1. Introduction
After the first use of infliximab in Crohn’s disease in 1995, the wide scale introduction of anti-tumour necrosis factor alfa (anti-TNF alfa) medications revolutionized the treatment of inflammatory bowel diseases (IBD’s) [
1].
During the last two decades an increased number of molecules were approved for the treatment of IBD with substantial increase of the costs related to therapy. The problem of increased costs and access to therapy became a preoccupation of payers and regulators but also for healthcare professionals.
There is a shift of costs from hospitalization and surgery to the cost related to biologic therapies. Anti-TNF use was the main cost driver, accounting for 64% and 31% of the total cost in CD and UC respectively, in the Netherlands, in 7 academic and 7 general hospitals [
2]. A review on biologic therapy access in CD in East European countries concluded that there is a strong correlation between the wealth of a country and the number of patients on biologics[
3].
Biosimilars are biologic medical products that are highly similar to their reference products and with no clinically meaningful differences in immunogenicity, safety or effectiveness.
The introduction of biosimilars was seen as a way to provide a lower-cost alternative to the originator ant TNF molecules and hence to increase treatment access and availability for patients [
3,
4].
Their introduction in gastroenterology was based on extrapolation, a decision to extend efficacy and safety data from an indication (usually from rheumatology) for which the biosimilar has been clinically tested to other indications for which the reference product is approved [
5].
Initially, due mainly to extrapolation, gastroenterologists were reserved on biosimilar use and adoption, as shown by an ECCO survey in 2013 which demonstrated that only a minority of IBD specialists was aware and confident about the benefits and issues generated by the use of biosimilars. It took only three years to increase the confidence in biosimilar use in gastroenterology, a new survey in 2016 demonstrating that
the originator and biosimilar were considered interchangeable by 44.4% of responders, as compared with 6% in 2013[
6]
.
However, among the patients with IBD still persists a certain lack of knowledge and confidence in biosimilars. In an Italian survey study among patients with IBD, the great majority of patients (73.9%) has not known if originators and biosimilars could be considered equivalent, or if efficacy or safety of biosimilars could be lower than those of originators[
7]. The authors conclude that substantial efforts from the scientific societies and IBD patients’ associations are required to overcome this issue [
7].
Another important concern is the nocebo effect which is defined as a negative effect of a medical treatment that is induced by patients’ expectations and is unrelated to the physiological action of the treatment itself. Offering the appropriate information about the concept of biosimilars and their advantage for increased accessibility to an effective treatment but also discussing the possibility of the nocebo effect is part of the education of the IBD patient, when therapeutic options are presented. This approach can lead to good clinical results as shown by a recent study on 210 patients with IBD that were non medically switched to a biosimilar where despite an increase of early nocebo complaints within the first 6 months after the switch, no significant changes were found in terms of clinical efficacy, biomarkers, therapeutic drug level, or anti-drug antibodies [
8].
Switching from originator molecule for patients who are in a stable clinical remission is currently acceptable by many IBD specialists and existing supported by position papers from different national associations. This switch appears as safe and as effective as treatment maintenance with the originator with no increased risk of immunogenicity [
9,
10].
Thus, the decision to start therapy with a biosimilar (naïve patients or switch) needs to be integrated with physicians’ knowledge about their patients and their disease on a case by-case basis, after extensive explanations and according to the local policies and reimbursement rules.
2. Aim
At the end of 2022 a decision of the Romanian National House of Insurance recommended the non-medical switch of patients treated with adalimumab to a biosimilar of the drug. The directive recommended that at least 50% of the existing patients treated with the originator Adalimumab to be switched in the first coming year.
We decided to evaluate a cohort of patients from two tertiary centres that had a non-medical switch from the originator adalimumab to a biosimilar, regarding efficacy, safety and side effects and persistence on treatment.
3. Materials and Methods
Study Design and Clinical Assessment
This is an observational, multicentric, prospective study conducted in two IBD centers in Bucharest on the non-medical switching of Adalimumab.
The study included 53 patients (27 male and 26 female) diagnosed with Ulcerative Colitis (UC) or Crohn’s Disease (CD) based on standard endoscopic, radiological, and histological criteria All patients were followed actively on a tight control approach[
11] . The extent of disease was determined using the Montreal classification[
12] and its severity was determined using the Mayo score for the UC patients[
13] and the Harvey Bradshaw Index(HBI) in CD patients[
14].
The patients included in the study had completed at least the induction treatment with the original Adalimumab biologic and were considered in clinical remission by their treating physician. As the prescription of adalimumab formulations is issued monthly by hospital physicians the patients were progressively non medically switched to one of the Adalimumab biosimilars based on the recommendation of the National insurance house at the moment of their first or second visit to the hospital for the prescription. The choice of the new treatment brand for Adalimumab was not based on any predetermined criteria, and the decision was made by the current physician after an extended discussion with the patient. The Adalimumab biosimilars included in the study were those available at that moment respectively Hukyndra, Imraldi, and Hyrimoz(
Table 1). [
15] All patients used injector pens for the administration of the biological treatment.
The original dose and timing of administration was not altered in any patient after the switch to the Adalimumab biosimilars. All included patients switching from the ADA originator to an ADA biosimilar were clinically assessed before switching to a biosimilar and then monthly for twelve months.
The following data were collected: demographics, previous and current therapy, clinical scores, adverse effects related to the therapy, as well as some biological markers (CRP, calprotectin), IBD disk, IBD daily life burden.
The primary objective was the maintenance of clinical remission after switching from the original Adalimumab treatment to the Adalimumab biosimilar. Remission was defined as no need for ADA optimization or the addition of steroids or immunosuppressants.
The secondary objectives evaluated the presence of adverse effects after switching treatments, as well as the persistence on therapy at 6 and 12 months.
4. Results
In our population, there were 42 patients with Crohn’s Disease (50% male and 50% female) and 11 with Ulcerative Colitis (54.54% male and 45.45% female) who switched from the original Adalimumab to a biosimilar Adalimumab.
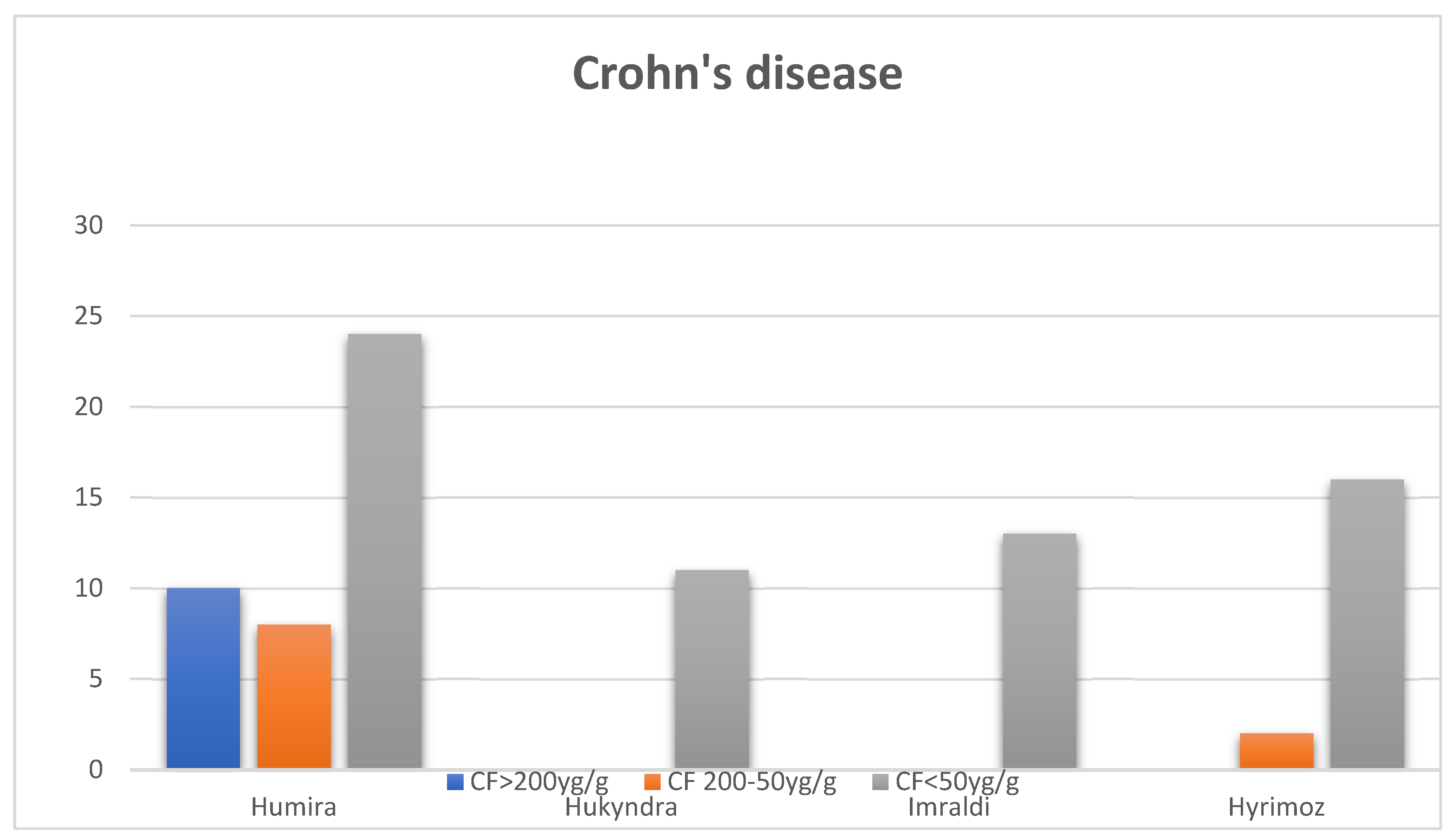
At the time of switching from the original Adalimumab to a biosimilar all patients were considered in clinical remission by their treating physician although 10 of the Crohn’s Disease patients had FC > 200 µg/g , 8 had FC between 200-50 µg/g, and 24 had FC < 50 µg/g. Six months after switching to one of the three biosimilars, patients had FC between 50-200 µg/g, and the rest had FC < 50 µg/g(Figure 1). One patient required a change to another biological treatment (Ustekinumab) due to the loss of clinical and biological response, increase in calprotectin levels above 1000, as well as active disease detected during colonoscopy (presence of deep ulcers and signs of inflammation in the colon and the ileo-colonic anastomosis).
Regarding the CF and CRP values in patients with Ulcerative Colitis (UC), during the treatment with original Adalimumab, 3 patients had CF >200 µg/g, 3 had CF between 200-50 µg/g, and 5 had CF <50 µg/g. Additionally, 2 had a CRP >5 mg/dL and the remaining 9 had a CRP <5 mg/dL. Six months after the initial period, 2 UC patients had CF >200 µg/g, 2 had CF between 50-200 µg/g, and the remaining 7 had CF <50 µg/g. The CRP values did not changed significantly after switching (Table 3).
The secondary objective was to evaluate the occurrence of side effects related to the biosimilar therapy. The main adverse effect reported by our patients was pain at the injection site.
Before switching the use of the new administration devices (pens) for the biosimilars was extensively explained to the patients using videos and sham models of the pens.
One patient encountered a problem with the pen at the second administration of Hukyndra when it failed to deliver the drug after lifting the pen too early from the skin surface. Of the 53 patients, only 2 reported pain at the injection site, but this was considered insignificant as the patients had a BMI under 20 and also reported the same symptoms with the original product.
Regarding other adverse effects, only one patient reported abdominal pain and rectal bleeding after the initiation of a biosimilar therapy. The adverse effects appeared after the first administration, were short-lived, and disappeared after two weeks. No clinical, biological or endoscopic recurrence was noticed in this patient.
5. Discussion
The advent of biosimilars has been a significant milestone in the treatment of IBD, significantly reducing the costs of advanced therapies and allowing their widespread use. Biosimilars are similar, but not identical, to the reference biologic, and although they may contain different substances not found in the original preparation, studies conducted to date have demonstrated high efficacy, safety, and comparable immunogenicity profile [15,16,17,18,19,20].
Switching from originator adalimumab to an adalimumab biosimilar, or even from switching from one biosimilar to another, for reasons driven by third parties, such as payers, in other words the non-medical switch, seems also safe and effective [
15,
16,
17,
18,
19]. Still the decision of non-medical switch is often seen as arbitrary by the physicians [
21].
When the recommendation of the national insurance house of insurance to switch to a adalimumab biosimilar was issued, a lot of gastroenterologists were still reluctant. We decided to present the existing treatment options to our patients and to discuss extensively on the concept of biosimilarity of the drugs allocating more time to the consultation in order to minimize the possible side effects after the switch including the nocebo effect. Offering the appropriate information about the biosimilar drugs and their effectivenes and side effects profile comparable to the originator with an advantage of increased accesibility and also discussing the possibility of the nocebo effect is part of the education of the IBD patient, when therapeutic options are presented[
15].
All patients but one in or cohort maintained clinical and biological remission at six and twelve months after switching treatments with minimal adverse reactions.
These findings correlate with data obtained in other studies. A remission rate of 74.5% and a treatment persistency of 81.6% at 12 months was shown in an Italian cohort of 98 patients switching from adalimumab originator to SB5. A higher number of patients reported mild adverse events after switch, most commonly injection site pain (maybe because SB5 formulation contained sodium citrate until 2023) [
15,
22].
In a study from Hungary, the subjective efficacy of switching to a biosimilar was proven in the case of adalimumab while a more reduced efficacy was experienced with the infliximab biosimilar. The perception of AEs was high and varied between biosimilars [
23].
The results of a real-life retrospective study of non-medical switch in Italy that included also patients in stable remission under ADA originator treatment, showed that all ADA biosimilars were effective and safe. Clinical remission was maintained in 124 out of 153 patients (81.0%) after a median follow-up of 12 months in patients switched from the originator [
24].
However, the Italian real-life experience with a rate of about 20% of patients losing remission, together with a significant rate of successful switch back to the ADA originator, is a finding that requires careful evaluation in prospective studies for both medical and ethical implications. This finding is particularly relevant for UC patients, who seem to have the worst performance when the ADA originator was replaced for a non-medical reason in this study [
25].
The patients non medically switched were in clinical stable remission while receiving the ADA originator treatment and despite the fact that all but one remained in clinical remission at one year, we cannot exclude a “nocebo” effect that could negatively influence the maintenance of remission after nonmedical switching as we also observed an increase of the IBD disk values and of the patients ‘scores of the visual analogue scale assessing the IBD daily life burden (0=perfect and 10=worst, >5 high burden) before and immediately after switch(unpublished data).
Regarding the safety profile, among the 53 patients, 2 experienced adverse reactions, such as pain at the injection site, but this is in line with the known side effects of Adalimumab. Switching to a biosimilar can also mean changing the delivery device and patient care process, which can cause anxiety for the patient. Ensuring and educating patients is essential to ensure their comfort with the use of new devices. Additionally, both the physician and the medical team must be familiar with the new types of Adalimumab biosimilar delivery devices. [15]. Factors contributing to pain during the administration of a subcutaneous product include the product’s composition, injection speed, viscosity, injection angle, injection site, and patient-related factors. The biosimilar pen devices used in our study have a needle thickness of 29G and a length of 4-8 mm, similar to the original Humira product. [15].
During follow-up, only one patient required discontinuation of the treatment due to secondary failure and switched to another biologic, no patients were optimized and remained in clinical remission without corticosteroids. The results are better or comparable with current literature reports [25,26,27,28,29,30], the small number of patients and the short duration of the study being a possible bias.
Our study has several limitations. Being an observational study, data collection is challenging by nature, and some data may be missing from medical records. Another limitation is the small sample size, and the relatively small number of UC patients. This can be explained by the preference for other biologic treatments (Infliximab or Vedolizumab) among UC patients. Also, the limited follow-up period of monitoring the patients of only 12 months is a limitation as we recognize that a longer follow-up might add insightful information on efficacy and persistence on treatment.
6. Conclusions
In this study on non-medical switch to a ADA biosimilar of 53 patients with IBD we managed to confirm the efficacy of the Adalimumab biosimilar in the management of IBD patients with excellent results at six and twelve months of follow up. Furthermore, it demonstrates that switching from the original product to a biosimilar is safe, cost-effective, and therapeutically effective. No difference was observed between the biosimilars used in the study.
This observational study is one of the first analyses conducted in Romania that shows the Adalimumab biosimilars are as effective as the originator Adalimumab in the clinical practice of IBD patients. These results support the idea that the widespread use of biosimilars does not affect therapy efficacy and patient safety.
References
- van Dullemen HM, van Deventer SJ, Hommes DW, Bijl HA, Jansen J, Tytgat GN, Woody J. Treatment of Crohn’s disease with anti-tumor necrosis factor chimeric monoclonal antibody (cA2). Gastroenterology. 1995 Jul;109(1):129-35. [CrossRef]
- van der Valk ME, Mangen MJ, Leenders M, et al Healthcare costs of inflammatory bowel disease have shifted from hospitalisation and surgery towards anti-TNFα therapy: results from the COIN study Gut 2014;63:72-79. [CrossRef]
- Péntek M, Lakatos PL, Oorsprong T, Gulácsi L, Pavlova M, Groot W, Rencz F, Brodszky V, Baji P, Crohn’s Disease Research Group. Access to biologicals in Crohn’s disease in ten European countries. World J Gastroenterol 2017; 23(34): 6294-6305 [PMID: 28974896. [CrossRef]
- Kirchhoff CF, Wang XM, Conlon HD, Anderson S, Ryan AM, Bose A. Biosimilars: key regulatory considerations and similarity assessment tools. Biotechnol. Bioeng. 114(12), 2696–2705 (2017). [CrossRef]
- The European commission. What you need to know about biosimilar medicinal products. 2013. Accessed June 2018.
- Danese S, Fiorino G, Michetti P. Changes in Biosimilar Knowledge among European Crohn’s Colitis Organization [ECCO] Members: An Updated Survey J Crohns Colitis 2016 Nov;10(11):1362-1365. [CrossRef]
- Macaluso FS, Leone S,Previtali E, Ventimiglia M, Armuzzi A, Orlando A; AMICI (Italian Inflammatory Bowel Disease Patients’ Association) and IG-IBD (Italian Group for the Study of Inflammatory Bowel Disease) Biosimilars: The viewpoint of Italian patients with inflammatory bowel disease Dig Liver Dis 2020 Nov;52(11):1304-1309. [CrossRef]
- Wetwittayakhlang, P.; Karkout, K.; Wongcha-Um, A.; Tselekouni, P.; Al-Jabri, R.; Afif, W.; Wild, G.; Bitton, A.; Bessissow, T.; Lakatos, P.L. Clinical Efficacy and Nocebo Effect Following Non-Medical Biosimilar Switch in Patients with Inflammatory Bowel Disease: A Prospective Observational Study. Dig. Liver Dis. 2024, 56, 35–42. [CrossRef]
- 2019; 9. Somers M,Bossuyt P, Ferrante M,Peeters H,Baert FBelgian IBD Research Group [BIRD] Position Statement 2019 on the Use of Adalimumab Biosimilars in Inflammatory Bowel Diseases Journal of Crohn’s and Colitis, 2020, 680–685.
- Fiorino G, Caprioli F, Daperno M,Mocciaro F, Principi M, Viscido A, Fantini MC, Orlando A, Papi C, Annese V, Danese S, Vecchi M, Rizzello F, Armuzzi A; National patients’ association representatives Use of biosimilars in inflammatory bowel disease: a position update of the Italian Group for the Study of Inflammatory Bowel Disease (IG-IBD) Dig Liver Dis 2019 May;51(5):632-639. [CrossRef]
- Ungaro RC, Yzet C, Bossuyt P, Baert FJ, Vanasek T, D’Haens GR, et al. Deep Remission at 1 Year Prevents Progression of Early Crohn’s Disease. Gastroenterology. 2020 Jul;159(1):139-147. Epub 2020 Mar 26. PMID: 32224129; PMCID: PMC7751802. [CrossRef]
- Odes S, Vardi H, Friger M, et al. European Collaborative Study Group on Inflammatory Bowel Disease. Effect of phenotype on health care costs in Crohn’s disease: A European study using the Montreal classification. J Crohns Colitis. 2007;1(2):87–96. [CrossRef]
- DignassA , EliakimR, MagroF, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 1: definitions and diagnosis J Crohns Colitis.2012;6:965–990. [CrossRef]
- Harvey RF , Bradshaw JM. A simple index of Crohn’s-disease activity Lancet.1980;1:514.
- Abitbol V, Benkhalifa S, Habauzit C, Marotte H. Navigating adalimumab biosimilars: an expert opinion. J Comp Eff Res. 2023 Nov;12(11):e230117. Epub 2023 Oct 19. Erratum in: J Comp Eff Res. 2024 Feb;13(2):e240003c1. doi: 10.57264/cer-2024-0003c1. PMID: 37855223; PMCID: PMC10690439. [CrossRef]
- Fiorino G, Gilardi D, Correale C, Furfaro F, Roda G, Loy L, Argollo M, Allocca M, Peyrin-Biroulet L, Danese S. Biosimilars of adalimumab: the upcoming challenge in IBD. Expert Opin Biol Ther. 2019 Oct;19(10):1023-1030. Epub 2019 Jan 8. PMID: 30601098. [CrossRef]
- Danese S, Bonovas S, Peyrin-Biroulet L. Biosimilars in IBD: from theory to practice. Nat Rev Gastroenterol Hepatol. 2017 Jan;14(1):22-31. Epub 2016 Oct 12. PMID: 27729659. [CrossRef]
- Angyal A, Bhat S. Biosimilars in IBD: What Every Clinician Needs to Know. Curr Gastroenterol Rep. 2024 Mar;26(3):77-85. Epub 2024 Jan 20. PMID: 38243154. [CrossRef]
- Solitano V, D’Amico F, Fiorino G, Peyrin-Biroulet L, Danese S. Biosimilar switching in inflammatory bowel disease: from evidence to clinical practice. Expert Rev Clin Immunol. 2020 Oct;16(10):1019-1028. Epub 2020 Sep 29. PMID: 32954893. [CrossRef]
- Ilias A, Gonczi L, Kurti Z, Lakatos PL. Biosimilars in ulcerative colitis: When and for who? Best Pract Res Clin Gastroenterol. 2018 Feb-Apr;32-33:35-42. Epub 2018 May 26. PMID: 30060937. [CrossRef]
- Salam T, Duhig A, Patel AA, Cameron A, Voelker J, Bookhart B, Coleman CI. Physicians’ perspectives regarding non-medical switching of prescription medications: Results of an internet e-survey. PLoS One. 2020 Jan 10;15(1):e0225867. PMID: 31923201; PMCID: PMC6953849. [CrossRef]
- Tapete G, Bertani L, Pieraccini A, et al. Effectiveness and Safety of Nonmedical Switch from Adalimumab Originator to SB5 Biosimilar in Patients With Inflammatory Bowel Diseases: Twelve-Month Follow- Up From the TABLET Registry. Inflamm Bowel Dis 2022; 28:62-69. [CrossRef]
- Sarlós P, Bikar A, Farkas N, Resál T, Szepes Z, Farkas K, Nagy F, Vincze Á, Miheller P, Molnár T.Self-reported efficacy and safety of infliximab and adalimumab biosimilars after non-medical switch in patients with inflammatory bowel disease: results of a multicenter survey. Expert Opin Biol Ther. 2023 Jul-Dec;23(8):827-832. [CrossRef]
- Tursi A, Mocci G, Allegretta L, Aragona G, Bianco MA, Colucci R, et al. Comparison of Performances of Adalimumab Biosimilars SB5, ABP501, GP2017, and MSB11022 in Treating Patients with Inflammatory Bowel Diseases: A Real-Life, Multicenter, Observational Study. Inflamm Bowel Dis. 2023 Mar 1;29(3):376-383. Erratum in: Inflamm Bowel Dis. 2022 Oct 3;28(10):e145. doi: 10.1093/ibd/izac163. Erratum in: Inflamm Bowel Dis. 2024 Mar 1;30(3):508. doi: 10.1093/ibd/izae017. PMID: 35579320. [CrossRef]
- Tursi A, Mocci G, Cuomo A, Ferronato A, Elisei W, Picchio M,et al..Replacement of Adalimumab Originator to Adalimumab Biosimilar for a Non-Medical Reason in Patients with Inflammatory Bowel Disease: A Real-life Comparison of Adalimumab Biosimilars Currently Available in Italy. J Gastrointestin Liver Dis. 2022 Dec 16;31(4):411-416. [CrossRef]
- Mocci, G.; Bodini, G.;Allegretta, L.; Cazzato, A.I.; Chiri, S.;Aragona, G.; Perazzo, P.;Ferronato, A.; Graziani, M.G.;Pagnini, C.; et al. Adalimumab Biosimilar GP2017 versus Adalimumab Originator in Treating Patients with Inflammatory Bowel Diseases: A Real-Life, Multicenter,Observational Study. Biomedicines 2022, 10, 1799. [CrossRef]
- M. Lukas, M. Kolar, J. Reissigova, D. Duricova, N. Machkova, V. Hruba,J. Jirsa, K. Pudilova & K. Malickova (2022) A switch from originator adalimumab to the biosimilar SB5 in patients with Crohn’s disease: an analysis of two propensity score-matched cohorts, Scandinavian Journal of Gastroenterology, 57:7, 814-824. [CrossRef]
- Vernero, M.; Bezzio, C.; Ribaldone, D.G.; Costa, S.; Scalvini, D.; Tribocco, E.; Manes, G.;Saibeni, S. Efficacy and Safety of Adalimumab Biosimilar GP2017 in Patients with Inflammatory Bowel Disease. J. Clin. Med. 2023, 12, 6839. [CrossRef]
- Kay J, Cross RK, Feldman SR, Park Y, Hanauer SB. Review of Adalimumab Biosimilar SB5 in Immune-Mediated Inflammatory Diseases. Adv Ther. 2024 Feb;41(2):509-533. Epub 2023 Dec 19. PMID: 38110655; PMCID: PMC10838831. [CrossRef]
- Jin R, Nduka C, Courmier D, Knight H, Meadows R, Piercy J, Cummings JRF, Radziszewski W. Real-World Experience of Adalimumab Biosimilar (ABP 501) Use in Patients with Inflammatory Bowel Disease in Europe. Adv Ther. 2024 Jan;41(1):331-348. Epub 2023 Nov 14. PMID: 37957522; PMCID: PMC10796661. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).