Submitted:
20 August 2024
Posted:
21 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Patients, Ethics and Data Retrieval
2.2. Comparative Measurement of Tumor Markers and Histological Findings before Surgery
2.3. Statistical Analysis
3. Results
3.1. Perioperative Parameters
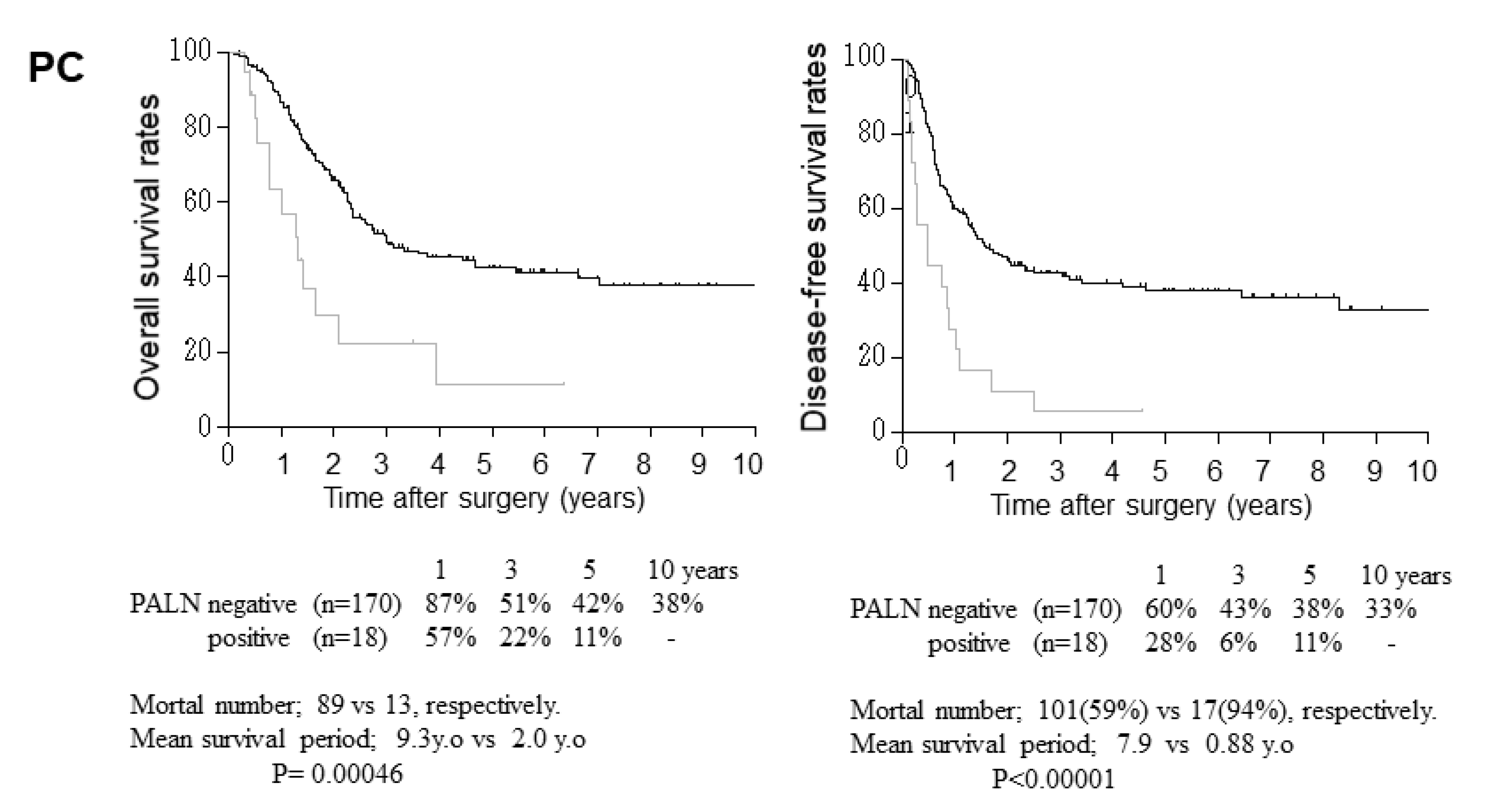
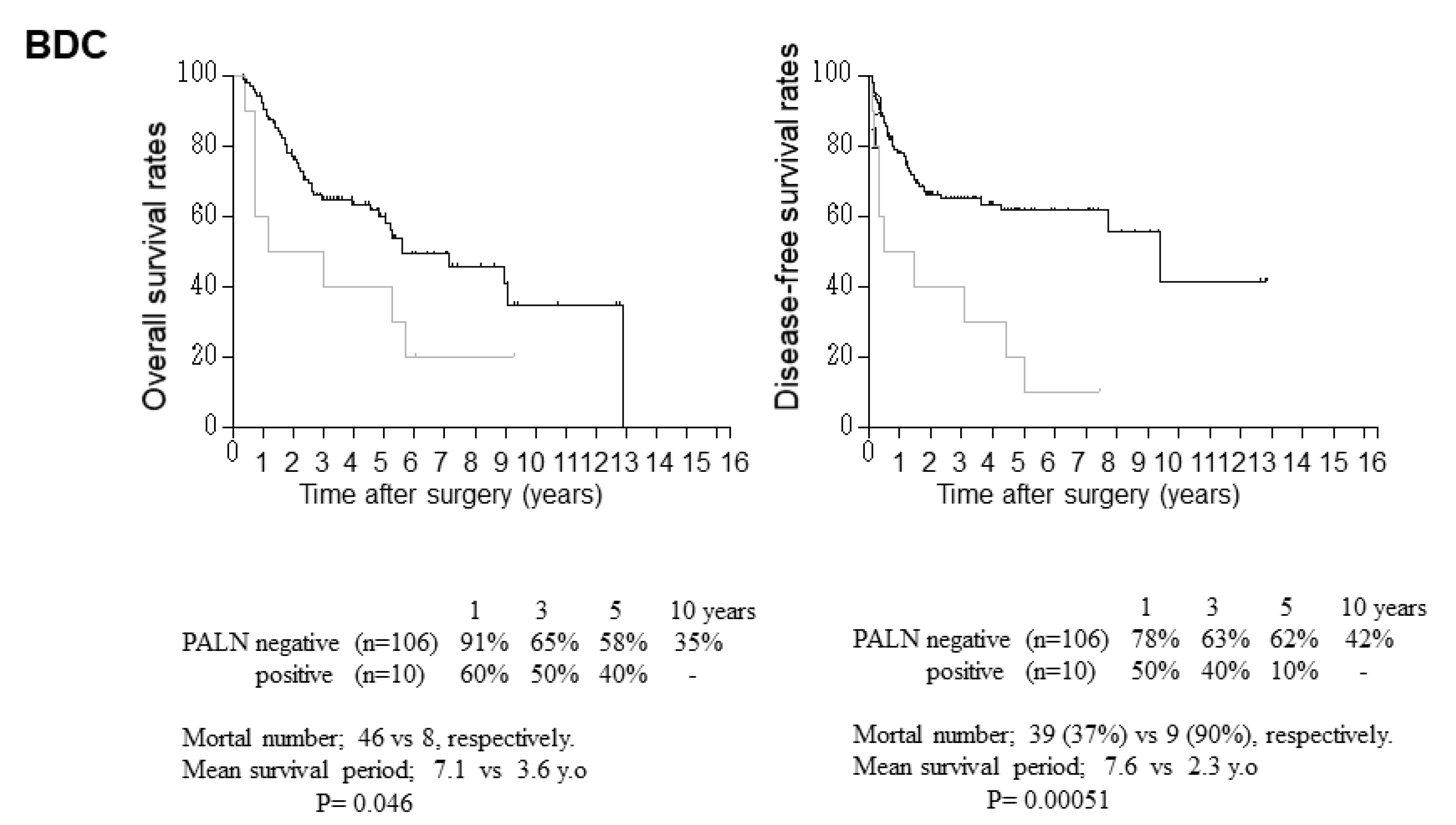
3.2. Relationship between Clinicopathological Parameters and Disease-Free and Overall Survival after Surgery
3.3. Relationship between Para-Aortic Lymph Node Metastasis and Other Clinicopathological Factors
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buanes, T.A. Role of surgery in pancreatic cancer. World J Gastroenterol. 2017, 23, 3765–3770. [Google Scholar] [CrossRef]
- Uenishi, T,; Ariizumi, S,; Aoki, T; , Ebata, T,; Ohtsuka, M,; Tanaka, E,; Yoshida, H,; Imura, S,; Ueno, M,; Kokudo, N,; Nagino, M,; Hirano, S,; Kubo, S,; Unno, M,; Shimada, M,; Yamaue, H,; Yamamoto, M,; Miyazaki, M,; Takada, T. Proposal of a new staging system for mass-forming intrahepatic cholangiocarcinoma: a multicenter analysis by the Study Group for Hepatic Surgery of the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci. 2014, 21, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Im, J,H,; Choi, G,H,; Lee, W,J,; Han, D,H,; Park, S,W,; Bang, S,; Choi, H,J,; Seong, J. Adjuvant radiotherapy and chemotherapy offer a recurrence and survival benefit in patients with resected perihilar cholangiocarcinoma. J Cancer Res Clin Oncol. 2021, 147, 2435–2445. [Google Scholar] [CrossRef] [PubMed]
- Maeta, T,; Ebata, T,; Hayashi, E,; Kawahara, T,; Mizuno, S,; Matsumoto, N,; Ohta, S,; Nagino, M,; Nagoya Surgical Oncology Group. Pancreatoduodenectomy with portal vein resection for distal cholangiocarcinoma. Br J Surg. 2017, 104, 1549–1557. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, L,; Lupinacci, R,M,; Svrcek, M,; Lesurtel, M,; Bubenheim, M,; Vuarnesson, H,; Balladur, P,; Paye, F. Para-aortic lymph node sampling in pancreatic head adenocarcinoma. Br J Surg. 2014, 101, 530–538. [Google Scholar] [CrossRef]
- Paiella, S,; Sandini, M,; Gianotti, L,; Butturini, G,; Salvia, R,; Bassi, C. The prognostic impact of para-aortic lymph node metastasis in pancreatic cancer: A systematic review and meta-analysis. Eur J Surg Oncol. 2016, 42, 616–624. [Google Scholar] [CrossRef]
- Petrova, E,; Mazzella, E,; Trojan, J,; Koch, C,; Schulze, F,; Bechstein, W,O,; Schnitzbauer, A,A. Prognostic value of paraaortic lymph node metastases in patients with ductal adenocarcinoma of the pancreatic head. Eur J Surg Oncol. 2023, 49, 996–1000. [Google Scholar] [CrossRef]
- Hempel, S,; Oehme, F,; Müssle, B,; Aust, D,E,; Distler, M,; Saeger, H,D,; Weitz, J,; Welsch, T. Prognostic impact of para-aortic lymph node metastases in non-pancreatic periampullary cancer. World J Surg Oncol. 2020, 18, 16. [Google Scholar] [CrossRef]
- Okada, K,; Uemura, K,; Kondo, N,; Sumiyoshi, T,; Seo, S,; Otsuka, H,; Serikawa, M,; Ishii, Y,; Tsuboi, T,; Murakami, Y,; Takahashi, S. Preoperative risk factors for para-aortic lymph node positivity in pancreatic cancer. Pancreatology. 2021, 21, 606–612. [Google Scholar] [CrossRef]
- Sho, M,; Murakami, Y,; Motoi, F,; Satoi, S,; Matsumoto, I,; Kawai, M,; Honda, G,; Uemura, K,; Yanagimoto, H,; Kurata, M,; Fukumoto, T,; Akahori, T,; Kinoshita, S,; Nagai, M,; Nishiwada, S,; Unno, M,; Yamaue, H,; Nakajima, Y. Postoperative prognosis of pancreatic cancer with para-aortic lymph node metastasis: a multicenter study on 822 patients. J Gastroenterol. 2015, 50, 694–702. [Google Scholar] [CrossRef]
- Doussot, A,; Bouvier, A,; Santucci, N,; Lequeu, J,B,; Cheynel, N,; Ortega-Deballon, P,; Rat, P,; Facy, O. Pancreatic ductal adenocarcinoma and paraaortic lymph nodes metastases: The accuracy of intraoperative frozen section. Pancreatology. 2019, 19, 710–715. [Google Scholar] [CrossRef]
- Paiella, S,; Malleo, G,; Maggino, L,; Bassi, C,; Salvia, R,; Butturini, G. Pancreatectomy with Para-Aortic Lymph Node Dissection for Pancreatic Head Adenocarcinoma: Pattern of Nodal Metastasis Spread and Analysis of Prognostic Factors. J Gastrointest Surg. 2015, 19, 1610–1613. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y,; Uemura, K,; Sudo, T,; Hashimoto, Y,; Nakashima, A,; Kondo, N,; Sakabe, R,; Kobayashi, H,; Sueda, T. Is para-aortic lymph node metastasis a contraindication for radical resection in biliary carcinoma? World J Surg. 2011, 35, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Nitta, N,; Ohgi, K,; Sugiura, T,; Okamura, Y,; Ito, T,; Yamamoto, Y,; Ashida, R,; Otsuka, S,; Sasaki, K,; Uesaka, K. Prognostic Impact of Paraaortic Lymph Node Metastasis in Extrahepatic Cholangiocarcinoma. World J Surg. 2021, 45, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Takagi, K,; Nagai, Y,; Umeda, Y,; Yoshida, R,; Yoshida, K,; Fuji, T,; Kumano, K,; Yasui, K,; Yagi, T,; Fujiwara, T. Prognostic Value of the Regional Lymph Node Station in Pancreatoduodenectomy for Ampullary Carcinoma. In Vivo. 2022, 36, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Asaoka, T,; Miyamoto, A,; Maeda, S,; Hama, N,; Tsujie, M,; Ikeda, M,; Sekimoto, M,; Nakamori, S. CA19-9 level determines therapeutic modality in pancreatic cancer patients with para-aortic lymph node metastasis. Hepatobiliary Pancreat Dis Int. 2018, 17, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Safi, S,A,; Rehders, A,; Haeberle, L,; Fung, S,; Lehwald, N,; Esposito, I,; Ziayee, F,; Krieg, A,; Knoefel, W,T,; Fluegen, G. Para-aortic lymph nodes and ductal adenocarcinoma of the pancreas: Distant neighbors? Surgery. 2021, 170, 1807–1814. [Google Scholar] [CrossRef]
- Kato, M,; Shimada, Y,; Tanaka, H,; Hosotani, R,; Ohshio, G,; Ishizaki, K,; Imamura, M. Characterization of six cell lines established from human pancreatic adenocarcinomas. Cancer. 1999, 85, 832–40. [Google Scholar] [CrossRef]
- Japan Pancreas Society. General rules for the study of pancreatic cancer, 7th edn, Revised and Enlarged version. In: Unno, M (eds.) Tokyo: Kanehara Co.; 2020: pp9-81 (in Japanese).
- Japanese Society of Hepato-Biliary-Pancreatic Surgery. General rules for clinical and pathological studies on cancer of the biliary tract, 7th edn. In: Sano, K (eds.) Tokyo: Kanehara Co.; 2021:pp15-74 (in Japanese).
- Ni, X,G,; Bai, X,F,; Mao, Y,L,; Shao, Y,F,; Wu, J,X,; Shan, Y,; Wang, C,F,; Wang, J,; Tian, Y,T,; Liu, Q,; Xu, D,K,; Zhao, P. The clinical value of serum CEA, CA19-9, and CA242 in the diagnosis and prognosis of pancreatic cancer. Eur J Surg Oncol. 2005, 31, 164–169. [Google Scholar] [CrossRef]
- Kurita, A,; Kodama, Y,; Nakamoto, Y,; Isoda, H,; Minamiguchi, S,; Yoshimura, K,; Kuriyama, K,; Sawai, Y,; Uza, N,; Hatano, E,; Uemoto, S,; Togashi, K,; Haga, H,; Chiba, T. Impact of EUS-FNA for preoperative para-aortic lymph node staging in patients with pancreatobiliary cancer. Gastrointest Endosc 2016, 84, 467–475. [Google Scholar] [CrossRef]
- De Gaetano, A,M,; Rufini, V,; Castaldi, P,; Gatto, A,M,; Filograna, L,; Giordano, A,; Bonomo, L. Clinical applications of (18)F-FDG PET in the management of hepatobiliary and pancreatic tumors. Abdom Imaging. 2012, 37, 983–1003. [Google Scholar] [CrossRef]
- Kim, J,S,; Hwang, H,K,; Lee, W,J,; Kang, CM. Unexpected Para-aortic Lymph Node Metastasis in Pancreatic Ductal Adenocarcinoma: a Contraindication to Resection? J Gastrointest Surg. 2020, 24, 2789–2799. [Google Scholar] [CrossRef] [PubMed]
- Kazami, Y,; Oba, A,; Ono, Y,; Sato, T,; Inoue, Y,; Saiura, A,; Takahashi, Y,; Ito, H. Intraoperative paraaortic lymph node sampling during resection for pancreatic cancer: evolving role in the modern chemotherapy era. HPB (Oxford). 2023, 25, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Lin, J,Y,; Zhang, X,M,; Kou, J,T,; Fa, H,; Zhang, X,X,; Dai, Y,; He, Q. Analysis of prognostic factors for pancreatic head cancer according to para-aortic lymph node. Cancer Med. 2016, 5, 2701–2707. [Google Scholar] [CrossRef]
- van Rijssen, L,B,; Narwade, P,; van Huijgevoort, N,C,; Tseng, D,S,; van Santvoort, H,C,; Molenaar, I,Q,; van Laarhoven, H,W,; van Eijck, C,H,; Busch, O,R,; Besselink, M,G; Dutch Pancreatic Cancer Group. Prognostic value of lymph node metastases detected during surgical exploration for pancreatic or periampullary cancer: a systematic review and meta-analysis. HPB (Oxford). 2016, 18, 559–566. [Google Scholar] [CrossRef]
- Hempel, S,; Plodeck, V,; Mierke, F,; Distler, M,; Aust, D,E,; Saeger, H,D,; Weitz, J,; Welsch, T. Para-aortic lymph node metastases in pancreatic cancer should not be considered a watershed for curative resection. Sci Rep. 2017, 7, 7688. [Google Scholar] [CrossRef] [PubMed]
- Geerinckx, B,; Teuwen, L,A,; Foo, T,; Vandamme, T,; Smith, A,; Peeters, M,; Price, T. Novel therapeutic strategies in pancreatic cancer: moving beyond cytotoxic chemotherapy. Expert Rev Anticancer Ther. 2023, 23, 1237–1249. [Google Scholar] [CrossRef]
- Hadfield, M,J,; DeCarli, K,; Bash, K,; Sun, G,; Almhanna, K. Current and Emerging Therapeutic Targets for the Treatment of Cholangiocarcinoma: An Updated Review. Int J Mol Sci. 2023, 25, 543. [Google Scholar] [CrossRef]
- Wahler, I,L,; Damanakis, A,; Große, Hokamp, N,; Bruns, C,; Schmidt, T. Therapy of Locally Advanced and Oligometastatic Pancreatic Adenocarcinoma. Cancers (Basel). 2023, 15, 5881. [Google Scholar] [CrossRef]
- Sperti, C,; Gruppo, M,; Blandamura, S,; Valmasoni, M,; Pozza, G,; Passuello, N,; Beltrame, V,; Moletta, L. Para-aortic node involvement is not an independent predictor of survival after resection for pancreatic cancer. World J Gastroenterol. 2017, 23, 4399–4406. [Google Scholar] [CrossRef]
- Hong, S,M,; Goggins, M,; Wolfgang, C,L,; Schulick, R,D,; Edil, B,H,; Cameron, J,L,; Handra-Luca, A,; Herman, J,M,; Hruban, R,H. Vascular invasion in infiltrating ductal adenocarcinoma of the pancreas can mimic pancreatic intraepithelial neoplasia: a histopathologic study of 209 cases. Am J Surg Pathol. 2012, 36, 235–241. [Google Scholar] [CrossRef]
- Chen, J,W,; Bhandari, M,; Astill, D,S,; Wilson, T,G,; Kow, L,; Brooke-Smith, M,; Toouli, J,; Padbury, R,T. Predicting patient survival after pancreaticoduodenectomy for malignancy: histopathological criteria based on perineural infiltration and lymphovascular invasion. HPB (Oxford). 2010, 12, 101–108. [Google Scholar] [CrossRef]
- Chatelain, D,; Farges, O,; Fuks, D,; Trouillet, N,; Pruvot, F,R,; Regimbeau, J,M. Assessment of pathology reports on hilar cholangiocarcinoma: the results of a nationwide, multicenter survey performed by the AFC-HC-2009 study group. J Hepatol. 2012, 56, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y,; Li, L,; Qu, C,; Liang, S,; Zeng, B,; Luo, Z. Endoscopic ultrasound-guided fine needle core biopsy for the diagnosis of pancreatic malignant lesions: a systematic review and Meta-Analysis. Sci Rep. 2016, 6, 22978. [Google Scholar] [CrossRef] [PubMed]
- Larghi, A,; Correale, L,; Ricci, R,; Abdulkader, I,; Monges, G,; Iglesias-Garcia, J,; Giovannini, M,; Attili, F,; Vitale, G,; Hassan, C,; Costamagna, G,; Rindi, G. Interobserver agreement and accuracy of preoperative endoscopic ultrasound-guided biopsy for histological grading of pancreatic cancer. Endoscopy. 2015, 47, 308–314. [Google Scholar]
- Asakura, Y,; Toyama, H,; Ishida, J,; Asari, S,; Terai, S,; Shirakawa, S,; Yamashita, H,; Shimizu, T,; Ogura, Y,; Matsumoto, I,; Gon, H,; Tsugawa, D,; Komatsu, S,; Kuramitsu, K,; Yanagimoto, H,; Kido, M,; Ajiki, T,; Fukumoto, T. Clinicopathological variables and risk factors for lung recurrence after resection of pancreatic ductal adenocarcinoma. Asian J Surg. 2023, 46, 207–212. [Google Scholar] [CrossRef]
- Agalianos, C,; Gouvas, N,; Papaparaskeva, K,; Dervenis, C. Positive para-aortic lymph nodes following pancreatectomy for pancreatic cancer. Systematic review and meta-analysis of impact on short term survival and association with clinicopathologic features. HPB (Oxford). 2016, 18, 633–641. [Google Scholar] [CrossRef]
- Birnbaum, D,J,; Viganò, L,; Russolillo, N,; Langella, S,; Ferrero, A,; Capussotti, L. Lymph node metastases in patients undergoing, surgery for a gallbladder cancer. Extension of the lymph node dissection and prognostic value of the lymph node ratio. Ann Surg Oncol. 2015, 22, 811–8. [Google Scholar] [CrossRef]
| a) Overall survival. | ||||||
|---|---|---|---|---|---|---|
| Univariate analysis | Multivariable analysis | |||||
| Probability (p-value) |
Risk ratio | 95% CI Lower - Upper |
Probability (p-value) |
Risk ratio | 95% CI Lower - Upper |
|
| Age, >70 years Sex, female CEA, >5 ng/ml CA199, >37 U/ml DUPAN-II, >150U/ml NAC, yes PD, yes Morphology, invasive Tumor size, >2cm Differentiation, moderately or poorly Histologic infiltration, yes lymphatic venous perineural Tumor involvement, yes retroperitoneal choledochal duodenal portal vein Node metastasis, yes Regional (RLN) para-aortic (PALN) Cancer positive at surgical margin, proximal bile duct exposed area Curability, R1 Adjuvant chemotherapy, yes Chemotherapy for cancer recurrence, yes |
.983 .428 .706 .004 .143 .021 .015 .004 .101 .000 .000 .000 .000 .000 .004 .000 .005 .000 .004 .022 .000 .011 .686 .770 |
.995 1.172 1.099 1.800 1.504 .459 1.711 1.489 1.485 2.837 3.289 4.582 4.036 2.145 1.653 1.781 1.879 3.325 2.447 3.874 4.966 2.208 1.085 1.063 |
.651 - 1.521 .792 - 1.735 .672 - 1.797 1.212 - 2.674 .871 - 2.597 .237 - .887 1.111 - 2.637 1.138 - 1.949 .925 - 2.383 1.768 - 4.555 2.094 - 5.166 2.365 - 8.876 2.339 - 6.964 1.400 - 3.287 1.178 - 2.321 1.363 - 2.326 1.208 - 2.922 2.162 - 5.113 1.332 - 4.493 1.212 - 12.385 2.572 - 9.591 1.197 - 4.072 .731 - 1.609 .706 - 1.599 |
.419 .003 .076 .075 .004 .028 .824 .005 .662 .259 .105 .199 .167 .234 .069 .041 .632 |
1.211 .269 1.760 .693 2.442 1.967 .824 2.841 .878 .736 1.395 1.461 1.481 1.597 3.268 2.483 1.195 |
.761 - 1.926 .114 - .641 .942 - 3.288 .462 - 1.038 1.326 - 4.499 1.077 - 3.594 .320 - 2.122 1.376 - 5.865 .489 - 1.574 .432 - 1.253 .932 - 2.088 .820 - 2.604 .849 - 2.582 .739 - 3.449 .912 - 11.704 1.036 - 5.948 .576 - 2.480 |
| b) Cancer-free survival. | ||||||
| Univariate analysis | Multivariable analysis | |||||
|
Probability (p-value) |
Risk ratio |
95% CI Lower - Upper |
Probability (p-value) |
Risk ratio |
95% CI Lower - Upper |
|
| Age, >70 years Sex, female CEA, >5 ng/ml CA199, >37 U/ml DUPAN-II, >150U/ml NAC, yes PD, yes Blood loss, >1500ml Morphology, invasive Tumor size, >2cm Differentiation, moderately or poorly Infiltration, yes lymph duct venous perineural Tumor involvement, yes retroperitoneal choledochal duodenal portal vein Node metastasis, yes Regional (RLN) para-aortic (PALN) Cancer positive at surgical margin, proximal bile duct exposed area Curability, R1 Adjuvant chemotherapy, yes Chemotherapy for cancer recurrence, yes |
.936 .279 .440 .001 .119 .051 .461 .098 .000 .540 .000 .000 .000 .000 .000 .033 .000 .007 .000 .004 .041 .000 .010 .360 .001 |
1.016 1.222 1.188 1.911 1.501 0.232 1.155 1.788 1.609 1.139 2.860 3.726 4.358 3.654 2.853 1.404 2.018 1.762 3.705 2.779 3.337 4.838 2.146 .840 1.862 |
.690 - 1.496 .850 - 1.757 .767 - 1.842 1.324 - 2.759 .901 - 2.501 0.061 - 1.020 .788 - 1.693 .897 - 2.033 1.243 - 2.082 .752 - 1.752 1.858 - 4.403 2.417 - 5.746 2.425 - 7.835 2.286 - 5.841 1.885 - 4.320 1.029 - 1.917 1.520 - 2.679 1.168 - 2.658 2.460 - 5.580 1.632 - 4.731 1.052 - 10.588 2.684 - 8.721 1.199 - 3.842 .579 - 1.219 1.283 - 2.702 |
.258 .801 .389 .005 .062 .870 .013 .970 .131 .051 .570 .110 .198 .217 .101 .749 .099 .077 |
1.279 1.078 .839 2.195 1.727 1.068 2.206 1.011 .674 1.522 1.173 1.536 1.578 2.209 2.049 1.133 .670 1.539 |
.835 - 1.960 .600 - 1.937 .562 - 1.251 1.271 - 3.789 .973 - 3.065 .486 - 2.349 1.180 - 4.121 .578 - 1.768 .404 - 1.125 .997 - 2.322 .676 - 2.036 .908 - 2.600 .788 -3.158 .627 - 7.785 .870 - 4.823 .527 - 2.440 .416 - 1.078 .954 - 2.485 |
| a) Overall survival. | ||||||
|---|---|---|---|---|---|---|
| Univariate analysis | Multivariable analysis | |||||
| Probability (p-value) |
Risk ratio |
95% CI Lower - Upper |
Probability (p-value) |
Risk ratio | 95% CI Lower - Upper |
|
| Age, >70 years Sex, female jaundice, yes Total bilirubin, >2mg/dL ALP, >400U/ml CEA, >5 ng/ml CA19-9, >37 U/ml Blood loss, >1500mL Morphology, invasive Tumor size, >2cm Differentiation, moderately or poorly Infiltration, yes lymph duct venous perineural Depth, beyond subserosa Tumor invasion liver gallbladder pancreas duodenum portal vein hepatic artery Cholangitis of bile duct, yes Node metastasis, yes Regional (RLN) para-aortic (PALN) Cancer positive at surgical margin proximal bile duct exposed area distal bile duct Curability, R1 PBMJ, yes Adjuvant chemotherapy, yes Chemotherapy for cancer recurrence, yes |
.052 .984 .115 .210 .087 .040 .005 .008 .150 .229 .056 .008 .001 .000 .000 .710 .257 .026 .416 .002 .046 .026 .003 .049 .080 .000 .348 .005 .002 .078 .000 |
.564 1.003 1.740 1.460 1.649 2.128 2.222 2.163 1.974 1.438 1.712 2.390 3.640 5.376 2.283 1.096 1.276 1.696 1.350 2.215 2.532 2.042 2.254 2.215 1.643 7.039 1.611 2.156 5.256 1.632 3.158 |
.316 - 1.006 .739 - 1.362 .874 - 3.466 .807 - 2.461 .929 - 2.927 1.034 - 4.379 1.272 - 3.883 1.220 - 3.836 .781 - 4.989 .795 - 2.603 .986 - 2.975 1.252 - 4.561 1.711 - 7.744 2.290 - 12.619 1.452 - 3.590 .676 - 1.776 .837 - 1.946 1.066 - 2.699 .655 - 2.780 1.340 - 3.660 1.017 - 6.301 1.087 - 3.835 1.310 - 3.877 1.010 - 4.614 .943 - 2.862 3.205 - 15.458 .595 - 4.361 1.259 - 3.691 1.834 - 15.068 .947 - 2.811 1.821 - 5.477 |
.660 .003 .051 .025 .025 .004 .654 .169 .864 .922 .495 .255 .049 .006 .672 .140 .000 |
1.280 3.325 2.817 4.042 5.290 7.529 1.195 2.012 1.188 1.152 1.398 1.625 6.896 18.114 .776 2.851 5.438 |
.427 - 3.839 1.103 - 10.019 .996 - 7.968 1.191 - 13.718 1.240 - 22.71 1.930 - 29.374 .548 - 2.604 .743 - 5.449 .166 - 8.506 .068 - 19.435 .535 - 3.652 .705 - 3.745 1.008 - 61.629 2.339 - 140.733 .239 - 2.517 .708 - 11.473 2.400 - 12.320 |
| b) Cancer-free survival. | ||||||
| Univariate analysis | Multivariable analysis | |||||
|
Probability (p-value) |
Risk ratio |
95% CI Lower - Upper |
Probability (p-value) |
Risk ratio |
95% CI Lower - Upper |
|
| Age, >70 years. Sex, female jaundice, yes Total bilirubin, >2mg/dL ALP, >400U/ml CEA, >5 ng/ml CA199, >37 U/ml Blood loss, >1500mL Morphology, invasive Tumor size, >2cm Differentiation, moderately or poorly Histologic infiltration, yes lymphatic venous perineural Depth, beyond subserosa Tumor invasion liver gallbladder pancreas duodenum portal vein hepatic artery Cholangitis of the proximal bile duct, yes Node metastasis, yes Regional (PLN) para-aortic (PALN) Cancer positive at surgical margin proximal bile duct exposed area distal bile duct Curability, R1 PBMJ, yes Adjuvant chemotherapy, yes Chemotherapy for cancer recurrence, yes |
.048 .563 .126 .434 .945 .105 .145 .056 .100 .118 .019 .009 .071 .001 .000 .863 .169 .007 .210 .001 .002 .008 .000 .001 .221 .000 .596 .048 .059 .249 .000 |
.544 .905 1.765 1.295 .978 1.854 1.561 1.455 2.436 1.642 1.996 2.485 2.760 4.165 2.646 .952 1.368 2.022 1.597 2.916 4.998 2.368 3.166 3.373 1.477 4.789 1.300 1.921 3.109 1.632 5.735 |
.298 - .994 .645 - 1.270 .853 - 3.652 .677 - 2.477 .524 - 1.826 .979 - 3.909 .858 - 2.838 .878 - 2.456 .844 - 7.034 .882 - 3.054 1.118 - 3.565 1.255 - 4.922 1.323 - 5.759 1.845 - 9.403 1.618 - 4.326 .545 - 1.665 .875 - 2.138 1.214 - 3.368 .769 - 3.317 1.536 - 5.535 1.813 - 13.781 1.248 - 4.491 1.769 - 5.667 1.626 - 6.999 .791 - 2.757 2.181 - 10.516 .493 - 3.425 1.003 - 3.024 .956 - 10.115 .786 - 2.530 3.147 - 10.450 |
.010 .110 .028 .350 .879 .097 .836 .321 .481 .013 .447 .242 .049 .000 |
3.662 2.027 3.352 1.704 .939 2.585 1.218 3.971 1.427 2.917 .577 2.551 2.763 12.944 |
1.370 - .3.661 1.143 - 9.831 1.560 - 8.792 .557 - 5.216 .418 - 2.109 .842 - 7.934 .190 - 7.823 .261 - 60.475 .531 - 3.836 1.258 - 6.765 .140 - 2.383 .530 - 12.260 1.005 - 7.597 4.640 - 36.104 |
| a) Univariate analysis. | ||||||||
|---|---|---|---|---|---|---|---|---|
| RLN metastasis | probability (p-value) |
PALN metastasis | probability (p-value) |
|||||
| negative (n=83) |
positive (n=98) |
negative (n=164) |
positive (n=17) |
|||||
| Age (years) | 69.3±8.8 | 67.0±9.9 | .190 | 68.5±9.4 | 64.0±9.2 | .103 | ||
| Gender, male/female | 47/36 | 44/54 | .846 | 91/73 | 9/8 | 1.0 | ||
| Operation., DP/ PD/ TP | 36/44/3 | 24/73/1 | .009 | 56/106/2 | 4/13/0 | .588 | ||
| CEA (ng/ml) | 14.6±86.5 | 7.7±24.1 | .884 | 6.8±20.5 | 50.6±189.8 | .886 | ||
| CA199 (U/ml) | 449±2280 | 557±1130 | <.001 | 406±982 | 1473±4710 | .115 | ||
| DUPAN-II (U/ml) | 543±2018 | 630±1188 | .004 | 569±1685 | 778±620 | .0012 | ||
| Neoadjuvant chemotherapy, no/yes | 78/5 | 93/4 | .810 | 92/72 | 13/4 | .056 | ||
| Morphology, Nodular/mixed/invasive/cystic/MPD |
14/1/40/26/2 | 27/1/68/2/0 | <.001 | 36/1/97/28/2 | 5/1/10/0/0 | .106 | ||
| Histological differentiation, papillary/well/moderately/poorly/other |
1/19/52/5/6 | 1/23/58/12/4 | .379 | 2/39/90/23/10 | 1/5/9/2/0 | .887 | ||
| Tumor size (cm) | 3.16±2.20 | 3.26±1.23 | .024 | 3.17±1.78 | 3.74±1.21 | .067 | ||
| Tumor infiltration, no/yes Lymphatic duct Venous Perineural |
55/28 37/46 36/47 |
19/79 8/90 10/88 |
<.001 <.001 <.001 |
73/91 45/119 43/121 |
1/16 1/16 2/15 |
.125 .126 .0047 |
||
| Extra-pancreatic involvement, no/yes Retro-pancreatic choledochal duodenal portal vein |
55/28 69/14 73/10 70/13 |
21/77 51/47 54/44 67/31 |
<.001 <.001 <.001 .022 |
75/89 113/51 116/48 126/38 |
0/17 6/11 10/7 10/7 |
<.001 .038 .436 .332 |
||
| PALN metastasis, no/yes | 81/2 | 83/15 | .0037 | - | - | - | ||
| R, 0/ 1 | 82/1 | 91/7 | .062 | 156/8 | 17/0 | <.001 | ||
| Adjuvant chemotherapy, no/yes | 48/35 | 59/39 | .864 | 92/72 | 15/2 | .021 | ||
| Cancer recurrence, no/yes | 49/34 | 15/83 | <.001 | 63/101 | 1/16 | .016 | ||
| Recurrence-free Survival (days) | 1385±472 | 472±553 | <.001 | 948±1129 | 333±416 | <.002 | ||
| Overall survival (days) | 1599±1275 | 815±802 | <.001 | 1235±1139 | 595±582 | .0028 | ||
| b) Multivariate logistic regression analysis. | ||||||||
| PLN | PALN | |||||||
|
Probability p-value |
Odds ratio | 95%CI lower | 95%CI upper |
Probability p-value |
Odds ratio | 95%CI lower | 95%CI upper | |
| Op., PD | .363 | .260 | .014 | 4.719 | ||||
| CA199, >37U/mL | .957 | 1.028 | .377 | 2.801 | ||||
| Dupan-II, >150u/mL | .598 | 1 | 1 | 1 | .023 | 4.921 | 1.243 | 19.475 |
| Morphology, invasive | .261 | 1.614 | .701 | 3.719 | ||||
| Size, >20mm | .406 | 1.637 | .511 | 5.244 | ||||
| Lymphatic invasion | .089 | 2.483 | .872 | 7.07 | ||||
| Venous invasion | .884 | .887 | .176 | 4.458 | ||||
| Perineural invasion | .104 | 3.222 | .786 | 13.217 | .141 | .2807 | .025 | 1.689 |
| Extra-pancreatic involvement Retro-pancreatic |
.080 |
2.681 |
.888 |
8.091 |
.997 |
5.760 |
.001 |
16.355 |
| Choledochal | .383 | 1.654 | .535 | 5.114 | .226 | 2.38 | .585 | 9.673 |
| Duodenal | .189 | 2.376 | .654 | 8.627 | ||||
| PALN metastasis, yes | .502 | 1.889 | .295 | 12.106 | ||||
| a) Univariate analysis. | ||||||||
|---|---|---|---|---|---|---|---|---|
| RLN metastasis | probability (p-value) |
PALN metastasis | probability (p-value) |
|||||
| negative (n=71) |
positive (n=45) |
negative (n=106) |
positive (n=10) |
|||||
| Age (years) | 69.8±10.6 | 66.4±12.3 | .092 | 69.5±10.2 | 58.2±17.3 | .027 | ||
| Gender, male/female | 55/16 | 30/15 | .286 | 79/27 | 6/4 | .454 | ||
| Operation, PD/HPD/Hepatectomy | 1944/4/23 | 2026/7/12 | .238 | 66/7/33 | 2004/4/2 | .0092 | ||
| Total bilirubin (mg/dl) | 1.49±2.40 | 1.45±1.39 | .120 | 1.51±2.14 | 1.10±0.40 | .593 | ||
| Alkaline phosphatase (U/ml) | 475±438 | 634±540 | .037 | 521±477 | 702±562 | .196 | ||
| CEA (ng/ml) | 6.0±24.3 | 3.8±3.6 | .089 | 5.3±19.8 | 3.0±2.2 | .928 | ||
| CA199 (U/ml) | 4394±33262 | 502±995 | .0019 | 3075±26940 | 324±729 | .310 | ||
| Jaundice, no/yes | 20/51 | 10/35 | .677 | 28/78 | 3/7 | .713 | ||
| PBMJ, no/yes | 69/2 | 43/2 | 1 | 103/3 | 9/1 | .809 | ||
| Morphology, Papillary/nodular/invasive/IPNB | 15/31/24/1 | 3/6/36/0 | .300 | 17/7/81/1 | 0/0/10/0/0 | .362 | ||
| Cholangitis of the proximal bile duct, no/yes | 52/19 | 31/14 | .484 | 81/25 | 6/4 | .078 | ||
| Histological differentiation, papillary/well/moderately/poorly/other |
12/31/19/9 | 2/19/19/5 | .134 | 13/43/36/14 | 1/5/3/1/0 | .535 | ||
| Tumor size (cm) | 1.56±1.55 | 2.34±1.69 | .036 | 1.72±1.57 | 3.54±1.62 | .0006 | ||
| Depth of invasion, m, fm/ss/se/si | 16/35/12/8 | 1/23/13/8 | .056 | 15/49/21/13 | 0/5/2/3 | .252 | ||
| Tumor infiltration, no/yes Lymphatic duct Venous Perineural |
37/34 31/40 30/41 |
5/40 6/39 6/39 |
<.001 .001 .002 |
41/65 37/69 35/71 |
0/10 0/10 1/9 |
.033 .052 .238 |
||
| Extra-pancreatic involvement, no/yes liver gallbladder pancreas duodenum portal vein hepatic artery |
63/8 68/3 52/19 63/8 68/3 70/1 |
38/7 43/2 19/26 3510 39/6 44/1 |
.017 .055 .0005 .196 .163 .333 |
87/19 104/2 67/39 88/18 100/6 104/2 |
6/4 6/4 3/7 10/0 7/3 10/0 |
.201 <.001 .029 .358 .011 .907 |
||
| Number of node metastasis | 0.82±4.09 | 1.94±1.56 | <.001 | 1.17±3.59 | 1.96±2.22 | .226 | ||
| PALN metastasis, no/yes | 69/2 | 37/8 | .0139 | - | - | - | ||
| Cancer positive at cutting edge, no/yes bile duct exposed area |
58/13 68/3 |
35/10 38/7 |
.578 .079 |
87/19 100/6 |
6/4 6/4 |
.202 .002 |
||
| R, 0/ 1 | 57/14 | 30/15 | .212 | 82/24 | 4/6 | .029 | ||
| Cancer recurrence, no/yes | 49/34 | 15/83 | <.001 | 67/39 | 1/9 | .00034 | ||
| Recurrence-free Survival (days) | 14195±1105 | 787±942 | <.001 | 1203±1096 | 836±941 | .171 | ||
| Overall survival (days) | 1605±1083 | 1059±943 | .0014 | 1411±1058 | 1206±1132 | .4 | ||
| b) Multivariate logistic regression analysis. | ||||||||
| RLN metastasis | PALN metastasis | |||||||
|
Probability p-value |
Odds ratio | 95%CI lower | 95%CI upper |
Probability p-value |
Odds ratio | 95%CI lower | 95%CI upper | |
| Age, >70 | .161 | .182 | .017 | 1.969 | ||||
| ALP, >400U/ml | .694 | .777 | .221 | 2.731 | ||||
| CA199, >37U/mL | .177 | 2.345 | .68 | 8.084 | ||||
| Op., PD | .286 | 4.345 | .292 | 64.649 | ||||
| Size, >20mm | .876 | 1.113 | .292 | 4.241 | .156 | 4.38 | .569 | 33.702 |
| Lymphatic invasion | .016 | 5.561 | 1.376 | 22.468 | .997 | 2.512 | .001 | 100.678 |
| Venous invasion | .361 | .487 | .104 | 2.282 | ||||
| Perineural invasion | .032 | 5.094 | 1.152 | 22.523 | ||||
| Organ involvement Liver Gallbladder Pancreas Portal vein |
.026 .002 |
4.582 7.204 |
2. 123 3.455 |
24.448 42.159 |
.290 .143 .204 |
3.325 7.843 2.788 |
.359 .497 .573 |
30.797 123.835 13.560 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





