Submitted:
21 August 2024
Posted:
22 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. The NMDA Receptor Function and Cognition
3. NMDA Receptor Hypofunction in Schizophrenia
3.1. Cellular and Molecular Level: Synaptic Plasticity
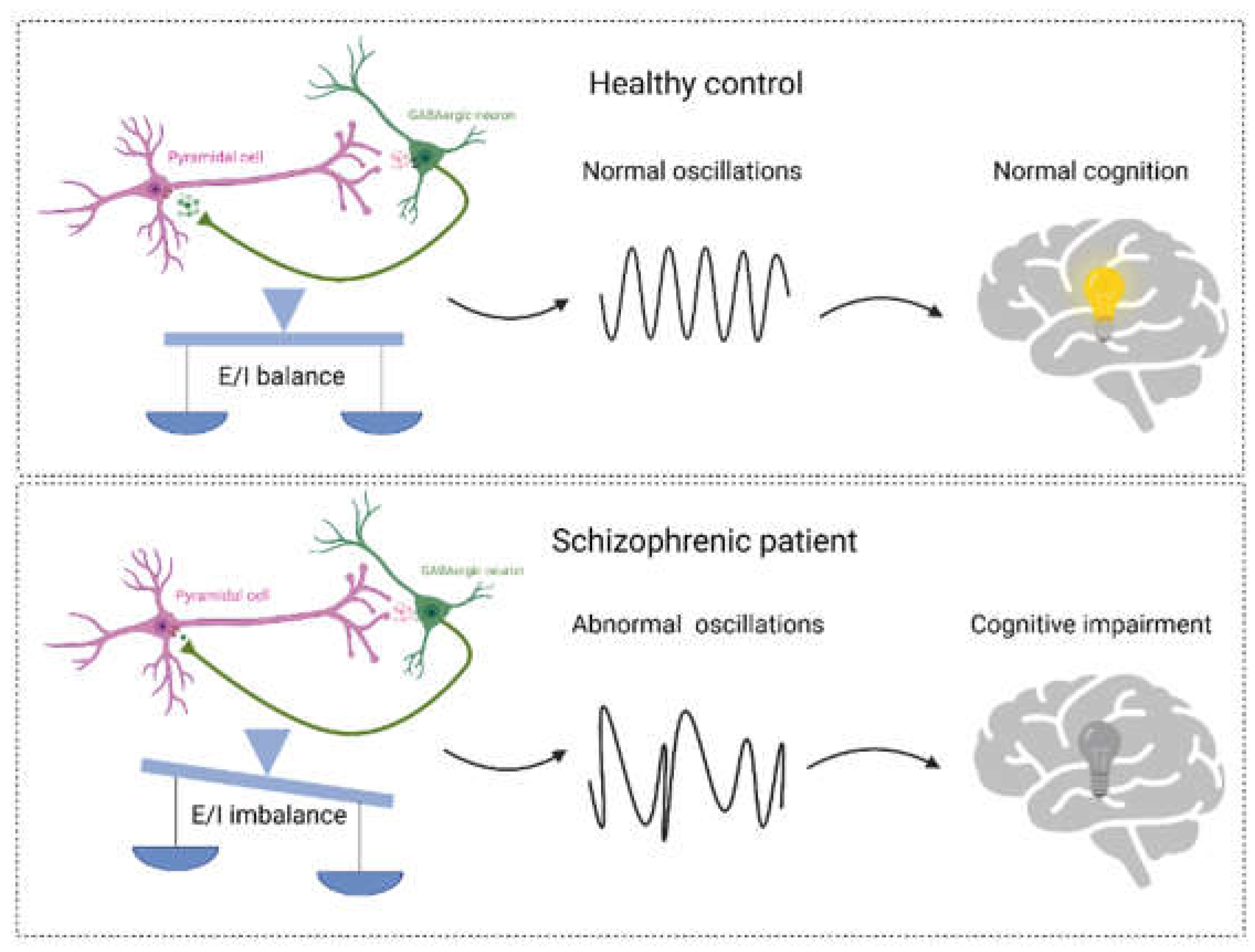
3.2. Neural network level: Excitation/Inhibition Balance and Neural Oscillation
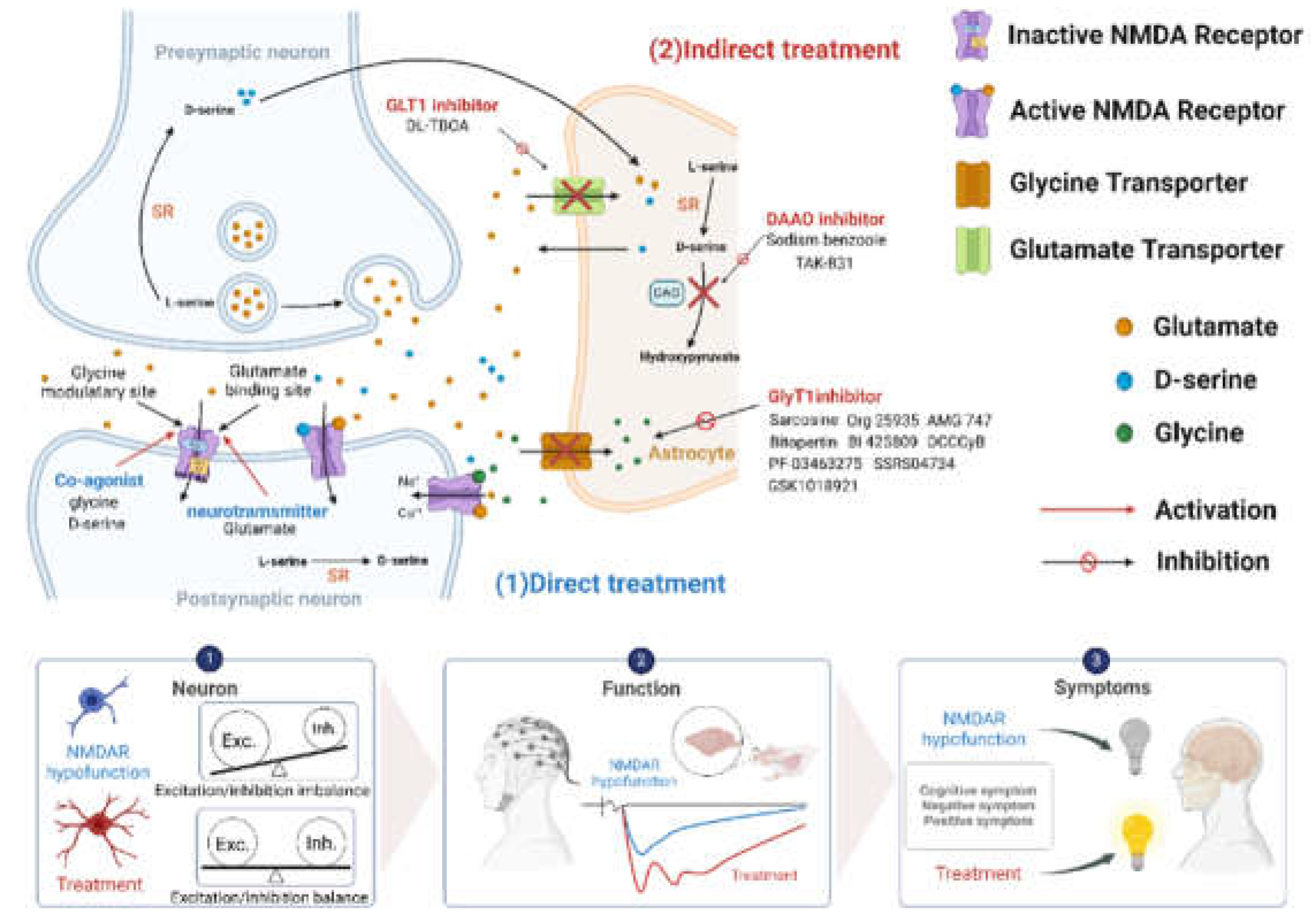
4. Targeting NMDA Receptors in Schizophrenia
4.1. Direct Enhancement of NMDA Receptor Function
4.1.1. Enhancement of NMDA Receptor Functions by Glycine
4.1.2. Enhancement of NMDA Receptor Functions by D-Serine
4.2. Indirect Enhancement of NMDA Receptor Function
4.2.1. Enhancement of NMDA Receptor Functions by GlyT1 Inhibitors
4.2.2. Enhancement of NMDA Receptor Functions by DAAO Inhibitors
4.2.3. Enhancement of NMDA Receptor Functions by other NMDAR Modulators
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Solmi, M.; Seitidis, G.; Mavridis, D.; Correll, C.U.; Dragioti, E.; Guimond, S.; Tuominen, L.; Dargél, A.; Carvalho, A.F.; Fornaro, M.; et al. Incidence, prevalence, and global burden of schizophrenia - data, with critical appraisal, from the Global Burden of Disease (GBD) 2019. Mol Psychiatry 2023, 28, 5319–5327. [Google Scholar] [CrossRef] [PubMed]
- Horan, W.P.; Catalano, L.T.; Green, M.F. An Update on Treatment of Cognitive Impairment Associated with Schizophrenia. Curr Top Behav Neurosci 2023, 63, 407–436. [Google Scholar] [CrossRef] [PubMed]
- Javitt, D.C. Cognitive Impairment Associated with Schizophrenia: From Pathophysiology to Treatment. Annu Rev Pharmacol Toxicol 2023, 63, 119–141. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P.; Dey, A.; Gopalakrishnan, A.V.; Swati, K.; Ojha, S.; Prakash, A.; Kumar, D.; Ambasta, R.K.; Jha, N.K.; Jha, S.K.; et al. Glutamatergic neurotransmission: A potential pharmacotherapeutic target for the treatment of cognitive disorders. Ageing Res Rev 2023, 85, 101838. [Google Scholar] [CrossRef]
- Moghaddam, B.; Javitt, D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology 2012, 37, 4–15. [Google Scholar] [CrossRef]
- Hansen, K.B.; Wollmuth, L.P.; Bowie, D.; Furukawa, H.; Menniti, F.S.; Sobolevsky, A.I.; Swanson, G.T.; Swanger, S.A.; Greger, I.H.; Nakagawa, T.; et al. Structure, Function, and Pharmacology of Glutamate Receptor Ion Channels. Pharmacol Rev 2021, 73, 298–487. [Google Scholar] [CrossRef]
- Zhou, C.; Tajima, N. Structural insights into NMDA receptor pharmacology. Biochem Soc Trans 2023, 51, 1713–1731. [Google Scholar] [CrossRef]
- Vyklicky, V.; Korinek, M.; Smejkalova, T.; Balik, A.; Krausova, B.; Kaniakova, M.; Lichnerova, K.; Cerny, J.; Krusek, J.; Dittert, I.; et al. Structure, function, and pharmacology of NMDA receptor channels. Physiol Res 2014, 63, S191–203. [Google Scholar] [CrossRef]
- Hansen, K.B.; Yi, F.; Perszyk, R.E.; Menniti, F.S.; Traynelis, S.F. NMDA Receptors in the Central Nervous System. Methods Mol Biol 2017, 1677, 1–80. [Google Scholar] [CrossRef]
- Lüscher, C.; Malenka, R.C. NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD). Cold Spring Harb Perspect Biol 2012, 4. [Google Scholar] [CrossRef]
- Dupuis, J.P.; Nicole, O.; Groc, L. NMDA receptor functions in health and disease: Old actor, new dimensions. Neuron 2023, 111, 2312–2328. [Google Scholar] [CrossRef] [PubMed]
- Veselinović, T.; Neuner, I. Progress and pitfalls in developing agents to treat neurocognitive deficits associated with schizophrenia. CNS Drugs 2022, 36, 819–858. [Google Scholar] [CrossRef]
- Nakazawa, K.; Sapkota, K. The origin of NMDA receptor hypofunction in schizophrenia. Pharmacol Ther 2020, 205, 107426. [Google Scholar] [CrossRef]
- Coyle, J.T.; Tsai, G.; Goff, D. Converging evidence of NMDA receptor hypofunction in the pathophysiology of schizophrenia. Ann N Y Acad Sci 2003, 1003, 318–327. [Google Scholar] [CrossRef]
- Jentsch, J.D.; Roth, R.H. The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology 1999, 20, 201–225. [Google Scholar] [CrossRef] [PubMed]
- Janus, A.; Lustyk, K.; Pytka, K. MK-801 and cognitive functions: Investigating the behavioral effects of a non-competitive NMDA receptor antagonist. Psychopharmacology (Berl) 2023, 240, 2435–2457. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, A.J. NR1 knockdown mice as a representative model of the glutamate hypothesis of schizophrenia. Prog Brain Res 2009, 179, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, K.; Jeevakumar, V.; Nakao, K. Spatial and temporal boundaries of NMDA receptor hypofunction leading to schizophrenia. NPJ Schizophr 2017, 3, 7. [Google Scholar] [CrossRef]
- Bygrave, A.M.; Masiulis, S.; Nicholson, E.; Berkemann, M.; Barkus, C.; Sprengel, R.; Harrison, P.J.; Kullmann, D.M.; Bannerman, D.M.; Kätzel, D. Knockout of NMDA-receptors from parvalbumin interneurons sensitizes to schizophrenia-related deficits induced by MK-801. Transl Psychiatry 2016, 6, e778. [Google Scholar] [CrossRef]
- Dalmau, J.; Gleichman, A.J.; Hughes, E.G.; Rossi, J.E.; Peng, X.; Lai, M.; Dessain, S.K.; Rosenfeld, M.R.; Balice-Gordon, R.; Lynch, D.R. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol 2008, 7, 1091–1098. [Google Scholar] [CrossRef]
- Snyder, M.A.; Gao, W.J. NMDA receptor hypofunction for schizophrenia revisited: Perspectives from epigenetic mechanisms. Schizophr Res 2020, 217, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Cotman, C.W.; Nieto-Sampedro, M. Cell biology of synaptic plasticity. Science 1984, 225, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Catts, V.S.; Lai, Y.L.; Weickert, C.S.; Weickert, T.W.; Catts, S.V. A quantitative review of the postmortem evidence for decreased cortical N-methyl-D-aspartate receptor expression levels in schizophrenia: How can we link molecular abnormalities to mismatch negativity deficits? Biol Psychol 2016, 116, 57–67. [Google Scholar] [CrossRef]
- Schwarcz, R.; Rassoulpour, A.; Wu, H.Q.; Medoff, D.; Tamminga, C.A.; Roberts, R.C. Increased cortical kynurenate content in schizophrenia. Biol Psychiatry 2001, 50, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Engberg, G.; Shimizu, E.; Nordin, C.; Lindström, L.H.; Iyo, M. Reduced D-serine to total serine ratio in the cerebrospinal fluid of drug naive schizophrenic patients. Prog Neuropsychopharmacol Biol Psychiatry 2005, 29, 767–769. [Google Scholar] [CrossRef]
- Psychiatric genome-wide association study analyses implicate neuronal, immune and histone pathways. Nat Neurosci 2015, 18, 199–209. [CrossRef]
- Kirov, G.; Pocklington, A.J.; Holmans, P.; Ivanov, D.; Ikeda, M.; Ruderfer, D.; Moran, J.; Chambert, K.; Toncheva, D.; Georgieva, L.; et al. De novo CNV analysis implicates specific abnormalities of postsynaptic signalling complexes in the pathogenesis of schizophrenia. Mol Psychiatry 2012, 17, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Mohn, A.R.; Gainetdinov, R.R.; Caron, M.G.; Koller, B.H. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell 1999, 98, 427–436. [Google Scholar] [CrossRef]
- Zhang, X.-Q.; Xu, L.; Zhu, X.-Y.; Tang, Z.-H.; Dong, Y.-B.; Yu, Z.-P.; Shang, Q.; Wang, Z.-C.; Shen, H.-W. D-serine reconstitutes synaptic and intrinsic inhibitory control of pyramidal neurons in a neurodevelopmental mouse model for schizophrenia. Nature Communications 2023, 14, 8255. [Google Scholar] [CrossRef]
- Sawa, A.; Snyder, S.H. Schizophrenia: diverse approaches to a complex disease. Science 2002, 296, 692–695. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Xu, L.; Ling, Y.; Hu, L.B.; Huang, J.; Shen, H.W. Diminished excitatory synaptic transmission correlates with impaired spatial working memory in neurodevelopmental rodent models of schizophrenia. Pharmacol Biochem Behav 2021, 10.1016/j.pbb.2021.173103, 173103. [CrossRef]
- Stefansson, H.; Sigurdsson, E.; Steinthorsdottir, V.; Bjornsdottir, S.; Sigmundsson, T.; Ghosh, S.; Brynjolfsson, J.; Gunnarsdottir, S.; Ivarsson, O.; Chou, T.T.; et al. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet 2002, 71, 877–892. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Jiang, Q.; Fu, A.K.; Ip, N.Y.; Yan, Z. Regulation of NMDA receptors by neuregulin signaling in prefrontal cortex. J Neurosci 2005, 25, 4974–4984. [Google Scholar] [CrossRef] [PubMed]
- Hahn, C.G.; Wang, H.Y.; Cho, D.S.; Talbot, K.; Gur, R.E.; Berrettini, W.H.; Bakshi, K.; Kamins, J.; Borgmann-Winter, K.E.; Siegel, S.J.; et al. Altered neuregulin 1-erbB4 signaling contributes to NMDA receptor hypofunction in schizophrenia. Nat Med 2006, 12, 824–828. [Google Scholar] [CrossRef] [PubMed]
- Levinson, J.N.; El-Husseini, A. Building excitatory and inhibitory synapses: balancing neuroligin partnerships. Neuron 2005, 48, 171–174. [Google Scholar] [CrossRef]
- Sohal, V.S.; Rubenstein, J.L.R. Excitation-inhibition balance as a framework for investigating mechanisms in neuropsychiatric disorders. Mol Psychiatry 2019, 24, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Sun, X.; Duan, J. NMDARs regulate the excitatory-inhibitory balance within neural circuits. Brain Science Advances 2023, 9, 3–14. [Google Scholar] [CrossRef]
- Balu, D.T. The NMDA receptor and schizophrenia: From pathophysiology to treatment. Adv Pharmacol 2016, 76, 351–382. [Google Scholar] [CrossRef]
- Gaebler, A.J.; Fakour, N.; Stöhr, F.; Zweerings, J.; Taebi, A.; Suslova, M.; Dukart, J.; Hipp, J.F.; Adhikari, B.M.; Kochunov, P.; et al. Functional connectivity signatures of NMDAR dysfunction in schizophrenia—integrating findings from imaging genetics and pharmaco-fMRI. Translational Psychiatry 2023, 13, 59. [Google Scholar] [CrossRef]
- Gonzalez-Burgos, G.; Lewis, D.A. NMDA receptor hypofunction, parvalbumin-positive neurons, and cortical gamma oscillations in schizophrenia. Schizophr Bull 2012, 38, 950–957. [Google Scholar] [CrossRef]
- Gonzalez-Burgos, G.; Fish, K.N.; Lewis, D.A. GABA neuron alterations, cortical circuit dysfunction and cognitive deficits in schizophrenia. Neural Plast 2011, 2011, 723184. [Google Scholar] [CrossRef]
- Sigurdsson, T. Neural circuit dysfunction in schizophrenia: Insights from animal models. Neuroscience 2016, 321, 42–65. [Google Scholar] [CrossRef] [PubMed]
- Jadi, M.P.; Behrens, M.M.; Sejnowski, T.J. Abnormal gamma oscillations in N-methyl-D-aspartate receptor hypofunction models of schizophrenia. Biol Psychiatry 2016, 79, 716–726. [Google Scholar] [CrossRef]
- Uhlhaas, P.J.; Singer, W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci 2010, 11, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Burgos, G.; Cho, R.Y.; Lewis, D.A. Alterations in cortical network oscillations and parvalbumin neurons in schizophrenia. Biol Psychiatry 2015, 77, 1031–1040. [Google Scholar] [CrossRef]
- Ellaithy, A.; Younkin, J.; González-Maeso, J.; Logothetis, D.E. Positive allosteric modulators of metabotropic glutamate 2 receptors in schizophrenia treatment. Trends Neurosci 2015, 38, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, S.; Arnone, D.; Cappai, A.; Howes, O. Alterations in the serotonin system in schizophrenia: a systematic review and meta-analysis of postmortem and molecular imaging studies. Neurosci Biobehav Rev 2014, 45, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Yuen, E.Y.; Jiang, Q.; Chen, P.; Feng, J.; Yan, Z. Activation of 5-HT2A/C receptors counteracts 5-HT1A regulation of n-methyl-D-aspartate receptor channels in pyramidal neurons of prefrontal cortex. J Biol Chem 2008, 283, 17194–17204. [Google Scholar] [CrossRef]
- Yuen, E.Y.; Jiang, Q.; Chen, P.; Gu, Z.; Feng, J.; Yan, Z. Serotonin 5-HT1A receptors regulate NMDA receptor channels through a microtubule-dependent mechanism. J Neurosci 2005, 25, 5488–5501. [Google Scholar] [CrossRef]
- Hanson, J.E.; Yuan, H.; Perszyk, R.E.; Banke, T.G.; Xing, H.; Tsai, M.C.; Menniti, F.S.; Traynelis, S.F. Therapeutic potential of N-methyl-D-aspartate receptor modulators in psychiatry. Neuropsychopharmacology 2024, 49, 51–66. [Google Scholar] [CrossRef]
- López-Corcuera, B.; Geerlings, A.; Aragón, C. Glycine neurotransmitter transporters: an update. Mol Membr Biol 2001, 18, 13–20. [Google Scholar] [CrossRef]
- Ishimaru, M.; Kurumaji, A.; Toru, M. Increases in strychnine-insensitive glycine binding sites in cerebral cortex of chronic schizophrenics: evidence for glutamate hypothesis. Biol Psychiatry 1994, 35, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Heresco-Levy, U.; Bar, G.; Levin, R.; Ermilov, M.; Ebstein, R.P.; Javitt, D.C. High glycine levels are associated with prepulse inhibition deficits in chronic schizophrenia patients. Schizophr Res 2007, 91, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Waziri, R.; Baruah, S. A hyperglycinergic rat model for the pathogenesis of schizophrenia: preliminary findings. Schizophr Res 1999, 37, 205–215. [Google Scholar] [CrossRef]
- Neeman, G.; Blanaru, M.; Bloch, B.; Kremer, I.; Ermilov, M.; Javitt, D.C.; Heresco-Levy, U. Relation of plasma glycine, serine, and homocysteine levels to schizophrenia symptoms and medication type. Am J Psychiatry 2005, 162, 1738–1740. [Google Scholar] [CrossRef] [PubMed]
- Mouri, A.; Noda, Y.; Noda, A.; Nakamura, T.; Tokura, T.; Yura, Y.; Nitta, A.; Furukawa, H.; Nabeshima, T. Involvement of a dysfunctional dopamine-D1/N-methyl-d-aspartate-NR1 and Ca2+/calmodulin-dependent protein kinase II pathway in the impairment of latent learning in a model of schizophrenia induced by phencyclidine. Mol Pharmacol 2007, 71, 1598–1609. [Google Scholar] [CrossRef]
- Hoffman, K.L.; Basurto, E. Clozapine and glycinamide prevent MK-801-induced deficits in the novel object recognition (NOR) test in the domestic rabbit (Oryctolagus cuniculus). Behav Brain Res 2014, 271, 203–211. [Google Scholar] [CrossRef]
- Waziri, R. Glycine therapy of schizophrenia. Biological psychiatry 1988, 23, 210–211. [Google Scholar] [CrossRef]
- Rosse, R.B.; Theut, S.K.; Banay-Schwartz, M.; Leighton, M.; Scarcella, E.; Cohen, C.G.; Deutsch, S.I. Glycine adjuvant therapy to conventional neuroleptic treatment in schizophrenia: an open-label, pilot study. Clinical neuropharmacology 1989, 12, 416–424. [Google Scholar] [CrossRef]
- Costa, J.; Khaled, E.; Sramek, J.; Bunney, W., Jr.; Potkin, S.G. An open trial of glycine as an adjunct to neuroleptics in chronic treatment-refractory schizophrenics. Journal of clinical psychopharmacology 1990, 10, 71–72. [Google Scholar] [CrossRef]
- Javitt, D.C.; Zylberman, I.; Zukin, S.R.; Heresco-Levy, U.; Lindenmayer, J.P. Amelioration of negative symptoms in schizophrenia by glycine. The American journal of psychiatry 1994, 151, 1234–1236. [Google Scholar]
- Leiderman, E.; Zylberman, I.; Zukin, S.R.; Cooper, T.B.; Javitt, D.C. Preliminary investigation of high-dose oral glycine on serum levels and negative symptoms in schizophrenia: An open-label trial. Biological Psychiatry 1996, 39, 213–215. [Google Scholar] [CrossRef]
- HerescoLevy, U.; Javitt, D.C.; Ermilov, M.; Mordel, C.; Horowitz, A.; Kelly, D. Double-blind, placebo-controlled, crossover trial of glycine adjuvant therapy for treatment-resistant schizophrenia. British Journal of Psychiatry 1996, 169, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Heresco-Levy, U.; Javitt, D.C.; Ermilov, M.; Mordel, C.; Silipo, G.; Lichtenstein, M. Efficacy of high-dose glycine in the treatment of enduring negative symptoms of schizophrenia. Archives of General Psychiatry 1999, 56, 29–36. [Google Scholar] [CrossRef]
- Potkin, S.G.; Jin, Y.; Bunney, B.G.; Costa, J.; Gulasekaram, B. Effect of clozapine and adjunctive high-dose glycine in treatment-resistant schizophrenia. American Journal of Psychiatry 1999, 156, 145–147. [Google Scholar] [CrossRef]
- Evins, A.E.; Fitzgerald, S.M.; Wine, L.; Rosselli, R.; Goff, D.C. Placebo-controlled trial of glycine added to clozapine in schizophrenia. American Journal of Psychiatry 2000, 157, 826–828. [Google Scholar] [CrossRef] [PubMed]
- E, E.A.; M, F.S.; L, W.; R, R.; C, G.D. Placebo-controlled trial of glycine added to clozapine in schizophrenia. The American journal of psychiatry 2000, 157, 826–828. [Google Scholar]
- Heresco-Levy, U.; Ermilov, M.; Lichtenberg, P.; Bar, G.; Javitt, D.C. High-dose glycine added to olanzapine and risperidone for the treatment of schizophrenia. Biological Psychiatry 2004, 55, 165–171. [Google Scholar] [CrossRef]
- Pablo, D.; Sreenivasa, B.; M, D.S.; Bill, D. Double-blind, placebo-controlled, crossover trial of clozapine plus glycine in refractory schizophrenia negative results. Journal of clinical psychopharmacology 2005, 25, 277–278. [Google Scholar]
- Greenwood, L.-M.; Leung, S.; Michie, P.T.; Green, A.; Nathan, P.J.; Fitzgerald, P.; Johnston, P.; Solowij, N.; Kulkarni, J.; Croft, R.J. The effects of glycine on auditory mismatch negativity in schizophrenia. Schizophrenia Research 2018, 191, 61–69. [Google Scholar] [CrossRef]
- McLean Hospital, B., Massachusetts; Department of Psychiatry, H.M.S., Boston, Massachusetts; McLean Hospital, B., Massachusetts; Department of Psychiatry, H.M.S., Boston, Massachusetts; McLean Hospital, B., Massachusetts; Molecular, D.o.; Human Genetics, B.C.o.M., Houston, Texas; Department of Biostatistics, U.o.A.a.B., Birmingham, Alabama; New York State Psychiatric Institute, N.Y.; McLean Hospital, B., Massachusetts, et al. Targeted Treatment of Individuals With Psychosis Carrying a Copy Number Variant Containing a Genomic Triplication of the Glycine Decarboxylase Gene. Biological Psychiatry 2019, 86, 523–535.
- Serrita, J.; Ralevski, E.; Yoon, G.; Petrakis, I. A Pilot Randomized, Placebo-Controlled Trial of Glycine for Treatment of Schizophrenia and Alcohol Dependence. Journal of Dual Diagnosis 2019, 15, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Strzelecki, D.; Kałuzyńska, O.; Józefowicz, O.; Pawełczyk, T.; Rabe-Jabłońska, J. Initial glycine serum level is not a predictor of the recovery resulting from glycine augmentation of antipsychotic treatment. Archives of Psychiatry and Psychotherapy 2011, 13, 5–11. [Google Scholar]
- Mothet, J.P.; Parent, A.T.; Wolosker, H.; Brady, R.O., Jr.; Linden, D.J.; Ferris, C.D.; Rogawski, M.A.; Snyder, S.H. D-serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor. Proc Natl Acad Sci U S A 2000, 97, 4926–4931. [Google Scholar] [CrossRef] [PubMed]
- Mothet, J.P.; Le Bail, M.; Billard, J.M. Time and space profiling of NMDA receptor co-agonist functions. J Neurochem 2015, 135, 210–225. [Google Scholar] [CrossRef] [PubMed]
- Papouin, T.; Ladépêche, L.; Ruel, J.; Sacchi, S.; Labasque, M.; Hanini, M.; Groc, L.; Pollegioni, L.; Mothet, J.P.; Oliet, S.H. Synaptic and extrasynaptic NMDA receptors are gated by different endogenous coagonists. Cell 2012, 150, 633–646. [Google Scholar] [CrossRef]
- Yang, Y.; Ge, W.; Chen, Y.; Zhang, Z.; Shen, W.; Wu, C.; Poo, M.; Duan, S. Contribution of astrocytes to hippocampal long-term potentiation through release of D-serine. Proc Natl Acad Sci U S A 2003, 100, 15194–15199. [Google Scholar] [CrossRef]
- Hardingham, G.E.; Bading, H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci 2010, 11, 682–696. [Google Scholar] [CrossRef]
- Li, S.T.; Ju, J.G. Functional roles of synaptic and extrasynaptic NMDA receptors in physiological and pathological neuronal activities. Curr Drug Targets 2012, 13, 207–221. [Google Scholar] [CrossRef]
- Hashimoto, K.; Fukushima, T.; Shimizu, E.; Komatsu, N.; Watanabe, H.; Shinoda, N.; Nakazato, M.; Kumakiri, C.; Okada, S.; Hasegawa, H.; et al. Decreased serum levels of D-serine in patients with schizophrenia: evidence in support of the N-methyl-D-aspartate receptor hypofunction hypothesis of schizophrenia. Arch Gen Psychiatry 2003, 60, 572–576. [Google Scholar] [CrossRef]
- Bendikov, I.; Nadri, C.; Amar, S.; Panizzutti, R.; De Miranda, J.; Wolosker, H.; Agam, G. A CSF and postmortem brain study of D-serine metabolic parameters in schizophrenia. Schizophr Res 2007, 90, 41–51. [Google Scholar] [CrossRef]
- Fujita, Y.; Ishima, T.; Hashimoto, K. Supplementation with D-serine prevents the onset of cognitive deficits in adult offspring after maternal immune activation. Sci Rep 2016, 6, 37261. [Google Scholar] [CrossRef] [PubMed]
- Verrall, L.; Burnet, P.W.; Betts, J.F.; Harrison, P.J. The neurobiology of D-amino acid oxidase and its involvement in schizophrenia. Mol Psychiatry 2010, 15, 122–137. [Google Scholar] [CrossRef] [PubMed]
- Carone, F.A.; Ganote, C.E. D-serine nephrotoxicity. The nature of proteinuria, glucosuria, and aminoaciduria in acute tubular necrosis. Arch Pathol 1975, 99, 658–662. [Google Scholar]
- Suzuki, M.; Gonda, Y.; Yamada, M.; Vandebroek, A.A.; Mita, M.; Hamase, K.; Yasui, M.; Sasabe, J. Serum D-serine accumulation after proximal renal tubular damage involves neutral amino acid transporter Asc-1. Sci Rep 2019, 9, 16705. [Google Scholar] [CrossRef]
- Mothet, J.P.; Billard, J.M.; Pollegioni, L.; Coyle, J.T.; Sweedler, J.V. Investigating brain d-serine: Advocacy for good practices. Acta Physiol (Oxf) 2019, 226, e13257. [Google Scholar] [CrossRef]
- Kantrowitz, J.T.; Woods, S.W.; Petkova, E.; Cornblatt, B.; Corcoran, C.M.; Chen, H.; Silipo, G.; Javitt, D.C. D-serine for the treatment of negative symptoms in individuals at clinical high risk of schizophrenia: a pilot, double-blind, placebo-controlled, randomised parallel group mechanistic proof-of-concept trial. Lancet Psychiatry 2015, 2, 403–412. [Google Scholar] [CrossRef]
- Tsai, G.; Yang, P.; Chung, L.C.; Lange, N.; Coyle, J.T. D-serine added to antipsychotics for the treatment of schizophrenia. Biol Psychiatry 1998, 44, 1081–1089. [Google Scholar] [CrossRef]
- Weiser, M.; Heresco-Levy, U.; Davidson, M.; Javitt, D.C.; Werbeloff, N.; Gershon, A.A.; Abramovich, Y.; Amital, D.; Doron, A.; Konas, S.; et al. A multicenter, add-on randomized controlled trial of low-dose d-serine for negative and cognitive symptoms of schizophrenia. J Clin Psychiatry 2012, 73, e728–734. [Google Scholar] [CrossRef]
- Kantrowitz, J.T.; Epstein, M.L.; Lee, M.; Lehrfeld, N.; Nolan, K.A.; Shope, C.; Petkova, E.; Silipo, G.; Javitt, D.C. Improvement in mismatch negativity generation during d-serine treatment in schizophrenia: Correlation with symptoms. Schizophr Res 2018, 191, 70–79. [Google Scholar] [CrossRef] [PubMed]
- da Silva Lde, B.; Leipnitz, G.; Seminotti, B.; Fernandes, C.G.; Beskow, A.P.; Amaral, A.U.; Wajner, M. D-serine induces lipid and protein oxidative damage and decreases glutathione levels in brain cortex of rats. Brain Res 2009, 1256, 34–42. [Google Scholar] [CrossRef]
- Katsuki, H.; Nonaka, M.; Shirakawa, H.; Kume, T.; Akaike, A. Endogenous D-serine is involved in induction of neuronal death by N-methyl-D-aspartate and simulated ischemia in rat cerebrocortical slices. J Pharmacol Exp Ther 2004, 311, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Ganote, C.E.; Peterson, D.R.; Carone, F.A. The nature of D-serine--induced nephrotoxicity. Am J Pathol 1974, 77, 269–282. [Google Scholar]
- Kantrowitz, J.T.; Malhotra, A.K.; Cornblatt, B.; Silipo, G.; Balla, A.; Suckow, R.F.; D'Souza, C.; Saksa, J.; Woods, S.W.; Javitt, D.C. High dose D-serine in the treatment of schizophrenia. Schizophr Res 2010, 121, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Tsai, G.E.; Yang, P.; Chung, L.C.; Tsai, I.C.; Tsai, C.W.; Coyle, J.T. D-serine added to clozapine for the treatment of schizophrenia. Am J Psychiatry 1999, 156, 1822–1825. [Google Scholar] [CrossRef]
- Heresco-Levy, U.; Javitt, D.C.; Ebstein, R.; Vass, A.; Lichtenberg, P.; Bar, G.; Catinari, S.; Ermilov, M. D-serine efficacy as add-on pharmacotherapy to risperidone and olanzapine for treatment-refractory schizophrenia. Biol Psychiatry 2005, 57, 577–585. [Google Scholar] [CrossRef]
- Lane, H.Y.; Lin, C.H.; Huang, Y.J.; Liao, C.H.; Chang, Y.C.; Tsai, G.E. A randomized, double-blind, placebo-controlled comparison study of sarcosine (N-methylglycine) and D-serine add-on treatment for schizophrenia. Int J Neuropsychopharmacol 2010, 13, 451–460. [Google Scholar] [CrossRef]
- Ermilov, M.; Gelfin, E.; Levin, R.; Lichtenberg, P.; Hashimoto, K.; Javitt, D.C.; Heresco-Levy, U. A pilot double-blind comparison of d-serine and high-dose olanzapine in treatment-resistant patients with schizophrenia. Schizophr Res 2013, 150, 604–605. [Google Scholar] [CrossRef] [PubMed]
- D'Souza, D.C.; Radhakrishnan, R.; Perry, E.; Bhakta, S.; Singh, N.M.; Yadav, R.; Abi-Saab, D.; Pittman, B.; Chaturvedi, S.K.; Sharma, M.P.; et al. Feasibility, safety, and efficacy of the combination of D-serine and computerized cognitive retraining in schizophrenia: an international collaborative pilot study. Neuropsychopharmacology 2013, 38, 492–503. [Google Scholar] [CrossRef]
- Heresco-Levy, U.; Durrant, A.R.; Ermilov, M.; Javitt, D.C.; Miya, K.; Mori, H. Clinical and electrophysiological effects of D-serine in a schizophrenia patient positive for anti-N-methyl-D-aspartate receptor antibodies. Biol Psychiatry 2015, 77, e27–29. [Google Scholar] [CrossRef]
- Kantrowitz, J.T.; Epstein, M.L.; Beggel, O.; Rohrig, S.; Lehrfeld, J.M.; Revheim, N.; Lehrfeld, N.P.; Reep, J.; Parker, E.; Silipo, G.; et al. Neurophysiological mechanisms of cortical plasticity impairments in schizophrenia and modulation by the NMDA receptor agonist D-serine. Brain 2016, 139, 3281–3295. [Google Scholar] [CrossRef]
- Sehatpour, P.; Iosifescu, D.V.; De Baun, H.M.; Shope, C.; Mayer, M.R.; Gangwisch, J.; Dias, E.; Sobeih, T.; Choo, T.H.; Wall, M.M.; et al. Dose-dependent augmentation of neuroplasticity-based auditory learning in schizophrenia: A double-blind, placebo-controlled, randomized, target engagement clinical trial of the NMDA glutamate receptor agonist d-serine. Biol Psychiatry 2023, 94, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Sur, C.; Kinney, G.G. Glycine transporter 1 inhibitors and modulation of NMDA receptor-mediated excitatory neurotransmission. Curr Drug Targets 2007, 8, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Balu, D.T.; Coyle, J.T. The NMDA receptor 'glycine modulatory site' in schizophrenia: D-serine, glycine, and beyond. Curr Opin Pharmacol 2015, 20, 109–115. [Google Scholar] [CrossRef]
- Piniella, D.; Zafra, F. Functional crosstalk of the glycine transporter GlyT1 and NMDA receptors. Neuropharmacology 2023, 232, 109514. [Google Scholar] [CrossRef] [PubMed]
- Rosenbrock, H.; Desch, M.; Wunderlich, G. Development of the novel GlyT1 inhibitor, iclepertin (BI 425809), for the treatment of cognitive impairment associated with schizophrenia. Eur Arch Psychiatry Clin Neurosci 2023, 273, 1557–1566. [Google Scholar] [CrossRef] [PubMed]
- Dudzik, P.; Lustyk, K.; Pytka, K. Beyond dopamine: Novel strategies for schizophrenia treatment. Med Res Rev 2024, 10.1002/med.22042. [CrossRef]
- Surti, T.S.; Ranganathan, M.; Johannesen, J.K.; Gueorguieva, R.; Deaso, E.; Kenney, J.G.; Krystal, J.H.; D'Souza, D.C. Randomized controlled trial of the glycine transporter 1 inhibitor PF-03463275 to enhance cognitive training and neuroplasticity in schizophrenia. Schizophr Res 2023, 256, 36–43. [Google Scholar] [CrossRef]
- Javitt, D.C.; Sershen, H.; Hashim, A.; Lajtha, A. Reversal of phencyclidine-induced hyperactivity by glycine and the glycine uptake inhibitor glycyldodecylamide. Neuropsychopharmacology 1997, 17, 202–204. [Google Scholar] [CrossRef]
- Hashimoto, K.; Fujita, Y.; Ishima, T.; Chaki, S.; Iyo, M. Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of the glycine transporter-1 inhibitor NFPS and D-serine. Eur Neuropsychopharmacol 2008, 18, 414–421. [Google Scholar] [CrossRef]
- Kopec, K.; Flood, D.G.; Gasior, M.; McKenna, B.A.; Zuvich, E.; Schreiber, J.; Salvino, J.M.; Durkin, J.T.; Ator, M.A.; Marino, M.J. Glycine transporter (GlyT1) inhibitors with reduced residence time increase prepulse inhibition without inducing hyperlocomotion in DBA/2 mice. Biochem Pharmacol 2010, 80, 1407–1417. [Google Scholar] [CrossRef]
- Chen, H.H.; Stoker, A.; Markou, A. The glutamatergic compounds sarcosine and N-acetylcysteine ameliorate prepulse inhibition deficits in metabotropic glutamate 5 receptor knockout mice. Psychopharmacology (Berl) 2010, 209, 343–350. [Google Scholar] [CrossRef]
- Kumar, A.; Akhtar, A.; Kuhad, A.; Sah, S.P. Sarcosine (glycine transporter inhibitor) attenuates behavioural and biochemical changes induced by ketamine, in the rat model of schizophrenia. Exp Brain Res 2023, 241, 451–467. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.C.; Hung, W.L.; Lin, B.X.; Shih, M.H.; Lu, L.Y.; Luo, D.Z.; Tai, H.C.; Studer, V.; Min, M.Y.; Lai, W.S. Therapeutic potential and underlying mechanism of sarcosine (N-methylglycine) in N-methyl-D-aspartate (NMDA) receptor hypofunction models of schizophrenia. J Psychopharmacol 2019, 33, 1288–1302. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Lin, C.H.; Liu, C.Y.; Chen, S.J.; Lane, H.Y. Efficacy and cognitive effect of sarcosine (N-methylglycine) in patients with schizophrenia: A systematic review and meta-analysis of double-blind randomised controlled trials. J Psychopharmacol 2020, 34, 495–505. [Google Scholar] [CrossRef]
- Zhang, H.X.; Hyrc, K.; Thio, L.L. The glycine transport inhibitor sarcosine is an NMDA receptor co-agonist that differs from glycine. J Physiol 2009, 587, 3207–3220. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Inoue, T.; Izumi, T.; Nakagawa, S.; Koyama, T. SSR504734, a glycine transporter-1 inhibitor, attenuates acquisition and expression of contextual conditioned fear in rats. Behav Pharmacol 2010, 21, 576–579. [Google Scholar] [CrossRef]
- Black, M.D.; Varty, G.B.; Arad, M.; Barak, S.; De Levie, A.; Boulay, D.; Pichat, P.; Griebel, G.; Weiner, I. Procognitive and antipsychotic efficacy of glycine transport 1 inhibitors (GlyT1) in acute and neurodevelopmental models of schizophrenia: latent inhibition studies in the rat. Psychopharmacology (Berl) 2009, 202, 385–396. [Google Scholar] [CrossRef]
- Tsai, G.; Ralph-Williams, R.J.; Martina, M.; Bergeron, R.; Berger-Sweeney, J.; Dunham, K.S.; Jiang, Z.; Caine, S.B.; Coyle, J.T. Gene knockout of glycine transporter 1: characterization of the behavioral phenotype. Proc Natl Acad Sci U S A 2004, 101, 8485–8490. [Google Scholar] [CrossRef]
- Cioffi, C.L. Glycine transporter-1 inhibitors: a patent review (2011-2016). Expert Opin Ther Pat 2018, 28, 197–210. [Google Scholar] [CrossRef]
- Hashimoto, K. Glycine transporter inhibitors as therapeutic agents for schizophrenia. Recent Pat CNS Drug Discov 2006, 1, 43–53. [Google Scholar] [CrossRef]
- Hashimoto, K. Glycine transport inhibitors for the treatment of schizophrenia. Open Med Chem J 2010, 4, 10–19. [Google Scholar] [CrossRef]
- Verrall, L.; Walker, M.; Rawlings, N.; Benzel, I.; Kew, J.N.; Harrison, P.J.; Burnet, P.W. d-Amino acid oxidase and serine racemase in human brain: normal distribution and altered expression in schizophrenia. Eur J Neurosci 2007, 26, 1657–1669. [Google Scholar] [CrossRef] [PubMed]
- Jagannath, V.; Theodoridou, A.; Gerstenberg, M.; Franscini, M.; Heekeren, K.; Correll, C.U.; Rössler, W.; Grünblatt, E.; Walitza, S. Prediction analysis for transition to schizophrenia in individuals at clinical High risk for psychosis: the relationship of DAO, DAOA, and NRG1 variants with negative symptoms and cognitive deficits. Front Psychiatry 2017, 8, 292. [Google Scholar] [CrossRef]
- Hall, J.; Whalley, H.C.; Moorhead, T.W.; Baig, B.J.; McIntosh, A.M.; Job, D.E.; Owens, D.G.; Lawrie, S.M.; Johnstone, E.C. Genetic variation in the DAOA (G72) gene modulates hippocampal function in subjects at high risk of schizophrenia. Biol Psychiatry 2008, 64, 428–433. [Google Scholar] [CrossRef]
- Lin, E.; Lin, C.H.; Lai, Y.L.; Huang, C.H.; Huang, Y.J.; Lane, H.Y. Combination of G72 genetic variation and G72 protein level to detect schizophrenia: machine learning approaches. Front Psychiatry 2018, 9, 566. [Google Scholar] [CrossRef] [PubMed]
- Iwasa, S.; Tabara, H.; Song, Z.; Nakabayashi, M.; Yokoyama, Y.; Fukushima, T. Inhibition of D-amino acid oxidase activity by antipsychotic drugs evaluated by a fluorometric assay using D-kynurenine as substrate. Yakugaku Zasshi 2011, 131, 1111–1116. [Google Scholar] [CrossRef]
- Krogmann, A.; Peters, L.; von Hardenberg, L.; Bödeker, K.; Nöhles, V.B.; Correll, C.U. Keeping up with the therapeutic advances in schizophrenia: a review of novel and emerging pharmacological entities. CNS Spectr 2019, 24, 38–69. [Google Scholar] [CrossRef]
- Adage, T.; Trillat, A.C.; Quattropani, A.; Perrin, D.; Cavarec, L.; Shaw, J.; Guerassimenko, O.; Giachetti, C.; Gréco, B.; Chumakov, I.; et al. In vitro and in vivo pharmacological profile of AS057278, a selective d-amino acid oxidase inhibitor with potential anti-psychotic properties. Eur Neuropsychopharmacol 2008, 18, 200–214. [Google Scholar] [CrossRef]
- Kuo, C.Y.; Lin, C.H.; Lane, H.Y. Targeting D-amino acid oxidase (DAAO) for the treatment of schizophrenia: rationale and current status of research. CNS Drugs 2022, 36, 1143–1153. [Google Scholar] [CrossRef]
- Hashimoto, K.; Fujita, Y.; Horio, M.; Kunitachi, S.; Iyo, M.; Ferraris, D.; Tsukamoto, T. Co-administration of a D-amino acid oxidase inhibitor potentiates the efficacy of D-serine in attenuating prepulse inhibition deficits after administration of dizocilpine. Biol Psychiatry 2009, 65, 1103–1106. [Google Scholar] [CrossRef] [PubMed]
- Almond, S.L.; Fradley, R.L.; Armstrong, E.J.; Heavens, R.B.; Rutter, A.R.; Newman, R.J.; Chiu, C.S.; Konno, R.; Hutson, P.H.; Brandon, N.J. Behavioral and biochemical characterization of a mutant mouse strain lacking D-amino acid oxidase activity and its implications for schizophrenia. Mol Cell Neurosci 2006, 32, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, M.; Watanabe, M.; Yamaguchi, S.; Konno, R.; Hori, Y. Spatial learning and long-term potentiation of mutant mice lacking D-amino-acid oxidase. Neurosci Res 2005, 53, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, M.; Okamura, T.; Kasai, N.; Hori, Y.; Summer, K.H.; Konno, R. D-amino-acid oxidase is involved in D-serine-induced nephrotoxicity. Chem Res Toxicol 2005, 18, 1678–1682. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.E.; Lock, E.A. Sodium benzoate attenuates D-serine induced nephrotoxicity in the rat. Toxicology 2005, 207, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Goff, D.C. D-cycloserine in schizophrenia: new strategies for improving clinical outcomes by enhancing plasticity. Curr Neuropharmacol 2017, 15, 21–34. [Google Scholar] [CrossRef]
- Goff, D.C.; Tsai, G.; Manoach, D.S.; Coyle, J.T. Dose-finding trial of D-cycloserine added to neuroleptics for negative symptoms in schizophrenia. Am J Psychiatry 1995, 152, 1213–1215. [Google Scholar] [CrossRef]
- Goff, D.C.; Tsai, G.; Levitt, J.; Amico, E.; Manoach, D.; Schoenfeld, D.A.; Hayden, D.L.; McCarley, R.; Coyle, J.T. A placebo-controlled trial of D-cycloserine added to conventional neuroleptics in patients with schizophrenia. Arch Gen Psychiatry 1999, 56, 21–27. [Google Scholar] [CrossRef] [PubMed]
- van Berckel, B.N.; Hijman, R.; van der Linden, J.A.; Westenberg, H.G.; van Ree, J.M.; Kahn, R.S. Efficacy and tolerance of D-cycloserine in drug-free schizophrenic patients. Biol Psychiatry 1996, 40, 1298–1300. [Google Scholar] [CrossRef]
- Tuominen, H.J.; Tiihonen, J.; Wahlbeck, K. Glutamatergic drugs for schizophrenia: a systematic review and meta-analysis. Schizophr Res 2005, 72, 225–234. [Google Scholar] [CrossRef]
- Vestring, S.; Dorner, A.; Scholliers, J.; Ehrenberger, K.; Kiss, A.; Arenz, L.; Theiss, A.; Rossner, P.; Frase, S.; Du Vinage, C.; et al. D-Cycloserine enhances the bidirectional range of NMDAR-dependent hippocampal synaptic plasticity. Transl Psychiatry 2024, 14, 18. [Google Scholar] [CrossRef]
- Hackos, D.H.; Lupardus, P.J.; Grand, T.; Chen, Y.; Wang, T.M.; Reynen, P.; Gustafson, A.; Wallweber, H.J.; Volgraf, M.; Sellers, B.D.; et al. Positive allosteric modulators of GluN2A-containing NMDARs with distinct modes of action and impacts on circuit function. Neuron 2016, 89, 983–999. [Google Scholar] [CrossRef]
- Ladagu, A.D.; Olopade, F.E.; Adejare, A.; Olopade, J.O. GluN2A and GluN2B N-methyl-D-aspartate receptor (NMDARs) subunits: their roles and therapeutic antagonists in neurological diseases. Pharmaceuticals (Basel) 2023, 16. [Google Scholar] [CrossRef] [PubMed]
- Preskorn, S.H.; Baker, B.; Kolluri, S.; Menniti, F.S.; Krams, M.; Landen, J.W. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol 2008, 28, 631–637. [Google Scholar] [CrossRef] [PubMed]

| Compound | Sample size (placebo vs. experiment) |
Dosage | Clinical results | References |
|---|---|---|---|---|
| Glycine | 11 (no placebo) | 5-25 (g/day) | No significant effects on symptoms | [58] |
| 6 (no placebo) | 10.8 (g/day) | No significant effects on symptoms | [59] | |
| 6 (no placebo) | 15 (g/day) | No significant effects on symptoms | [60] | |
| 7 vs. 7 | 2-30 (g/day) | Significant improvements in negative symptoms | [61] | |
| 5 (no placebo) | 0.14-0.8 (g/kg/day) | Significant improvements in negative symptoms | [62] | |
| 11 vs. 11 | 0.8 (g/kg/day) | Significant improvements in depressive, cognitive and negative symptoms | [63] | |
| 22 vs. 22 | 0.8 (g/kg/day) | Significant improvements in depressive, cognitive and negative symptoms | [64] | |
| 10 vs. 9 | 30 (g/day) | No significant effects on symptoms | [65] | |
| 13 vs. 14 | 60 (g/day) | No significant effects on symptoms | [66] | |
| 6 vs. 6 | 0.2-0.8 (g/kg/day) | Significant improvements in negative symptoms | [67] | |
| 12 vs. 12 | 60 (g/day) | No significant effects on symptoms | [68] | |
| 45 (55) vs. 42 (54) | 15-60 (g/day) | No significant effects on symptoms | [69] | |
| 2 vs. 2 | 6-48 (g/day) | Significant improvements in Clinical symptoms | [70] | |
| 2 (no placebo) | 5.4-86.5 (g/day) | Significant improvements in Clinical symptoms | [71] | |
| 10 vs.10 | 0.8 (g/kg/day) | No significant effects on symptoms | [72] | |
| 29 (no placebo) | 0.8 (g/kg/day) | Significant improvements in positive and negative symptoms | [73] |
| Compound | Sample size (placebo vs. experiment) |
Dosage | Clinical results | References |
|---|---|---|---|---|
| D-serine | 15 vs. 14 | 30 (mg/kg/day) | Significant improvements in positive, negative, and cognitive symptoms | [88] |
| 10 vs. 10 | 30 (mg/kg/day) | No significant effects on symptoms | [95] | |
| 38 vs.37 | 20-30 (mg/kg/day) | Significant improvements in negative, positive, cognitive, and depression symptoms | [96] | |
| 16 (20)vs.16 (20) | 2 (g/day) | No significant effects on symptoms | [97] | |
| 12 vs.19 vs.16 | 30,60,120 (mg/kg/day) | Significant improvements in symptoms | [94] | |
| 69 (98)vs. 73 (97) | 2 (g/day) | No significant effects on symptoms | [89] | |
| 5 (10)vs. 3 (8) | 1.5-3 (g/day) | Significant improvements in negative symptom | [98] | |
| 23 (26)vs. 25 (27) | 30 (mg/kg/day) | No significant effects on symptoms | [99] | |
| 17 | 1.5-4 (g/day) | Significant improvements in symptoms | [100] | |
| 20 (24)vs. 15 (20) | 60 (mg/kg/day) | Significant improvements in negative symptoms | [87] | |
| 13 | 60 (mg/kg/day) | Improvements in auditory plasticity | [101] | |
| 16 vs.16 | 60 (mg/kg/day) | Significant improvements in symptoms | [90] | |
| 9 vs. 12 | 100 mg/kg | Significant plasticity improvement | [102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





