Submitted:
21 August 2024
Posted:
22 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
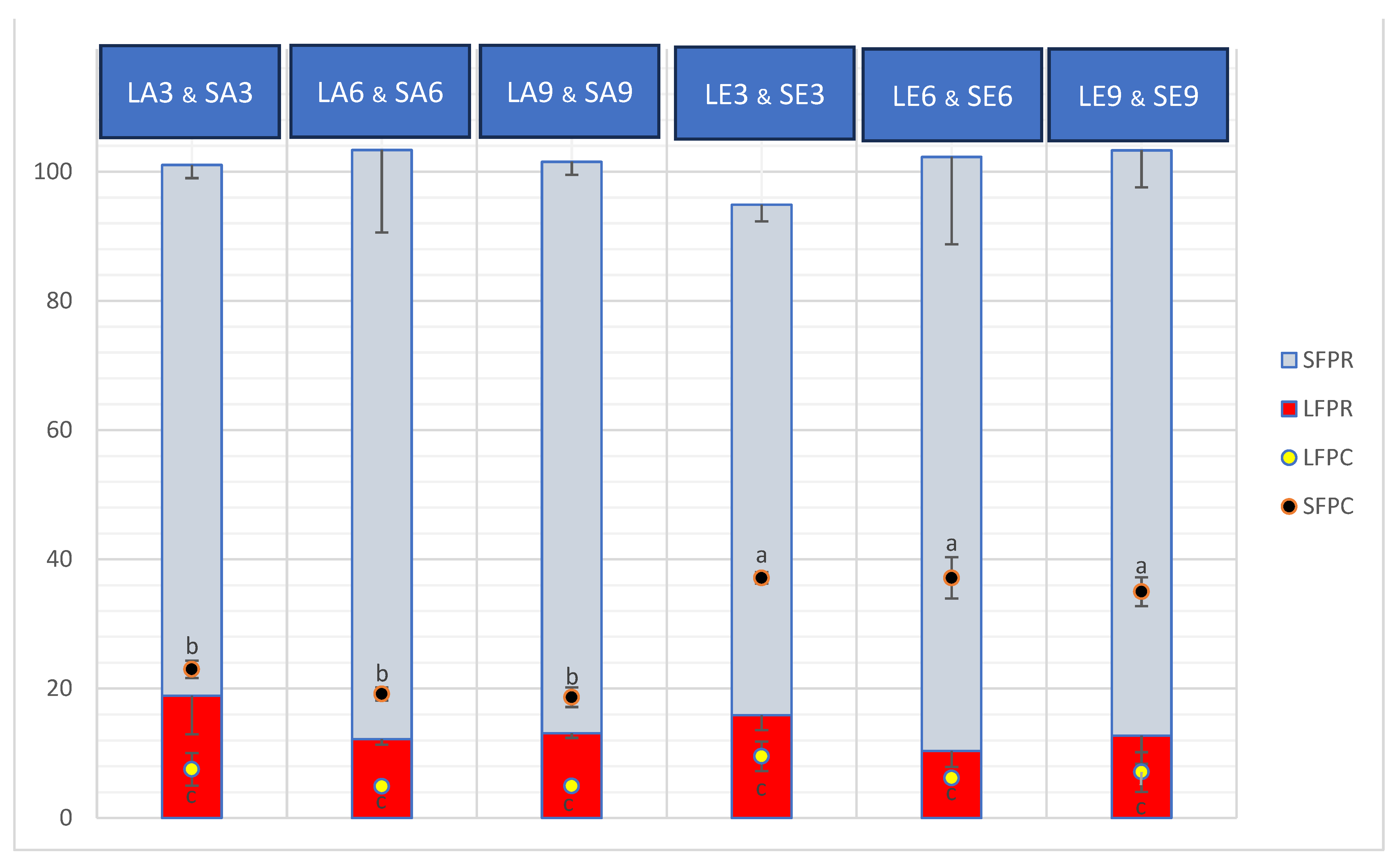
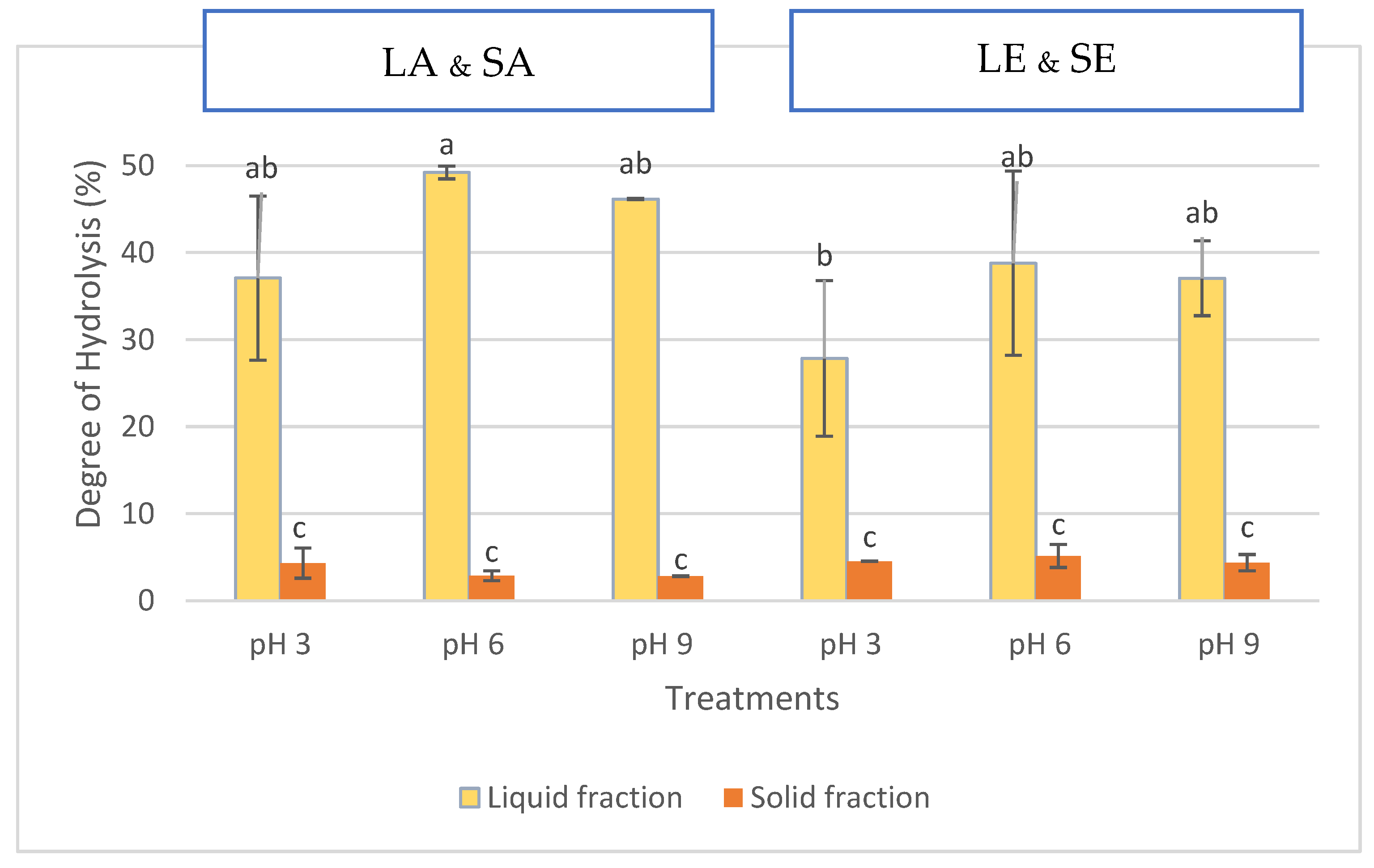
2.1. Protein Content, Protein Recovery, and Degree of Hydrolysis
2.2. Amino Acid Composition
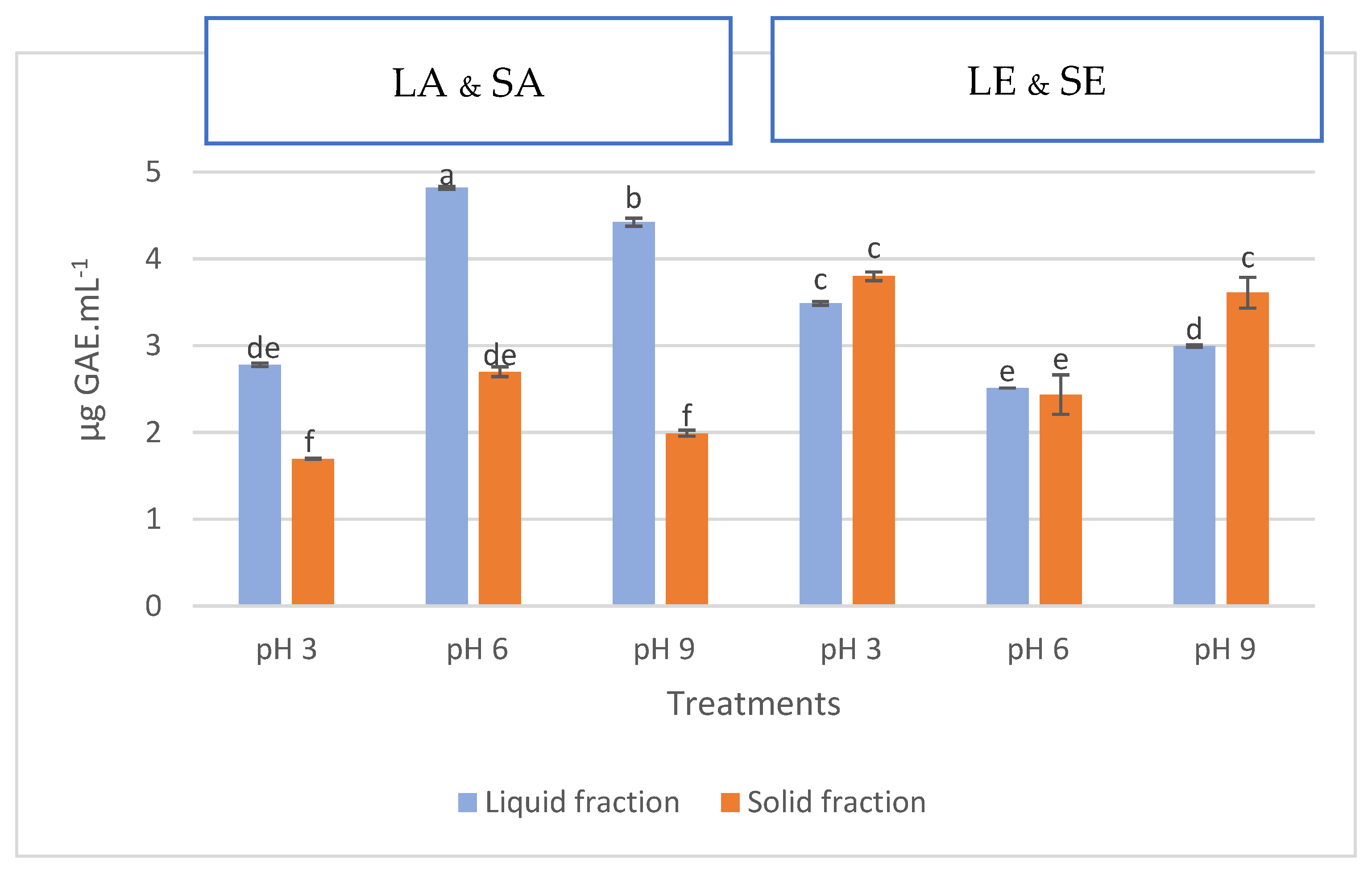
2.3. Total Phenolic Content
2.4. Antioxidant Properties
3. Discussion
3.1. Protein Content, Protein Recovery, and DH
3.2. Amino Acid Composition
3.3. Total Phenolic Content
3.4. Antioxidant Properties
4. Materials and Methods
4.1. Seaweed Biomass Preparation
4.2. Enzymes and Chemicals
4.3. Aqueous Extraction
4.4. Enzymatic Hydrolysis
4.5. Protein Content and Recovery
4.6. DH
4.7. Amino Acid Profile
4.8. Total Phenolic Content
4.9. DPPH Radical Scavenging Activity
4.10. Fe2+ Chelating Activity
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lomartire, S.; Gonçalves, A.M.M. An Overview of Potential Seaweed-Derived Bioactive Compounds for Pharmaceutical Applications. Marine Drugs 2022, 20, 141. [Google Scholar] [CrossRef]
- Chakraborty, K. Seaweeds as Prospective Marine Resources for the Development of Bioactive Pharmacophores and Nutraceuticals. In Sustainable Global Resources of Seaweeds Volume 2: Food, Pharmaceutical and Health Applications; Ambati, R.R., Ravishankar, G.A., Eds.; Springer International Publishing: Cham, 2022; ISBN 978-3-030-92174-3. [Google Scholar]
- Lomartire, S.; Gonçalves, A.M.M. Marine Macroalgae Polyphenols as Potential Neuroprotective Antioxidants in Neurodegenerative Diseases. Mar Drugs 2023, 21. [Google Scholar] [CrossRef]
- Amaro, H.M.; Pagels, F.; Tavares, T.G.; Costa, I.; Sousa-Pinto, I.; Guedes, A.C. Antioxidant and Anti-Inflammatory Potential of Seaweed Extracts as Functional Ingredients. Hydrobiology 2022, 1, 469–482. [Google Scholar] [CrossRef]
- Seca, A.M.L.; Pinto, D.C.G.A. Overview on the Antihypertensive and Anti-Obesity Effects of Secondary Metabolites from Seaweeds. Mar Drugs 2018, 16. [Google Scholar] [CrossRef]
- Purcell, D.; Packer, M.A.; Hayes, M. Angiotensin-I-Converting Enzyme Inhibitory Activity of Protein Hydrolysates Generated from the Macroalga Laminaria Digitata (Hudson) JV Lamouroux 1813. Foods 2022, 11. [Google Scholar] [CrossRef]
- Lee, H.; Selvaraj, B.; Lee, J.W. Anticancer Effects of Seaweed-Derived Bioactive Compounds. Applied Sciences 2021, 11. [Google Scholar] [CrossRef]
- Cho, C.-H.; Lu, Y.-A.; Kim, M.-Y.; Jeon, Y.-J.; Lee, S.-H. Therapeutic Potential of Seaweed-Derived Bioactive Compounds for Cardiovascular Disease Treatment. Applied Sciences 2022, 12. [Google Scholar] [CrossRef]
- Echave, J.; Otero, P.; Garcia-Oliveira, P.; Munekata, P.E.S.; Pateiro, M.; Lorenzo, J.M.; Simal-Gandara, J.; Prieto, M.A. Seaweed-Derived Proteins and Peptides: Promising Marine Bioactives. Antioxidants 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Cotas, J.; Gonçalves, A.M. Seaweed Proteins: A Step towards Sustainability? Nutrients 2024, 16. [Google Scholar] [CrossRef]
- Ghelichi, S.; Sørensen, A.-D.M.; Hajfathalian, M.; Jacobsen, C. Effect of Post-Extraction Ultrasonication on Compositional Features and Antioxidant Activities of Enzymatic/Alkaline Extracts of Palmaria Palmata. Mar Drugs 2024, 22. [Google Scholar] [CrossRef]
- Sabeena Farvin, K.H.; Jacobsen, C. Phenolic Compounds and Antioxidant Activities of Selected Species of Seaweeds from Danish Coast. Food Chem 2013, 138, 1670–1681. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Gayen, K.; Bhowmick, T.K. Green Extraction of Biomolecules from Algae Using Subcritical and Supercritical Fluids. Biomass Convers Biorefin 2022, 1, 1–23. [Google Scholar] [CrossRef]
- Sumampouw, G.A.; Jacobsen, C.; Getachew, A.T. Optimization of Phenolic Antioxidants Extraction from Fucus Vesiculosus by Pressurized Liquid Extraction. J Appl Phycol 2021, 33, 1195–1207. [Google Scholar] [CrossRef]
- Gisbert, M.; Barcala, M.; Rosell, C.M.; Sineiro, J.; Moreira, R. Aqueous Extracts Characteristics Obtained by Ultrasound-Assisted Extraction from Ascophyllum Nodosum Seaweeds: Effect of Operation Conditions. J Appl Phycol 2021, 33, 3297–3308. [Google Scholar] [CrossRef]
- Lee, Z.J.; Xie, C.; Duan, X.; Ng, K.; Suleria, H.A.R. Optimization of Ultrasonic Extraction Parameters for the Recovery of Phenolic Compounds in Brown Seaweed: Comparison with Conventional Techniques. Antioxidants 2024, 13. [Google Scholar] [CrossRef]
- Sasaki, C.; Tamura, S.; Suzuki, M.; Etomi, K.; Nii, N.; Hayashi, J.; Kanemaru, K. Continuous Microwave-Assisted Step-by-Step Extraction of Bioactive Water-Soluble Materials and Fucoidan from Brown Seaweed Undaria Pinnatifida Waste. Biomass Convers Biorefin 2024, 14, 7673–7682. [Google Scholar] [CrossRef]
- Vásquez, V.; Martínez, R.; Bernal, C. Enzyme-Assisted Extraction of Proteins from the Seaweeds Macrocystis Pyrifera and Chondracanthus Chamissoi: Characterization of the Extracts and Their Bioactive Potential. J Appl Phycol 2019, 31, 1999–2010. [Google Scholar] [CrossRef]
- Naseri, A.; Marinho, G.S.; Holdt, S.L.; Bartela, J.M.; Jacobsen, C. Enzyme-Assisted Extraction and Characterization of Protein from Red Seaweed Palmaria Palmata. Algal Res 2020, 47, 101849. [Google Scholar] [CrossRef]
- Veide Vilg, J.; Undeland, I. PH-Driven Solubilization and Isoelectric Precipitation of Proteins from the Brown Seaweed Saccharina Latissima—Effects of Osmotic Shock, Water Volume and Temperature. J Appl Phycol 2017, 29, 585–593. [Google Scholar] [CrossRef]
- Sun, Z.; Chi, Q.; Sun, L.; Liu, Y. Protein Extraction from Microalgae Residue and Nutritional Assessment. Bioprocess Biosyst Eng 2022, 45, 1879–1888. [Google Scholar] [CrossRef]
- Stévant, P.; Schmedes, P.S.; Le Gall, L.; Wegeberg, S.; Dumay, J.; Rebours, C. Concise Review of the Red Macroalga Dulse, Palmaria Palmata (L.) Weber & Mohr. Journal of Applied Phycology 2023, 35, 523–550. [Google Scholar] [CrossRef]
- Bjarnadóttir, M.; Aðalbjörnsson, B.V.; Nilsson, A.; Slizyte, R.; Roleda, M.Y.; Hreggviðsson, G.Ó.; Friðjónsson, Ó.H.; Jónsdóttir, R. Palmaria Palmata as an Alternative Protein Source: Enzymatic Protein Extraction, Amino Acid Composition, and Nitrogen-to-Protein Conversion Factor. J Appl Phycol 2018, 30, 2061–2070. [Google Scholar] [CrossRef]
- Veide Vilg, J.; Undeland, I. PH-Driven Solubilization and Isoelectric Precipitation of Proteins from the Brown Seaweed Saccharina Latissima—Effects of Osmotic Shock, Water Volume and Temperature. J Appl Phycol 2017, 29, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Tahergorabi Reza and Jaczynski, J. Isoelectric Solubilization/Precipitation as a Means to Recover Protein and Lipids from Seafood By-Products. In Seafood Processing By-Products: Trends and Applications; Kim, S.-K., Ed.; Springer New York: New York, NY, 2014; ISBN 978-1-4614-9590-1. [Google Scholar]
- Liu, Z.; Sun, X. A Critical Review of the Abilities, Determinants, and Possible Molecular Mechanisms of Seaweed Polysaccharides Antioxidants. International Journal of Molecular Sciences 2020, 21, 7774. [Google Scholar] [CrossRef] [PubMed]
- Echave, J.; Fraga-Corral, M.; Garcia-Perez, P.; Popović-Djordjević, J.; Avdović, E.H.; Radulović, M.; Xiao, J.; Prieto, M.A.; Simal-Gandara, J. Seaweed Protein Hydrolysates and Bioactive Peptides: Extraction, Purification, and Applications. Marine Drugs 2021, 19, 500. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Bandyopadhyay, P. Polysaccharide-Protein Interactions and Their Relevance in Food Colloids. In The Complex World of Polysaccharides; Karunaratne, D.N., Ed.; IntechOpen: Rijeka, 2012. [Google Scholar]
- Zheng, J.; Van der Meeren, P.; Sun, W. New Insights into Protein–Polysaccharide Complex Coacervation: Dynamics, Molecular Parameters, and Applications. Aggregate 2024, 5, e449. [Google Scholar] [CrossRef]
- Comert, F.; Malanowski, A.J.; Azarikia, F.; Dubin, P.L. Coacervation and Precipitation in Polysaccharide–Protein Systems. Soft Matter 2016, 12, 4154–4161. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, H.; Li, S.; Song, C.; Zhang, S.; Ren, J.; Udenigwe, C.C. Maillard-Type Protein–Polysaccharide Conjugates and Electrostatic Protein–Polysaccharide Complexes as Delivery Vehicles for Food Bioactive Ingredients: Formation, Types, and Applications. Gels 2022, 8, 135. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.N.; Nickerson, M.T. Review on Plant Protein–Polysaccharide Complex Coacervation, and the Functionality and Applicability of Formed Complexes. J Sci Food Agric 2018, 98, 5559–5571. [Google Scholar] [CrossRef]
- Soussi Hachfi, R.; Hamon, P.; Rousseau, F.; Famelart, M.H.; Bouhallab, S. Ionic Strength Dependence of the Complex Coacervation between Lactoferrin and β-Lactoglobulin. Foods 2023, 12, 1040. [Google Scholar] [CrossRef]
- Stévant, P.; Schmedes, P.S.; Le Gall, L.; Wegeberg, S.; Dumay, J.; Rebours, C. Concise Review of the Red Macroalga Dulse, Palmaria Palmata (L.) Weber & Mohr. Journal of Applied Phycology 2023, 35, 523–550. [Google Scholar] [CrossRef]
- Dong, Z.; Yu, S.; Zhai, K.; Bao, N.; Rashed, M.M.A.; Wu, X. Fabrication and Characterization of Complex Coacervation: The Integration of Sesame Protein Isolate-Polysaccharides. Foods 2023, 12, 3696. [Google Scholar] [CrossRef]
- Chiu, T. hsin; Chen, M. lun; Chang, H. chia Comparisons of Emulsifying Properties of Maillard Reaction Products Conjugated by Green, Red Seaweeds and Various Commercial Proteins. Food Hydrocoll 2009, 23, 2270–2277. [Google Scholar] [CrossRef]
- Thiviya, P.; Gamage, A.; Gama-Arachchige, N.S.; Merah, O.; Madhujith, T. Seaweeds as a Source of Functional Proteins. Phycology 2022, 2, 216–243. [Google Scholar] [CrossRef]
- Masson, P.; Lushchekina, S. Conformational Stability and Denaturation Processes of Proteins Investigated by Electrophoresis under Extreme Conditions. Molecules 2022, 27, 6861. [Google Scholar] [CrossRef]
- Rovelli, G.; Wilson, K.R. Elucidating the Mechanism for the Reaction of O-Phthalaldehyde with Primary Amines in the Presence of Thiols. Journal of Physical Chemistry B 2023, 127, 3257–3265. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, Y.; Li, X. Ortho-Phthalaldehyde (OPA)-Based Chemoselective Protein Bioconjugation and Peptide Cyclization. Methods Enzymol 2020, 639, 237–261. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Solis, I.; Ibarra-Herrera, C.C.; Del, M.; Rocha-Pizaña, R.; Luna-Vital, D. Alkaline Extraction–Isoelectric Precipitation of Plant Proteins. Green Protein Processing Technologies from Plants 2023, 1–29. [Google Scholar] [CrossRef]
- Ying, Y.; Li, H. Recent Progress in the Analysis of Protein Deamidation Using Mass Spectrometry. Methods 2022, 200, 42–57. [Google Scholar] [CrossRef]
- Hadidi, M.; Aghababaei, F.; McClements, D.J. Enhanced Alkaline Extraction Techniques for Isolating and Modifying Plant-Based Proteins. Food Hydrocoll 2023, 145, 109132. [Google Scholar] [CrossRef]
- Lewis, C.; Hughes, B.H.; Vasquez, M.; Wall, A.M.; Northrup, V.L.; Witzleb, T.J.; Billiot, E.J.; Fang, Y.; Billiot, F.H.; Morris, K.F. Effect of PH on the Binding of Sodium, Lysine, and Arginine Counterions to l-Undecyl Leucinate Micelles. J Surfactants Deterg 2016, 19, 1175–1188. [Google Scholar] [CrossRef]
- Kuang, J.; Tao, Y.; Song, Y.; Chemmalil, L.; Mussa, N.; Ding, J.; Li, Z.J. Understanding the Pathway and Kinetics of Aspartic Acid Isomerization in Peptide Mapping Methods for Monoclonal Antibodies. Anal Bioanal Chem 2021, 413, 2113–2123. [Google Scholar] [CrossRef]
- Chen, Y.; Tao, K.; Ji, W.; Kumar, V.B.; Rencus-Lazar, S.; Gazit, E. Histidine as a Key Modulator of Molecular Self-Assembly: Peptide-Based Supramolecular Materials Inspired by Biological Systems. Materials Today 2022, 60, 106–127. [Google Scholar] [CrossRef]
- Liao, S.M.; Du, Q.S.; Meng, J.Z.; Pang, Z.W.; Huang, R.B. The Multiple Roles of Histidine in Protein Interactions. Chem Cent J 2013, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Schendel, R.R.; Pandeya, P.R. Determination of (Total) Phenolics and Antioxidant Capacity in Food and Ingredients. 2024, 419–429. [Google Scholar] [CrossRef]
- Bock, A.; Kieserling, H.; Steinhäuser, U.; Rohn, S. Impact of Phenolic Acid Derivatives on the Oxidative Stability of β-Lactoglobulin-Stabilized Emulsions. Antioxidants 2023, 12, 182. [Google Scholar] [CrossRef] [PubMed]
- Vuolo, M.M.; Lima, V.S.; Maróstica Junior, M.R. Phenolic Compounds: Structure, Classification, and Antioxidant Power. Bioactive Compounds: Health Benefits and Potential Applications 2019, 33–50. [Google Scholar] [CrossRef]
- Friedman, M.; Jürgens, H.S. Effect of PH on the Stability of Plant Phenolic Compounds. J Agric Food Chem 2000, 48, 2101–2110. [Google Scholar] [CrossRef]
- Abd El-Maksoud, A.A.; Abd El-Ghany, I.H.; El-Beltagi, H.S.; Anankanbil, S.; Banerijee, C.; Petersen, S. V.; Pérez, B.; Guo, Z. Adding Functionality to Milk-Based Protein: Preparation, and Physico-Chemical Characterization of β-Lactoglobulin-Phenolic Conjugates. Food Chem 2018, 241, 281–289. [Google Scholar] [CrossRef]
- Mohammed, H.O.; O’grady, M.N.; O’sullivan, M.G.; Hamill, R.M.; Kilcawley, K.N.; Kerry, J.P. An Assessment of Selected Nutritional, Bioactive, Thermal and Technological Properties of Brown and Red Irish Seaweed Species. Foods 2021, 10, 2784. [Google Scholar] [CrossRef]
- Wołosiak, R.; Drużyńska, B.; Derewiaka, D.; Piecyk, M.; Majewska, E.; Ciecierska, M.; Worobiej, E.; Pakosz, P. Verification of the Conditions for Determination of Antioxidant Activity by ABTS and DPPH Assays—A Practical Approach. Molecules 2021, 27, 50. [Google Scholar] [CrossRef] [PubMed]
- Aluko, R.E. Amino Acids, Peptides, and Proteins as Antioxidants for Food Preservation. Handbook of Antioxidants for Food Preservation 2015, 105–140. [Google Scholar] [CrossRef]
- Andjelković, M.; Van Camp, J.; De Meulenaer, B.; Depaemelaere, G.; Socaciu, C.; Verloo, M.; Verhe, R. Iron-Chelation Properties of Phenolic Acids Bearing Catechol and Galloyl Groups. Food Chem 2006, 98, 23–31. [Google Scholar] [CrossRef]
- Maleki, N.; Roomiani, L.; Tadayoni, M. Microwave-Assisted Extraction Optimization, Antimicrobial and Antioxidant Properties of Carrageenan from Red Algae (Gracilaria Acerosa). Journal of Food Measurement and Characterization 2023, 17, 1156–1166. [Google Scholar] [CrossRef]
- Ali, K.A.; Wahba, M.I.; Abou-Zeid, R.E.; Kamel, S. Development of Carrageenan Modified with Nanocellulose-Based Materials in Removing of Cu2+, Pb2+, Ca2+, Mg2+, and Fe2+. International Journal of Environmental Science and Technology 2019, 16, 5569–5576. [Google Scholar] [CrossRef]
- Lawson, M.K.; Valko, M.; Cronin, M.T.D.; Jomová, K. Chelators in Iron and Copper Toxicity. Curr Pharmacol Rep 2016, 2, 271–280. [Google Scholar] [CrossRef]
- Milach, O.A.; Mel’sitova, I. V.; Yurkova, I.L. Pro(Anti)Oxidant Properties of Amino Acids and Their Derivatives in The Presence of Fe2+ and Cu2+ Ions. Russ J Gen Chem 2020, 90, 987–993. [Google Scholar] [CrossRef]
- Hau, E.H.; Teh, S.S.; Yeo, S.K.; Chua, B.L.; Owatworakit, A.; Xiao, J.; Mah, S.H. Physicochemical and Functional Properties of Flavourzyme-Extracted Protein Hydrolysate from Oil Palm Leaves. Biomass Convers Biorefin 2022, 1, 1–15. [Google Scholar] [CrossRef]
- Bjørlie, M.; Hartmann, J.C.; Rasmussen, L.H.; Yesiltas, B.; Sørensen, A.D.M.; Gregersen Echers, S.; Jacobsen, C. Screening for Metal-Chelating Activity in Potato Protein Hydrolysates Using Surface Plasmon Resonance and Peptidomics. Antioxidants 2024, 13, 346. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am J Enol Vitic 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative Properties of Xanthan on the Autoxidation of Soybean Oil in Cyclodextrin Emulsion. J Agric Food Chem 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Dinis, T.C.P.; Madeira, V.M.C.; Almeida, L.M. Action of Phenolic Derivatives (Acetaminophen, Salicylate, and 5-Aminosalicylate) as Inhibitors of Membrane Lipid Peroxidation and as Peroxyl Radical Scavengers. Arch Biochem Biophys 1994, 315, 161–169. [Google Scholar] [CrossRef] [PubMed]
| Aqueous extraction on seaweed | Enzymatic extraction on solid fractions from aqueous extraction | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Liquid fraction (LA) | Solid fraction (SA) | Liquid fraction (LE) | Solid fraction (SE) | |||||||||
| pH 3 | pH 6 | pH 9 | pH 3 | pH 6 | pH 9 | pH 3 | pH 6 | pH 9 | pH 3 | pH 6 | pH 9 | |
| PHE* | 0.40 ± 0.01c | 0.63 ± 0.04c | 0.63 ± 0.03c | 6.21 ± 0.59b | 5.53 ± 0.31b | 5.02 ± 0.52b | 1.09 ± 0.14c | 0.73 ± 0.18c | 0.77 ± 0.46c | 12.04 ± 1.13a | 11.89 ± 1.43a | 10.91 ± 0.72a |
| LEU* | 0.75 ± 0.04c | 0.92 ± 0.11c | 0.94 ± 0.09c | 11.59 ± 1.30b | 10.38 ± 0.50b | 9.23 ± 1.01b | 1.66 ± 0.23c | 1.15 ± 0.32c | 1.30 ± 0.52c | 21.18 ± 2.28a | 21.22 ± 2.58a | 19.34 ± 1.62a |
| ILE* | 0.42 ± 0.03c | 0.55 ± 0.06c | 0.54 ± 0.06c | 6.40 ± 0.69b | 5.69 ± 0.37b | 5.19 ± 0.62b | 0.98 ± 0.16c | 0.64 ± 0.18c | 0.79 ± 0.34c | 11.85 ± 1.14a | 11.91 ± 1.68a | 10.92 ± 0.92a |
| MET* | 0.19 ± 0.03c | 0.28 ± 0.06c | 0.28 ± 0.03c | 2.96 ± 0.35b | 2.68 ± 0.19b | 2.42 ± 0.31b | 0.40 ± 0.04c | 0.27 ± 0.04c | 0.32 ± 0.12c | 5.63 ± 0.55a | 5.73 ± 0.68a | 5.24 ± 0.39a |
| TYR* | 0.41 ± 0.04c | 0.71 ± 0.27c | 0.71 ± 0.04c | 6.88 ± 0.70b | 5.99 ± 0.31b | 5.51 ± 0.55b | 1.14 ± 0.08c | 0.65 ± 0.18c | 0.74 ± 0.40c | 14.08 ± 0.32a | 13.65 ± 0.73a | 12.64 ± 0.70a |
| PRO | 2.71 ± 0.05c | 2.91 ± 0.29c | 2.86 ± 0.10c | 8.39 ± 0.85b | 7.40 ± 0.29b | 6.95 ± 0.77b | 2.74 ± 0.11c | 2.22 ± 0.28c | 1.76 ± 0.55c | 15.45 ± 1.48a | 15.84 ± 1.66a | 14.38 ± 0.86a |
| VAL* | 1.04 ± 0.05c | 1.13 ± 0.13c | 1.12 ± 0.08c | 12.35 ± 1.34b | 10.95 ± 0.46b | 10.11 ± 1.09b | 2.22 ± 0.30c | 1.88 ± 0.31c | 1.95 ± 0.28c | 24.00 ± 2.34a | 23.88 ± 2.33a | 21.83 ± 1.70a |
| ALA | 2.49 ± 0.15c | 2.41 ± 0.32c | 2.27 ± 0.28c | 13.88 ± 1.28b | 12.45 ± 0.67b | 11.69 ± 1.30b | 4.25 ± 0.62c | 3.80 ± 0.29c | 4.11 ± 0.24c | 25.36 ± 2.32a | 26.33 ± 2.94a | 23.76 ± 1.53a |
| THR* | 1.21 ± 0.07c | 1.40 ± 0.23c | 1.38 ± 0.11c | 8.55 ± 1.05b | 7.83 ± 0.39b | 6.88 ± 1.43b | 1.57 ± 0.22c | 1.59 ± 0.25c | 1.59 ± 0.36c | 18.34 ± 1.65a | 18.89 ± 1.97a | 17.16 ± 1.09a |
| GLY | 2.39 ± 0.38c | 3.00 ± 0.41c | 2.93 ± 0.31c | 13.02 ± 1.12b | 11.80 ± 0.90b | 10.92 ± 1.09b | 3.56 ± 0.45c | 2.76 ± 0.55c | 2.75 ± 0.73c | 22.82 ± 1.86a | 23.23 ± 1.99a | 21.33 ± 1.29a |
| SER | 3.12 ± 0.75c | 2.62 ± 0.53c | 2.59 ± 0.29c | 13.20 ± 1.28b | 12.32 ± 0.64b | 11.18 ± 1.07b | 3.11 ± 0.58c | 3.35 ± 0.54c | 2.91 ± 0.75c | 22.58 ± 2.12a | 22.74 ± 2.27a | 21.32 ± 1.22a |
| ARG | 0.68 ± 0.24e | 0.74 ± 0.24e | 0.83 ± 0.18e | 10.90 ± 0.94c | 9.21 ± 0.45cd | 8.24 ± 0.74d | 1.03 ± 0.11e | 0.99 ± 0.35e | 1.06 ± 0.40e | 20.39 ± 1.80a | 20.37 ± 1.98a | 18.10 ± 1.10b |
| HIS* | ND** | ND | ND | 3.31 ± 0.64b | 2.76 ± 0.29bc | 2.32 ± 0.30c | ND | ND | ND | 5.06 ± 0.59a | 5.46 ± 0.70a | 4.83 ± 0.39a |
| GLU | 9.52 ± 0.24def | 11.80 ± 0.89d | 11.03 ± 0.44de | 23.09 ± 1.98b | 20.24 ± 0.82bc | 18.86 ± 2.15c | 7.85 ± 0.41ef | 6.08 ± 0.86f | 6.23 ± 1.44f | 35.15 ± 2.72a | 35.44 ± 3.66a | 32.13 ± 2.31a |
| C-C* | ND | ND | ND | ND | ND | ND | ND | ND | ND | 2.09 ± 0.67a | 1.41 ± 0.50b | 1.27 ± 0.27b |
| ASP | 8.62 ± 0.67d | 8.43 ± 0.62d | 9.22 ± 0.43d | 24.19 ± 3.10b | 21.68 ± 1.30bc | 20.06 ± 2.21c | 5.95 ± 0.51de | 4.46 ± 0.67e | 5.55 ± 1.34de | 36.18 ± 2.79a | 37.29 ± 3.41a | 34.27 ± 1.77a |
| TAA*** | 33.95 ± 1.57d | 37.51 ± 2.97d | 37.34 ± 1.27d | 164.92 ± 16.40b | 146.90 ± 6.90bc | 134.58 ± 14.70c | 37.54 ± 3.57d | 30.56 ± 3.30d | 31.84 ± 7.19d | 292.20 ± 25.73a | 295.29 ± 30.84a | 269.45 ± 16.81a |
| EAA | 0.42 ± 0.14c | 5.61 ± 0.69c | 5.60 ± 0.20c | 58.26 ± 6.15b | 51.80 ± 2.05b | 46.67 ± 5.60b | 9.05 ± 1.08c | 6.91 ± 1.22c | 7.46 ± 2.45c | 114.26 ± 10.94a | 114.04 ± 13.12a | 104.15 ± 7.05a |
| EAA/TAA | 0.130 ± 0.002d | 0.149 ± 0.009d | 0.150 ± 0.005d | 0.353 ± 0.004b | 0.353 ± 0.003b | 0.346 ± 0.004b | 0.241 ± 0.006c | 0.224 ± 0.016c | 0.230 ± 0.029c | 0.391 ± 0.005a | 0.386 ± 0.005a | 0.386 ± 0.003a |
| Extraction method | pH value | IC50 (mg.mL-1) for DPPH radical scavenging activity | IC50 (mg.mL-1) for Fe2+ chelating activity | ||
|---|---|---|---|---|---|
| Liquid fraction | Solid fraction | Liquid fraction | Solid fraction | ||
| Aqueous extraction on seaweed (LA and SA) | 3 | 9.31 ± 0.19c | 3.97 ± 0.07a | NR* | 4.81 ± 0.05bc |
| 6 | 9.03 ± 2.72c | 2.85 ± 0.08a | 5.06 ± 0.55c | 11.84 ± 2.50ef | |
| 9 | NR | 8.15 ± 0.02bc | 1.26 ± 0.07a | 8.97 ± 0.36de | |
| Enzymatic extraction on solid fraction from aqueous extraction (LE and SE) | 3 | 10.41 ± 0.51c | 2.29 ± 1.00a | 5.52 ± 0.60cd | 0.63 ± 0.04a |
| 6 | 10.09 ± 0.57c | 2.92 ± 0.02a | 14.60 ± 0.15f | 0.89 ± 0.07a | |
| 9 | NR | 4.38 ± 0.17ab | 8.92 ± 1.26de | 1.35 ± 0.11ab | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





