1. Introduction
Electrodeposited composite coatings offer a superior alternative to single metal coatings, as they combine the beneficial properties of multiple materials. By incorporating dispersed phases like ceramic nanoparticles, these coatings enhance mechanical strength, corrosion resistance, and tailored functionalities [
1,
2]. Ongoing advancements in material combinations and deposition techniques position composite coatings to significantly influence a broad spectrum of industrial applications. Electrodeposited nickel composite coatings are widely used in various industrial applications due to their unique combination of properties. These coatings offer high corrosion resistance, wear resistance, and improved mechanical properties compared to pure nickel coatings. Understanding the characteristics and properties of these composite coatings is crucial for optimizing their performance in specific applications. In this review, the aim is to explore the key characteristics of electrodeposited nickel composite coatings, including their microstructure, hardness, and adhesion properties. By gaining a deeper insight into these characteristics, we can enhance our knowledge of how these coatings function and contribute to their improved design and performance. This review will provide valuable insights into the development and application of electrodeposited nickel composite coatings and their various properties.
The electrodeposition process of nickel composite coatings represents a significant advancement in surface modification techniques, offering enhanced properties crucial for various applications. Pulse electrodeposition and pulse reverse electrodeposition have emerged as transformative methods, enabling improved surface characteristics such as reduced porosity, minimal inclusions, and higher deposition rates compared to traditional direct current methods. These processes provide flexibility in adjusting key parameters like pulse current density, on time, and off time to tailor the composition and microstructure of the coatings. Incorporating nanoparticles, such as Al2O3, SiC, and ZrO2, into the nickel matrix through electrodeposition yields composite coatings with superior microhardness, wear resistance, and unique grain textures. A detailed investigation into the effects of processing parameters on the microstructure and mechanical properties of these coatings reveals promising results, with Ni-Al2O3 composites exhibiting exceptional hardness and wear resistance. Furthermore, tribo-corrosion studies on NiP and NiP–SiC coatings highlight the impact of SiC particle dispersion on wear volume loss and current density during testing, emphasizing the intricate interplay between composition and performance in electrodeposited nickel composite coatings.
2. Electrodeposition
Electrodeposition involves using an applied current or potential to deposit a film of metal or alloy onto a conductive substrate by reducing metallic ions. When particulates are co-deposited with the metal or alloy, a composite is formed. This process is used to apply a thin layer of metal to a workpiece, enhancing properties such as corrosion resistance, wear characteristics, and aesthetics. By coating inexpensive base materials with layers of metals that have superior properties, electrodeposition extends their applications and makes them more cost-effective. This versatile technology offers various methods to create thin films and coatings on target substrates [
3,
4]. Below is a breakdown of the different electrodeposition methods.
2.1. Direct Current Electrodeposition
This is the simplest, most basic, and widely used method of electrodeposition. A constant direct current is applied, causing positively charged ions (cations) in the electrolyte solution to migrate towards the negatively charged electrode (cathode), where the coating is desired. At the cathode, the cations are reduced to their elemental form, depositing as a thin film on the substrate. This technique is further divided based on the orientation of the electrodes in the electrolyte bath during the electrodeposition process: conventional electro co-deposition (CECD) and sediment co-deposition (SCD). In conventional electrodeposition, the electrodes are placed vertically in the electrolyte bath, while in SCD, they are placed horizontally. These orientations significantly influence the adsorption of nanoparticles into the alloy matrix during electrodeposition.
In a study investigating the effect of particle size and co-deposition technique on the hardness and corrosion properties of Ni–Co/SiC composite coatings, it was found that these factors greatly impact the final properties of the coatings [
5]. Here it was found out that the two main forces involved during SCD electrodeposition (gravitational pull and the electrophoresis force) produced higher corrosion resistance compared to conventional deposition (CECD) which exclusively relies on gravitational pull. Direct current electrodeposition has comparative advantages over other coating techniques due to its simplicity in terms of setup and operation as well as availability of well-established vast technical and reliable process. Another study [
6] demonstrated that DC setups are easier to design, operate, and maintain compared to those requiring precise control of pulse parameters in PC and PRC methods. The results indicated that PC electrodeposited films exhibit a porous morphology with smaller crystallite sizes and higher donor density compared to DC electrodeposited films, which feature equiaxed particles in their morphology. This simplicity of DC setups translates to lower initial investment and operational costs. Additionally, all DC-deposited Ni and Ni nanocomposite coatings displayed significantly stronger (more uniform) crystallographic textures compared to those deposited using PC and PRC techniques [
7].
Direct current electrodeposition is the traditional method where a constant current or potential is continuously applied during the deposition process to coat the desired materials.
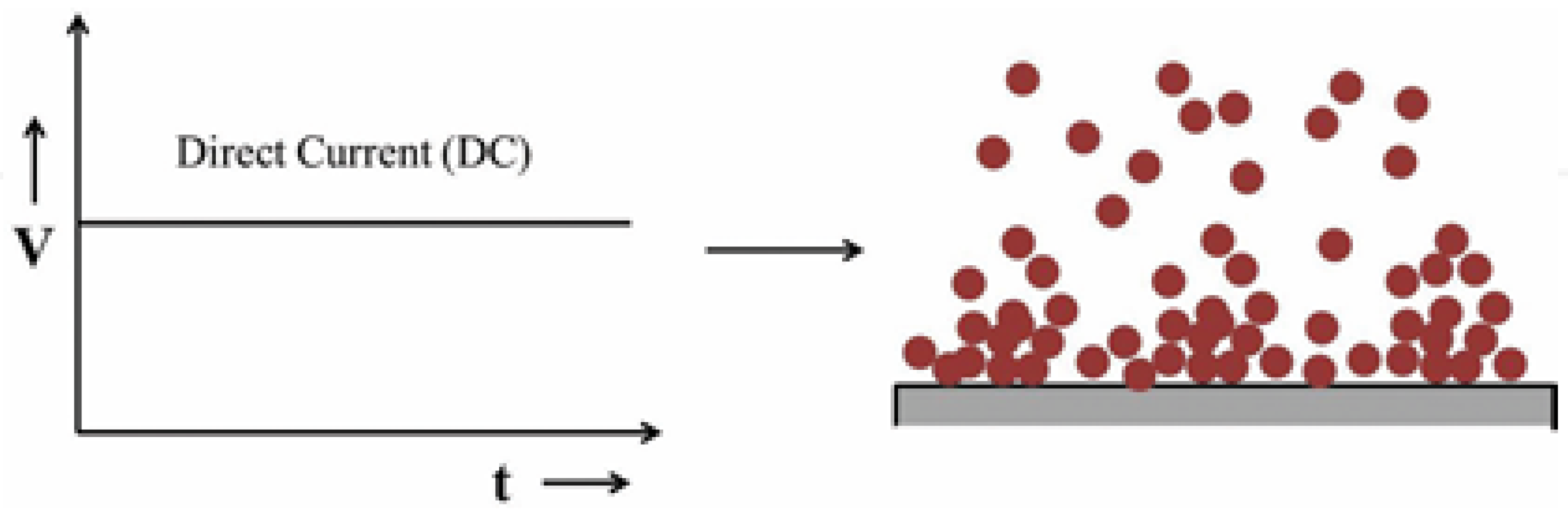
Figure 1 below illustrates the schematic of the direct current application and the typical growth process associated with it. The DC technique primarily involves two variables: the applied potential/current and the deposition time, while precursor concentration and electrolyte pH are also important factors. By adjusting these parameters, the morphology, composition, and thickness of the deposit can be modified. In DC deposition, the continuous application of constant potential/current results in film deposition without any relaxation, promoting the growth of existing nuclei rather than generating new nucleation sites, which leads to a rough and porous deposit.
Figure 1.
Schematic of direct current electrodeposition and expected growth process of the deposit [
8].
Figure 1.
Schematic of direct current electrodeposition and expected growth process of the deposit [
8].
2.2. Pulse Electrodeposition
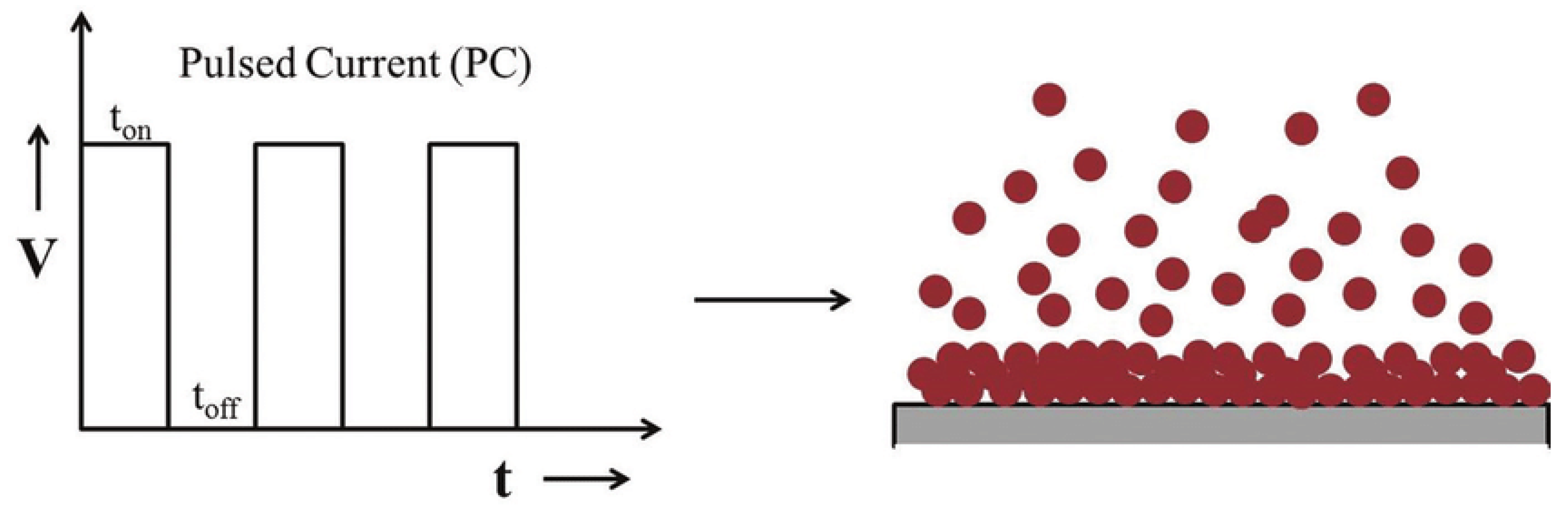
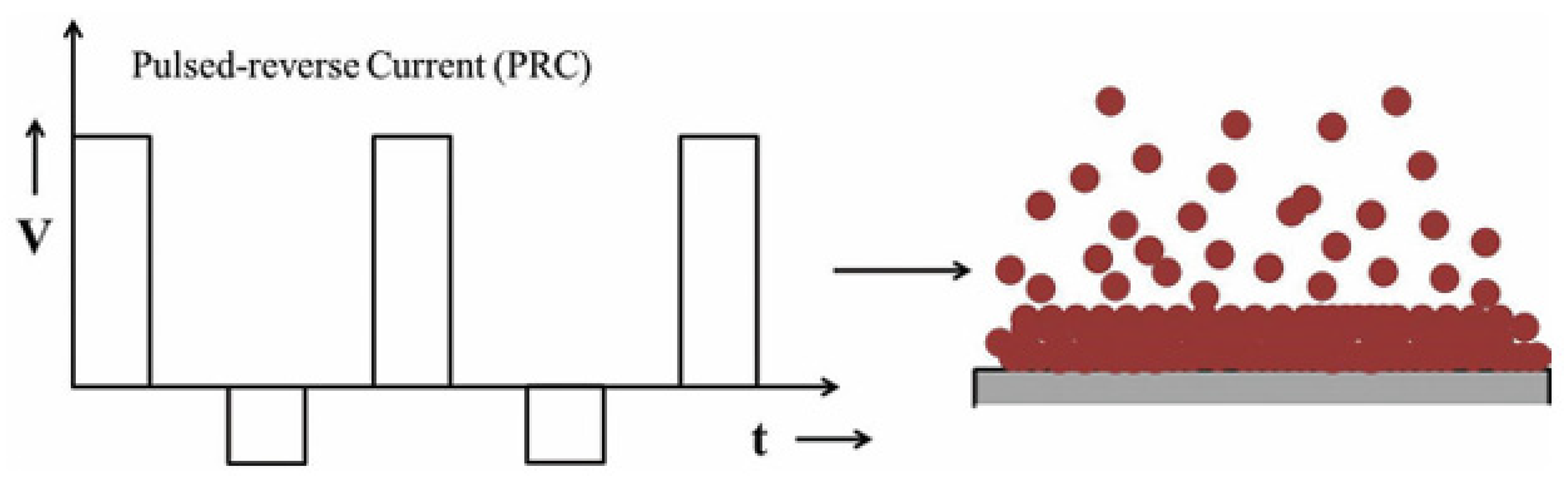
Pulse Electrodeposition (PED) represents an advancement in the electrodeposition of metals, alloys, and metal matrix composites (MMCs). Unlike traditional direct current (DC) electrodeposition, PED provides enhanced flexibility in adjusting key deposition parameters such as peak current density (Ip), pulse current on-time (ton), and pulse current off-time (toff). This flexibility enables the creation of tailored compositions and microstructures in the deposited coatings. Achieving the desired average current density (Ia) involves utilizing different combinations of peak current densities, pulse current on-times, and pulse current off-times [
9]. Additionally, pulse electrodeposition current can be effectively alternated between two different values, creating a series of pulses with equal amplitude, duration, and polarity, separated by zero current [
10].
Figure 2 below illustrates a typical pulse current waveform where the current alternates between a positive current value (Ip) for the duration of ton and a zero-current value (0) for the duration of t
off.
Concepts of pulse and pulse reverse techniques are demonstrated below:
During the toff period in pulse electrodeposition, the electric double layer around the cathode discharges, allowing ions to pass through it and reach the cathode surface. In contrast, during DC electrodeposition, this electric double layer hinders ions from reaching the cathode surface.
During electrodeposition, regions with high current density in the electrolyte bath experience greater ion depletion compared to areas with low current density. During the toff period, ions migrate to these depleted regions, ensuring a more uniform ion distribution for deposition when the ton pulse occurs.
Pulse electrodeposition enables the synthesis of coatings with controlled thickness, composition, and microstructure by adjusting pulse parameters. PC (Pulse Current) and PRC (Pulse Reverse Current) electrodeposition techniques offer several advantages and disadvantages compared to the DC electrodeposition technique.[
4,
11]. The advantages are:
Limiting current density significantly increases by replenishing metal ions in the diffusion layer during the off time.
Flexibility in pulse parameters reduces process limitations.
Results in fine-grained deposits with lower porosity and reduced stress.
Improves adhesion of the deposit and creates uniform thickness.
Enhances the rate of deposition and improves physical and mechanical properties.
On the other hand, pulse electrodeposition method has downsides like:
Pulse generators are more expensive than DC units.
This technique requires careful advance planning and a series of procedures to achieve optimal results.
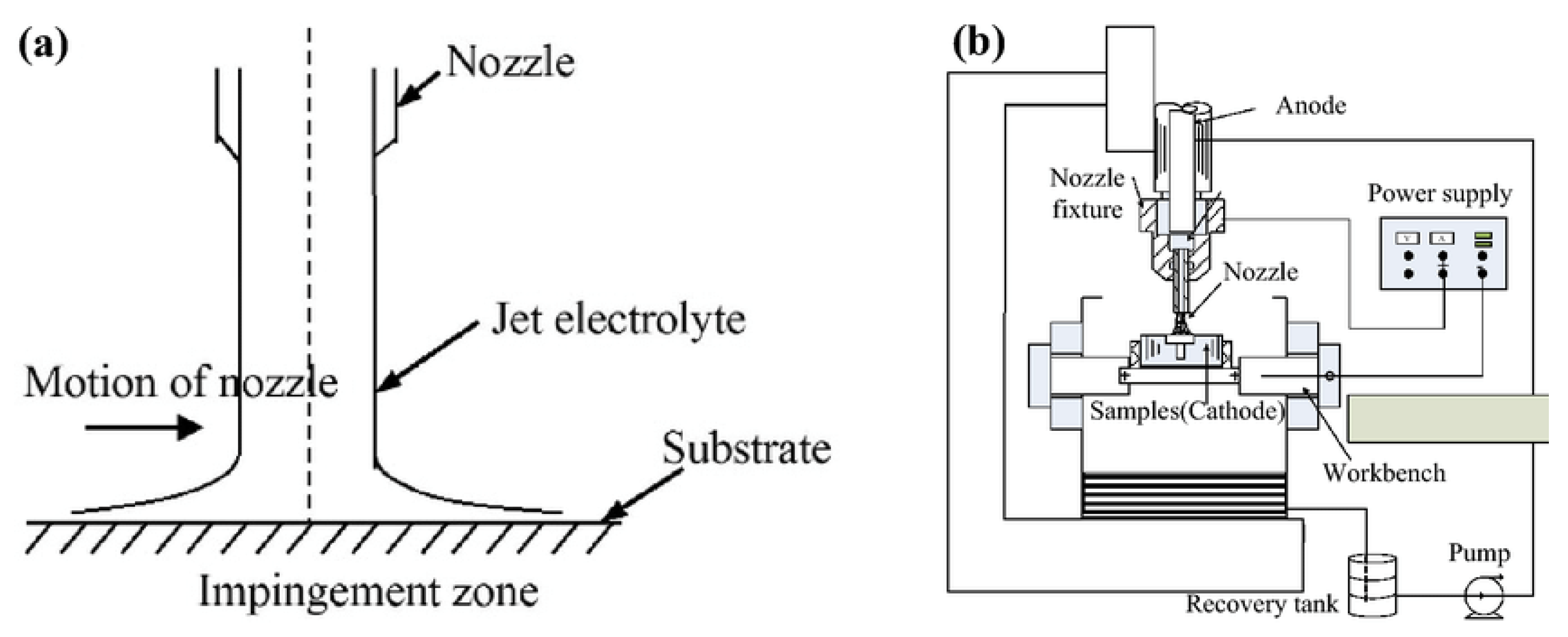
2.3. Jet Electrodeposition
In this type of electrodeposition method, a stream of plating solution is aimed directly at the cathode surface. An electrical field exists between the anode (situated in the nozzle) and the cathode (substrate).
Figure 3 below illustrates the setup configuration.
As the plating solution flows, electric current is conducted through the fluid stream to the substrate surface. This facilitates deposition on the cathode surface where the jet flows over. Jet electrodeposition is a rapid electroplating technique that offers numerous advantages over other coating methods, such as high deposition rates and effective refinement of grain size [
13,
14].
2.4. Ni-Al2O3 Composite Coatings
Electrodeposited Ni composite coatings containing ceramic particles have been widely investigated due to their improved mechanical, wear and corrosion resistant properties over plain nickel coatings [
15,
16,
17,
18]. Ni-Al
2O
3 composite coatings, consisting of nickel (Ni) and alumina (Al
2O
3), have emerged as a powerful technology for enhancing the properties of underlying substrate materials. These coatings offer a unique blend of properties derived from their individual constituents. Nickel provides excellent ductility, strength, and electrical conductivity, while alumina contributes superior wear resistance, high-temperature stability, and outstanding corrosion resistance [
19]. Consequently, this synergy makes Ni-Al
2O
3 coatings ideal for various applications demanding protection against harsh environments. It is observed in [
20] the effect of current density on electrodeposited Ni-Al
2O
3 composite coatings. Wear resistance as well as microhardness of this composite coatings showed a significant improvement at a current density of 0.01A/dm2. This significant improvement in hardness was mainly due to the combined effects of dispersion strengthening as well as refined grain sizes. The abrasive strength of this composite coatings was found out to be 57 MPa. By varying alumina content in the electrolyte bath, improved wear and corrosion resistance was achieved [
3].
Here, the wear rate of the coatings has decreased from 5.96*10
-5 to 3.16 * 10
-5 mm
3 /Nm with the increase of Al
2O
3 particles in the composite coating which indicates approximately two times improvement in the wear resistance. The corrosion resistance was also increased and an improvement in corrosion rate was achieved from 392 value to 063 µm/year value with increasing particle concentration. It was found out in [
21] that, with pulse electrodeposition method, roughness properties of Ni-Al
2O
3 composite coatings were improved with varying duty cycles from 20% - 100%. Results demonstrated that with decrease in duty cycle, there was an enhancement in surface roughness properties from 0.779 to 0.245 µm. The change in surface roughness was due to the variation of grain size, resulting from the varying time intervals during pulse coatings.
Hardness and wear resistance of composite coatings as well as microstructure and surface morphology are strongly influenced by current density as well [
22]. The microhardness values of deposits were found to increase with the increasing of current density. This observed higher microhardness may be due to the larger amounts of incorporated alumina particles. When current density is increased from 3 A/dm
2 to 5 A/dm
2 the polarization resistance value improved and decreased the corrosion current density. Moreover, the corrosion potential of these samples has shifted toward more positive side, indicating improved corrosion resistance. It was found out that the coating deposited at 5 A/dm
2 was characterized by homogeneous distribution of the ceramic phase, relatively small values of the residual stresses as well as showed the smallest corrosion current density and the highest polarization resistance.
In Ni-Al
2O
3 composite coatings, the Al
2O
3 content plays a decisive role in the corrosion resistance, so that the corrosion rate of the coating decreases with increasing Al
2O
3 content [
23]. Increasing the current above 50 mA decreases the influence of the current on the corrosion rate and the degradation potential becomes higher for the coating developed at a current of 60 mA than for the other composite coatings. Increasing the bath temperature up to 40 °C the corrosion current density has decreased. However, an increase in temperature above 40 °C caused an increase in the corrosion current density and consequently a decrease in the corrosion resistance of the composite coatings.
Furthermore, microstructure and wear resistance of nickel-alumina composite coatings are influenced by pulse frequency. At lower frequencies, nickel-alumina composite coatings exhibited better hardness and wear resistance compared to higher frequencies. Due to the presence of adhesive wear, wear resistance of composite coatings is largely influenced by microstructure and less influenced by reinforced alumina particles [
24].
2.5. Ni-SiC Composite Coatings
Ni-SiC composite coatings have emerged as a promising material for surface engineering applications due to their ability to combine the desirable properties of nickel (Ni) with the exceptional hardness and wear resistance of silicon carbide (SiC) [
25,
26].
Electrodeposition is a widely used technique for creating Ni-SiC coatings. Recent studies have explored optimizing this process to achieve better control over SiC particle distribution and content within the nickel matrix. For instance, research by [
27] investigated the use of binary non-ionic surfactants to improve the incorporation of SiC nanoparticles during electrodeposition. Their findings demonstrated that surfactants improved the dispersion of SiC nanoparticles, leading to more uniform and higher hardness coatings compared to pure nickel coatings.
The primary motivation for incorporating SiC nanoparticles into nickel is to improve its mechanical properties. Studies have shown that Ni-SiC composites exhibit enhanced micro-hardness, wear resistance, and corrosion resistance compared to pure nickel coatings [
28]. This makes them suitable for applications in various tribological environments, such as cutting tools, gears, and bearings. For instance, a study by investigated the effect of SiC nanoparticle concentration on the properties of Ni-SiC coatings fabricated via high-frequency inductive cladding [
28]. They observed that increasing the SiC content resulted in a significant improvement in wear and corrosion resistance, making these coatings ideal for protecting carbon steel components. This method offers rapid deposition rates but requires further investigation into optimizing parameters for achieving uniform and well-distributed SiC particles.
The microstructure of Ni-SiC composites plays a crucial role in determining their performance. The size, distribution, and interfacial bonding between Ni and SiC particles significantly impact the mechanical properties. Studies by [
28] employed electrodeposition to fabricate Ni-SiC coatings with varying SiC particle concentrations. They observed that increasing the SiC content led to grain refinement and enhanced corrosion resistance in the composite coating. The incorporation of SiC nanoparticles into the nickel matrix generally improves the micro-hardness and wear resistance of the coating. Research by [
27] showed a significant increase in micro-hardness compared to pure nickel coatings. This improvement can be attributed to the presence of hard SiC particles that act as load-bearing reinforcements within the nickel matrix.
Recent research efforts in Ni-SiC composites focus on improving the control over deposition processes and understanding the effect of SiC particle characteristics (size, morphology) on the final properties of the coating. Additionally, researchers are exploring novel fabrication techniques like laser cladding to achieve superior properties and address limitations associated with conventional methods.
2.6. Ni-ZrO2 Composite Coatings
Nickel and zirconium dioxide (zirconia) have good compatibility due to their similar thermal expansion coefficient and elastic modulus. Several techniques are employed to create Ni-ZrO
2 composite coatings. Electrodeposition is a widely used method, allowing for the incorporation of ZrO
2 particles within a nickel matrix [
29,
30,
31]. Studies have explored optimizing the ZrO
2 content in the plating bath to achieve a balance between particle incorporation and distribution within the coating [
29]. Sol-enhanced electroplating has also been investigated, demonstrating improved dispersion of ZrO
2 nanoparticles compared to traditional powder addition methods [
32].
Recent research highlights the importance of achieving a uniform distribution of ZrO
2 particles within the nickel matrix [
29,
32]. This distribution is influenced by the ZrO
2 concentration in the fabrication process and can significantly impact properties like hardness and wear resistance. The incorporation of ZrO
2 into nickel coatings leads to substantial improvements in various properties. A key area of focus is enhanced wear resistance, achieved through the dispersion of hard ZrO
2 particles within the softer nickel matrix [
32,
33]. Additionally, Ni-ZrO
2 composites demonstrate improved corrosion resistance compared to pure nickel coatings [
29]. This study explores the impact of ZrO
2 concentration in the electrolytic bath on the particle distribution and corrosion resistance of the coatings. Their findings suggest an optimal ZrO
2 concentration for achieving the best combination of these properties. This enhancement is attributed to the barrier effect of ZrO
2 particles, hindering the diffusion of corrosive species towards the substrate. It was also found out that the microhardness and tribo-corrosion properties of composite coatings were significantly improved due to reinforcement of uniformly dispersed ZrO2 particles in the nickel matrix followed by combined effect of dispersion strengthening and structural modification [
34].
Current research efforts in Ni-ZrO
2 composites are directed towards optimizing fabrication techniques for superior control over microstructure and achieving the desired balance between different properties. Sol-enhanced electroplating is a promising approach for achieving better dispersion of ZrO
2 nanoparticles [
32]. It has investigated a sol-enhanced electroplating method, demonstrating superior improvements in mechanical properties compared to traditional ZrO
2 powder incorporation. Furthermore, studies are exploring the influence of ZrO
2 particle size and morphology on the overall performance of the composite coatings.
Ni-ZrO2 composite coatings hold immense potential for various industrial applications due to their combined advantages of good mechanical properties, corrosion resistance, and compatibility with different substrates. Further research focused on optimizing fabrication techniques, exploring alternative ZrO2 dopants, and investigating the tribological behavior of these coatings will pave the way for their wider adoption in fields like automotive, aerospace, and machine tools.
The microstructure of Ni-ZrO
2 composite coatings plays a crucial role in determining their performance. Studies [
32,
33] utilize Scanning Electron Microscopy (SEM) and X-ray Diffraction (XRD) to analyze the distribution and crystallographic phases within the coatings These studies emphasize the importance of achieving a uniform dispersion of ZrO
2 nanoparticles within the Ni matrix for optimal properties.
The primary advantage of Ni-ZrO
2 composite coatings lies in their enhanced functionalities compared to individual materials. A study by [
35] demonstrates a significant improvement in wear resistance with increasing ZrO
2 content in the coatings. Similarly, other researches highlight the enhanced corrosion resistance achieved through Ni-ZrO
2 composite coatings.
2.7. Ni-TiO2 Composite Coatings
Ni-TiO2 composite coatings have gained significant attention in recent years due to their potential applications in various industrial sectors. These coatings consist of a mixture of nickel (Ni) and titanium dioxide (TiO2) nanoparticles, which are deposited onto a substrate surface using techniques such as electrodeposition or chemical vapor deposition. The unique combination of properties offered by both Ni and TiO2, such as corrosion resistance, mechanical strength, and photocatalytic activity, makes these composite coatings highly versatile and suitable for a wide range of applications.
One of the key advantages of Ni-TiO2 composite coatings is their enhanced corrosion resistance compared to traditional coatings. The addition of TiO2 nanoparticles improves the overall chemical stability of the coating, making it more resistant to harsh environments and corrosive substances [
36]. This property is particularly beneficial in industries such as aerospace, automotive, and marine, where components are constantly exposed to corrosive elements. Additionally, the photocatalytic activity of TiO2 nanoparticles can also provide self-cleaning properties to the coating, making it easier to maintain and prolonging its lifespan.
In a study carried out to investigate the effects of pulse electrodeposition parameters on the properties of nickel–titania composite coatings electrodeposited from watts bath [
37], it was found out microhardness of composite coatings increased with increasing current density from 2 to 5 A/dm
2 and after that it decreased as current density increases. Microhardness and particle reinforcement in composite coatings found maximum value for coatings deposited at 10 Hz frequency and 10% duty cycle.
Furthermore, the mechanical strength of Ni-TiO2 composite coatings makes them suitable for applications where wear and abrasion resistance are crucial. The Ni component provides excellent adhesion to the substrate surface, while the TiO2 nanoparticles act as reinforcements, improving the overall hardness and durability of the coating [
38]. This makes them ideal for use in components subjected to high levels of mechanical stress, such as cutting tools, bearings, and medical implants. Overall, the unique combination of properties offered by Ni-TiO2 composite coatings makes them a promising material for various industrial applications.
2.8. Ni-WC Composite Coatings
Ni-WC composite coatings have gained significant attention in recent years due to their exceptional mechanical and tribological properties. These coatings are formed by incorporating tungsten carbide (WC) particles into a nickel (Ni) matrix, resulting in a material that combines the hardness of WC with the ductility of Ni. The unique combination of properties exhibited by Ni-WC composite coatings makes them ideal for a wide range of industrial applications, including cutting tools, wear-resistant surfaces, and protective coatings.
One of the key advantages of Ni-WC composite coatings is their exceptional hardness and wear resistance. Tungsten carbide is one of the hardest materials known, with a Mohs hardness of 9, while nickel provides the necessary toughness and ductility to prevent cracking and delamination. This combination of hardness and toughness makes Ni-WC composite coatings highly resistant to wear, abrasion, and erosion, making them ideal for applications in which components are subjected to severe mechanical stresses. In addition to their outstanding mechanical properties, Ni-WC composite coatings also exhibit excellent corrosion resistance [
39,
40]. The nickel matrix in these coatings acts as a barrier to prevent corrosive elements from reaching the substrate material, while the tungsten carbide particles provide additional protection against chemical attack. As a result, components coated with Ni-WC composites are able to withstand harsh operating environments, such as those found in the aerospace, automotive, and oil and gas industries.
Another key advantage of Ni-WC composite coatings is their versatility and ease of application. These coatings can be deposited using a variety of techniques, including thermal spray, electroplating, and chemical vapor deposition, allowing for customization based on the specific requirements of the application [
40]. Additionally, the composition and microstructure of Ni-WC coatings can be tailored to optimize properties such as hardness, wear resistance, and corrosion resistance, making them highly versatile and adaptable to a wide range of applications. Despite their many advantages, Ni-WC composite coatings do have some limitations that must be considered. For example, the high hardness of tungsten carbide particles can result in increased tool wear during machining and grinding processes, which may require specialized equipment and techniques for processing. Additionally, the high cost of tungsten carbide can make Ni-WC coatings more expensive than alternative coating materials, which may limit their use in cost-sensitive applications [
41].
Generally, Ni-WC composite coatings offer a unique combination of mechanical, tribological, and corrosion-resistant properties that make them ideal for a wide range of industrial applications. Their exceptional hardness, wear resistance, and corrosion resistance, coupled with their versatility and ease of application, make Ni-WC coatings a highly attractive option for components subjected to severe mechanical and environmental stresses. While they do have some limitations, the many advantages of Ni-WC composite coatings make them a valuable and promising material for future research and development in the field of surface engineering.
2.9. Properties of Nickel Composite Coatings
Nickel composite coatings have gained significant attention in various industries due to their unique properties and wide range of applications. As researchers continue to delve into this field, numerous recent publications have shed light on the advancements and discoveries related to the properties of nickel composite coatings. Nickel composite coatings are composed of a nickel matrix with dispersed second-phase particles, including Al2O3, Si3N4, SiC, Cr2O3, WC, TiO2, diamond, PTFE, graphite, or even microcapsules containing liquid. Researches show these particles enhance the mechanical, tribological, and corrosion resistance properties of the composite coatings. They act as physical barriers to dislocation movement and grain boundary sliding, leading to a significant improvement in the mechanical properties of the composite coatings.
By incorporating a second phase, typically non-metallic particles, into a nickel matrix, nickel composite coatings offer improved properties compared to conventional nickel coatings. Here are some key properties of nickel composite coatings:
2.9.1. Hardness
Hardness is a measure of a material’s resistance to localized plastic deformation, such as indentation or scratching, when subjected to mechanical forces like pressing or abrasion [
42]. Hardness is an important property in engineering and materials science because it directly correlates to a material’s performance and suitability for various applications. Hardness is generally related to tensile stress yield resulting from a known amount deformation. Nickel composite coatings reinforced with Al
2O
3 [
43], SiC [
44] and TiO
2 [
45,
46] showed improved microhardness properties.
2.9.2. Corrosion Resistance
Nickel composite coatings are known for their corrosion resistance properties. These coatings are used to protect various components from corrosion in a wide range of environments, including offshore and harsh chemical environments. Studies show Nickel composite coatings exhibit better corrosion resistance than pure nickel coatings. It was found out increasing Al2O3 concentration resulted in halving of corrosion rates demonstrating Al
2O
3 particle incorporation is effective to enhance corrosion resistance [
47]. By Varying the concentrations of Al
2O
3 on steel substrates from the electroplating bath, tests conducted under working conditions demonstrated that adding Al
2O
3 particles to the bath positively affects the electrochemical behavior of the steel, reducing its susceptibility to corrosion and the optimal concentration was found out to be 20 g/L [
48].
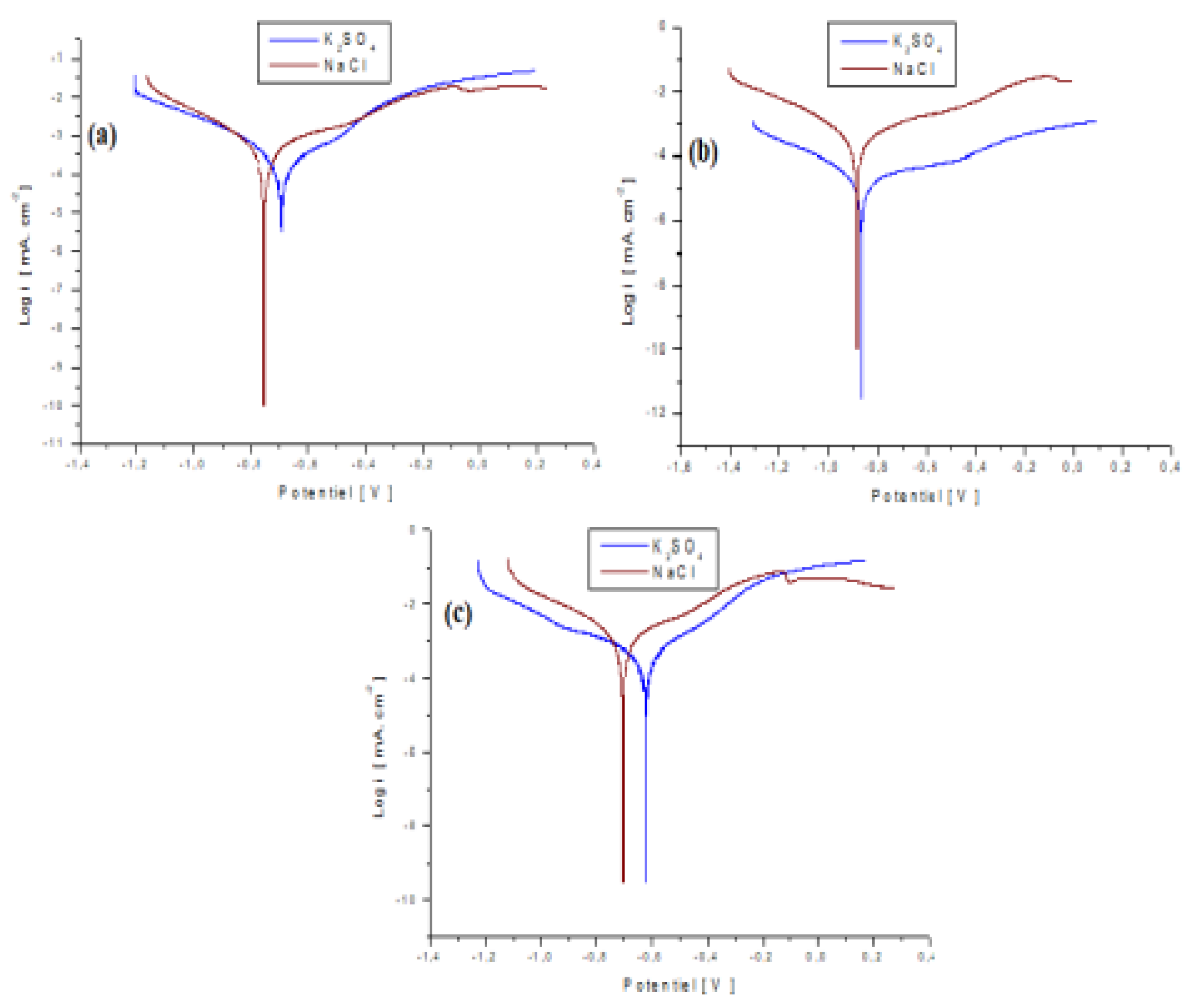
Figure 5.
Polarization curves for a steel plate coated in a bath without alumina (a), with 20 g/L (b) and 30 g/L (c) of Al2O3 in 0.5M K2SO4 and 0.5 NaCl.
Figure 5.
Polarization curves for a steel plate coated in a bath without alumina (a), with 20 g/L (b) and 30 g/L (c) of Al2O3 in 0.5M K2SO4 and 0.5 NaCl.
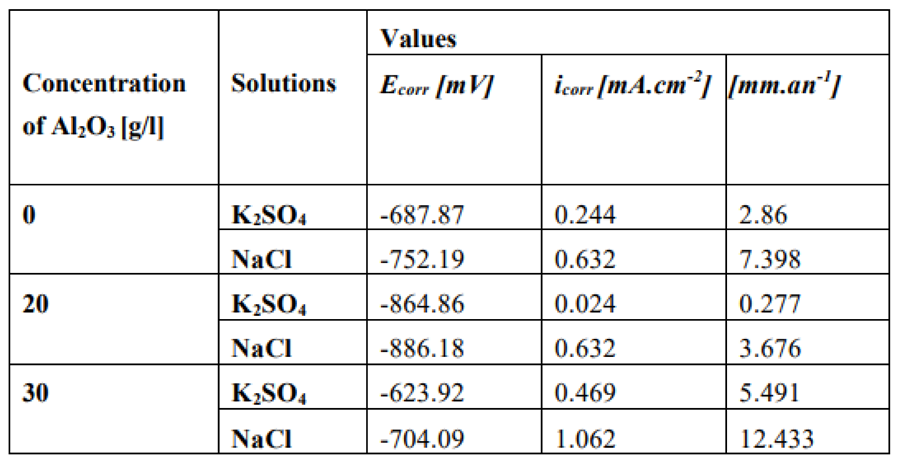
Table 1.
Values of electrochemical parameters in a bath of 0; 20 and 30 g/L Al2O3 in K2SO4 and NaCl solutions [
48].
Ni–SiC composite coatings with improved wear and corrosion resistance can be obtained by electrodeposition method as well. Values of I
corr and E
corr of Ni–SiC composite coatings deposited were found 1.13 × 10−3 mA/cm
2 and −0.311 V, respectively, indicating levels of optimal corrosion resistance [
49]. Another study [
50] revealed that Ni-SiC composite coatings prepared on stainless steel 410 by pulse electrodeposition method produced best corrosion resistance at a coating time of 60 minutes.
Studies show that Ni-ZrO₂ composite coatings are known for their excellent corrosion resistance, making them suitable for various industrial applications. The incorporation of ZrO₂ (zirconia) particles into a Ni (nickel) matrix enhances the coating’s properties. Studies comparing Ni-ZrO₂ composite coatings with pure Ni coatings or other composite coatings (such as Ni-Al₂O₃) have shown that Ni-ZrO₂ coatings generally exhibit superior corrosion resistance due to the combined effects of the nickel matrix and the zirconia particles. In a study conducted to assess the microstructural, mechanical and corrosion characterization of electroless Ni-P composite coatings modified with ZrO2 reinforcing nanoparticles, it was found out that introduction of ZrO2 particles enhance corrosion resistance of coatings [
51]. Moreover, Ni composite reinforced with addition of TiO2 [
52] and WC [
53] showed enhanced corrosion resistance when compared to pure Ni coatings on various substrates.
2.9.3. Wear Resistance
Nickel composite coatings reinforced with Al
2O
3 [
17,
54], WC [
40,
55,
56], TiO
2 [
38,
57,
58], ZrO2 [
59,
60], SiC [
25,
44,
61] showed enhanced wear resistance properties compared to pure Ni coatings on substrates. The enhancement in microhardness and wear resistance of Ni composite coatings primarily depends on the added reinforcing particles within the nickel matrix. These particles act as a physical barrier, inhibiting nickel grain growth and reducing plastic deformation under various loads. This apparently promotes grain refinement and a dispersive strengthening effect, thereby improving the microhardness and wear resistance of the composite coatings [
62].
2.10. Conclusion
Nickel electrodeposition is a widely used technique for producing thin films with a variety of desirable properties. In recent years, there has been a growing interest in the development of nickel composite coatings, which incorporate additional elements or particles into the nickel matrix to achieve enhanced properties. These composite coatings can offer improved wear resistance, corrosion resistance, hardness, and other functional properties compared to pure nickel coatings. Some of the recent advancements in electrodeposited nickel composite coatings include:
Improved wear resistance: researchers have found that incorporating Al, Si, Zr, C, W and Ti particles into nickel coatings can significantly improve wear resistance. The uniform distribution of the particles within the nickel matrix acts as a barrier to wear and tear.
Enhanced mechanical properties: studies have shown that electrodeposited nickel composite coatings with Al, Si, Zr, C, W and Ti particles exhibit superior mechanical properties, including increased hardness. The presence of these particles refines the grain size of the nickel matrix and hinders the movement of defects, leading to enhanced mechanical strength.
Better corrosion resistance: Nickel composite coatings can offer improved protection against corrosion compared to pure nickel coatings.
References
- Saini, A.; Singh, G.; Mehta, S.; Singh, H.; Dixit, S. A review on mechanical behaviour of electrodeposited Ni-composite coatings. Int. J. Interact. Des. Manuf. (IJIDeM) 2022, 17, 2247–2258. [CrossRef]
- Walsh, F.; Larson, C. Towards improved electroplating of metal-particle composite coatings. Trans. IMF 2020, 98, 288–299. [CrossRef]
- Nasirpouri, F., et al., Electrodeposition of anticorrosion nanocoatings, in Corrosion Protection at the Nanoscale. 2020, Elsevier. p. 473-497. [CrossRef]
- Kale, M.B.; Borse, R.A.; Mohamed, A.G.A.; Wang, Y. Electrocatalysts by Electrodeposition: Recent Advances, Synthesis Methods, and Applications in Energy Conversion. Adv. Funct. Mater. 2021, 31, 2101313. [CrossRef]
- Bakhit, B.; Akbari, A. Effect of particle size and co-deposition technique on hardness and corrosion properties of Ni–Co/SiC composite coatings. Surf. Coatings Technol. 2012, 206, 4964–4975. [CrossRef]
- Hessam, R.; Najafisayar, P.; Rasouli, S.S. A comparison between growth of direct and pulse current electrodeposited crystalline SnO2 films; electrochemical properties for application in lithium-ion batteries. Mater. Renew. Sustain. Energy 2022, 11, 259–266. [CrossRef]
- Borkar, T., Electrodeposition of nickel composite coatings. 2010: Oklahoma State University.
- Mandati, S., et al., Pulsed Electrochemical Deposition of CuInSe2 and Cu (In, Ga) Se2 Semiconductor Thin-Films. Semiconductors–Growth and Characterization, 109–132. 2018.
- Akbarpour, M.; Asl, F.G. Fabrication of high-performance graphene/nickel-cobalt composite coatings using ultrasonic-assisted pulse electrodeposition. Ceram. Int. 2023, 49, 13829–13835. [CrossRef]
- Xia, F., et al., Effect of pulse current density on microstructure and wear property of Ni-TiN nanocoatings deposited via pulse electrodeposition. Applied Surface Science, 2021. 538: p. 148139. [CrossRef]
- Krajaisri, P.; Puranasiri, R.; Chiyasak, P.; Rodchanarowan, A. Investigation of pulse current densities and temperatures on electrodeposition of tin-copper alloys. Surf. Coatings Technol. 2022, 435, 128244. [CrossRef]
- Fan, H., et al., Effects of deposition parameters on the microstructure and properties of Jet-electrodeposited Ni-La2O3 composite coatings. Journal of Materials Science: Materials in Electronics, 2020. 31(16): p. 13919-13925. [CrossRef]
- Deshmukh, V.K.; Rajput, M.S.; Narang, H. A systematic review on high speed selective jet electrodeposition manufacturing. World J. Eng. 2022, 21, 275–292. [CrossRef]
- Chen, Y.; Wen, X.; Li, H.; Zhu, F.; Fang, C.; Li, Z.; Zhou, Z.; Jiang, W. Effects of deposition current density, time and scanning velocity on scanning jet electrodeposition of Ni-Co alloy coating. J. Manuf. Process. 2023, 101, 458–468. [CrossRef]
- Karthik, R., R. Mani, and P. Manikandan, Tribological studies of Ni-SiC and Ni-Al2O3 composite coatings by pulsed electrodeposition. Materials Today: Proceedings, 2021. 37: p. 701-706. [CrossRef]
- Kumar, N.; Kishore, K.; Yadav, S.; Sharma, P. Characterisation of Ni-Al2O3 composite coatings at different Al2O3 concentrations. Mater. Today: Proc. 2024. [CrossRef]
- Fan, H., et al., Improvement of microstructure and properties of Ni–Al2O3 composite coating via jet electrodeposition. International Journal of Modern Physics B, 2020. 34(27): p. 2050243. [CrossRef]
- Xie, W., et al., Electrodeposition of Ni and Ni-Al2O3 Composite Coatings on Q345 Steels under Different Current Densities. 2024.
- Gao, M.; Pei, Z.; Song, G.; Liu, Z.; Li, H.; Gong, J. Wear resistance of Ni/nano-Al2O3 composite coatings by brush electroplating. J. Mater. Sci. 2024, 59, 7009–7027. [CrossRef]
- Saha, R.; Khan, T. Effect of applied current on the electrodeposited Ni–Al2O3 composite coatings. Surf. Coatings Technol. 2010, 205, 890–895. [CrossRef]
- John, A.; Saeed, A.; Khan, Z.A. Influence of the Duty Cycle of Pulse Electrodeposition-Coated Ni-Al2O3 Nanocomposites on Surface Roughness Properties. Materials 2023, 16, 2192. [CrossRef]
- Góral, A.; Nowak, M.; Berent, K.; Kania, B. Influence of current density on microstructure and properties of electrodeposited nickel-alumina composite coatings. J. Alloy. Compd. 2014, 615, S406–S410. [CrossRef]
- Rezgui, I., A. Belloufi, and A. Mihi, Experimental investigation of the corrosion resistance of Ni-Al2O3 composite coatings obtained by electrodeposition. UPB Sci. Bull. B Chem. Mater. Sci, 2020. 82(1): p. 221-237.
- Chen, L.; Wang, L.; Zeng, Z.; Xu, T. Influence of pulse frequency on the microstructure and wear resistance of electrodeposited Ni–Al2O3 composite coatings. Surf. Coatings Technol. 2006, 201, 599–605. [CrossRef]
- Huang, P.-C.; Hou, K.-H.; Hong, J.-J.; Lin, M.-H.; Wang, G.-L. Study of fabrication and wear properties of Ni–SiC composite coatings on A356 aluminum alloy. Wear 2021, 477, 203772. [CrossRef]
- Li, J.; Lin, O.; Cheng, C.; Wang, W.; Xu, C.; Ren, L. Fabrication of a Ni/SiC composite coating on steel surface with excellent corrosion inhibition performance. J. Mech. Work. Technol. 2021, 290. [CrossRef]
- Rao, H.; Li, W.; Zhao, F.; Song, Y.; Liu, H.; Zhu, L.; Chen, H. Electrodeposition of High-Quality Ni/SiC Composite Coatings by Using Binary Non-Ionic Surfactants. Molecules 2023, 28, 3344. [CrossRef]
- Li, G., et al., Investigation on Microstructure and Properties of the Electrodeposited Ni-SiC Composite Coating. Coatings, 2023. 13(4): p. 695. [CrossRef]
- Arghavanian, R.; Ahmadi, N.P. Electrodeposition of Ni–ZrO2 composite coatings and evaluation of particle distribution and corrosion resistance. Surf. Eng. 2011, 27, 649–654. [CrossRef]
- Parida, G., D. Chaira, and A. Basu, Ni–ZrO 2 Composite Coating by Electro-Co-Deposition. Transactions of the Indian Institute of Metals, 2013. 66: p. 5-11. [CrossRef]
- Algailani, H.M., A.K. Mahmoud, and H.A. Al-Kaisy, Fabrication of Ni-ZrO2 nanocomposite coating by electroless deposition technique. Engineering and Technology Journal, 2020. 38(5A): p. 649-655.
- Xiong, C., et al., Improving the properties of Ni-zro2 composite coatings by sol-enhanced electroplating. International Journal of Electrochemical Science, 2018. 13(11): p. 11049-11057. [CrossRef]
- Zhang, Y., et al., Microstructure, wear and corrosion resistances of Ni–ZrO2–CeO2 nanocoatings. Ceramics International, 2024. [CrossRef]
- Benea, L. Electrodeposition and tribocorrosion behaviour of ZrO2–Ni composite coatings. J. Appl. Electrochem. 2009, 39, 1671–1681. [CrossRef]
- Laszczyńska, A., et al., Electrodeposition and characterization of Ni–Mo–ZrO2 composite coatings. Applied Surface Science, 2016. 369: p. 224-231. [CrossRef]
- Novytska, N. and I. Zaverach, Composition, topography and electrocatalytic properties of Ni-TiO2 composite coatings. Materials Today: Proceedings, 2022. 50: p. 442-447. [CrossRef]
- Lajevardi, S.A.; Shahrabi, T. Effects of pulse electrodeposition parameters on the properties of Ni–TiO2 nanocomposite coatings. Appl. Surf. Sci. 2010, 256, 6775–6781. [CrossRef]
- Salehi, M., et al., The role of TiO2 nanoparticles on the topography and hydrophobicity of electrodeposited Ni-TiO2 composite coating. Surface Topography: Metrology and Properties, 2020. 8(2): p. 025008. [CrossRef]
- Tehrani, H.M.; Shoja-Razavi, R.; Erfanmanesh, M.; Hashemi, S.H.; Barekat, M. Evaluation of the mechanical properties of WC-Ni composite coating on an AISI 321 steel substrate. Opt. Laser Technol. 2020, 127, 106138. [CrossRef]
- Li, Y.; Song, P.; Wang, W.; Lei, M.; Li, X. Microstructure and wear resistance of a Ni-WC composite coating on titanium grade 2 obtained by electroplating and electron beam remelting. Mater. Charact. 2020, 170, 110674. [CrossRef]
- Zhao, S.; Yang, L.; Huang, Y.; Xu, S. A novel method to fabricate Ni/WC composite coatings by laser wire deposition: Processing characteristics, microstructural evolution and mechanical properties under different wire transfer modes. Addit. Manuf. 2021, 38. [CrossRef]
- Oganov, A.R.; Lyakhov, A.O. Towards the theory of hardness of materials. J. Superhard Mater. 2010, 32, 143–147. [CrossRef]
- Shakoor, R., et al., Properties of electrodeposited Ni–B–Al2O3 composite coatings. Materials & Design, 2014. 64: p. 127-135. [CrossRef]
- Kumar, D., G. Shree, and V.K. Dwivedi, Wear and hardness evaluation of electrodeposited Ni-SiC nanocomposite coated copper. International Journal of Microstructure and Materials Properties, 2020. 15(2): p. 87-106. [CrossRef]
- Parida, G., et al., Synthesis and characterization of Ni-TiO2 composite coatings by electro-co-deposition. Surface and Coatings Technology, 2011. 205(21-22): p. 4871-4879. [CrossRef]
- Chen, W.; Gao, W. Sol-enhanced electroplating of nanostructured Ni–TiO2 composite coatings—The effects of sol concentration on the mechanical and corrosion properties. Electrochimica Acta 2010, 55, 6865–6871. [CrossRef]
- Rezgui, I.; Belloufi, A.; Abdelkrim, M. Investigating corrosion resistance in Ni–Al2O3 composite coatings: a fuzzy logic-based predictive study. Int. J. Adv. Manuf. Technol. 2024, 132, 4363–4381. [CrossRef]
- Mouna, R., A. Noureddine, and M. Ferkhi, Anticorrosive Properties of Nickel-Alumina Composite Coatings on A Steel Substrate. Analytical and Bioanalytical Electrochemistry, 2023. 15(5): p. 382-393. [CrossRef]
- Zhang, H.; Wang, J.; Chen, S.; Wang, H.; He, Y.; Ma, C. Ni–SiC composite coatings with improved wear and corrosion resistance synthesized via ultrasonic electrodeposition. Ceram. Int. 2020, 47, 9437–9446. [CrossRef]
- Nikitasari, A., et al. The influence of coating time on corrosion resistance of Ni/Ni-SiC composite coating. in AIP Conference Proceedings. 2020. AIP Publishing. [CrossRef]
- Pedrizzetti, G.; Paglia, L.; Genova, V.; Cinotti, S.; Bellacci, M.; Marra, F.; Pulci, G. Microstructural, mechanical and corrosion characterization of electroless Ni-P composite coatings modified with ZrO2 reinforcing nanoparticles. Surf. Coatings Technol. 2023, 473. [CrossRef]
- Abdel Hamid, Z., et al., Use of a Ni-TiO2 nanocomposite film to enhance agricultural cutting knife surfaces by electrodeposition technology. Journal of Materials Science, 2021. 56(25): p. 14096-14113. [CrossRef]
- Lemya, L.; Hachemi, B.T.; Elhachmi, G.T. Microstructural, surface and electrochemical properties of electrodeposited Ni-WC nanocomposites coatings. Main Group Chem. 2022, Preprint, 1–10. [CrossRef]
- Qu, G., et al. Microstructure and Wear Resistance of Ni-Al2O3 Nano-composite Coating. in Journal of Physics: Conference Series. 2021. IOP Publishing. [CrossRef]
- Wang, Q.; Li, Q.; Zhang, L.; Chen, D.X.; Jin, H.; Li, J.D.; Zhang, J.W.; Ban, C.Y. Microstructure and properties of Ni-WC gradient composite coating prepared by laser cladding. Ceram. Int. 2021, 48, 7905–7917. [CrossRef]
- Alidokht, S.A.; Chromik, R.R. Sliding wear behavior of cold-sprayed Ni-WC composite coatings: Influence OF WC content. Wear 2021, 477. [CrossRef]
- Natarajan, P., A. Jegan, and M. Mohanraj, Wear Behavior of Ni-TiO2 Nano-Composite Coating on AISI 1022 CS by Pulse Electrodeposition Wear Behavior of Ni-TiO 2 Nano-Composite Coating on AISI 1022 CS by Pulse Electrodeposition. JN Mater. Electrochem. Syst., 2020. 23. [CrossRef]
- Gül, H., The effect of TiO2 concentration on morphology, wear and corrosion properties of NiB-TiO2 composite coatings by ultrasonic-assisted pulse electrodeposition. Surface Topography: Metrology and Properties, 2023. 11(1): p. 015001. [CrossRef]
- Aruna, S., S. Latha, and R. Lakshmi, Multifunctional properties of electrodeposited nickel composite coating containing nanosized monoclinic zirconia particles. Journal of Metallurgy and Materials Science, 2020. 66(1and2): p. 77-91.
- Qiu, Y.; Jiang, G.-Y. Study on friction, wear and thermal insulation properties of laser deposition Ni/ZrO2. Optik 2021, 247, 167783. [CrossRef]
- Wang, H., et al., Ni-SiC composite coatings with good wear and corrosion resistance synthesized via ultrasonic electrodeposition. Journal of Materials Engineering and Performance, 2021. 30(2): p. 1535-1544. [CrossRef]
- Vaezi, M.; Sadrnezhaad, S.; Nikzad, L. Electrodeposition of Ni–SiC nano-composite coatings and evaluation of wear and corrosion resistance and electroplating characteristics. Colloids Surfaces A: Physicochem. Eng. Asp. 2008, 315, 176–182. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).