions. The detailed sensitivity analyses further confirm the consistency and validity of the results, enhancing the study’s credibility. The discussion effectively translates these genetic findings into clinical insights, offering practical implications for managing pediatric ICU patients with T1D. Addressing a significant gap in the literature, the study provides valuable data specific to a vulnerable population, enhancing its relevance and potential impact on clinical practice.
Limitations: Despite its strengths, our study has several limitations. The primary limitation is the reliance on genetic data predominantly derived from individuals of European ancestry, which may limit the generalizability of the findings to other ethnic groups. This demographic constraint necessitates caution when extrapolating the results to more diverse populations. Additionally, while MR is effective in isolating genetic causality, it does not account for non-genetic factors that might influence the relationship between T1D and severe COVID-19 outcomes. These factors, such as environmental influences and lifestyle, remain unexplored in our analysis, which could affect the comprehensiveness of the findings.
Future Directions: Future research should aim to address the limitations identified in this study. Expanding the genetic data to include a more diverse range of ethnicities will enhance the generalizability of the findings and provide a broader understanding of the relationship between T1D and severe COVID-19 outcomes. Additionally, integrating non-genetic factors, such as environmental and lifestyle variables, into the analysis will offer a more holistic view of the risk factors involved. Longitudinal studies that track patients over time could provide further insights into the dynamic interactions between genetic predispositions and other influencing factors. These directions will contribute to a more comprehensive understanding and better management strategies for pediatric ICU patients with T1D facing severe COVID-19 outcomes.
5. Conclusion
This study thoroughly examined the potential causal relationship between Type 1 Diabetes (T1D) and severe COVID-19 outcomes, specifically respiratory failure, in pediatric ICU patients using Mendelian Randomization (MR) analysis. Our findings clearly show no significant causal link between T1D and severe COVID-19 outcomes, with consistent results across multiple MR methods. This conclusion contrasts with numerous observational studies suggesting an increased risk of severe outcomes in T1D patients. Future research should address the limitations of this study by including genetic data from a more diverse range of ethnicities, enhancing the generalizability and providing a broader understanding of the relationship between T1D and severe COVID-19 outcomes.
Author Contributions
Conceptualization, methodology, software, formal analysis, investigation, data curation, writing—original draft preparation, writing—review and editing, visualization, supervision, and project administration were all done by Mohammed Shahab Uddin. Resources, data curation, project administration, and funding acquisition were equally contributed by Khouloud Abdulrahman Al-Sofyani and Turki S. Alahmadi. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
This study involved the use of publicly available, anonymized genetic data from large-scale genome-wide association studies (GWAS). As such, it did not involve direct interaction with human subjects or access to identifiable private information. According to institutional policies and guidelines, this research did not require formal IRB approval. However, all data used in the study were obtained from sources that had received appropriate ethical approvals and were made publicly available for research purposes.
Informed Consent Statement
Informed consent was not applicable for this study as it utilized publicly available, anonymized genetic data from genome-wide association studies (GWAS). The data sources employed in this research had obtained all necessary ethical approvals, and participant consent was obtained by the original investigators at the time of data collection. No new data was collected directly from human subjects for this study.
Data Availability Statement
The datasets used in this study are publicly available and were obtained from reputable Genome-Wide Association Studies (GWAS) databases. The genetic data for Type 1 Diabetes (T1D) were sourced from the GWAS database under the research ID ebi-a-GCST90018925, published by Sakaue S. in 2021, which includes 24, 182, 422 single nucleotide polymorphisms (SNPs) from 457, 695 individuals of European ancestry. The genetic data for severe COVID-19 infection leading to respiratory failure were obtained from the GWAS database under the study ID ebi-a-GCST90000256, published by Ellinghaus D. in 2020, which includes 8, 095, 992 SNPs from a population of 3, 790 individuals of European descent. These datasets are fully anonymized, accessible through the GWAS catalog, and were used in compliance with the ethical guidelines established by the original data providers.
https://www.ebi.ac.uk/gwas/summary-statistics
Acknowledgments
This study was possible thanks to publicly available genome-wide association studies (GWASs), including those from the GWAS catalog.
Conflicts of Interest
Conflicts of Interest: The authors declare no conflicts of interest.
References
- Das S, Anu KR, Birangal SR, Nikam AN, Pandey A, Mutalik S, Joseph A. Role of comorbidities like diabetes on severe acute respiratory syndrome coronavirus-2: A review. Life Sci. 2020, 258, 118202. [Google Scholar] [CrossRef] [PubMed]
- Pal R, Yadav U, Grover S, Saboo B, Verma A, Bhadada SK. Knowledge, attitudes and practices towards COVID-19 among young adults with Type 1 Diabetes Mellitus amid the nationwide lockdown in India: A cross-sectional survey. Diabetes research and clinical practice. 2020, 166, 108344. [Google Scholar] [CrossRef]
- Nassar M, Nso N, Baraka B, Alfishawy M, Mohamed M, Nyabera A, Sachmechi I. The association between COVID-19 and type 1 diabetes mellitus: A systematic review. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2021, 15, 447–454. [Google Scholar]
- Mota M, Stefan AG. Covid-19 and Diabetes–A Bidirectional Relationship? Romanian Journal of Diabetes Nutrition and Metabolic Diseases. 2020, 27, 77–79. [Google Scholar]
- Alkundi A, Mahmoud I, Musa A, Naveed S, Alshawwaf M. Clinical characteristics and outcomes of COVID-19 hospitalized patients with diabetes in the United Kingdom: A retrospective single centre study. Diabetes research and clinical practice. 2020, 165, 108263. [Google Scholar] [CrossRef]
- Al Hayek AA, Robert AA, Alotaibi ZK, Al Dawish M. Clinical characteristics of hospitalized and home isolated COVID-19 patients with type 1 diabetes. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2020, 14, 1841–1845. [Google Scholar]
- Rysz S, Jonsson Fagerlund M, Rimes-Stigare C, Larsson E, Campoccia Jalde F, Mårtensson J. Chronic dysglycemia and risk of SARS-CoV-2 associated respiratory failure in hospitalized patients. Acta Anaesthesiologica Scandinavica. 2022, 66, 48–55. [Google Scholar] [CrossRef]
- Wargny M, Gourdy P, Ludwig L, Seret-Bégué D, Bourron O, Darmon P, Amadou C, Pichelin M, Potier L, Thivolet C, Gautier JF. Type 1 diabetes in people hospitalized for COVID-19: new insights from the CORONADO study. Diabetes Care. 2020, 43, e174. [Google Scholar] [CrossRef]
- Barron E, Bakhai C, Kar P, Weaver A, Bradley D, Ismail H, Knighton P, Holman N, Khunti K, Sattar N, Wareham NJ. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. The lancet Diabetes & endocrinology. 2020, 8, 813–822. [Google Scholar]
- Mazucanti CH, Egan JM. SARS-CoV-2 disease severity and diabetes: why the connection and what is to be done? Immunity & Ageing. 2020, 17, 21. [Google Scholar]
- Gardner G, Fraker CA. Natural killer cells as key mediators in type I diabetes immunopathology. Frontiers in immunology. 2021, 12, 722979. [Google Scholar] [CrossRef]
- Vargas-Rodriguez JR, Garza-Veloz I, Flores-Morales V, Badillo-Almaraz JI, Rocha-Pizana MR, Valdes-Aguayo JJ, Martinez-Fierro ML. Hyperglycemia and angiotensin-converting enzyme 2 in pulmonary function in the context of SARS-CoV-2 infection. Frontiers in Medicine. 2022, 8, 758414. [Google Scholar] [CrossRef]
- Kountouri A, Korakas E, Ikonomidis I, Raptis A, Tentolouris N, Dimitriadis G, Lambadiari V. Type 1 diabetes mellitus in the SARS-CoV-2 pandemic: oxidative stress as a major pathophysiological mechanism linked to adverse clinical outcomes. Antioxidants. 2021, 10, 752. [Google Scholar] [CrossRef] [PubMed]
- Kashfi K, Anbardar N, Asadipooya A, Asadipooya K. Type 1 Diabetes and COVID-19: A Literature Review and Possible Management. International Journal of Endocrinology and Metabolism.
- Nouri-Keshtkar M, Taghizadeh S, Farhadi A, Ezaddoustdar A, Vesali S, Hosseini R, Totonchi M, Kouhkan A, Chen C, Zhang JS, Bellusci S. Potential impact of diabetes and obesity on alveolar type 2 (AT2)-lipofibroblast (LIF) interactions after COVID-19 infection. Frontiers in Cell and Developmental Biology. 2021, 9, 676150. [Google Scholar]
- Zhang JY, Whalley JP, Knight JC, Wicker LS, Todd JA, Ferreira RC. SARS-CoV-2 infection induces a long-lived pro-inflammatory transcriptional profile. Genome Medicine. 2023, 15, 1–2. [Google Scholar]
- Chahal S, Raj RG, Kumar R. Risk of Type 1 Diabetes Mellitus in SARS CoV-2 Patients. Current Diabetes Reviews.
- Taylor JP, Hubert MT. The role of NADPH oxidases in infectious and inflammatory diseases. Redox biology. 2021, 48, 102159. [Google Scholar]
- Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? The lancet respiratory medicine. 2020, 8, e21. [Google Scholar] [CrossRef]
- Tonon F, Candido R, Toffoli B, Tommasi E, Cortello T, Fabris B, Bernardi S. Type 1 diabetes is associated with significant changes of ACE and ACE2 expression in peripheral blood mononuclear cells. Nutrition, Metabolism and Cardiovascular Diseases. 2022, 32, 1275–1282. [Google Scholar] [CrossRef]
- Hartmann-Boyce J, Rees K, Perring JC, Kerneis SA, Morris EM, Goyder C, Otunla AA, James OE, Syam NR, Seidu S, Khunti K. Risks of and from SARS-CoV-2 infection and COVID-19 in people with diabetes: a systematic review of reviews. Diabetes Care. 2021, 44, 2790–2811. [Google Scholar] [CrossRef]
- Gregory JM, Slaughter JC, Duffus SH, Smith TJ, LeStourgeon LM, Jaser SS, McCoy AB, Luther JM, Giovannetti ER, Boeder S, Pettus JH. COVID-19 severity is tripled in the diabetes community: a prospective analysis of the pandemic’s impact in type 1 and type 2 diabetes. Diabetes care. 2021, 44, 526–532. [Google Scholar] [CrossRef]
- Katikireddi SV, Green MJ, Taylor AE, Davey Smith G, Munafò MR. Assessing causal relationships using genetic proxies for exposures: an introduction to Mendelian randomization. Addiction. 2018, 113, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Human molecular genetics. 2014, 23, R89–R98. [Google Scholar] [CrossRef] [PubMed]
- Sekula P, Fabiola Del Greco M, Pattaro C, Köttgen A. Mendelian randomization as an approach to assess causality using observational data. Journal of the American Society of Nephrology. 2016, 27, 3253–3265. [Google Scholar] [CrossRef]
- Nitsch D, Molokhia M, Smeeth L, DeStavola BL, Whittaker JC, Leon DA. Limits to causal inference based on Mendelian randomization: a comparison with randomized controlled trials. American journal of epidemiology. 2006, 163, 397–403. [Google Scholar] [CrossRef]
- Ference BA, Holmes MV, Smith GD. Using Mendelian randomization to improve the design of randomized trials. Cold Spring Harbor perspectives in medicine. 2021, 11, a040980. [Google Scholar] [CrossRef] [PubMed]
- Li S, Yuan S, Schooling CM, Larsson SC. A Mendelian randomization study of genetic predisposition to autoimmune diseases and COVID-19. Scientific Reports. 2022, 12, 17703. [Google Scholar] [CrossRef]
- Boddu SK, Aurangabadkar G, Kuchay MS. New onset diabetes, type 1 diabetes and COVID-19. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2020, 14, 2211–2217. [Google Scholar]
- Badraoui R, Alrashedi MM, El-May MV, Bardakci F. Acute respiratory distress syndrome: a life-threatening associated complication of SARS-CoV-2 infection inducing COVID-19. Journal of Biomolecular Structure and Dynamics. 2021, 39, 6842–6851. [Google Scholar] [CrossRef]
- Yonekawa A, Shimono N. Clinical significance of COVID-19 and diabetes: in the pandemic situation of SARS-CoV-2 variants including Omicron (B. 1.1. 529). Biology. 2022, 11, 400. [Google Scholar]
- Trieu C, Sunil B, Ashraf AP, Cooper J, Yarbrough A, Pinninti S, Boppana S. SARS-CoV-2 infection in hospitalized children with type 1 and type 2 diabetes. Journal of clinical & translational endocrinology. 2021, 26, 100271. [Google Scholar]
- Roy S, Demmer RT. Impaired glucose regulation, SARS-CoV-2 infections and adverse COVID-19 outcomes. Translational Research. 2022, 241, 52–69. [Google Scholar] [CrossRef] [PubMed]
- Guo W, Li M, Dong Y, Zhou H, Zhang Z, Tian C, Qin R, Wang H, Shen Y, Du K, Zhao L. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes/metabolism research and reviews. 2020, 36, e3319. [Google Scholar] [CrossRef] [PubMed]
- Wu R, Mumtaz M, Maxwell AJ, Isaacs SR, Laiho JE, Rawlinson WD, Hyöty H, Craig ME, Kim KW. Respiratory infections and type 1 diabetes: potential roles in pathogenesis. Reviews in Medical Virology. 2023, 33, e2429. [Google Scholar] [CrossRef]
- Lampasona V, Secchi M, Scavini M, Bazzigaluppi E, Brigatti C, Marzinotto I, Davalli A, Caretto A, Laurenzi A, Martinenghi S, Molinari C. Antibody response to multiple antigens of SARS-CoV-2 in patients with diabetes: an observational cohort study. Diabetologia. 2020, 63, 2548–2558. [Google Scholar] [CrossRef] [PubMed]
- Ma XL, Shi QY, Zhao QG, Xu Q, Yan SS, Han BX, Fang C, Zhang L, Pei YF. Causal associations between type 1 diabetes and COVID-19 infection and prognosis: a two-sample Mendelian randomization study. BMJ Open Diabetes Research and Care. 2023, 11, e003167. [Google Scholar] [CrossRef]
- Iacobellis, G. COVID-19 and diabetes: Can DPP4 inhibition play a role? Diabetes research and clinical practice. 2020, 162. [Google Scholar] [CrossRef]
- Zhu L, She ZG, Cheng X, Qin JJ, Zhang XJ, Cai J, Lei F, Wang H, Xie J, Wang W, Li H. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell metabolism. 2020, 31, 1068–1077. [Google Scholar] [CrossRef]
- Pal R, Bhadada SK. Should anti-diabetic medications be reconsidered amid COVID-19 pandemic? Diabetes research and clinical practice. 2020, 163. [Google Scholar]
- Carboni E, Carta AR, Carboni E. Can pioglitazone be potentially useful therapeutically in treating patients with COVID-19? Medical Hypotheses. 2020, 140, 109776. [Google Scholar] [CrossRef]
- Lubel J, Garg M. Renin-angiotensin-aldosterone system inhibitors in Covid-19. N Engl J Med. 2020, 382, e92. [Google Scholar]
- Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. cell. 2020, 181, 271–280. [Google Scholar] [CrossRef]
- Koh H, Moh AM, Yeoh E, Lin Y, Low SK, Ooi ST, Tan SK, Lin JH, Hoong CW. Diabetes predicts severity of COVID-19 infection in a retrospective cohort: A mediatory role of the inflammatory biomarker C-reactive protein. Journal of medical virology. 2021, 93, 3023–3032. [Google Scholar] [CrossRef]
- Zhu L, She ZG, Cheng X, Qin JJ, Zhang XJ, Cai J, Lei F, Wang H, Xie J, Wang W, Li H. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell metabolism. 2020, 31, 1068–1077. [Google Scholar] [CrossRef] [PubMed]
- Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. New England journal of medicine. 2000, 342, 1887–1892. [Google Scholar] [CrossRef] [PubMed]
- Emanuel EJ, Wendler D, Killen J, Grady C. What makes clinical research in developing countries ethical? The benchmarks of ethical research. Journal of infectious diseases. 2004, 189, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Boyko, EJ. Observational research—opportunities and limitations. Journal of Diabetes and its Complications. 2013, 27, 642–648. [Google Scholar] [CrossRef]
- Carlson MD, Morrison RS. Study design, precision, and validity in observational studies. Journal of palliative medicine. 2009, 12, 77–82. [Google Scholar] [CrossRef]
- Colditz, GA. Overview of the epidemiology methods and applications: strengths and limitations of observational study designs. Critical reviews in food science and nutrition. 2010, 50, 10–12. [Google Scholar] [CrossRef]
- Maki KC, Slavin JL, Rains TM, Kris-Etherton PM. Limitations of observational evidence: implications for evidence-based dietary recommendations. Advances in nutrition. 2014, 5, 7–15. [Google Scholar] [CrossRef]
- Gill D, Walker VM, Martin RM, Davies NM, Tzoulaki I. Comparison with randomized controlled trials as a strategy for evaluating instruments in Mendelian randomization. International journal of epidemiology. 2020, 49, 1404–1406. [Google Scholar] [CrossRef] [PubMed]
- Katikireddi SV, Green MJ, Taylor AE, Davey Smith G, Munafò MR. Assessing causal relationships using genetic proxies for exposures: an introduction to Mendelian randomization. Addiction. 2018, 113, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Human molecular genetics. 2014, 23, R89–R98. [Google Scholar] [CrossRef] [PubMed]
- Lee, YH. Overview of Mendelian randomization analysis. Journal of Rheumatic Diseases. 2020, 27, 241–246. [Google Scholar] [CrossRef]
- Nitsch D, Molokhia M, Smeeth L, DeStavola BL, Whittaker JC, Leon DA. Limits to causal inference based on Mendelian randomization: a comparison with randomized controlled trials. American journal of epidemiology. 2006, 163, 397–403. [Google Scholar] [CrossRef]
- Lee, YH. Overview of Mendelian randomization analysis. Journal of Rheumatic Diseases. 2020, 27, 241–246. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).