Submitted:
20 August 2024
Posted:
22 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Aerobic Composting and cucumber Potting Experiment
2.2. Basic physical and chemical indicators.
2.3. Microbial Diversity Determination and Analysis
2.3.1. DNA Extraction and Purification
2.3.2. High-Throughput Sequencing
2.4. Statistics and Data Analysis
3. Results and Discussion
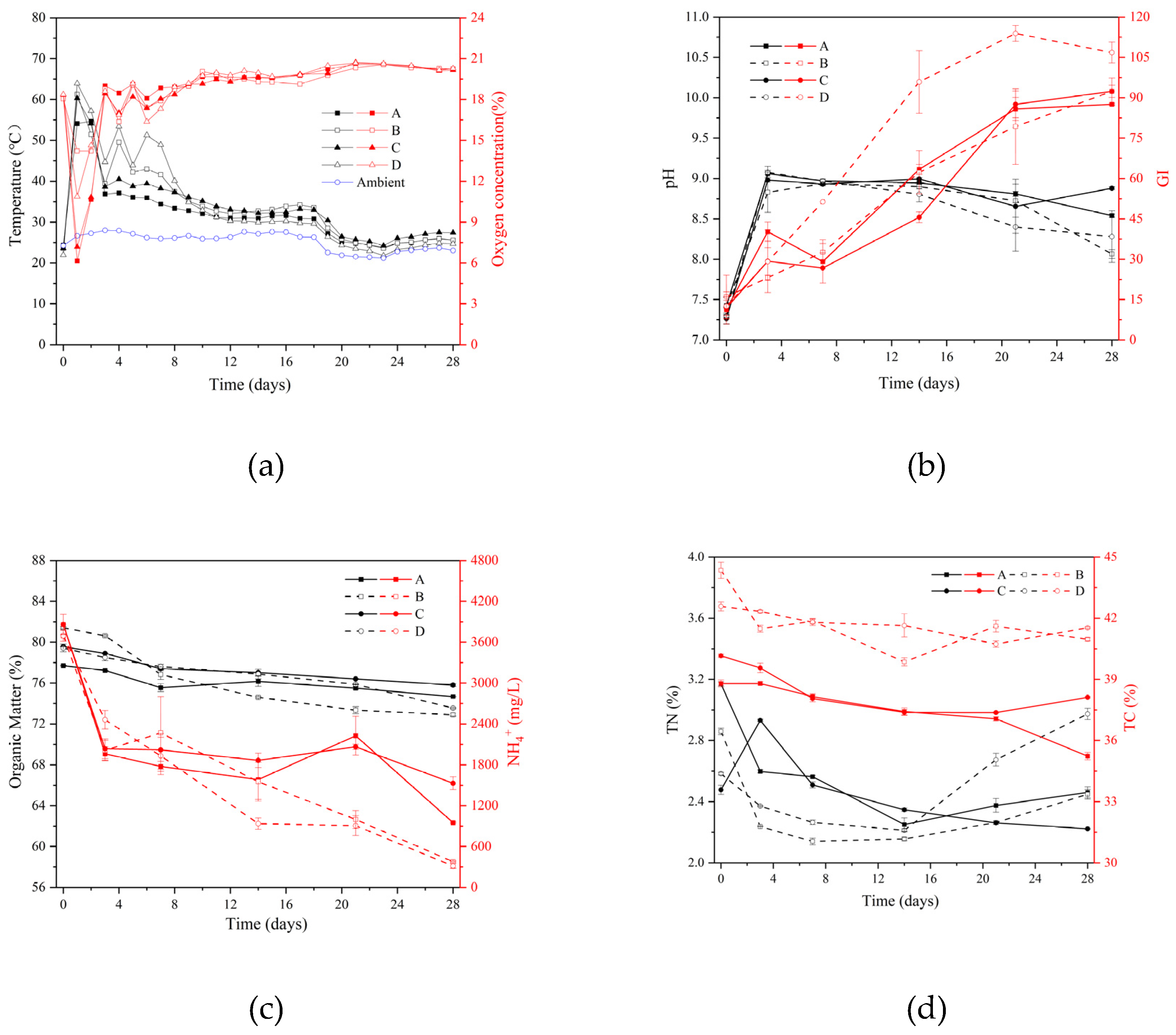
3.1. Physicochemical Changes in the Manure–Compost–Soil Process
3.1.1. Manure–Compost during Aerobic Composting
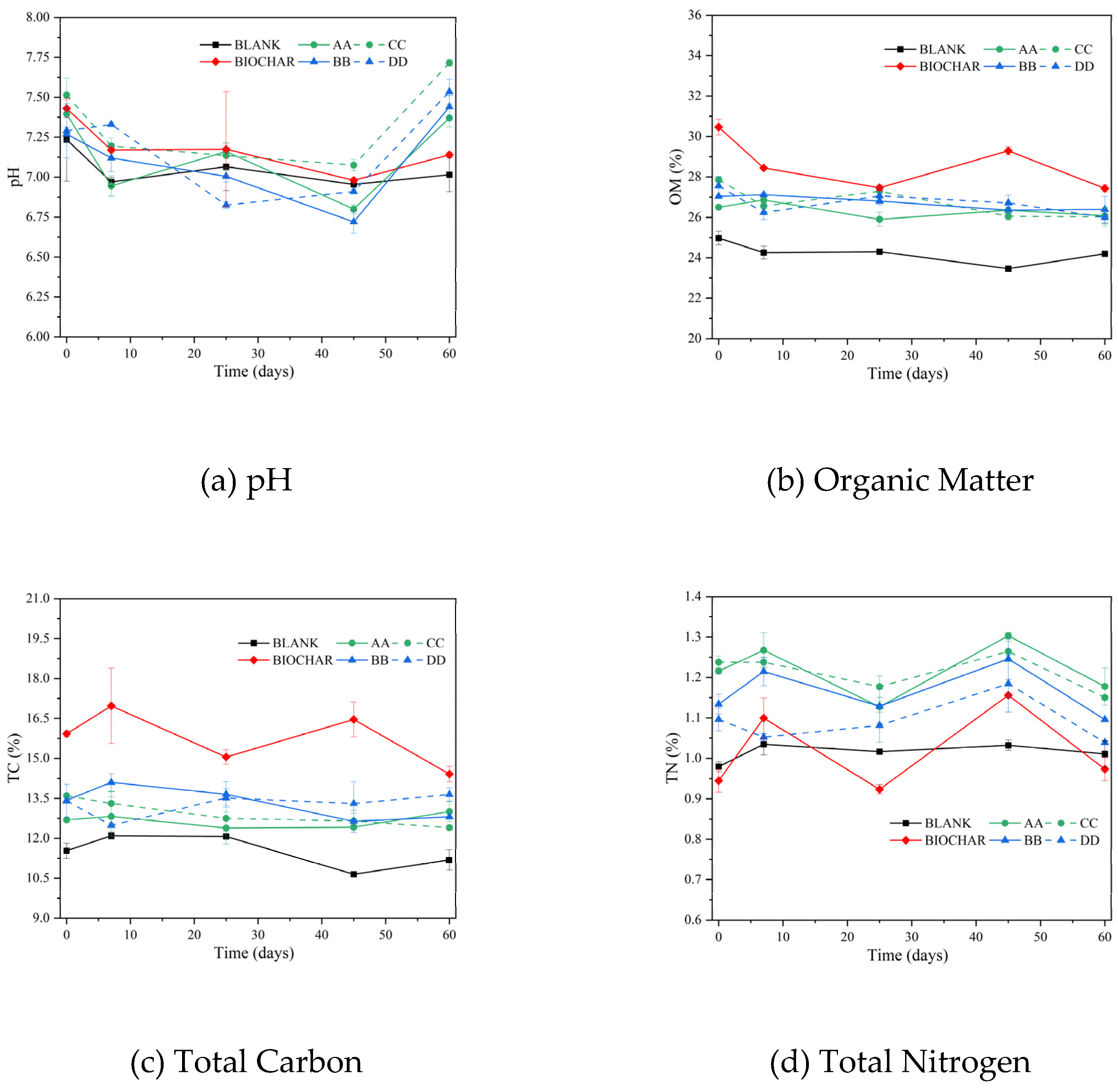
3.1.2. Compost–Soil in the Potting Experiment
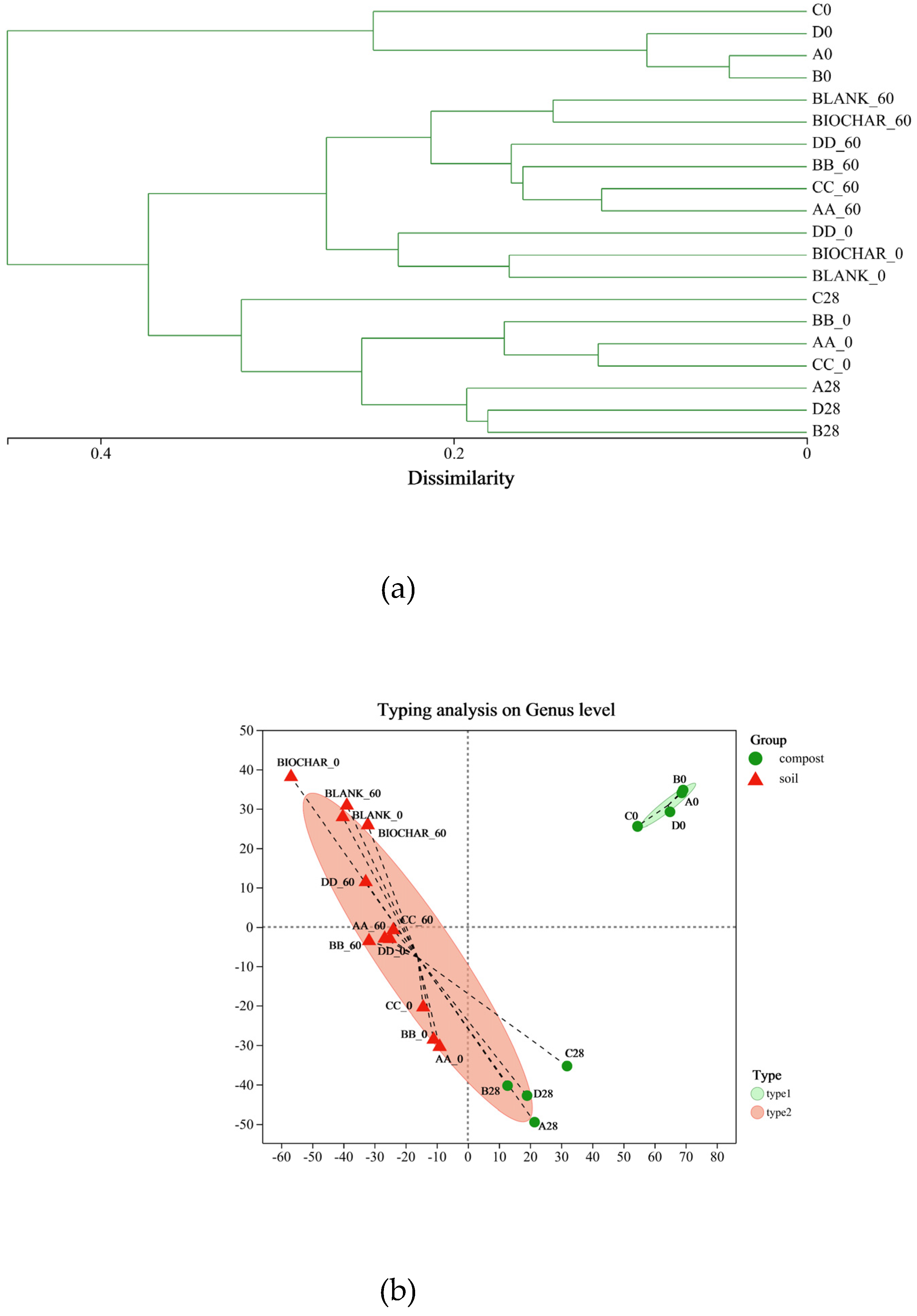
3.2. Analysis of the Bacterial Diversity in the Manure–Compost–Soil Process
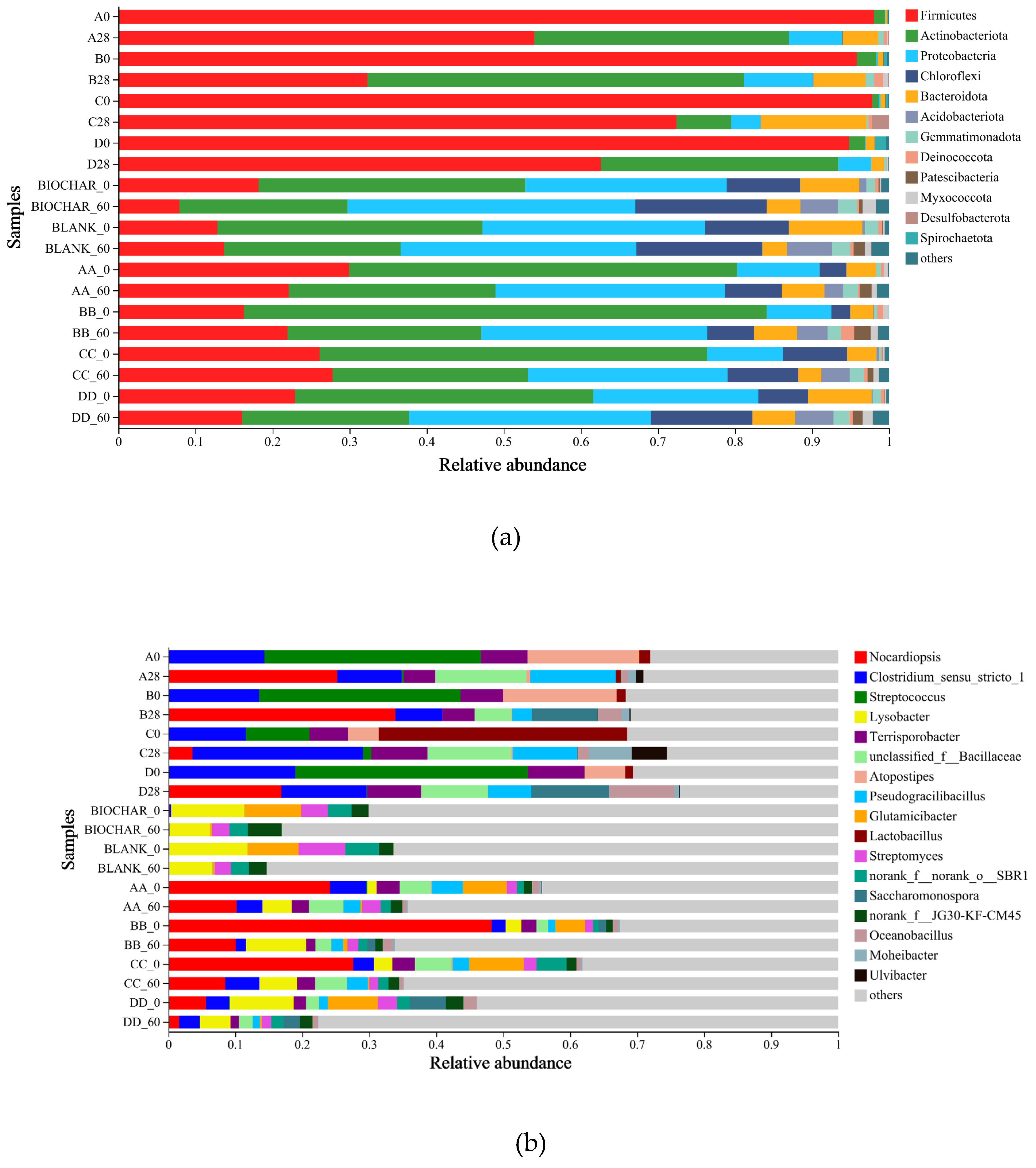
3.3. Bacterial Community Composition in the Manure–Compost–Soil Process
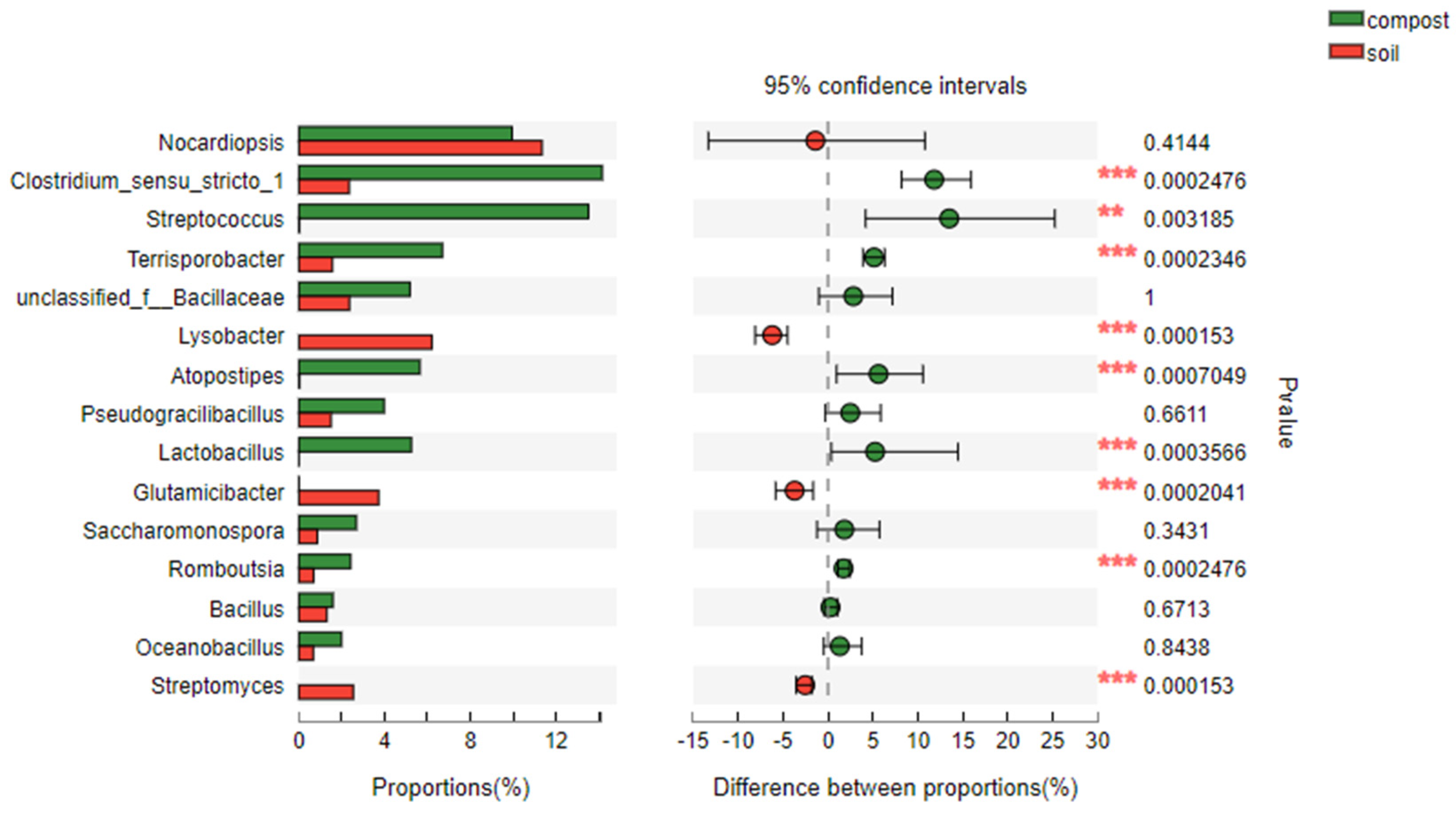
3.4. Difference Analysis of Bacteria from Pig Manure–Compost to Compost–Soil
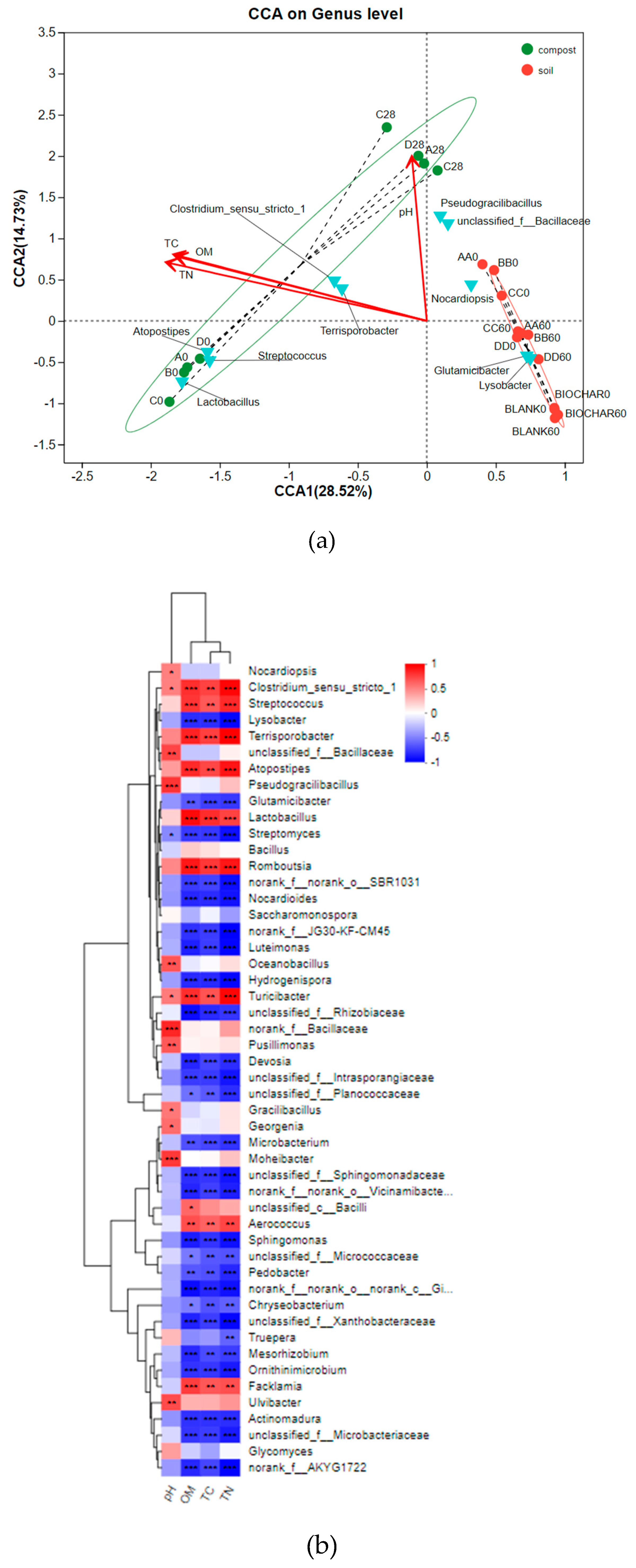
3.5. Correlation Analysis of Bacteria and Physicochemical Properties in the Manure-Compost-Soil Process
4. Conclusion
- Biochar addition during aerobic composting increased the C/N ratio and total carbon content of the compost, as well as improved the germination index.
- When applied to soil, composts containing biochar increased the soil's pH, organic matter, total carbon, and total nitrogen contents compared to composts without biochar.
- Microbial diversity decreased during the high-temperature phase of aerobic composting but increased significantly in the final compost products. Soil samples amended with composts showed higher microbial diversity than unamended soil.
- The microbial communities in the composts and soil samples were distinct, but some bacterial genera (e.g., Nocardiopsis, Clostridium_sensu_stricto_1) persisted and contributed positively to the microbial diversity of the soil.
- Correlation analysis revealed significant relationships between bacterial genera and soil physicochemical properties, providing insights into the microbial mechanisms underlying the observed effects of biochar on soil quality.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Awasthi, S.K.; Duan, Y.; Liu, T.; Zhang, Z.; Pandey, A.; Varjani, S.; Awasthi, M.K.; Taherzadeh, M.J. Can biochar regulate the fate of heavy metals (Cu and Zn) resistant bacteria community during the poultry manure composting? J Hazard Mater 2021, 406, 124593. [CrossRef]
- Ma, S.; Sun, X.; Fang, C.; He, X.; Han, L.; Huang, G. Exploring the mechanisms of decreased methane during pig manure and wheat straw aerobic composting covered with a semi-permeable membrane. Waste Manage 2018, 78, 393-400. [CrossRef]
- Ravindran, B.; Nguyen, D.D.; Chaudhary, D.K.; Chang, S.W.; Kim, J.; Lee, S.R.; Shin, J.; Jeon, B.; Chung, S.; Lee, J. Influence of biochar on physico-chemical and microbial community during swine manure composting process. J Environ Manage 2019, 232, 592-599. [CrossRef]
- Haug, R. The practical handbook of compost engineering. CRC Press: 1993; p. [CrossRef]
- Zhou, S.; Chang, T.; Zhang, Y.; Shaghaleh, H.; Zhang, J.; Yang, X.; Qin, H.; Talpur, M.M.A.; Alhaj Hamoud, Y. Organic fertilizer compost alters the microbial composition and network structure in strongly acidic soil. Appl Soil Ecol 2024, 195, 105263. [CrossRef]
- Morra, L.; Bilotto, M.; Baldantoni, D.; Alfani, A.; Baiano, S. A seven-year experiment in a vegetable crops sequence: Effects of replacing mineral fertilizers with Biowaste compost on crop productivity, soil organic carbon and nitrates concentrations. Sci Hortic-Amsterdam 2021, 290, 110534. [CrossRef]
- Scotti, R.; Pane, C.; Spaccini, R.; Palese, A.M.; Piccolo, A.; Celano, G.; Zaccardelli, M. On-farm compost: a useful tool to improve soil quality under intensive farming systems. Appl Soil Ecol 2016, 107, 13-23. [CrossRef]
- Awasthi, M.K.; Zhang, Z.; Wang, Q.; Shen, F.; Li, R.; Li, D.; Ren, X.; Wang, M.; Chen, H.; Zhao, J. New insight with the effects of biochar amendment on bacterial diversity as indicators of biomarkers support the thermophilic phase during sewage sludge composting. Bioresource Technol 2017, 238, 589-601. [CrossRef]
- He, X.; Yin, H.; Han, L.; Cui, R.; Fang, C.; Huang, G. Effects of biochar size and type on gaseous emissions during pig manure/wheat straw aerobic composting: Insights into multivariate-microscale characterization and microbial mechanism. Bioresource Technol 2019, 271, 375-382. [CrossRef]
- Ma, S.; Fang, C.; Sun, X.; Han, L.; He, X.; Huang, G. Bacterial community succession during pig manure and wheat straw aerobic composting covered with a semi-permeable membrane under slight positive pressure. Bioresource Technol 2018, 259, 221-227. [CrossRef]
- Babin, D.; Leoni, C.; Neal, A.L.; Sessitsch, A.; Smalla, K. Editorial to the Thematic Topic "Towards a more sustainable agriculture through managing soil microbiomes". Fems Microbiol Ecol 2021, 97. [CrossRef]
- Fierer, N.; Wood, S.A.; Bueno De Mesquita, C.P. How microbes can, and cannot, be used to assess soil health. Soil Biology and Biochemistry 2021, 153, 108111. [CrossRef]
- Bol, R.; Amelung, W.; Friedrich, C.; Ostle, N. Tracing dung-derived carbon in temperate grassland using 13C natural abundance measurements. Soil Biol Biochem 2000, 32, 1337-1343. [CrossRef]
- Duan, M.; Li, H.; Gu, J.; Tuo, X.; Sun, W.; Qian, X.; Wang, X. Effects of biochar on reducing the abundance of oxytetracycline, antibiotic resistance genes, and human pathogenic bacteria in soil and lettuce. Environ Pollut 2017, 224, 787-795. [CrossRef]
- Mawof, A.; Prasher, S.; Bayen, S.; Nzediegwu, C. Effects of Biochar and Biochar-Compost Mix as Soil Amendments on Soil Quality and Yield of Potatoes Irrigated with Wastewater. J Soil Sci Plant Nut 2021, 21, 2600-2612. [CrossRef]
- Purakayastha, T.J.; Bera, T.; Bhaduri, D.; Sarkar, B.; Mandal, S.; Wade, P.; Kumari, S.; Biswas, S.; Menon, M.; Pathak, H.; et al. A review on biochar modulated soil condition improvements and nutrient dynamics concerning crop yields: Pathways to climate change mitigation and global food security. Chemosphere 2019, 227, 345-365. [CrossRef]
- Nguyen, M.K.; Lin, C.; Hoang, H.G.; Sanderson, P.; Dang, B.T.; Bui, X.T.; Nguyen, N.S.H.; Vo, D.N.; Tran, H.T. Evaluate the role of biochar during the organic waste composting process: A critical review. Chemosphere (Oxford) 2022, 299, 134488. [CrossRef]
- Sanchez-Monedero, M.A.; Cayuela, M.L.; Roig, A.; Jindo, K.; Mondini, C.; Bolan, N. Role of biochar as an additive in organic waste composting. Bioresource Technol 2018, 247, 1155-1164. [CrossRef]
- Agegnehu, G.; Srivastava, A.K.; Bird, M.I. The role of biochar and biochar-compost in improving soil quality and crop performance: A review. Appl Soil Ecol 2017, 119, 156-170. [CrossRef]
- Fu, T.; Shangguan, H.; Wu, J.; Tang, J.; Yuan, H.; Zhou, S. Insight into the synergistic effects of conductive biochar for accelerating maturation during electric field-assisted aerobic composting. Bioresource Technol 2021, 337, 125359. [CrossRef]
- Guo, X.; Liu, H.; Zhang, J. The role of biochar in organic waste composting and soil improvement: A review. Waste Manage 2020, 102, 884-899. [CrossRef]
- Kung, C.; Mu, J.E. Prospect of China's renewable energy development from pyrolysis and biochar applications under climate change. Renewable and Sustainable Energy Reviews 2019, 114, 109343. [CrossRef]
- Sun, X.; Ma, S.; Han, L.; Li, R.; Schlick, U.; Chen, P.; Huang, G. The effect of a semi-permeable membrane-covered composting system on greenhouse gas and ammonia emissions in the Tibetan Plateau. J Clean Prod 2018, 204, 778-787. [CrossRef]
- Xiao, L.; Feng, L.R.; Yuan, G.D.; Wei, J. Low-cost field production of biochars and their properties. Environ Geochem Hlth 2020, 42, 1569-1578. [CrossRef]
- Yin, Y.; Tao, X.; Du, Y.; Li, M.; Yang, S.; Zhang, W.; Yang, C.; Li, H.; Wang, X.; Chen, R. Biochar improves the humification process during pig manure composting: Insights into roles of the bacterial community and metabolic functions. J Environ Manage 2024, 355, 120463. [CrossRef]
- Xu, N.; Tan, G.C.; Wang, H.Y.; Gai, X.P. Effect of biochar additions to soil on nitrogen leaching, microbial biomass and bacterial community structure. Eur J Soil Biol 2016, 74, 1-8. [CrossRef]
- Liu, N.; Zhou, J.; Han, L.; Ma, S.; Sun, X.; Huang, G. Role and multi-scale characterization of bamboo biochar during poultry manure aerobic composting. Bioresource Technol 2017, 241, 190-199. [CrossRef]
- He, X.; Yin, H.; Fang, C.; Xiong, J.; Han, L.; Yang, Z.; Huang, G. Metagenomic and q-PCR analysis reveals the effect of powder bamboo biochar on nitrous oxide and ammonia emissions during aerobic composting. Bioresource Technol 2021, 323, 124567. [CrossRef]
- He, X.; Chen, L.; Han, L.; Liu, N.; Cui, R.; Yin, H.; Huang, G. Evaluation of biochar powder on oxygen supply efficiency and global warming potential during mainstream large-scale aerobic composting. Bioresource Technol 2017, 245, 309-317. [CrossRef]
- Xiong, J.; Ma, S.; He, X.; Han, L.; Huang, G. Nitrogen transformation and dynamic changes in related functional genes during functional-membrane covered aerobic composting. Bioresource Technol 2021, 332, 125087. [CrossRef]
- Fang, C.; Yuan, X.; Liao, K.; Qu, H.; Han, L.; He, X.; Huang, G. Micro-aerobic conditions based on membrane-covered improves the quality of compost products: Insights into fungal community evolution and dissolved organic matter characteristics. Bioresource Technol 2022, 362, 127849. [CrossRef]
- Mori, H.; Maruyama, F.; Kato, H.; Toyoda, A.; Dozono, A.; Ohtsubo, Y.; Nagata, Y.; Fujiyama, A.; Tsuda, M.; Kurokawa, K. Design and Experimental Application of a Novel Non-Degenerate Universal Primer Set that Amplifies Prokaryotic 16S rRNA Genes with a Low Possibility to Amplify Eukaryotic rRNA Genes. Dna Res 2014, 21, 217-227. [CrossRef]
- López-Cano, I.; Roig, A.; Cayuela, M.L.; Alburquerque, J.A.; Sánchez-Monedero, M.A. Biochar improves N cycling during composting of olive mill wastes and sheep manure. Waste Management (Elmsford) 2016, 49, 553-559. [CrossRef]
- Sánchez-García, M.; Alburquerque, J.A.; Sánchez-Monedero, M.A.; Roig, A.; Cayuela, M.L. Biochar accelerates organic matter degradation and enhances N mineralisation during composting of poultry manure without a relevant impact on gas emissions. Bioresource Technol 2015, 192, 272-279. [CrossRef]
- Wei, Y.; Zhao, Y.; Shi, M.; Cao, Z.; Lu, Q.; Yang, T.; Fan, Y.; Wei, Z. Effect of organic acids production and bacterial community on the possible mechanism of phosphorus solubilization during composting with enriched phosphate-solubilizing bacteria inoculation. Bioresource Technol 2018, 247, 190-199. [CrossRef]
- Xie, T.; Zhang, Z.; Zhang, D.; Wei, C.; Lin, Y.; Feng, R.; Nan, J.; Feng, Y. Effect of hydrothermal pretreatment and compound microbial agents on compost maturity and gaseous emissions during aerobic composting of kitchen waste. Sci Total Environ 2023, 854, 158712. [CrossRef]
- Tiquia, S.M.; Tam, N.F.Y. Elimination of phytotoxicity during co-composting of spent pig-manure sawdust litter and pig sludge. Bioresource Technol 1998, 65, 43-49. [CrossRef]
- Kong, Y.; Zhang, J.; Yang, Y.; Liu, Y.; Zhang, L.; Wang, G.; Liu, G.; Dang, R.; Li, G.; Yuan, J. Determining the extraction conditions and phytotoxicity threshold for compost maturity evaluation using the seed germination index method. Waste Management (Elmsford) 2023, 171, 502-511. [CrossRef]
- Zhang, L.; Sun, X. Changes in physical, chemical, and microbiological properties during the two-stage co-composting of green waste with spent mushroom compost and biochar. Bioresource Technol 2014, 171, 274-284. [CrossRef]
- Ottani, F.; Parenti, M.; Santunione, G.; Moscatelli, G.; Kahn, R.; Pedrazzi, S.; Allesina, G. Effects of different gasification biochar grain size on greenhouse gases and ammonia emissions in municipal aerated composting processes. J Environ Manage 2023, 331, 117257. [CrossRef]
- Yu, H.; Xie, B.; Khan, R.; Shen, G. The changes in carbon, nitrogen components and humic substances during organic-inorganic aerobic co-composting. Bioresource Technol 2019, 271, 228-235. [CrossRef]
- Naeem, M.A.; Khalid, M.; Aon, M.; Abbas, G.; Amjad, M.; Murtaza, B.; Khan, W.; Ahmad, N. Combined application of biochar with compost and fertilizer improves soil properties and grain yield of maize. J Plant Nutr 2018, 41, 112-122. [CrossRef]
- Liu, H.; Awasthi, M.K.; Zhang, Z.; Syed, A.; Bahkali, A.H. Evaluation of gases emission and enzyme dynamics in sheep manure compost occupying with peach shell biochar. Environmental Pollution (1987) 2024, 351, 124065. [CrossRef]
- Aina Najwa Mohd Nor Azman, N.; Inderakusumowati Md Khalid, P.; Aishah Saidina Amin, N.; Yamani Zakaria, Z.; Mohammad Zainol, M.; Ilham, Z.; Phaiboonsilpa, N.; Asmadi, M. Effects of biochar, compost, and composted biochar soil amendments on okra plant growth. Materials Today : Proceedings 2023. [CrossRef]
- Neville, F.; Seyfaee, A. Real-Time Monitoring of in Situ Polyethyleneimine-Silica Particle Formation. Langmuir 2013, 29, 14681-14690. [CrossRef]
- He, X.; Han, L.; Fu, B.; Du, S.; Liu, Y.; Huang, G. Effect and microbial reaction mechanism of rice straw biochar on pore methane production during mainstream large-scale aerobic composting in China. J Clean Prod 2019, 215, 1223-1232. [CrossRef]
- Mao, H.; Lv, Z.; Sun, H.; Li, R.; Zhai, B.; Wang, Z.; Awasthi, M.K.; Wang, Q.; Zhou, L. Improvement of biochar and bacterial powder addition on gaseous emission and bacterial community in pig manure compost. Bioresource Technol 2018, 258, 195-202. [CrossRef]
- Wang, P.; Wang, S.; Chen, F.; Zhang, T.; Kong, W. Preparation of two types plant biochars and application in soil quality improvement. Sci Total Environ 2024, 906, 167334. [CrossRef]
- Bennur, T.; Kumar, A.R.; Zinjarde, S.; Javdekar, V. Nocardiopsis species: Incidence, ecological roles and adaptations. Microbiol Res 2015, 174, 33-47. [CrossRef]
- Ibrahim, A.H.; Desoukey, S.Y.; Fouad, M.A.; Kamel, M.S.; Gulder, T.A.M.; Abdelmohsen, U.R. Natural Product Potential of the Genus Nocardiopsis. Mar Drugs 2018, 16. [CrossRef]
- Jepsen, S.E.; Krause, M.; Gruttner, H. Reduction of Fecal Streptococcus and Salmonella by selected treatment methods for sludge and organic waste. Water Sci Technol 1997, 36, 203-210. [CrossRef]
- Li, X.; Shi, X.; Lu, M.; Zhao, Y.; Li, X.; Peng, H.; Guo, R. Succession of the bacterial community and functional characteristics during continuous thermophilic composting of dairy manure amended with recycled ceramsite. Bioresource Technol 2019, 294, 122044. [CrossRef]
- Wang, J.; Gu, J.; Wang, X.; Song, Z.; Dai, X.; Guo, H.; Yu, J.; Zhao, W.; Lei, L. Enhanced removal of antibiotic resistance genes and mobile genetic elements during swine manure composting inoculated with mature compost. J Hazard Mater 2021, 411, 125135. [CrossRef]
- Exposito, R.G.; Postma, J.; Raaijmakers, J.M.; De Bruijn, I. Diversity and Activity of Lysobacter Species from Disease Suppressive Soils. Front Microbiol 2015, 6. [CrossRef]
- Puopolo, G.; Tomada, S.; Pertot, I. The impact of the omics era on the knowledge and use of Lysobacter species to control phytopathogenic micro-organisms. J Appl Microbiol 2018, 124, 15-27. [CrossRef]
- Qin, S.; Feng, W.; Zhang, Y.; Wang, T.; Xiong, Y.; Xing, K. Diversity of Bacterial Microbiota of Coastal Halophyte Limonium sinense and Amelioration of Salinity Stress Damage by Symbiotic Plant Growth-Promoting Actinobacterium Glutamicibacter halophytocola KLBMP 5180. Appl Environ Microb 2018, 84. [CrossRef]
- Cordovez, V.; Carrion, V.J.; Etalo, D.W.; Mumm, R.; Zhu, H.; van Wezel, G.P.; Raaijmakers, J.M. Diversity and functions of volatile organic compounds produced by Streptomyces from a disease-suppressive soil. Front Microbiol 2015, 6. [CrossRef]
| Experiment | Group name | Ingredients a |
|---|---|---|
| Aerobic Composting | Control groups (A and C) | Swine manure and wheat straw (Ratio of 5:1) |
| Experimental groups (B and D) | Swine manure, wheat straw, and wheat straw biochar (Ratio of 10:2:1) |
|
| Potting b | BLANK | 0.25 kg nutrient soil |
| BIOCHAR | 0.225 kg nutrient soil + 0.025 kg biochar | |
| AA | 0.225 kg nutrient soil + 0.025 kg compost from the Group A composting | |
| BB | 0.225 kg nutrient soil + 0.025 kg biochar-based compost from Group B | |
| CC | 0.225 kg nutrient soil + 0.025 kg compost from Group C | |
| DD | 0.225 kg nutrient soil + 0.025 kg biochar-based compost from Group D |
| Name | MC a | pH a | OM b | TC b | TN b |
|---|---|---|---|---|---|
| Pig manure | 66.00 ± 0.72 | / | 74.70 ± 0.01 | 37.41 ± 0.14 | 3.19 ± 0.01 |
| Wheat straw | 6.07 ± 0.14 | / | 89.81 ± 0.03 | 43.43 ± 0.09 | 0.99 ± 0.06 |
| Biochar | 3.21 ± 0.15 | 10.21 ± 0.07 | 80.99 ± 0.28 | 70.60 ± 0.16 | 1.08 ± 0.01 |
| Black soil | 2.71 ± 0.15 | 6.87 ± 0.01 | 4.51 ± 0.06 | 12.60 ± 0.24 | 1.12 ± 0.02 |
| Vermiculite | 5.62 ± 0.03 | 7.25 ± 0.01 | 25.05 ±0.68 | 0.16 ± 0.01 | 0.20 ± 0.01 |
| Compost A | 13.32 ± 0.06 | 7.96 ± 0.01 | 65.57 ± 0.07 | 33.36 ± 0.59 | 3.97 ± 0.06 |
| Compost C | 13.38 ±0.15 | 8.07 ± 0.03 | 65.30 ± 0.22 | 32.51 ± 0.02 | 3.86 ± 0.01 |
| Compost B | 10.80 ± 0.36 | 8.54 ± 0.04 | 67.98 ± 0.09 | 41.41 ± 0.15 | 3.34 ± 0.01 |
| Compost D | 10.31 ± 0.10 | 8.15 ± 0.03 | 71.47 ± 0.28 | 43.20 ± 0.06 | 2.67 ± 0.02 |
| Sample name | Chao | Shannon | Simpson | Shannoneven | Simpsoneven |
|---|---|---|---|---|---|
| A0 | 252 | 3.314 | 0.111 | 0.599 | 0.036 |
| C0 | 243 | 3.131 | 0.155 | 0.570 | 0.026 |
| B0 | 294 | 3.542 | 0.098 | 0.623 | 0.035 |
| D0 | 260 | 3.273 | 0.128 | 0.589 | 0.030 |
| A28 | 199 | 3.840 | 0.060 | 0.725 | 0.084 |
| B28 | 136 | 3.231 | 0.123 | 0.658 | 0.060 |
| C28 | 268 | 4.092 | 0.058 | 0.732 | 0.064 |
| D28 | 145 | 3.430 | 0.068 | 0.689 | 0.101 |
| BLANK_0 | 635 | 5.449 | 0.014 | 0.844 | 0.114 |
| AA_0 | 351 | 4.466 | 0.045 | 0.762 | 0.063 |
| BB_0 | 278 | 3.372 | 0.199 | 0.599 | 0.018 |
| CC_0 | 330 | 4.515 | 0.031 | 0.778 | 0.096 |
| DD_0 | 436 | 4.967 | 0.019 | 0.817 | 0.121 |
| BIOCHAR_0 | 1035 | 5.739 | 0.014 | 0.827 | 0.070 |
| BLANK_60 | 896 | 6.259 | 0.004 | 0.921 | 0.298 |
| AA_60 | 761 | 5.830 | 0.008 | 0.879 | 0.171 |
| BB_60 | 856 | 5.771 | 0.014 | 0.855 | 0.083 |
| CC_60 | 661 | 5.739 | 0.008 | 0.884 | 0.179 |
| DD_60 | 987 | 6.288 | 0.004 | 0.912 | 0.243 |
| BIOCHAR_60 | 758 | 6.145 | 0.004 | 0.927 | 0.332 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




