1. Introduction
Acute pancreatitis (AP) is a life-threatening condition with an inflammatory onset that presents a wide range of severity [
1] which is closely linked to the presence of necrosis and to the development of infection, known as infected pancreatic necrosis (IPN). The Atlanta criteria, revised in 2012, standardise the diagnosis and classification of AP based on clinical symptoms, enzyme levels and imaging findings [
2].
Bacterial translocation, in which bacteria migrate from the intestine to the pancreas, contributes to the systemic inflammatory response in AP and is considered the cause of infection of necrotic material [
3]. In recent decades, the use of antibiotics to prevent or treat IPN become a controversial topic. Although early randomised studies and meta-analyses suggested benefits of antibiotic prophylaxis, subsequent trials and systematic reviews have not confirmed these findings. Currently, most clinical guidelines only recommend antibiotics when infection is confirmed or strongly suspected. Despite these recommendations, recent studies have found a high rate of inappropriate antibiotic use in AP [
4,
5].
This scoping review aims to update the available scientific evidence on the use of antibiotics in the prophylaxis and treatment of AP. It assesses the microbiology of IPN, the criteria for IPN diagnosis and antibiotic prescription, the type of antimicrobials recommended, and the duration of treatment.
2. Results
Figure 1 shows the PRISMA flowchart of the review. In total, 1016 publications were identified. After removing duplicates and screening by title/abstract, 813 publications were excluded and 203 were selected for full-text screening. Of these, 133 were subsequently retrieved, leaving 70 studies for evaluation. In addition, ten publications from other sources were included. After adding these studies, 80 articles were analysed, of which 13 were systematic reviews or meta-analyses and 22 were randomised controlled studies (RCTs).
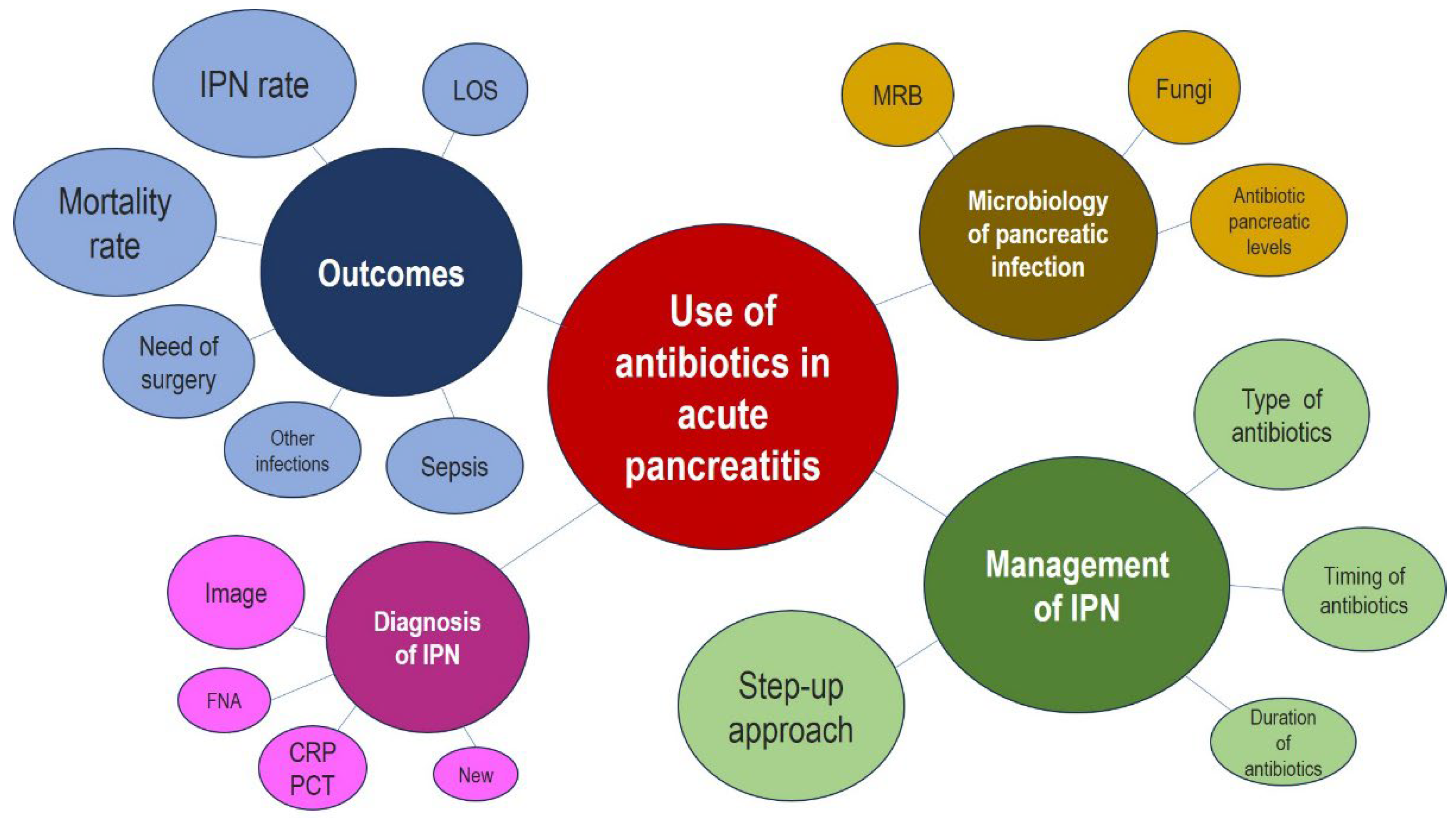
Figure 2 shows the data map of the key issues reviewed.
2.1. Definitions, Classification and Diagnosis
AP is an acute inflammatory process of the pancreas with a mortality ranging from 1 % in oedematous interstitial AP to up to 28 % in severe AP [
6,
7,
8]. According to the 2012 Atlanta classification [
2], acute necrotising pancreatitis is characterised by inflammation associated with pancreatic parenchymal necrosis and/or peripancreatic necrosis. Between 5-10% of patients develop necrosis of the pancreatic parenchyma, peripancreatic tissue, or both. Necrotising pancreatitis can usually only be detected 72 to 96 hours after the onset of symptoms [
9], and is associated with high rates of early organ failure (38 %), need for intervention (38 %) and death (15 %) [
10].
2.2. Prediction of Severity
The numerous scores designed to predict AP severity have little clinical impact and most clinical guidelines recommend strict monitoring of systemic inflammatory response syndrome (SIRS) and organ failure parameters during the first 48 h (especially renal, respiratory and cardiovascular failure) [
2,
10,
11], using the Marshall [
12] or SOFA [
13] scales.
The Atlanta criteria use the existence of organ failure to classify severity, categorising AP as mild (absence of organ failure and local or systemic complications), moderate (no organ failure or transient organ failure <48 hours and/or local complications) and severe (persistent organ failure >48 hours that may involve one or more organs).
2.3. Local Complications
Local complications should be suspected when there is persistent pain, lipase or amylase upsurge, increased organ failure, fever and leukocytosis [
2].
Infected pancreatic necrosis (IPN). Pancreatic and peripancreatic necrosis can remain sterile or become infected, with no absolute correlation between the extent of necrosis and the risk of infection [
2,
8]. Necrosis presents an infection rate of 33% and mortality ranges from 15% to 35% [
14]. IPN is rare during the first week, when sepsis is mainly due to pneumonia or bacteraemia [
8]. There is no correlation between the extent of necrosis and the risk of infection. Although infection can occur early in the course of necrotising pancreatitis, it is most often seen late in the clinical course, after 10 days, and peaks between the second and fourth week of AP [
8,
15,
16].
2.4. Infections and Microbiology of Acute Pancreatitis
The mean time from onset of AP to diagnosis of IPN in the PROPATRIA study was 26 days [
17]. However, other authors using routine fine needle aspiration (FNA) detect it in most cases at two weeks, so it is possible that IPN may develop before the clinical manifestation of infection [
18,
19]. In addition to IPN, up to 20% of patients with AP develop extrapancreatic infections (bacteraemia, sepsis, pneumonia or urinary tract infections)[
8], which are associated with increased mortality [
20].
Previous bacteraemia in patients with necrosis is associated with an increased risk of IPN and in half of patients the same germ is isolated in both sources [
6]. Bacteraemia is an independent mortality and risk factor for IPN and can be used as a prognostic factor in patients with known pancreatic necrosis [
6].
Most infections (approximately 75%) are monomicrobial, caused by organisms of enteric origin [
3]. The main source of infection is thought to be bacterial translocation from the small intestine rather than from the colon [
21]. At the time of initial infection, Gram-negative bacteria (especially
Escherichia coli, Klebsiella spp. and
Pseudomonas aeruginosa), Gram-positive bacteria (
Enterococcus faecium, Ente
rococcus faecalis, Staphylococcus epidermidis, Staphylococcus aureus) and fungi (
Candida spp.), with some regional variation [
8,
22,
23] should be considered. Initial fungal infection can double the mortality rate [
8].
The presence of multi-drug resistant organisms (MDRO) is of concern in this patient group, especially if prolonged empirical treatments have been performed. MDRO rates of 21%-32% have been reported in necrotic tissue from patients treated prophylactically with carbapenem [
24,
25]. In a 2018 study, 50% of patients acquired an extensively drug-resistant bacterial infection at some point, which was the main reason for prolonged intensive care unit stay [
26].
Gut Microbiota Dysbiosis in AP. Manipulation of the gut microbiome may be a new line of treatment, as a dysbiosis of the gut microbiota in AP has been identified [
27,
28,
29], based on an apparent reduction in bacterial diversity in this site [
29]. The gut microbiome may be altered in AP patients, characterised by a decrease in Bifidobacterium and bacteria that produce short-chain fatty acids with anti-inflammatory properties and, in contrast, an increase in harmful bacteria such as Enterobacteriaceae and
Enterococcus [
28,
29].
2.5. Management of Infected Pancreatic Necrosis; Research Questions
Table 1 summarises the main findings of the scoping review.
2.5.1. Does Preventive Antibiotic Treatment Reduce Mortality in Acute Pancreatitis with Necrosis?
The possibility of preventing IPN and its associated morbidity and mortality by prophylactic administration of antibiotics has been a matter of debate for 40 years. This preventive antibiotic treatment (PAT) has been the subject of at least 21 RCTs and 21 meta-analyses and is a topic of discussion in all AP clinical practice guidelines. Although early RCTs and meta-analyses showed reductions in mortality, incidence of IPN and extrapancreatic infections, the subsequent development of higher quality studies and the inclusion of larger numbers of cases have changed the paradigm. The most recent guidelines [
1,
9,
30,
31,
32,
33,
34,
35]do not recommend PAT in AP of any degree of severity, nor when pancreatic necrosis is found; this is because of the results of meta-analyses published since 2008, which find no difference in mortality or incidence of IPN with the use of antibiotics [
36,
37,
38,
39,
40,
41,
42].
As mentioned, an overall analysis of the published meta-analyses on PAT shows no reduction in the incidence of necrotising infection or mortality, although most of this studies show a fall in extrapancreatic infections [
37,
38,
40,
41,
42,
43,
44,
45], especially due to a decrease in sepsis and urinary tract infections.
However, a rigorous assessment of the results of the published meta-analyses, should take two factors into account: the methodological quality of the RCTs on which they are based, and the sample size of. A study by De Vries et al. analysed the quality of RCTs on the effect of PAT in acute pancreatitis prior to 2009 and found an inverse relationship between the methodological quality of the RCTs and the reduction in mortality risk they showed, categorising their overall quality as moderate [
46]. To address the sample size factor, an important reference point is the systematic review and meta-analysis by Poropat et al. published in 2022 [
42], which demonstrated that current meta-analyses of mortality, sepsis and urinary tract infection outcomes are underpowered, and as a result are unable to provide strong evidence of the impact of PAT on these conditions. The only positive results with the use of PAT found in this study are reductions in hospital stay, overall infection rates and extrapancreatic infections, findings that are in agreement with most previous meta-analyses.
In summary, there is no evidence that PAT decreases the main outcomes of infection prevention and mortality in pancreatic necrosis, but we cannot be sure that this lack of evidence is not due to the possibility that the sample of available RCTs and meta-analyses is insufficient. Nevertheless, the authors believe that the recommendations of current guidelines should be followed and that signs of severity or necrosis are not sufficient grounds for routine TAP. However, subtle warning signs that may raise suspicion of IPN should be recognised early and antibiotic treatment should be initiated in these situations.
Further high-quality studies and with adequate sample sizes are therefore needed to reach the targets calculated by Poropat et al. of 1638 cases to analyse mortality, 1291 to analyse sepsis and 871 to analyse urinary tract infection [
42].
2.5.2. How Is Infected Pancreatic Necrosis Diagnosed?
Suspected diagnosis. A study of the value of routine laboratory tests to differentiate infected and sterile necrosis found only C-reactive protein (CRP) and leukocyte counts to be discriminatory, with cut-off values of 81 mg/l for CRP and 13×109/l for leukocytes [
47]. However, in the early phase of AP, the elevation of these parameters is indistinguishable from that caused by other infectious complications or the inflammatory state of the disease itself [
1,
48,
49] and is not considered sufficient to diagnose IPN and thus indicate antibiotic treatment.
IPN may be suspected in patients with pancreatic or extrapancreatic necrosis with clinical deterioration (clinical instability, persistent sepsis, new or prolonged organ failure, increased need for intensive support, leucocytosis, rising CRP or fever) or who do not improve after 7-10 days of hospitalisation. In this context and in the absence of an alternative cause of infection, a retrospective study showed that IPN caused the condition in 80% of cases, with a false positive rate of 20% [
50].
For its part, a 2014 systematic review considered procalcitonin (PCT) to be the best biological predictor of IPN, with a cut-off value of 3.5 ng/mL, offering a sensitivity of 90% and a specificity of 89%, although its values appear to be slightly higher than the upper normal limit [
51]. Most of the evidence in favour of PCT does not come from RCTs; a 2017 Cochrane study could not reach conclusions on the usefulness of CRP and PCT due to the paucity of RCTs and the methodological shortcomings of those available [
52]. Subsequently, an RCT analysed a PCT-based algorithm for deciding the initiation, continuation and termination of antibiotic treatment, finding a decrease in antibiotic use in the PCT-guided group, and no increase in the rate of infections and mortality [
53]. Recent guidelines consider that PCT may be the most useful marker for predicting the risk of developing infected pancreatic necrosis [
1,
10,
35,
54].
Certainty diagnosis. When IPN is suspected, the first examination performed is a CT scan, in search of extraluminal gas in the pancreatic or peripancreatic tissues. The presence of gas in these collections suggests a direct diagnosis of infection with a very high degree of certainty; however, gas formations occur in only half of patients with IPN (sensitivity 56%, specificity 97%) [
1,
2,
19,
55].
Diffusion-weighted MRI also visualises air in the retroperitoneum [
56], but it is not always available and is difficult to perform in critically ill patients. Furthermore, few studies are available on its superiority over CT [
10,
55].
There is no doubt that the diagnosis can be established with the detection of bacteria or fungi by Gram stain or culture on material obtained by FNA, although it presents false negative rates of 20-25% and false positive rates of 4-15% [
50,
57]. Furthermore, FNA may be associated with iatrogenic infectious complications such as peritoneal contamination and gastrointestinal perforation and haemorrhagic complications. It is therefore no longer routinely performed at many centres [
1] and current guidelines do not consider it essential for the diagnosis of IPN [
1,
9,
10,
35].
However, new methodologies ancillary to microbiology, such as metagenomic next-generation sequencing (mNGS) may revolutionise the management of IPN by facilitating earlier diagnosis, as well as broadening the range of pathogens identifiable in the sample. In a retrospective study of 40 patients with suspected IPN undergoing CT-guided FNA, culture and mNGS were used simultaneously. The mNGS result was obtained earlier than the culture result (42 h (36-62 h) vs. 60 h (42-124 h), P = 0.032); furthermore, traditional cultures isolated seven bacterial species and two fungal species, while mNGS detected 22 bacterial species and two fungal species. The sensitivity, specificity, NPV and PPV of mNGS were 88.0%, 100%, 83.3% and 100%, respectively [
58].
This same mNGS technique has even been used to analyse circulating microbial cell-free DNA. In plasma from 44 patients with suspected IPN, the mNGS positivity rate was 54.6%. In patients in whom IPN was confirmed, mNGS was compared with microbiological results, which were considered the reference standard. Of the 24 cases with positive mNGS, 20 (83.3%, 95%CI 68.42-98.2%) matched the results with the IPN drainage culture. The positive and negative percentage agreements of plasma mNGS for IPN were 80.0% (95%CI, 64.32-95.68) and 89.5% (95%CI, 75.67-100), respectively. Furthermore, compared with the mNGS-negative group, patients in the positive group had more new-onset septic shock [
59]. Further experience is probably needed before this technology can be integrated into practice.
2.5.3. When Should Antibiotic Treatment be Prescribed in Acute Pancreatitis?
When infection is suspected, antibiotics should be started promptly while the source of infection is being determined. Using data from RCTs prior to 2015, two meta-analyses suggest that, in the presence of IPN, early initiation of treatment is a key determinant in reducing mortality [
44,
60]. As described above, certain clinical signs and the presence of retroperitoneal gas are reasonably suggestive of IPN and empirical antibiotic treatment can be initiated without FNA, especially if percutaneous drainage is to be part of the management algorithm [
1,
9,
31,
61]. However, if cultures are subsequently negative and no source of infection is identified, antibiotics should be discontinued.
2.5.4. Which Antibiotics Are Indicated in the Treatment of Infected Pancreatic Necrosis?
The choice of antibiotic should be based on the results of in vitro antimicrobial sensitivity and in vivo bioavailability, including penetration into pancreatic tissue. Pancreatic necrosis, in which vascular supply is lacking, would be expected to have minimal penetration. However, some studies have detected significant levels of piperacillin/tazobactam, metronidazole, imipenem, ciprofloxacin and ofloxacin in necrotic pancreatic tissue samples [
62,
63,
64]. It should be remembered that these broad-spectrum molecules may be responsible for the emergence of MDRO and that there is little literature analysing other antibiotics with less ecological impact [
35].
If empirical antibiotics are initiated, agents with Gram-negative and Gram-positive coverage should be used (e.g., a carbapenem alone; or piperacillin/tazobactam, ceftazidime or cefepime combined with an anaerobic agent such as metronidazole). In any case, antibiotic selection can be adjusted according to local microbial flora and resistance patterns.
Adaptation of antibiotic therapy. When microbiology results are available, antibiotic therapy should be tailored according to the pathogens identified and their susceptibility patterns. This personalised approach helps to optimise therapeutic efficacy and minimise the development of antibiotic resistance.
Antifungals. Antifungal prophylaxis has been suggested for patients receiving broad-spectrum antibiotics. The use of prophylactic antibacterial therapy, and especially its long duration, increase the incidence of pancreatic fungal infection. A 2021 meta-analysis reported an incidence of fungal infections of 26.6% in IPN [
65], with Candida albicans being the most frequently isolated fungus, but it should be noted that many of the included patients had prolonged prior antibiotic therapy.
It seems clear that fungal infection negatively affects the prognosis of patients with pancreatitis and is associated with increased morbidity, mortality, ICU admission rate and length of hospital stay. However, antifungal agents may not reach therapeutic levels in poorly perfused pancreatic or peripancreatic tissues, and current guidelines do not recommend the routine administration of prophylactic antifungal therapy in conjunction with therapeutic antibiotics [
1,
10,
31,
34,
35,
66].
2.5.5. What Is the Role of the Step-up Approach?
The step-up approach has been proposed as an effective strategy in the treatment of IPN. It is based on a combination of therapies, starting with conservative measures, such as antibiotics and supportive management, and gradually escalating to more invasive interventions, such as ultrasound- or CT-directed percutaneous drainage, video-assisted retroperitoneal necrosectomy (VARD), transgastric necrosectomy or open surgery, if necessary.
The pioneering PANTER (Percutaneous Step-Up Approach in Necrotising Pancreatitis) trial showed that image-guided percutaneous drainage reduces the rate of serious complications or death when compared to open surgery for IPN [
61]. Upon suspicion or confirmation of IPN, the step-up approach starts with antibiotic treatment. The next step is the insertion of an image-directed pig-tail drain through the left retroperitoneum, facilitating minimally invasive retroperitoneal necrosectomy, if necessary. If there is no clinical improvement, the third step, left retroperitoneal surgical step-up approach (LRRSA) is performed with continuous postoperative high-volume lavage.
In 2018, the TENSION trial showed that the endoscopic step-up approach is not superior to the surgical approach in terms of mortality or major complications, although the pancreatic fistula rate and hospital stay were lower with endoscopy [
67]. The authors concluded that the endoscopic step-up approach is the treatment of choice for transgastric approachable necrosis and that the left retroperitoneal surgical step-up approach should be reserved for IPN far from the stomach, alone or in combination with the endoscopic approach. Whether performed endoscopically or surgically, it is best to delay the step-up approach as long as possible for optimal results [
68].
2.5.6. What Is the Adequate Duration of Antibiotic Treatment?
There are no data on the optimal duration of antibiotic treatment in IPN. Although early and aggressive antibiotic therapy is essential, its prolonged use should be justified based on the clinical response and ongoing infection. Antibiotic therapy also entails risks, such as the increased prevalence of MDRO and the incidence of fungal infections, which correlate with mortality.
Duration of antibiotic treatment can be guided by factors such as extent of necrosis, clinical improvement and the resolution of systemic inflammatory markers. Although still a matter of debate, it is common to discontinue antimicrobials 48 h after removal of the last drain if all cultures are negative [
10]. Individualised patient assessment, taking into account factors such as immunocompetence and comorbidities, is crucial in determining the appropriate duration of antibiotic treatment.
3. Discussion
This paper provides a comprehensive overview of the existing literature on the role of antibiotics in the management of AP. It summarises the available evidence related to risk profiles, diagnosis of IPN, microbiology of infection, the most appropriate antibiotics and criteria for their prescription, operative and non-operative management, and duration of treatment. The paper demonstrates the need for further high-level studies assessing most aspects of practice.
The main findings of this review favour basing predictions of severity on the existence of SIRS criteria and organ failure. AP mortality is strongly correlated with extrapancreatic infections and IPN. These complications may occur at any time during the course of the disease but are most frequent after 8-10 days of admission, although IPN probably appears earlier than is suggested in current reports. Obtaining microbiological samples by FNA is not always essential for its diagnosis, and a high level of clinical suspicion or CT signs may be sufficient to initiate antibiotic therapy. The diagnostic suspicion of IPN in patients with pancreatic necrosis could be based on further clinical deterioration with worsening organ failure, accompanied by increased CRP and leukocyte levels, while the most useful biomarker in this context seems to be PCT. Promising microbiological methods are currently emerging such as metagenomic sequencing that may allow an prompt diagnosis of IPN and the establishment of early antibiotic therapy, which has been correlated with therapeutic efficacy. Emerging research on intestinal dysbiosis in AP, the possible manipulation of the intestinal microbiome and the effect of selective decontamination of the intestinal tract raise novel issues in the antibiotic management of AP that deserve further investigation.
Based on updated information from meta-analyses, all current clinical guidelines recommend antibiotic therapy only in cases of highly suspected or confirmed IPN. This recommendation seems to be supported by the present review, although the issue remains open for further research and cannot be considered settled. Thus, although the currently available meta-analyses do not present evidence that PAT decreases pancreatic necrosis infection and mortality, this possibility cannot be ruled out due to the insufficient sample size in the published RCTs and the low statistical power of meta-analyses assessing mortality and infections of all types in PC. As discussed, the conclusions of the Poropat et al.[
42] meta-analysis are illustrative. The study uses the trial sequential analysis technique to calculate the sample size necessary for the results of a meta-analysis to be considered reliable. This technique and the estimation of the optimal sample size is described in detail in García-Alamino et al. [
69]. Calculating the optimal sample size reduces the risk of false results in meta-analyses and determines whether more RCTs are needed to address the effects of the intervention or, conversely, whether a given meta-analysis can be considered as providing the definitive evidence. Poropat et al. believed that no distinction can be made between the lack of efficacy of PAT and the lack of power of meta-analyses, a circumstance that may lead to the erroneous rejection of an effective intervention because of a type 2 statistical error (i.e., a false negative result). This may be one of these situations in which absence of evidence is not evidence of absence. Some beneficial effects have, however, been demonstrated with PAT, such as reductions in hospital stay and in rates of general and extra-pancreatic infections.
Despite the theoretical agreement between meta-analyses and guidelines in terms of knowledge of scientific evidence, there is a notable gap with regard to the practice of care. A British study reported the prescription of PAT in 62% of acute pancreatitis without a clear explanation of its indication [
5]. Another retrospective study, conducted in 23 countries, showed an overall overuse of antibiotics in AP, ranging from 31% to 82%, with higher rates in Asia [
54]. No consensus was detected for the indication of antibiotic therapy, which was mostly initiated on the basis of white blood cell count, lipase, amylases and CRP levels. The same study found that these parameters were always elevated in the early stages of pancreatitis, but were not related to infection, unlike PCT, which was a good predictor of infection at this stage.
As regards IPN source control, this is one of the areas that has been studied the most in the last decade, over the course of which the concept of the step-up approach has been consolidated. Antibiotic treatment with Gram-positive, Gram-negative and anaerobic coverage should be considered as the first stage of the step-up approach, gradually followed by image-guided percutaneous drainage and retroperitoneal or transgastric necrosectomy, which should be postponed as long as the patient's condition allows.
As in all intra-abdominal infections, the duration of antibiotic treatment should be as short as possible, depending on the quality of septic source control and the patient’s clinical condition. Although outside the time period of this review, recent Surgical Infection Society guidelines recommend limiting therapy to four days for low and high risk patients even when source control has been achieved by a percutaneous drainage procedure [
70].
Limitations of the study. Studies written in languages other than English or Spanish were not included; nor were studies that reported qualitative data that might have been relevant to the findings. Other relevant limitations, which need to be addressed in future research, include the heterogeneity of the studies, in terms of differences in protocols, outcomes, and the small sample size of the trials. Finally, due to the use of the scoping review methodology [
71], no quality assessment of the studies included was performed.
4. Materials and Methods
This study is based on the guidance framework for conducting scoping reviews developed by the Joanna Briggs Institute [
72], and is reported in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) extension for scoping reviews (PRISMA-ScR) [
71]. The project has been preregistered on the OSF Database (
https://osf.io/fsk7a/?view_only=). Information sources. PubMed, Scopus and Cochrane databases were searched using a predefined strategy. The reference lists of all the studies included were manually reviewed to identify other relevant papers. The records retrieved were double-screened for eligibility.
4.1. Eligibility Criteria
Inclusion criteria were studies of patients with AP and interventions such as antibiotic prophylaxis, and the treatment and management of pancreatic infection. The studies included were systematic reviews with or without meta-analysis and randomised clinical trials. Narrative reviews, case reports, observational case-control studies, editorials, expert opinions, pre-clinical studies, conference proceedings and studies not published in English or in Spanish were excluded.
4.2. Search Strategy
The studies were searched using relevant keywords and Medical Subject Heading (MeSH) terms, spanning the period from January 2000 to December 2023. Additional references were identified through manual searches from the grey literature, analysing key articles and reference lists of relevant studies; searches of guidelines of professional associations and societies; Google Scholar, ResearchGate and Academia.edu for reports and preprints.
4.3. Selection of Sources of Evidence
Screening was carried out independently by two reviewers (JMB and SA), with full texts of potentially relevant articles considered for inclusion. Disagreements between reviewers were resolved by consensus or with a third reviewer (MJ). Study descriptors were extracted including title, first author, year of publication, country of origin, study design, sample size, study setting (single or multicentre) and key findings. In order to group the papers identified, key themes from the studies were identified and used to create a framework corresponding to the clinical pathway, addressing aspects such as diagnoses, treatments and outcomes.
4.4. Synthesis of Results
A narrative synthesis of the studies included was produced. In line with the methodology of scoping reviews, a formal assessment of the risk of bias in the studies was not performed [
71]. Identification of gaps was undertaken by reviewing areas addressed by the studies according to the aspect of care addressed. Study findings were considered by the research team, and questions relevant to the gaps in the evidence were proposed. A concept map was generated with a data mapping tool to visualize the evidence and demonstrate any gaps in the evidence (MapForce
® 2024).
4.5. Research Questions
A summary of the published evidence on definitions, severity criteria, complications and microbiology of pancreatic infection was performed. In addition, a number of research questions were formulated: (1) Does antibiotic prophylaxis reduce mortality in AP with necrosis? (2) How should be IPN diagnosed? (3) When should antibiotic treatment be prescribed in AP? (4) Which antibiotics are indicated in the treatment of IPN? (5) What is the role of the step-up approach? (6) What is the adequate duration of antibiotic treatment?
4.6. Endpoints
The following endpoints were used in the analysis of the selected articles: mortality rate; pancreatic necrosis infection rate; non-pancreatic infection rate; sepsis; need for surgical intervention; length of hospital stay (LOS).
5. Conclusions and Implications for Research and Practice
Early initiation of antibiotic therapy is crucial in the setting of IPN, but the current evidence does not favour the routine use of preventive antibiotics in severe AP or AP with necrosis. Current guidelines advise treatment only when IPN is confirmed or strongly suspected. Nevertheless, the results of available RCTs and meta-analyses should be considered inconclusive due to the heterogeneity of the studies, with variations in antibiotic classes, timing of treatment, outcomes analysed, study designs and, above all, sample sizes. Our understanding of the use of antibiotics in AP seems incomplete; evidence is lacking on the overall efficacy of prophylactic or preventive antibiotics in IPN, the criteria for initiation of treatment, the type of antibiotics to be used and their timing. Determination of PCT and CRP levels and some novel microbiological techniques may be useful to predict the likelihood of developing IPN and to identify patients who might benefit from early antibiotic treatment.
Although it is advisable to adhere to the current guidelines for sparing antibiotic use in AP, high-quality RCTs are now needed to shed light on the unclarified issues regarding the role of antibiotics and antifungals in AP.
Author Contributions
Conceptualization, J.M.B., C.G.S., A.M.V., I.R.P., E.M., J.M.Ba.,and X.G.; methodology, J.M.B., C.G.S. and X.G.; software, J.M.B.; validation, J.M.B., and S.A.; formal analysis, J.M.B., and S.A.; investigation, J.M.B., and S.A.; resources J.M.B., and S.A.; data curation, J.M.B., and S.A.; writing—original draft preparation, J.M.B.; writing—review and editing, J.M.B., C.G.S., A.M.V., I.R.P., E.M., J.M.Ba.,and X.G.; supervision, J.M.B..,and X.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study is part of a research project of the Surgical Infection Section of the Spanish Association of Surgeons and the Spanish Observatory of Infection in Surgery, who approved its methodology.
Data Availability Statement
All data will be made available on request.
Acknowledgments
The authors thank Michael Maudsley for his help with the English.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Leppäniemi, A.; Tolonen, M.; Tarasconi, A.; Segovia-Lohse, H.; Gamberini, E.; Kirkpatrick, A.W.; Ball, C.G.; Parry, N.; Sartelli, M.; Wolbrink, D.; et al. 2019 WSES Guidelines for the Management of Severe Acute Pancreatitis. World Journal of Emergency Surgery 2019, 14. [Google Scholar] [CrossRef] [PubMed]
- Banks, P.A.; Bollen, T.L.; Dervenis, C.; Gooszen, H.G.; Johnson, C.D.; Sarr, M.G.; Tsiotos, G.G.; Vege, S.S. Classification of Acute Pancreatitis-2012: Revision of the Atlanta Classification and Definitions by International Consensus. [CrossRef]
- Mowbray, N.G.; Ben-Ismaeil, B.; Hammoda, M.; Shingler, G.; Al-Sarireh, B. The Microbiology of Infected Pancreatic Necrosis. Hepatobiliary and Pancreatic Diseases International 2018, 17, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Timmerhuis, H.C.; Van Den Berg, F.F.; Noorda, P.C.; Van Dijk, S.M.; Van Grinsven, J.; Sperna Weiland, C.J.; Umans, D.S.; Mohamed, Y.A.; Curvers, W.L.; Bouwense, S.A.W.; et al. Overuse and Misuse of Antibiotics and the Clinical Consequence in Necrotizing Pancreatitis: An Observational Multicenter Study. Ann Surg 2023, 278. [Google Scholar] [CrossRef] [PubMed]
- Barrie, J.; Jamdar, S.; Smith, N.; McPherson, S.J.; Siriwardena, A.K.; O’Reilly, D.A. Mis-Use of Antibiotics in Acute Pancreatitis: Insights from the United Kingdom’s National Confidential Enquiry into Patient Outcome and Death (NCEPOD) Survey of Acute Pancreatitis. Pancreatology 2018, 18. [Google Scholar] [CrossRef]
- Singh, V.K.; Bollen, T.L.; Wu, B.U.; Repas, K.; Maurer, R.; Yu, S.; Mortele, K.J.; Conwell, D.L.; Banks, P.A. An Assessment of the Severity of Interstitial Pancreatitis. Clinical Gastroenterology and Hepatology 2011, 9. [Google Scholar] [CrossRef]
- Van Santvoort, H.C.; Bakker, O.J.; Bollen, T.L.; Besselink, M.G.; Ahmed Ali, U.; Schrijver, A.M.; Boermeester, M.A.; Van Goor, H.; Dejong, C.H.; Van Eijck, C.H.; et al. A Conservative and Minimally Invasive Approach to Necrotizing Pancreatitis Improves Outcome. Gastroenterology 2011, 141. [Google Scholar] [CrossRef]
- Besselink, M.G.; Van Santvoort, H.C.; Boermeester, M.A.; Nieuweohuijs, V.B.; Van Goor, H.; Dejong, C.H.C.; Schaapherder, A.F.; Gooszen, H.G. Timing and Impact of Infections in Acute Pancreatitis. British Journal of Surgery 2009, 96. [Google Scholar] [CrossRef]
- IAP/APA Evidence-Based Guidelines for the Management of Acute Pancreatitis. Pancreatology 2013, 13. [CrossRef]
- Arvanitakis, M.; Dumonceau, J.M.; Albert, J.; Badaoui, A.; Bali, M.A.; Barthet, M.; Besselink, M.; Deviere, J.; Ferreira, A.O.; Gyökeres, T.; et al. Endoscopic Management of Acute Necrotizing Pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) Evidence-Based Multidisciplinary Guidelines. Endoscopy 2018, 50, 524–546. [Google Scholar] [CrossRef]
- Boxhoorn, L.; Voermans, R.P.; Bouwense, S.A.; Bruno, M.J.; Verdonk, R.C.; Boermeester, M.A.; van Santvoort, H.C.; Besselink, M.G. Acute Pancreatitis. The Lancet 2020, 396, 726–734. [Google Scholar] [CrossRef]
- Marshall, J.C.; Cook, D.J.; Christou, N. V.; Bernard, G.R.; Sprung, C.L.; Sibbald, W.J. Multiple Organ Dysfunction Score: A Reliable Descriptor of a Complex Clinical Outcome. Crit Care Med 1995, 23. [Google Scholar] [CrossRef]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-Related Organ Failure Assessment) Score to Describe Organ Dysfunction/Failure. Intensive Care Med 1996, 22. [Google Scholar] [CrossRef] [PubMed]
- Van DIjk, S.M.; Hallensleben, N.D.L.; Van Santvoort, H.C.; Fockens, P.; Van Goor, H.; Bruno, M.J.; Besselink, M.G. Acute Pancreatitis: Recent Advances through Randomised Trials. Gut 2017, 66. [Google Scholar] [CrossRef]
- Beger, H.G.; Bittner, R.; Block, S.; Büchler, M. Bacterial Contamination of Pancreatic Necrosis. A Prospective Clinical Study. Gastroenterology 1986, 91. [Google Scholar] [CrossRef] [PubMed]
- Bradley, E.L.; Allen, K. A Prospective Longitudinal Study of Observation versus Surgical Intervention in the Management of Necrotizing Pancreatitis. The American Journal of Surgery 1991, 161. [Google Scholar] [CrossRef] [PubMed]
- Besselink, M.G.H.; Timmerman, H.M.; Buskens, E.; Nieuwenhuijs, V.B.; Akkermans, L.M.A.; Gooszen, H.G. Probiotic Prophylaxis in Patients with Predicted Severe Acute Pancreatitis (PROPATRIA): Design and Rationale of a Double-Blind, Placebocontrolled Randomised Multicenter Trial [ISRCTN38327949]. BMC Surg 2004, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rau, B.; Pralle, U.; Mayer, J.M.; Beger, H.G. Role of Ultrasonographically Guided Fine-Needle Aspiration Cytology in the Diagnosis of Infected Pancreatic Necrosis. British Journal of Surgery 1998, 85. [Google Scholar] [CrossRef]
- Gerzof, S.G.; Banks, P.A.; Robbins, A.H.; Johnson, W.C.; Spechler, S.J.; Wetzner, S.M.; Snider, J.M.; Langevin, R.E.; Jay, M.E. Early Diagnosis of Pancreatic Infection by Computed Tomography-Guided Aspiration. Gastroenterology 1987, 93. [Google Scholar] [CrossRef]
- Wu, B.U.; Johannes, R.S.; Kurtz, S.; Banks, P.A. The Impact of Hospital-Acquired Infection on Outcome in Acute Pancreatitis. Gastroenterology 2008, 135. [Google Scholar] [CrossRef]
- Fritz, S.; Hackert, T.; Hartwig, W.; Rossmanith, F.; Strobel, O.; Schneider, L.; Will-Schweiger, K.; Kommerell, M.; Büchler, M.W.; Werner, J. Bacterial Translocation and Infected Pancreatic Necrosis in Acute Necrotizing Pancreatitis Derives from Small Bowel Rather than from Colon. Am J Surg 2010, 200. [Google Scholar] [CrossRef]
- Lu, J. Di; Cao, F.; Ding, Y.X.; Wu, Y.D.; Guo, Y.L.; Li, F. Timing, Distribution, and Microbiology of Infectious Complications after Necrotizing Pancreatitis. World J Gastroenterol 2019, 25. [Google Scholar] [CrossRef]
- Li, F.; Zhang, F.; Wan, X.; Wu, K.; Liu, Q.; Qiu, C.; Yin, H.; Lyu, J. Infections in Acute Pancreatitis: Organisms, Resistance-Patterns and Effect on Mortality. Dig Dis Sci 2023, 68, 630–643. [Google Scholar] [CrossRef]
- Gloor, B.; Müller, C.A.; Worni, M.; Stahel, P.F.; Redaelli, C.; Uhl, W.; Büchler, M.W. Pancreatic Infection in Severe Pancreatitis: The Role of Fungus and Multiresistant Organisms. Archives of Surgery 2001, 136. [Google Scholar] [CrossRef] [PubMed]
- Maraví-Poma, E.; Gener, J.; Alvarez-Lerma, F.; Olaechea, P.; Blanco, A.; Domínguez-Muñoz, J.E. Early Antibiotic Treatment (Prophylaxis) of Septic Complications in Severe Acute Necrotizing Pancreatitis: A Prospective, Randomized, Multicenter Study Comparing Two Regimens with Imipenem-Cilastatin. Intensive Care Med 2003, 29, 1974–1980. [Google Scholar] [CrossRef] [PubMed]
- Moka, P.; Goswami, P.; Kapil, A.; Xess, I.; Sreenivas, V.; Saraya, A. Impact of Antibiotic-Resistant Bacterial and Fungal Infections in Outcome of Acute Pancreatitis. Pancreas 2018, 47. [Google Scholar] [CrossRef]
- Tan, C.; Ling, Z.; Huang, Y.; Cao, Y.; Liu, Q.; Cai, T.; Yuan, H.; Liu, C.; Li, Y.; Xu, K. Dysbiosis of Intestinal Microbiota Associated with Inflammation Involved in the Progression of Acute Pancreatitis. Pancreas 2015, 44. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Mei, Q.; Fu, Y.; Zeng, Y. Alteration of Gut Microbiota in Acute Pancreatitis and Associated Therapeutic Strategies. Biomedicine and Pharmacotherapy 2021, 141. [Google Scholar] [CrossRef] [PubMed]
- Van Den Berg, F.F.; Van Dalen, D.; Hyoju, S.K.; Van Santvoort, H.C.; Besselink, M.G.; Wiersinga, W.J.; Zaborina, O.; Boermeester, M.A.; Alverdy, J. Western-Type Diet Influences Mortality from Necrotising Pancreatitis and Demonstrates a Central Role for Butyrate. Gut 2021, 70. [Google Scholar] [CrossRef]
- Uhl, W.; Warshaw, A.; Imrie, C.; Bassi, C.; McKay, C.J.; Lankisch, P.G.; Carter, R.; Di Magno, E.; Banks, P.A.; Whitcomb, D.C.; et al. IAP Guidelines for the Surgical Management of Acute Pancreatitis. Pancreatology 2002, 2, 565–573. [Google Scholar] [CrossRef]
- Tenner, S.; Baillie, J.; Dewitt, J.; Vege, S.S. American College of Gastroenterology Guideline: Management of Acute Pancreatitis. American Journal of Gastroenterology 2013, 108. [Google Scholar] [CrossRef]
- Koizumi, M.; Takada, T.; Kawarada, Y.; Hirata, K.; Mayumi, T.; Yoshida, M.; Sekimoto, M.; Hirota, M.; Kimura, Y.; Takeda, K.; et al. JPN Guidelines for the Management of Acute Pancreatitis: Diagnostic Criteria for Acute Pancreatitis. J Hepatobiliary Pancreat Surg 2006, 13, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Pezzilli, R.; Zerbi, A.; Campra, D.; Capurso, G.; Golfieri, R.; Arcidiacono, P.G.; Billi, P.; Butturini, G.; Calculli, L.; Cannizzaro, R.; et al. Consensus Guidelines on Severe Acute Pancreatitis. Digestive and Liver Disease 2015, 47. [Google Scholar] [CrossRef]
- Crockett, S.D.; Wani, S.; Gardner, T.B.; Falck-Ytter, Y.; Barkun, A.N.; Crockett, S.; Feuerstein, J.; Flamm, S.; Gellad, Z.; Gerson, L.; et al. American Gastroenterological Association Institute Guideline on Initial Management of Acute Pancreatitis. Gastroenterology 2018, 154, 1096–1101. [Google Scholar] [CrossRef]
- Jaber, S.; Garnier, M.; Asehnoune, K.; Bounes, F.; Buscail, L.; Chevaux, J.B.; Dahyot-Fizelier, C.; Darrivere, L.; Jabaudon, M.; Joannes-Boyau, O.; et al. Guidelines for the Management of Patients with Severe Acute Pancreatitis, 2021. Anaesth Crit Care Pain Med 2022, 41. [Google Scholar] [CrossRef]
- Bai, Y.; Gao, J.; Zou, D.W.; Li, Z.S. Prophylactic Antibiotics Cannot Reduce Infected Pancreatic Necrosis and Mortality in Acute Necrotizing Pancreatitis: Evidence from a Meta-Analysis of Randomized Controlled Trials. American Journal of Gastroenterology 2008, 103, 104–110. [Google Scholar] [CrossRef]
- Xu, T.; Cai, Q. Prophylactic Antibiotic Treatment in Acute Necrotizing Pancreatitis: Results from a Meta-Analysis. Scand J Gastroenterol 2008, 43, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Jafri, N.S.; Mahid, S.S.; Idstein, S.R.; Hornung, C.A.; Galandiuk, S. Antibiotic Prophylaxis Is Not Protective in Severe Acute Pancreatitis: A Systematic Review and Meta-Analysis. Am J Surg 2009, 197. [Google Scholar] [CrossRef]
- Villatoro, E.; Mulla, M.; Larvin, M. Antibiotic Therapy for Prophylaxis against Infection of Pancreatic Necrosis in Acute Pancreatitis. Cochrane Database of Systematic Reviews 2010, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Sun, Y.H.; Wen, L.M.; Wang, J.H.; Yang, J.H.; Cheng, K.; Lin, H.; Chen, Q.L. Assessment of Prophylactic Antibiotics Administration for Acute Pancreatitis: A Meta-Analysis of Randomized Controlled Trials. Chin Med J (Engl) 2020, 133, 212–220. [Google Scholar] [CrossRef]
- Guo, D.; Dai, W.; Shen, J.; Zhang, M.; Shi, Y.; Jiang, K.; Guo, L. Assessment of Prophylactic Carbapenem Antibiotics Administration for Severe Acute Pancreatitis: An Updated Systematic Review and Meta-Analysis. Digestion 2022, 183–191. [Google Scholar] [CrossRef]
- Poropat, G.; Goričanec, K.; Lacković, A.; Kresović, A.; Lončarić, A.; Marušić, M. Systematic Review with Trial Sequential Analysis of Prophylactic Antibiotics for Acute Pancreatitis. Antibiotics 2022, 11. [Google Scholar] [CrossRef]
- Golub, R.; Siddiqi, F.; Pohl, D. Role of Antibiotics in Acute Pancreatitis: A Meta-Analysis. Journal of Gastrointestinal Surgery 1998, 2, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Dambrauskas, Z.; Gulbinas, A.; Pundzius, J.; Barauskas, G. Meta-Analysis of Prophylactic Parenteral Antibiotic Use in Acute Necrotizing Pancreatitis. Medicina (Kaunas) 2007, 43, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Howden, C.W. Prophylactic Antibiotic Administration Reduces Sepsis and Mortality in Acute Necrotizing Pancreatitis: A Meta-Analysis. Pancreas 2001, 22, 28–31. [Google Scholar] [CrossRef] [PubMed]
- De Vries, A.C.; Besselink, M.G.H.; Buskens, E.; Ridwan, B.U.; Schipper, M.; Van Erpecum, K.J.; Gooszen, H.G. Randomized Controlled Trials of Antibiotic Prophylaxis in Severe Acute Pancreatitis: Relationship between Methodological Quality and Outcome. Pancreatology 2007, 7, 531–538. [Google Scholar] [CrossRef]
- Dambrauskas, Z.; Gulbinas, A.; Pundzius, J.; Barauskas, G. Value of Routine Clinical Tests in Predicting the Development of Infected Pancreatic Necrosis in Severe Acute Pancreatitis. Scand J Gastroenterol 2007, 42, 1256–1264. [Google Scholar] [CrossRef]
- De Waele, J.J. Rational Use of Antimicrobials in Patients with Severe Acute Pancreatitis. Semin Respir Crit Care Med 2011, 32. [Google Scholar] [CrossRef]
- Stigliano, S.; Sternby, H.; de Madaria, E.; Capurso, G.; Petrov, M.S. Early Management of Acute Pancreatitis: A Review of the Best Evidence. Digestive and Liver Disease 2017, 49. [Google Scholar] [CrossRef] [PubMed]
- Van Baal, M.C.; Bollen, T.L.; Bakker, O.J.; Van Goor, H.; Boermeester, M.A.; Dejong, C.H.; Gooszen, H.G.; Van Der Harst, E.; Van Eijck, C.H.; Van Santvoort, H.C.; et al. The Role of Routine Fine-Needle Aspiration in the Diagnosis of Infected Necrotizing Pancreatitis. Surgery (United States) 2014, 155, 442–448. [Google Scholar] [CrossRef]
- Adachi, T.; Kishihara, Y.; Okano, H.; Honzawa, H.; Hirayama, M.; Higashi, H.; Yasuda, H.; Minami, Y.; Hara, S.; Harada, N.; et al. The Utility of Procalcitonin for the Patients with Infected Pancreatic Necrotic and Pancreatic Abscess. Intensive Care Medicine Experimental 2015, 3. [Google Scholar] [CrossRef]
- Komolafe, O.; Pereira, S.P.; Davidson, B.R.; Gurusamy, K.S. Serum C-Reactive Protein, Procalcitonin, and Lactate Dehydrogenase for the Diagnosis of Pancreatic Necrosis. Cochrane Database of Systematic Reviews 2017, 2017. [Google Scholar] [CrossRef]
- Siriwardena, A.K.; Jegatheeswaran, S.; Mason, J.M.; Baltatzis, M.; Sheen, A.J.; O’Reilly, D.A.; Jamdar, S.; Deshpande, R.; De Liguori Carino, N.; Satyadas, T.; et al. A Procalcitonin-Based Algorithm to Guide Antibiotic Use in Patients with Acute Pancreatitis (PROCAP): A Single-Centre, Patient-Blinded, Randomised Controlled Trial. Lancet Gastroenterol Hepatol 2022, 7, 913–921. [Google Scholar] [CrossRef]
- Párniczky, A.; Lantos, T.; Tóth, E.M.; Szakács, Z.; Gódi, S.; Hágendorn, R.; Illés, D.; Koncz, B.; Márta, K.; Mikó, A.; et al. Antibiotic Therapy in Acute Pancreatitis: From Global Overuse to Evidence Based Recommendations. Pancreatology 2019, 19, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Wolbrink, D.R.J.; Kolwijck, E.; Ten Oever, J.; Horvath, K.D.; Bouwense, S.A.W.; Schouten, J.A. Management of Infected Pancreatic Necrosis in the Intensive Care Unit: A Narrative Review. Clinical Microbiology and Infection 2020, 26, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Brizi, M.G.; Perillo, F.; Cannone, F.; Tuzza, L.; Manfredi, R. The Role of Imaging in Acute Pancreatitis. Radiologia Medica 2021, 126. [Google Scholar] [CrossRef]
- Rodriguez, J.R.; Razo, A.O.; Targarona, J.; Thayer, S.P.; Rattner, D.W.; Warshaw, A.L.; Fernández-Del Castillo, C. Debridement and Closed Packing for Sterile or Infected Necrotizing Pancreatitis: Insights into Indications and Outcomes in 167 Patients. Ann Surg 2008, 247. [Google Scholar] [CrossRef]
- Hong, D.; Wang, P.; Chen, Y.; Zhang, J.; Jiang, W.; Ye, B.; Li, G.; Zhou, J.; Mao, W.; Tong, Z.; et al. Detection of Potential Pathogen in Pancreatic Fluid Aspiration with Metagenomic Next-Generation Sequencing in Patients with Suspected Infected Pancreatic Necrosis. Digestive and Liver Disease 2023, 55. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.; Wang, P.; Zhang, J.; Li, K.; Ye, B.; Li, G.; Zhou, J.; Tong, Z.; Ke, L.; Shi, S.; et al. Plasma Metagenomic Next-Generation Sequencing of Microbial Cell-Free DNA Detects Pathogens in Patients with Suspected Infected Pancreatic Necrosis. BMC Infect Dis 2022, 22. [Google Scholar] [CrossRef]
- Ukai, T.; Shikata, S.; Inoue, M.; Noguchi, Y.; Igarashi, H.; Isaji, S.; Mayumi, T.; Yoshida, M.; Takemura, Y.C. Early Prophylactic Antibiotics Administration for Acute Necrotizing Pancreatitis: A Meta-Analysis of Randomized Controlled Trials. J Hepatobiliary Pancreat Sci 2015, 22, 316–321. [Google Scholar] [CrossRef]
- van Santvoort, H.C.; Besselink, M.G.; Bakker, O.J.; Hofker, H.S.; Boermeester, M.A.; Dejong, C.H.; van Goor, H.; Schaapherder, A.F.; van Eijck, C.H.; Bollen, T.L.; et al. A Step-up Approach or Open Necrosectomy for Necrotizing Pancreatitis. New England Journal of Medicine 2010, 362. [Google Scholar] [CrossRef]
- Otto, W.; Komorzycki, K.; Krawczyk, M. Efficacy of Antibiotic Penetration into Pancreatic Necrosis. HPB 2006, 8. [Google Scholar] [CrossRef] [PubMed]
- Bassi, C.; Pederzoli, P.; Vesentini, S.; Falconi, M.; Bonora, A.; Abbas, H.; Benini, A.; Bertazzoni, E.M. Behavior of Antibiotics during Human Necrotizing Pancreatitis. Antimicrob Agents Chemother 1994, 38. [Google Scholar] [CrossRef]
- Büchler, M.; Malfertheiner, P.; Frieß, H.; Isenmann, R.; Vanek, E.; Grimm, H.; Schlegel, P.; Friess, T.; Beger, H.G. Human Pancreatic Tissue Concentration of Bactericidal Antibiotics. Gastroenterology 1992, 103. [Google Scholar] [CrossRef]
- Singh, R.R.; Mitchell, W.; David, Y.; Cheesman, A.; Dixon, R.E.; Nagula, S.; Dimaio, C.J.; Greenwald, D.A.; Kumta, N.A. Pancreatic Fungal Infection in Patients with Necrotizing Pancreatitis: A Systematic Review and Meta-Analysis. J Clin Gastroenterol 2021, 55. [Google Scholar] [CrossRef]
- Baron, T.H.; DiMaio, C.J.; Wang, A.Y.; Morgan, K.A. American Gastroenterological Association Clinical Practice Update: Management of Pancreatic Necrosis. Gastroenterology 2020, 158, 67–75e1. [Google Scholar] [CrossRef] [PubMed]
- van Brunschot, S.; van Grinsven, J.; van Santvoort, H.C.; Bakker, O.J.; Besselink, M.G.; Boermeester, M.A.; Bollen, T.L.; Bosscha, K.; Bouwense, S.A.; Bruno, M.J.; et al. Endoscopic or Surgical Step-up Approach for Infected Necrotising Pancreatitis: A Multicentre Randomised Trial. The Lancet 2018, 391, 51–58. [Google Scholar] [CrossRef]
- Boxhoorn, L.; van Dijk, S.M.; van Grinsven, J.; Verdonk, R.C.; Boermeester, M.A.; Bollen, T.L.; Bouwense, S.A.W.; Bruno, M.J.; Cappendijk, V.C.; Dejong, C.H.C.; et al. Immediate versus Postponed Intervention for Infected Necrotizing Pancreatitis. New England Journal of Medicine 2021, 385. [Google Scholar] [CrossRef]
- Garcia-Alamino, J.M.; Lopez-Cano, M. Systematic Reviews With Meta-Analysis of Clinical Trials: Is There Enough Evidence? Cirugía Española (English Edition) 2020, 98. [CrossRef]
- Huston, J.M.; Barie, P.S.; Dellinger, E.P.; Forrester, J.D.; Duane, T.M.; Tessier, J.M.; Sawyer, R.G.; Cainzos, M.A.; Rasa, K.; Chipman, J.G.; et al. The Surgical Infection Society Guidelines on the Management of Intra-Abdominal Infection: 2024 Update. Surg Infect (Larchmt) 2024. [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med 2018, 169. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.; Godfrey, C.; McInerney, P.; Munn, Z.; Tricco, A.; Khalil, H. Scoping Reviews - JBI Manual for Evidence Synthesis - JBI GLOBAL WIKI. JBI Reviewer’s Manual, JBI, 2020.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).