Submitted:
21 August 2024
Posted:
21 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Region
2.2. Experimental Colonies
2.3. V. destructor Infestation in Adult bees, Brood, and Daily Mite Fall
2.4. Bee Population, Brood, and Food Stores of Experimental Colonies
2.5. Statistical Analyses
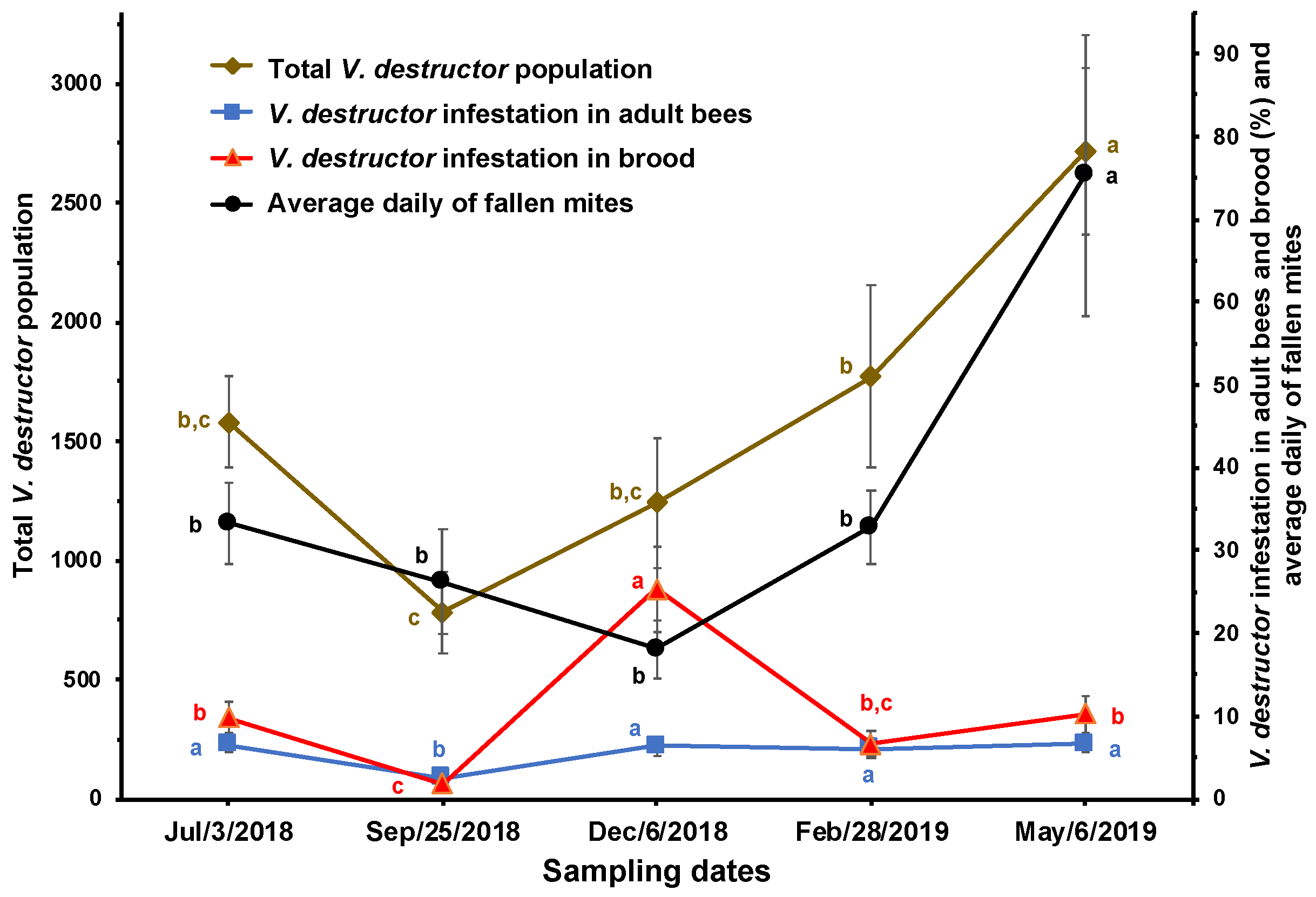
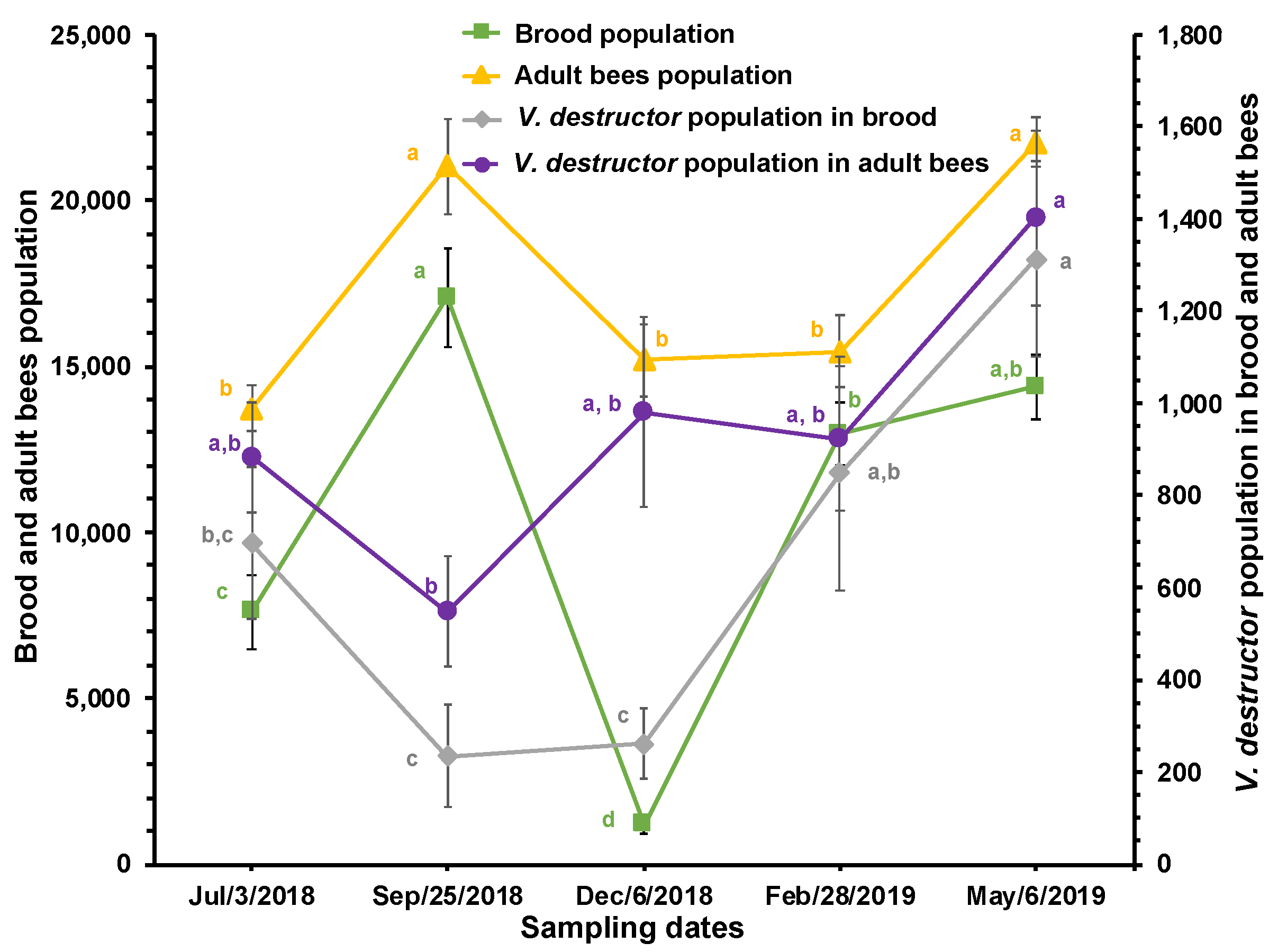
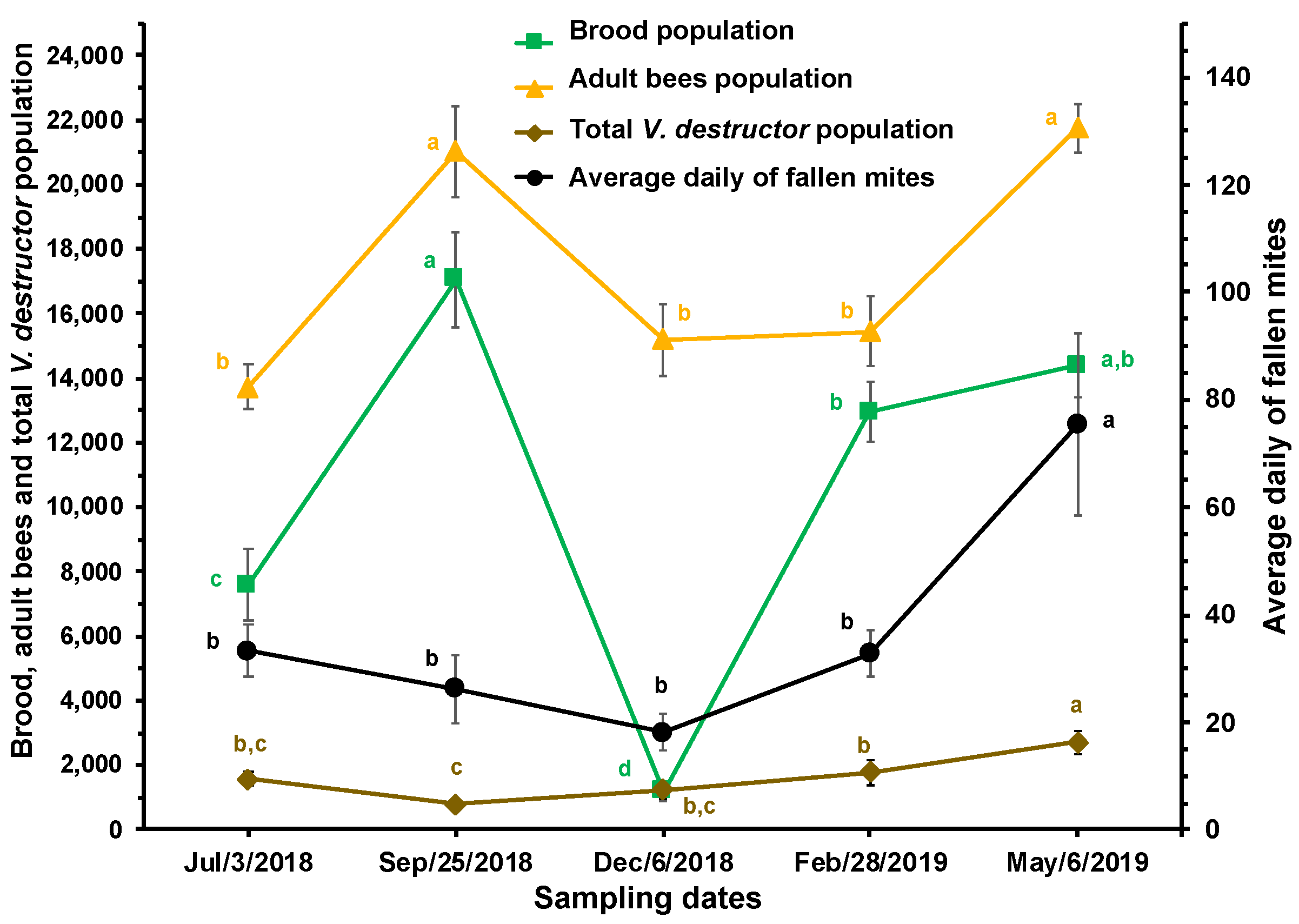
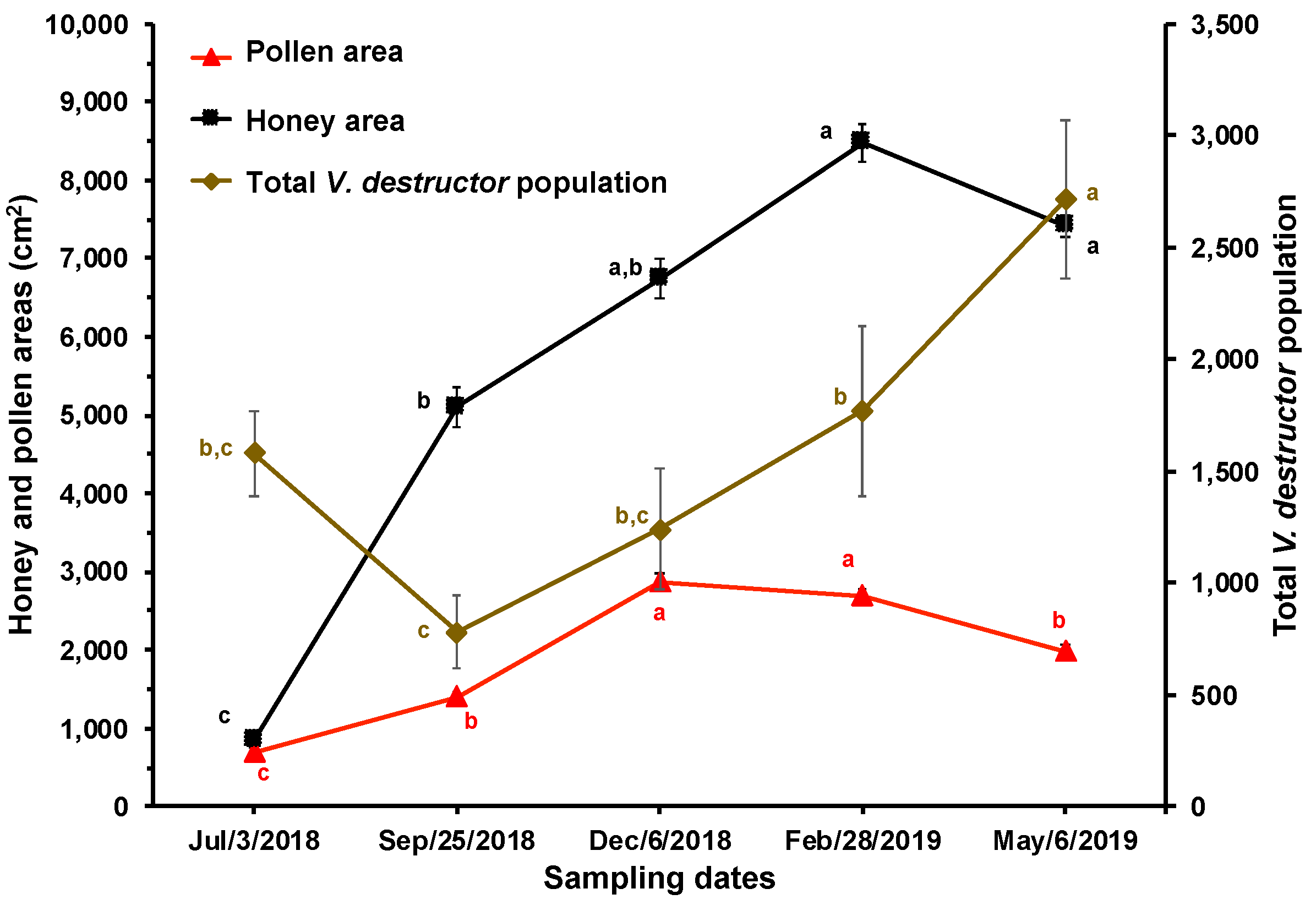
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Anderson, D.L.; Trueman, J.W.H. Varroa jacobsoni (Acari: Varroidae) Is More than One Species. Exp. Appl. Acarol. 2000, 24, 165–189. [Google Scholar] [CrossRef]
- Nazzi, F.; Le Conte, Y. Ecology of Varroa destructor, the major ectoparasite of the western honey bee, Apis mellifera. Annu. Rev. Entomol. 2016, 61, 417–432. [Google Scholar] [CrossRef] [PubMed]
- Traynor, K.S.; Mondet, F.; de Miranda, J.R.; Techer, M.; Kowallik, V.; Oddie, M.A.Y.; Chantawannakul, P.; McAfee, A. Varroa destructor: A complex parasite, crippling honey bees worldwide. Trends Parasitol. 2020, 36, 592–606. [Google Scholar] [CrossRef] [PubMed]
- Chapman, N.C.; Colin, T.; Cook, J.; da Silva, C.R.B.; Gloag, R.; Hogendoorn, K.; Howard, S.R.; Remnant, E.J.; Roberts, J.M.K.; Tierney, S.M.; et al. The final frontier: Ecological and evolutionary dynamics of a global parasite invasion. Biol. Lett. 2023, 19, 20220589. [Google Scholar] [CrossRef] [PubMed]
- Bowen-Walker, P.L.; Gunn, A. The effect of the ectoparasitic mite, Varroa destructor on adult worker honeybee (Apis mellifera) emergence weights, water, protein, carbohydrate, and lipid levels. Entomol. Exp. Appl. 2001, 101, 207–217. [Google Scholar] [CrossRef]
- vanEngelsdorp, D.; Meixner, M.D. A historical review of managed honey bee populations in europe and the United States and the factors that may affect them. J. Invertebr. Pathol. 2010, 103, S80–S95. [Google Scholar] [CrossRef]
- Koleoglu, G.; Goodwin, P.H.; Reyes-Quintana, M.; Hamiduzzaman, M.M; Guzman-Novoa, E. Effect of Varroa destructor, wounding and Varroa homogenate on gene expression in brood and adult honey bees. PLoS ONE 2017, 12, e0169669. [Google Scholar] [CrossRef]
- Koleoglu, G.; Goodwin, P.H.; Reyes-Quintana, M.; Hamiduzzaman, M.M.; Guzman-Novoa, E. Varroa destructor parasitism reduces hemocyte concentrations and prophenol oxidase gene expression in bees from two populations. Parasitol. Res. 2018, 117, 1175–1183. [Google Scholar] [CrossRef]
- Morfin, N.; Anguiano-Baez, R.; Guzman-Novoa, E. Honey Bee (Apis mellifera) Immunity. Vet. Clin. N. Am. Food Anim. 2021, 37, 521–533. [Google Scholar] [CrossRef]
- Chen, Y.; Pettis, J.S.; Evans, J.D.; Kramer, M.; Feldlaufer, M.F. Transmission of kashmir bee virus by the ectoparasitic mite Varroa destructor. Apidologie 2004, 35, 441–448. [Google Scholar] [CrossRef]
- Martin, S.J.; Ball, B.V.; Carreck, N.L. Prevalence and persistence of Deformed wing virus (DWV) in untreated or acaricide-treated Varroa destructor infested honey bee (Apis mellifera) colonies. J. Apic. Res. 2010, 49, 72–79. [Google Scholar] [CrossRef]
- Ryabov, E.V.; Childers, A.K.; Chen, Y.; Madella, S.; Nessa, A.; vanEngelsdorp, D.; Evans, J.D. Recent spread of Varroa destructor virus-1, a honey bee pathogen, in the United States. Sci. Rep. 2017, 7, 17447. [Google Scholar] [CrossRef]
- Dainat, B.; Evans, J.D.; Chen, Y.P.; Gauthier, L.; Neumanna, P. Dead or alive: Deformed wing virus and Varroa destructor reduce the life span of winter honeybees. Appl. Environ. Microbiol. 2012, 78, 981–987. [Google Scholar] [CrossRef]
- Arechavaleta-Velasco, M.E.; Guzman-Novoa, E. Producción de miel de colonias de abejas (Apis mellifera L.) tratadas y no tratadas con fluvalinato contra Varroa jacobsoni Oudemans en Valle de Bravo, Estado de México. Vet. Méx. 2000, 31, 379–382. [Google Scholar]
- Medina-Flores, C.A.; Guzmán-Novoa, E.; Aréchiga-Flores, C.; Aguilera-Soto, J.; Gutiérrez-Piña, F. Efecto del nivel de infestación de Varroa destructor sobre la producción de miel de colonias de Apis mellifera en el altiplano semiárido de México. Rev. Mex. Cienc. Pecu. 2011, 2, 313–317. [Google Scholar]
- Emsen, B.; Guzman-Novoa, E.; Kelly, P.G. Honey production of honey bee (Hymenoptera: Apidae) colonies with high and low Varroa destructor (Acari: Varroidae) infestation rates in eastern Canada. Can. Entomol. 2014, 146, 236–240. [Google Scholar] [CrossRef]
- Chauzat, M.-P.; Martel, A.-C.; Zeggane, S.; Drajnudel, P.; Schurr, F.; Clément, M.-C.; Ribière-Chabert, M.; Aubert, M.; Faucon, J.-P. A case control study and a survey on mortalities of honey bee colonies (Apis mellifera) in France during the winter of 2005-6. J. Apic. Res. 2010, 49, 40–51. [Google Scholar] [CrossRef]
- Guzmán-Novoa, E.; Eccles, L.; Calvete, Y.; Mcgowan, J.; Kelly, P.G.; Correa-Benítez, A. Varroa destructor is the main culprit for the death and reduced populations of overwintered honey bee (Apis mellifera) colonies in Ontario, Canada. Apidologie 2010, 41, 443–450. [Google Scholar] [CrossRef]
- Medina-Flores, C.A.; López-Carlos, M.; Carrillo-Muro, O.; Gray, A. Honey Bee Colony Losses in Mexico’s Semi-Arid High Plateau for the Winters 2016–2017 to 2021–2022. Insects 2023, 14, 453. [Google Scholar] [CrossRef]
- Rodríguez-Dehaibes, S.R.; Otero-Colina, G.; Villanueva-Jiménez, J.A.; Corcuera, P. ; Susceptibility of Varroa destructor (Gamasida: Varroidae) to four pesticides used in three Mexican apicultural regions under two different management systems. Int. J. Acarol. 2011, 37, 441–447. [Google Scholar] [CrossRef]
- Berry, J.A.; Hood, W.M.; Pietravalle, S.; Delaplane, K.S. Field-level sublethal effects of approved bee hive chemicals on Honey Bees (Apis mellifera L). PLoS ONE 2013, 8, e76536. [Google Scholar] [CrossRef] [PubMed]
- Martínez, P.J.F.; Medina, M.L.A. Evaluación de la resistencia del ácaro Varroa destructor al fluvalinato en colonias de abejas (Apis mellifera) en Yucatán, México. Rev. Mex. Cienc. Pecu. 2011, 2, 93–99. [Google Scholar]
- Kamler, M.; Nesvorna, M.; Stara, J.; Erban, T.; Hubert, J. Comparison of tau-fluvalinate, acrinathrin, and amitraz effects on susceptible and resistant populations of Varroa destructor in a vial test. Exp. Appl. Acarol. 2016, 69, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gashout, H.A.; Goodwin, P.H.; Guzman-Novoa, E. Lethality of synthetic and natural acaricides to worker honey bees (Apis mellifera) and their impact in the expression of health and detoxification-related genes. Environ. Sci. Pollut. Res. 2018, 25, 34730–34739. [Google Scholar] [CrossRef]
- Delaplane, K.S.; Hood, W.M. Effects of delayed acaricide treatment in honey bee colonies parasitized by Varroa jacobsoni and a late-season treatment threshold for the South-Eastern USA. J. Apic. Res. 1997, 36, 125–132. [Google Scholar] [CrossRef]
- Delaplane, K.S.; Hood, W.M. Economic Threshold for Varroa jacobsoni Oud. in the Southeastern USA. Apidologie 1999, 30, 383–395. [Google Scholar] [CrossRef]
- Delaplane, K.S. Varroa control: timing is everything. Am. Bee J 1998, 138, 575–576. [Google Scholar]
- Caron, D. Delaware bee mites survey. Am. Bee J. 1999, 139, 631–633. [Google Scholar]
- González-Cabrera, J.; Rodríguez-Vargas, S.; Davies, T. G E.; Field, L.M.; Schmehl, D.; Ellis, J.D.; Krieger, K.; Williamson, M.S. Novel Mutations in the Voltage-Gated Sodium Channel of Pyrethroid-Resistant Varroa destructor Populations from the Southeastern USA. PloS ONE 2016, 11, e0155332. [Google Scholar] [CrossRef]
- Gatien, P.; Currie, R.W. Timing of acaracide treatments for control of low-level populations of Varroa destructor (Acari: Varroidae) and implications for colony performance of honey bees. Can. Entomol. 2003, 135, 749–763. [Google Scholar] [CrossRef]
- Kokkinis, M.; Liakos, V. Population dynamics of Varroa destructor in colonies of Apis mellifera macedonica in Greece. J. Apic. Res. 2004, 43, 150–154. [Google Scholar] [CrossRef]
- Calderón, R.A.; van Veen, J.W. Varroa destructor (Mesostigmata: Varroidae) in Costa Rica: population dynamics and its influence on the colony condition of Africanized honey bees (Hymenoptera: Apidae). Rev. Biol. Trop. 2008, 56, 1741–1747. [Google Scholar] [CrossRef] [PubMed]
- Strauss, U.; Pirk, C.W.W.; Crewe, R.M.; Human, H.; Dietemann, V. Impact of Varroa destructor on honeybee (Apis mellifera scutellata) colony development in South Africa. Exp. Appl. Acarol. 2015, 65, 89–106. [Google Scholar] [CrossRef]
- da Silva, L.A.; da Silva, A.D.; Domingos, H.G.T.; Bergamo, G.C.; Message, D.; Gramacho, K.P. Varroa destructor mite population dynamics in africanized honeybee (Apis mellifera) colonies in a semi-arid region. Exp. Appl. Acarol. 2024, 1–11. [Google Scholar] [CrossRef]
- INEGI. Instituto Nacional de Estadística Geografía e Informática. Aspectos geográficos de Zacatecas. Available online: https://www.inegi.org.mx/contenidos/app/areasgeograficas/resumen/resumen_32.pdf (accessed on 8 June 2024).
- Guzman-Novoa, E.; Morfin, N.; Dainat, B.; Williams, G.R.; van der Steen, J.; Correa-Benítez, A.; Delaplane, K.S. Standard methods to estimate strength parameters, flight activity, comb construction, and fitness of Apis mellifera colonies 2.0. J. Apic. Res. 2024. [Google Scholar] [CrossRef]
- De Jong, D.; De Andrea Roma, D.; Gonçalves, L.S. A comparative analysis of shaking solutions for the detection of Varroa jacobsoni on adult honeybees. Apidologie 1982, 13, 297–306. [Google Scholar] [CrossRef]
- Dietemann, V.; Nazzi, F.; Martin, S.J.; Anderson, D.L.; Locke, B.; Delaplane, K.S.; Wauquiez, Q.; Tannahill, C.; Frey, E.; Ziegelmann, B.; et al. Standard methods for varroa research. J. Apic. Res. 2013, 52, 1–54. [Google Scholar] [CrossRef]
- SAS. Statistical Analysis Software, version 9.0; SAS Institute: Raleigh, NC, USA, 2002. [Google Scholar]
- Fries, I.; Hansen, H.; Imdorf, A.; Rosenkranz, P. Swarming in honey bees (Apis mellifera) and Varroa destructor population development in Sweden. Apidologie 2003, 34, 389–397. [Google Scholar] [CrossRef]
- Moretto, G.; Goncalves, L.S.; De Jong, D. Relationship between food availability and the reproductive ability of the mite Varroa jacobsoni in africanized bee colonies. Am Bee J. 1997, 137, 67–9. [Google Scholar]
- Branco, M.R.; Kidd, N.A.C.; Pickard, R.S. A comparative evaluation of sampling methods for Varroa destructor (Acari: Varroidae) population estimation. Apidologie 2006, 37, 452–461. [Google Scholar] [CrossRef]
- Moretto, G.; Goncalves, L.S.; De Jong, D. Africanized bees are more efficient at removing Varroa jacobsoni: preliminary report. Am. Bee J. 1991, 131, 434. [Google Scholar]
- Rosenkranz, P.; Aumeier, P.; Ziegelmann, B. Biology and control of Varroa destructor. J. Invertebr. Pathol. 2010, 103, S96–S119. [Google Scholar] [CrossRef]
- SAGARPA (Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación). Modificación a la Norma Oficial Mexicana NOM-001-ZOO-1994, Campaña Contra la Varroasis de las Abejas. 2005. Available online: https://www.agricultura.gob.mx/sites/default/files/sagarpa/document/2018/08/07/1274/180919-nom-001-zoo-1994-y-nom-002-zoo-1994.pdf (accessed on 24 July 2024).
- Maya-Martínez, O.; Medina-Flores, C.; Aquino-Pérez, G.; Olmos-Oropeza, G.; López-Carlos, M. Seasonal treatment with amitraz against Varroa destructor and its effects in honey bee colonies of Apis mellifera. Abanico Vet. 2020, 10, e–129. [Google Scholar] [CrossRef]
- Medina, L.M.; Martin, S.J.; Espinosa-Montaño, L.; Ratnieks, F.L. Reproduction of Varroa destructor in worker brood of Africanized honey bees (Apis mellifera). Exp. Appl. Acarol. 2002, 27, 79–88. [Google Scholar] [CrossRef]
- Dynes, T.L.; Berry, J.A.; Delaplane, K.S.; De Roode, J.C.; Brosi, B.J. Assessing virulence of Varroa destructor mites from different honey bee management regimes. Apidologie 2020, 51, 276–289. [Google Scholar] [CrossRef]
- Calis, J.N.M.; Fries, I.; Ryrie, S.C. Population modelling of Varroa jacobsoni Oud. Apidologie 1999, 30, 111–124. [Google Scholar] [CrossRef]
- Frey, E.; Rosenkranz, P. Autumn Invasion Rates of Varroa destructor (Mesostigmata: Varroidae) Into Honey Bee (Hymenoptera: Apidae) Colonies and the Resulting Increase in Mite Populations. J. Econ. Entomol. 2014, 107, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Seeley, T.D.; Smith, M.L. Crowding Honey bee Colonies in Apiaries Can Increase Their Vulnerability to the Deadly Ectoparasite Varroa destructor. Apidologie 2015, 46, 716–727. [Google Scholar] [CrossRef]
- Messan, K.; DeGrandi-Hoffman, G.; Castillo-Chavez, C.; Kang, Y. Migration effects on population dynamics of the honybee-mite interactions. Math. Model. Nat. Phenom. 2017, 12, 84–115. [Google Scholar] [CrossRef]
- de Guzman, L.I.; Rinderer, T.E.; Frake, A.M. Growth of Varroa destructor (acari: Varroidae) populations in Russian honey bee (hymenoptera: Apidae) colonies. Ann. Entomol. Soc. Am. 2007, 100, 187–195. [Google Scholar] [CrossRef]
- Medina-Flores, C.A.; Guzman-Novoa, E.; Hamiduzzaman, M.M.; Aréchiga-Flores, C.F.; López-Carlos, M.A. Africanized honey bees (Apis mellifera) have low infestation levels of the mite Varroa destructor in different ecological regions in Mexico. Genet. Mol. Res. 2014, 13, 7282–7293. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Cuellar, A.K.; De la Mora, A.; Contreras-Escareño, F.; Morfin, N.; Tapia-González, J.M.; Macías-Macías, J.O.; Petukhova, T.; Correa-Benítez, A.; Guzman-Novoa, E. Genotype, but not climate, affects the resistance of honey bees (Apis mellifera) to viral infections and to the mite Varroa destructor. Vet. Sci. 2022, 9, 358. [Google Scholar] [CrossRef] [PubMed]
- Morfin, N.; Goodwin, P.H.; Guzman-Novoa, E. Varroa destructor and its impacts on honey bee biology. Front. Bee Sci. 2023, 1, 1272937. [Google Scholar] [CrossRef]
- Guzman-Novoa, E.; Corona, M.; Alburaki, M.; Reynaldi, F.J.; Invernizzi, C.; Fernández de Landa, G.; Maggi, M. Honey bee populations surviving Varroa destructor parasitism in Latin America and their mechanisms of resistance. Front. Ecol. Evol. 2024, 12, 1434490. [Google Scholar] [CrossRef]
| Variable | Infestation adult | Infestation brood | Fallen of mites | Total mites | Brood amount | Adult population | Pollen area |
|---|---|---|---|---|---|---|---|
| Infestation brood | 0.48*** | ||||||
| Fallen mites | 0.43** | 0.12ns | |||||
| Total mites | 0.73*** | 0.40** | 0.62*** | ||||
| Brood amount | -0.30* | -0.65*** | 0.13ns | 0.04ns | |||
| Adult population | -0.23ns | -0.18ns | 0.17ns | 0.15ns | 0.59*** | ||
| Pollen area | 0.20ns | 0.19ns | 0.18ns | 0.10ns | -0.12ns | -0.02ns | |
| Honey area | 0.17ns | 0.18ns | 0.10ns | 0.30ns | 0.15ns | 0.21ns | 0.45*** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





