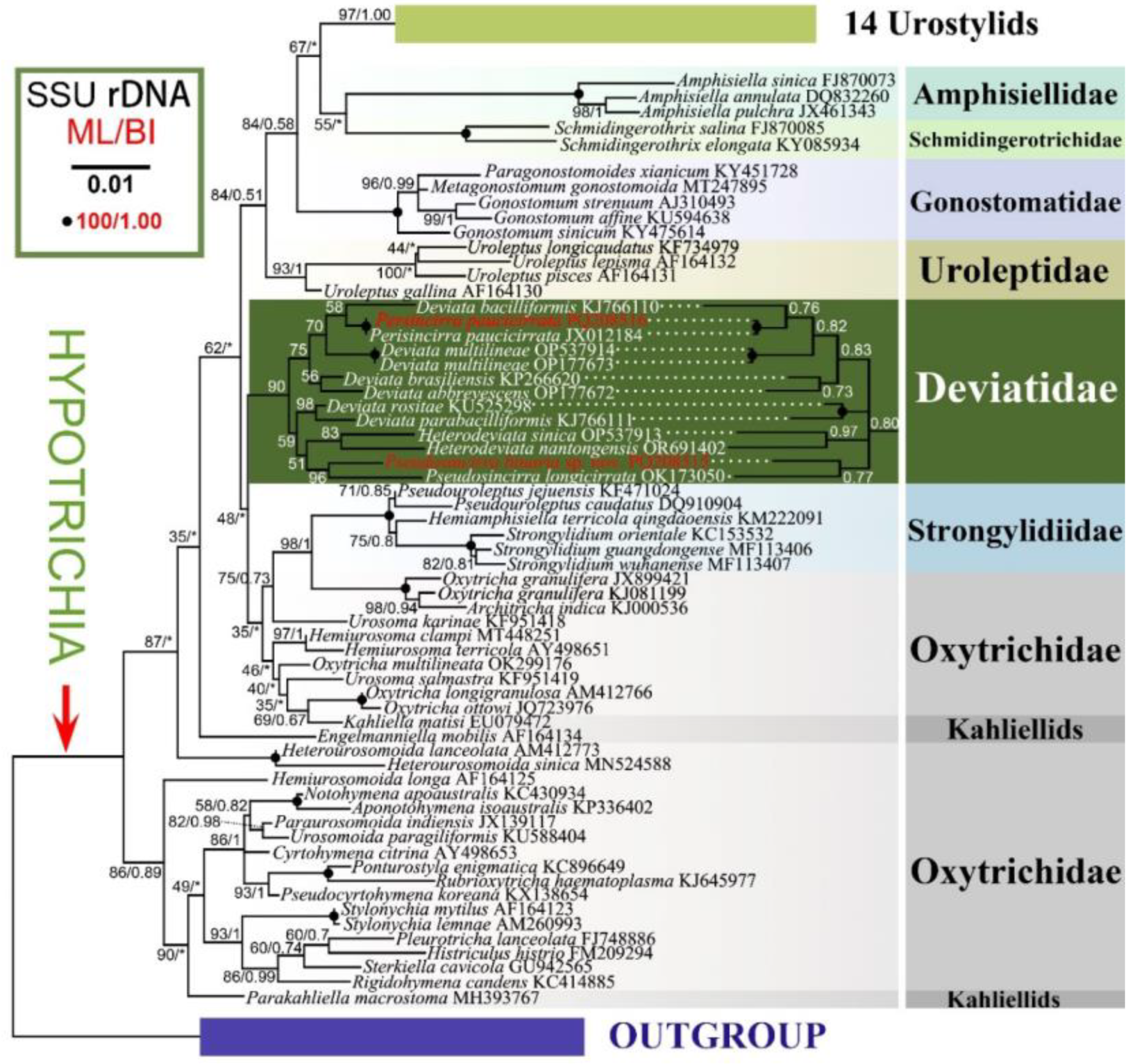
Figure 1.
Morphology of Pseudosincirra binaria sp. nov. from life (A, F–K) and after protargol staining (B–E, L–P). A Ventral view of a representative individual, arrow indicates the contractile vacuole. B, C Ventral (B) and dorsal (C) views of the holotype, showing the infraciliature and nuclear apparatus, arrowhead and arrow in (B) mark the buccal cirrus and pharyngeal fibers, respectively, arrows and arrowheads in (C) denote micronuclei and caudal cirri, separately. D, E Ventral (D) and dorsal (E) views of the same specimen, to show the general infraciliature and nuclear apparatus, arrowhead showing buccal cirrus. F Dorsal view, showing the contractile vacuole (arrowhead). G Ventral view of typical well-nourished cultured individual. H Detail of the anterior end of body, arrowhead denotes the adoral membranelles. I Detail of the posterior portion of cell, arrowhead indicates the marginal cirri. J Detail of the posterior end of body, arrowheads show caudal cirri. K Showing the cytoplasmic granules, macronuclear nodules and contractile vacuole (arrow). L, M Ventral views of the anterior portion of two cells, showing oral apparatus, frontal cirri and the buccal cirrus (arrowheads), and part of frontoventral row. N Ventral view of the anterior portion of cell, showing paroral and endoral membranes, and parabuccal cirrus (arrow). O Two macronuclear nodules and micronucleus in between (arrowhead). P Detail of the posterior portion of cell, showing dorsal kineties and caudal cirri. AZM, adoral zone of membranelles; CC, caudal cirri; DK1, 2, 3, dorsal kineties 1, 2, 3; EM, endoral membrane; FC, frontal cirri; FVR, frontoventral row; L1, 2, 3, the inner, middle and outer left marginal row; Ma, macronuclear nodules; Mi, micronuclei; PBC, parabuccal cirrus; PM, paroral membrane; R1, 2, the inner and outer right marginal row. Scale bars=50 μm.
Figure 1.
Morphology of Pseudosincirra binaria sp. nov. from life (A, F–K) and after protargol staining (B–E, L–P). A Ventral view of a representative individual, arrow indicates the contractile vacuole. B, C Ventral (B) and dorsal (C) views of the holotype, showing the infraciliature and nuclear apparatus, arrowhead and arrow in (B) mark the buccal cirrus and pharyngeal fibers, respectively, arrows and arrowheads in (C) denote micronuclei and caudal cirri, separately. D, E Ventral (D) and dorsal (E) views of the same specimen, to show the general infraciliature and nuclear apparatus, arrowhead showing buccal cirrus. F Dorsal view, showing the contractile vacuole (arrowhead). G Ventral view of typical well-nourished cultured individual. H Detail of the anterior end of body, arrowhead denotes the adoral membranelles. I Detail of the posterior portion of cell, arrowhead indicates the marginal cirri. J Detail of the posterior end of body, arrowheads show caudal cirri. K Showing the cytoplasmic granules, macronuclear nodules and contractile vacuole (arrow). L, M Ventral views of the anterior portion of two cells, showing oral apparatus, frontal cirri and the buccal cirrus (arrowheads), and part of frontoventral row. N Ventral view of the anterior portion of cell, showing paroral and endoral membranes, and parabuccal cirrus (arrow). O Two macronuclear nodules and micronucleus in between (arrowhead). P Detail of the posterior portion of cell, showing dorsal kineties and caudal cirri. AZM, adoral zone of membranelles; CC, caudal cirri; DK1, 2, 3, dorsal kineties 1, 2, 3; EM, endoral membrane; FC, frontal cirri; FVR, frontoventral row; L1, 2, 3, the inner, middle and outer left marginal row; Ma, macronuclear nodules; Mi, micronuclei; PBC, parabuccal cirrus; PM, paroral membrane; R1, 2, the inner and outer right marginal row. Scale bars=50 μm.
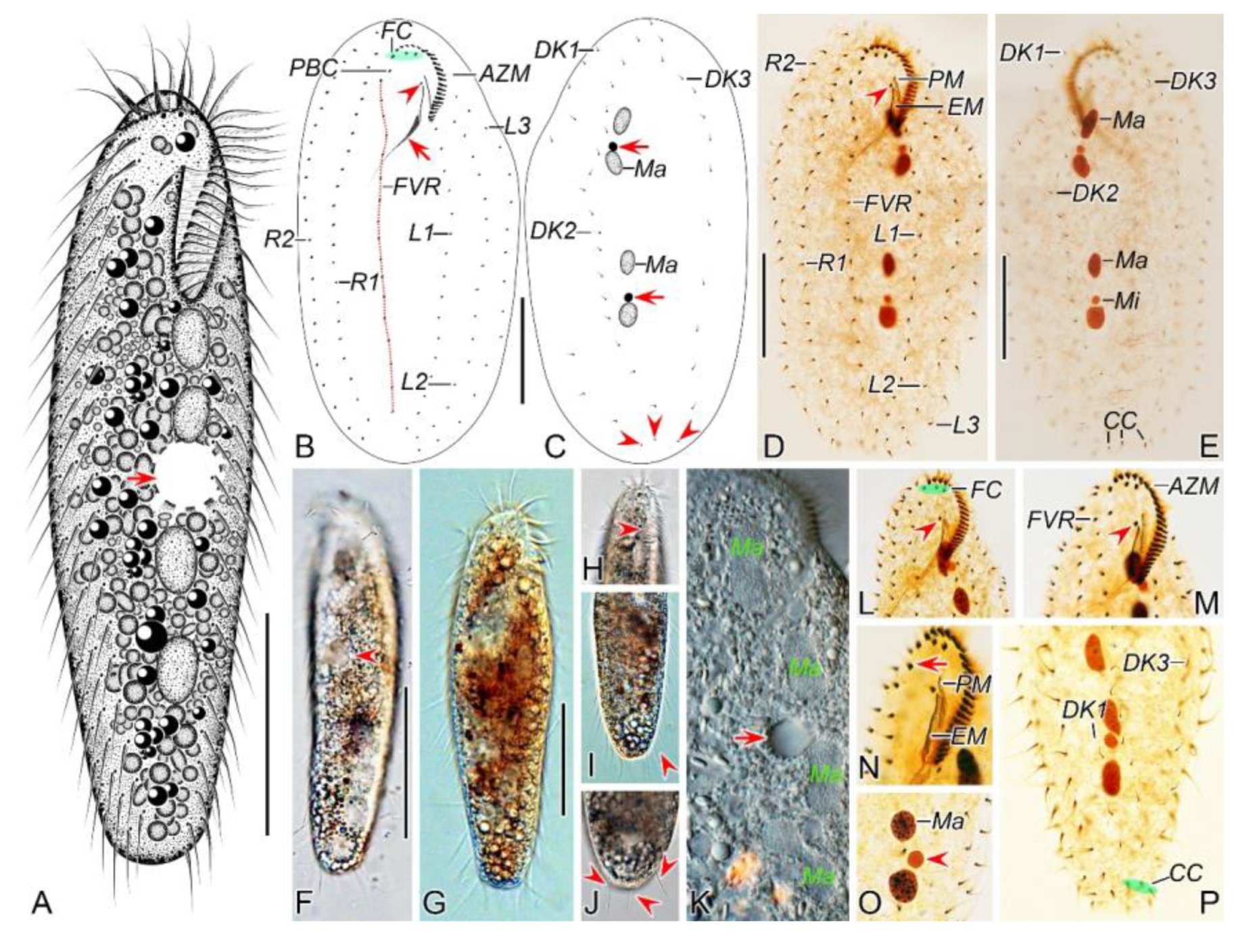
Figure 2.
Morphogenetic photomicrographs of Pseudsincirra binaria sp. nov. after protargol staining. A Detail of early divider, arrowheads indicate the replication bands and arrows denote the micronuclei. B Ventral view of a very early divider, showing the newly formed oral primordium between frontoventral row and the inner left marginal row. C, D With the proliferation of basal bodies, the oral primordium of the opisthe lengthens, arrowheads and arrow in (D) indicate macronuclear nodules and the micronucleus, respectively. E, F Ventral views of early dividers, arrowheads denote several newly formed adoral zone of membranes and arrows point to undulating membranes anlage, in this stage, streaks are formed anteriorly to the right of the oral primordium. G, H Ventral views of early dividers, arrowhead in (H) demonstrates incompletely formed adoral zone of membranes, in this stage, anlagen Ⅰ–Ⅲ of the opisthe are formed. I, J Ventral views of early dividers, arrowhead in (I) showing the posterior majority of anlage I splits longitudinally to form paroral and endoral membranes and arrowhead in (J) marks the almost completely formed adoral zone of membranes. K, L Ventral views of early dividers, showing the anlage for the inner right marginal row of the opisthe (arrowheads) formed de novo to the right of the parental row. M Dorsal view of an early divider, showing dorsal kineties anlagen 1–3 (number). N Ventral view of the anterior portion of an early divider, showing the anterior portion of the parental paroral dedifferentiating to anlage I, buccal cirrus and parabuccal cirrus dedifferentiating to anlage Ⅱ and anlage Ⅲ, respectively. O Ventral view of the anterior portion of an early divider, showing the parental frontal cirri (ellipse) and frontal ventral cirral anlagen I–Ⅲ of the proter, arrowhead shows the parental undulating membranes begin to disintegrate. P Ventral view of the anterior portion of an early divider, arrowheads mark newly formed frontal cirri and arrow points to frontal ventral cirral anlage Ⅳ of the proter. Q Ventral view of the anterior portion of an early divider, showing the anlage of inner right marginal row develops to the right of the parental inner right marginal row, arrowhead indicates anlage V of the proter appears within the parental inner right marginal row and arrow denotes frontal ventral cirral anlage Ⅳ. R, S Ventral (R) and dorsal (S) views of the same early divider, arrow and arrowhead in (R) indicate the left marginal row anlage I and intrakinetally formed frontal ventral cirral anlage Ⅳ of the opisthe, respectively, ellipse encircles the newly formed frontal cirri of the opisthe, numbers in (S) demonstrate dorsal kineties anlagen 1–3 formed within old kineties. T Ventral view of an early-middle divider, arrowheads denote the intrakinetally formed marginal row anlagen, arrows mark the micronuclei, double arrowhead indicates frontal oventral cirral anlage Ⅳ of the opisthe and ellipse encircles the newly formed frontal cirri of the opisthe, in this stage, the macronuclear nodules begin to fuse into a single globular mass. U Ventral view of an early divider, arrowheads point to left marginal row anlagen, in this stage, only anlagen for the inner and middle left marginal rows appear. 1–3, dorsal kineties anlagen 1–3; AZM, adoral zone of membranelles; FC, frontal cirri; FVR, frontoventral row; L1, inner left marginal row; Ma, macronuclear nodules; OP, oral primordium; R1, inner right marginal row; RMA1, anlage for the inner right marginal row; Ⅰ–Ⅲ, frontal ventral cirral anlagen Ⅰ–Ⅲ. Scale bars=30 μm.
Figure 2.
Morphogenetic photomicrographs of Pseudsincirra binaria sp. nov. after protargol staining. A Detail of early divider, arrowheads indicate the replication bands and arrows denote the micronuclei. B Ventral view of a very early divider, showing the newly formed oral primordium between frontoventral row and the inner left marginal row. C, D With the proliferation of basal bodies, the oral primordium of the opisthe lengthens, arrowheads and arrow in (D) indicate macronuclear nodules and the micronucleus, respectively. E, F Ventral views of early dividers, arrowheads denote several newly formed adoral zone of membranes and arrows point to undulating membranes anlage, in this stage, streaks are formed anteriorly to the right of the oral primordium. G, H Ventral views of early dividers, arrowhead in (H) demonstrates incompletely formed adoral zone of membranes, in this stage, anlagen Ⅰ–Ⅲ of the opisthe are formed. I, J Ventral views of early dividers, arrowhead in (I) showing the posterior majority of anlage I splits longitudinally to form paroral and endoral membranes and arrowhead in (J) marks the almost completely formed adoral zone of membranes. K, L Ventral views of early dividers, showing the anlage for the inner right marginal row of the opisthe (arrowheads) formed de novo to the right of the parental row. M Dorsal view of an early divider, showing dorsal kineties anlagen 1–3 (number). N Ventral view of the anterior portion of an early divider, showing the anterior portion of the parental paroral dedifferentiating to anlage I, buccal cirrus and parabuccal cirrus dedifferentiating to anlage Ⅱ and anlage Ⅲ, respectively. O Ventral view of the anterior portion of an early divider, showing the parental frontal cirri (ellipse) and frontal ventral cirral anlagen I–Ⅲ of the proter, arrowhead shows the parental undulating membranes begin to disintegrate. P Ventral view of the anterior portion of an early divider, arrowheads mark newly formed frontal cirri and arrow points to frontal ventral cirral anlage Ⅳ of the proter. Q Ventral view of the anterior portion of an early divider, showing the anlage of inner right marginal row develops to the right of the parental inner right marginal row, arrowhead indicates anlage V of the proter appears within the parental inner right marginal row and arrow denotes frontal ventral cirral anlage Ⅳ. R, S Ventral (R) and dorsal (S) views of the same early divider, arrow and arrowhead in (R) indicate the left marginal row anlage I and intrakinetally formed frontal ventral cirral anlage Ⅳ of the opisthe, respectively, ellipse encircles the newly formed frontal cirri of the opisthe, numbers in (S) demonstrate dorsal kineties anlagen 1–3 formed within old kineties. T Ventral view of an early-middle divider, arrowheads denote the intrakinetally formed marginal row anlagen, arrows mark the micronuclei, double arrowhead indicates frontal oventral cirral anlage Ⅳ of the opisthe and ellipse encircles the newly formed frontal cirri of the opisthe, in this stage, the macronuclear nodules begin to fuse into a single globular mass. U Ventral view of an early divider, arrowheads point to left marginal row anlagen, in this stage, only anlagen for the inner and middle left marginal rows appear. 1–3, dorsal kineties anlagen 1–3; AZM, adoral zone of membranelles; FC, frontal cirri; FVR, frontoventral row; L1, inner left marginal row; Ma, macronuclear nodules; OP, oral primordium; R1, inner right marginal row; RMA1, anlage for the inner right marginal row; Ⅰ–Ⅲ, frontal ventral cirral anlagen Ⅰ–Ⅲ. Scale bars=30 μm.
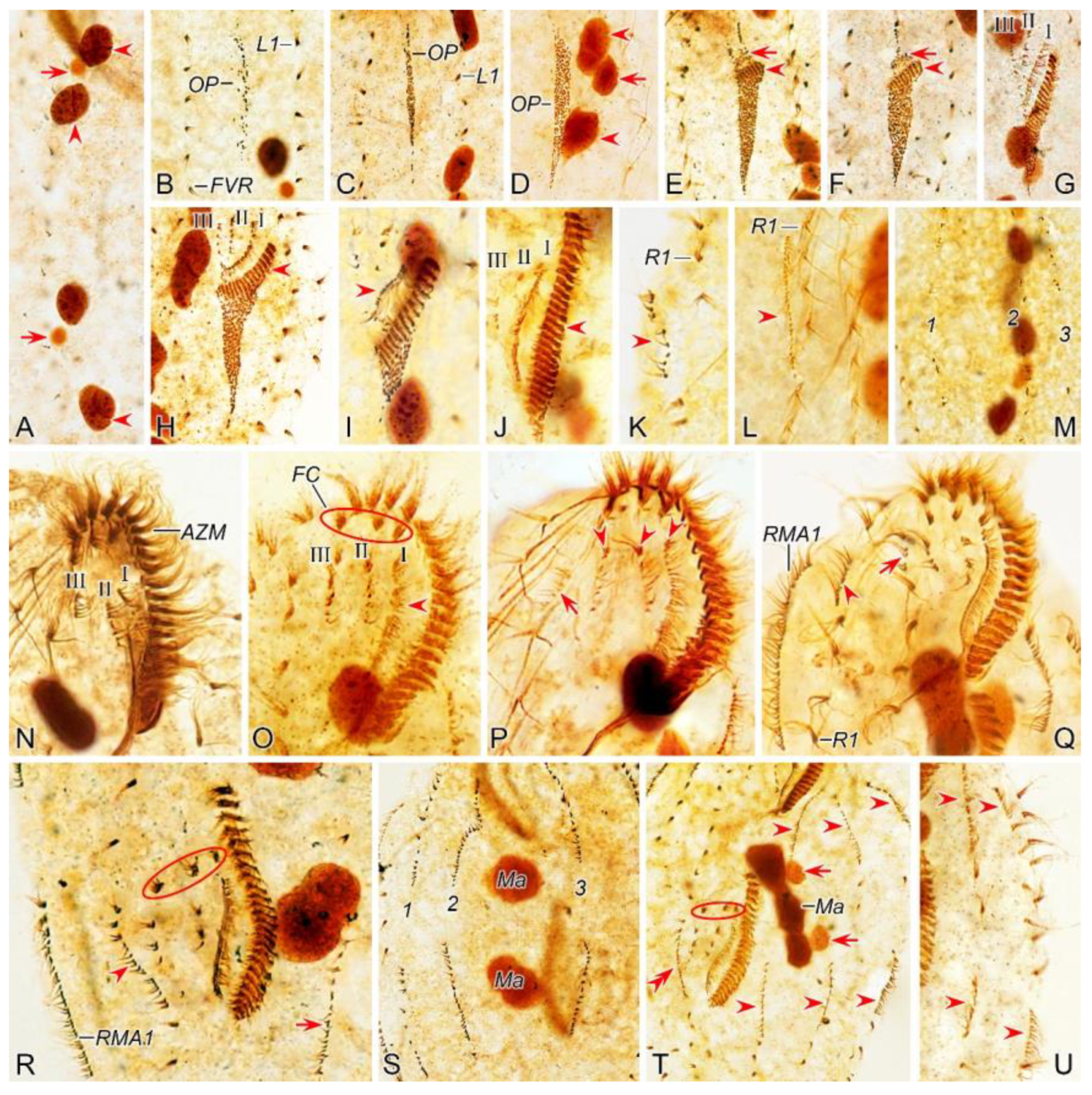
Figure 3.
Morphogenesis of Pseudosincirra binaria sp. nov. after protargol staining. A Detail of early dividers, arrowheads indicate the replication bands and arrows denote the micronuclei. B Ventral view of a very early divider, showing the newly formed oral primordium between frontoventral row and the inner left marginal row. C, D With the proliferation of basal bodies, the oral primordium of the opisthe lengthens. E, F Ventral views of early divider, arrowheads denote several newly formed adoral zone of membranes and arrows point to undulating membranes anlage, in this stage, streaks are formed anteriorly to the right of the oral primordium. G, H Ventral views of early divider, arrowhead in (H) demonstrates incompletely formed adoral zone of membranes, in this stage, anlagen Ⅰ–Ⅲ of the opisthe are formed. I, J Ventral views of early divider, arrowhead in (I) showing the posterior majority of anlage I splits longitudinally to form paroral and endoral membranes and arrowhead in (J) marks the almost completely formed adoral zone of membranes. K, L Ventral views of early divider, showing the anlage for the inner right marginal row of the opisthe (arrowheads) formed de novo to the right of the parental row. M Dorsal view of an early divider, showing dorsal kinety anlagen 1–3 (number). N Ventral view of the anterior portion of an early divider, showing the anterior portion of the parental paroral dedifferentiating to anlage I, buccal cirrus and parabuccal cirrus dedifferentiating to anlage Ⅱ and anlage Ⅲ, respectively. O Ventral view of the anterior portion of an early divider, showing the parental frontal cirri (ellipse) and frontal ventral cirral anlagen I–Ⅲ of the proter, arrowhead shows the parental undulating membranes begin to disintegrate. P Ventral view of the anterior portion of an early divider, arrowheads mark newly formed frontal cirri and arrow points to frontal ventral cirral anlage Ⅳ of the proter. Q Ventral view of the anterior portion of an early divider, showing the anlage of inner right marginal row develops to the right of the parental inner right marginal row, arrowhead indicates anlage V of the proter appears within the parental inner right marginal row and arrow denotes frontal ventral cirral anlage Ⅳ. R, S Ventral (R) and dorsal (S) views of the same early divider, arrow and arrowhead in (R) indicate the left marginal row anlage I and intrakinetally formed frontal ventral cirral anlage Ⅳ of the opisthe, respectively, ellipse encircles the newly formed frontal cirri of the opisthe, numbers in (S) demonstrate dorsal kineties anlagen 1–3 formed within old kineties. T Ventral view of an early-middle divider, arrowheads denote the intrakinetally formed marginal cirral anlagen, arrows mark the micronuclei, double arrowhead indicates frontal ventral cirral anlage Ⅳ of the opisthe and ellipse encircles the newly formed frontal cirri of the opisthe, in this stage, the macronuclear nodules begin to fuse into a single globular mass. U Ventral view of an early divider, arrowheads point to left marginal row anlagen, in this stage, only anlagen for the inner and middle left marginal rows appear. 1–3, dorsal kinety anlagen 1–3; AZM, adoral zone of membranelles; FC, frontal cirri; FVR, frontoventral row; L1, inner left marginal row; Ma, macronuclear nodules; OP, oral primordium; R1, inner right marginal row; RMA1, anlage for the inner right marginal row; Ⅰ–Ⅲ, frontal ventral cirral anlagen Ⅰ–Ⅲ. Scale bars=30 μm.
Figure 3.
Morphogenesis of Pseudosincirra binaria sp. nov. after protargol staining. A Detail of early dividers, arrowheads indicate the replication bands and arrows denote the micronuclei. B Ventral view of a very early divider, showing the newly formed oral primordium between frontoventral row and the inner left marginal row. C, D With the proliferation of basal bodies, the oral primordium of the opisthe lengthens. E, F Ventral views of early divider, arrowheads denote several newly formed adoral zone of membranes and arrows point to undulating membranes anlage, in this stage, streaks are formed anteriorly to the right of the oral primordium. G, H Ventral views of early divider, arrowhead in (H) demonstrates incompletely formed adoral zone of membranes, in this stage, anlagen Ⅰ–Ⅲ of the opisthe are formed. I, J Ventral views of early divider, arrowhead in (I) showing the posterior majority of anlage I splits longitudinally to form paroral and endoral membranes and arrowhead in (J) marks the almost completely formed adoral zone of membranes. K, L Ventral views of early divider, showing the anlage for the inner right marginal row of the opisthe (arrowheads) formed de novo to the right of the parental row. M Dorsal view of an early divider, showing dorsal kinety anlagen 1–3 (number). N Ventral view of the anterior portion of an early divider, showing the anterior portion of the parental paroral dedifferentiating to anlage I, buccal cirrus and parabuccal cirrus dedifferentiating to anlage Ⅱ and anlage Ⅲ, respectively. O Ventral view of the anterior portion of an early divider, showing the parental frontal cirri (ellipse) and frontal ventral cirral anlagen I–Ⅲ of the proter, arrowhead shows the parental undulating membranes begin to disintegrate. P Ventral view of the anterior portion of an early divider, arrowheads mark newly formed frontal cirri and arrow points to frontal ventral cirral anlage Ⅳ of the proter. Q Ventral view of the anterior portion of an early divider, showing the anlage of inner right marginal row develops to the right of the parental inner right marginal row, arrowhead indicates anlage V of the proter appears within the parental inner right marginal row and arrow denotes frontal ventral cirral anlage Ⅳ. R, S Ventral (R) and dorsal (S) views of the same early divider, arrow and arrowhead in (R) indicate the left marginal row anlage I and intrakinetally formed frontal ventral cirral anlage Ⅳ of the opisthe, respectively, ellipse encircles the newly formed frontal cirri of the opisthe, numbers in (S) demonstrate dorsal kineties anlagen 1–3 formed within old kineties. T Ventral view of an early-middle divider, arrowheads denote the intrakinetally formed marginal cirral anlagen, arrows mark the micronuclei, double arrowhead indicates frontal ventral cirral anlage Ⅳ of the opisthe and ellipse encircles the newly formed frontal cirri of the opisthe, in this stage, the macronuclear nodules begin to fuse into a single globular mass. U Ventral view of an early divider, arrowheads point to left marginal row anlagen, in this stage, only anlagen for the inner and middle left marginal rows appear. 1–3, dorsal kinety anlagen 1–3; AZM, adoral zone of membranelles; FC, frontal cirri; FVR, frontoventral row; L1, inner left marginal row; Ma, macronuclear nodules; OP, oral primordium; R1, inner right marginal row; RMA1, anlage for the inner right marginal row; Ⅰ–Ⅲ, frontal ventral cirral anlagen Ⅰ–Ⅲ. Scale bars=30 μm.
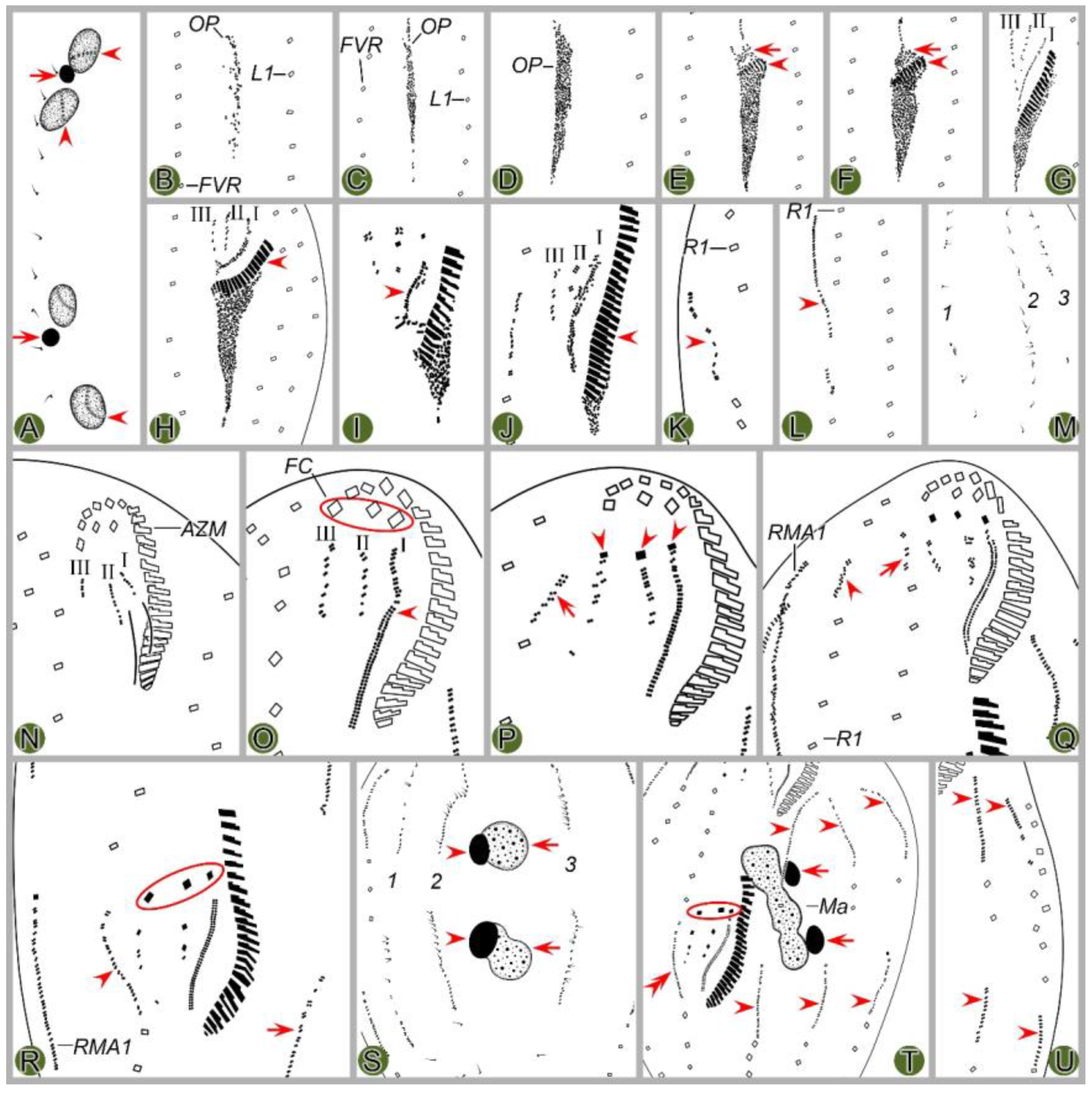
Figure 4.
Morphogenetic photomicrographs of Pseudsincirra binaria sp. nov. after protargol staining. A, B Ventral (A) and dorsal (B) views of the same middle divider, solid arrow marks the anlage V of the proter develops into new cirri posteriad, hollow arrow denotes new cirri from the anlage Ⅳ of the proter, double-arrowhead indicates the newly formed parabuccal cirri of the opisthe, ellipse encircles the newly formed frontal cirri of the opisthe, arrowheads in (A) and (B) show the conspicuous gap in the middle part of inner dorsal kinety and the parental caudal cirri, respectively, in this stage, the macronuclear nodules fuse into a single mass. C, D Ventral (C) and dorsal (D) views of the same middle-late divider, arrows in (C) denote the micronuclei, arrowhead indicates the fuse macronuclear mass begins to divide, arrowheads in (D) demonstrate the newly formed caudal cirri of the proter and opisthe, arrows indicate the conspicuous gap in the middle part of the left dorsal kinety, ellipses encircle the cirri developed from the anlage Ⅳ in (C) and the parental caudal cirri in (D). E, F Ventral (E) and dorsal (F) views of the same late divider, arrow and arrowhead in (E) indicate the newly formed buccal cirrus of the opisthe and the parabuccal cirrus of the proter, ellipse encircles the cirri developed from the anlage Ⅳ, arrows and arrowheads in (F) point to the new caudal cirri and micronuclei, respectively, in this stage, the macronucleus complete its first division. G, H Ventral (G) and dorsal (H) views of the same late divider, arrowheads in (G) indicate frontoventral row formed from cirri that developed from the anlagen Ⅳ and V in proter, and from anlage IV in opisthe, arrows and arrowheads in (H) denote micronuclei and caudal cirri, respectively, double-arrowheads indicate macronuclear nodules, ellipse encircles the old caudal cirri, in this stage, the macronucleusr complete its second division. 1–3, dorsal kineties anlagen 1–3; AZM, adoral zone of membranelles; L1, 2, 3, the inner, middle and outer left marginal rows; Ma, fused macronuclear mass; R1, 2, the inner and outer right marginal rows. Scale bars=30 μm.
Figure 4.
Morphogenetic photomicrographs of Pseudsincirra binaria sp. nov. after protargol staining. A, B Ventral (A) and dorsal (B) views of the same middle divider, solid arrow marks the anlage V of the proter develops into new cirri posteriad, hollow arrow denotes new cirri from the anlage Ⅳ of the proter, double-arrowhead indicates the newly formed parabuccal cirri of the opisthe, ellipse encircles the newly formed frontal cirri of the opisthe, arrowheads in (A) and (B) show the conspicuous gap in the middle part of inner dorsal kinety and the parental caudal cirri, respectively, in this stage, the macronuclear nodules fuse into a single mass. C, D Ventral (C) and dorsal (D) views of the same middle-late divider, arrows in (C) denote the micronuclei, arrowhead indicates the fuse macronuclear mass begins to divide, arrowheads in (D) demonstrate the newly formed caudal cirri of the proter and opisthe, arrows indicate the conspicuous gap in the middle part of the left dorsal kinety, ellipses encircle the cirri developed from the anlage Ⅳ in (C) and the parental caudal cirri in (D). E, F Ventral (E) and dorsal (F) views of the same late divider, arrow and arrowhead in (E) indicate the newly formed buccal cirrus of the opisthe and the parabuccal cirrus of the proter, ellipse encircles the cirri developed from the anlage Ⅳ, arrows and arrowheads in (F) point to the new caudal cirri and micronuclei, respectively, in this stage, the macronucleus complete its first division. G, H Ventral (G) and dorsal (H) views of the same late divider, arrowheads in (G) indicate frontoventral row formed from cirri that developed from the anlagen Ⅳ and V in proter, and from anlage IV in opisthe, arrows and arrowheads in (H) denote micronuclei and caudal cirri, respectively, double-arrowheads indicate macronuclear nodules, ellipse encircles the old caudal cirri, in this stage, the macronucleusr complete its second division. 1–3, dorsal kineties anlagen 1–3; AZM, adoral zone of membranelles; L1, 2, 3, the inner, middle and outer left marginal rows; Ma, fused macronuclear mass; R1, 2, the inner and outer right marginal rows. Scale bars=30 μm.
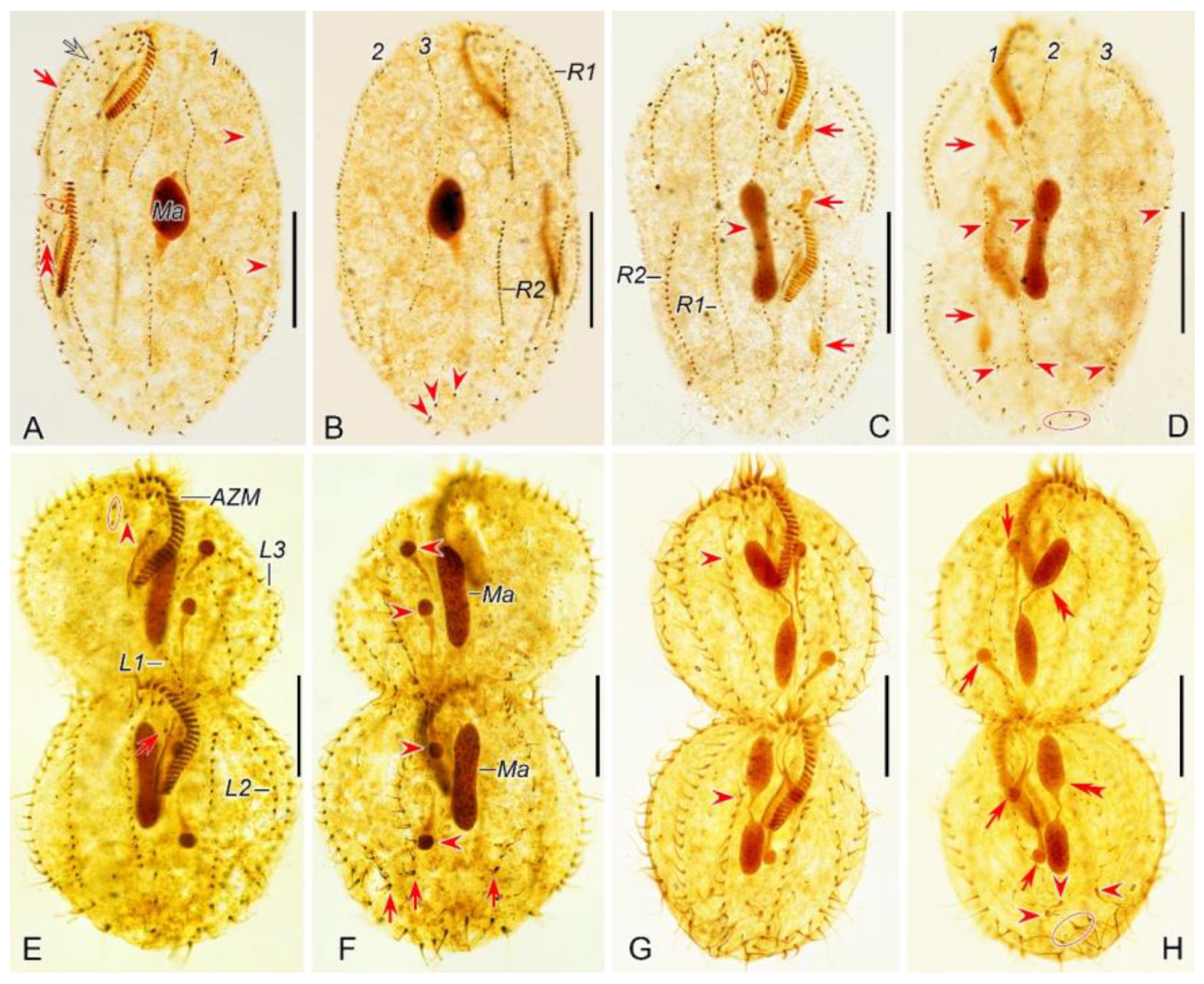
Figure 5.
Middle to late morphogenetic stages of Pseudsincirra binaria sp. nov. after protargol staining. A, B Ventral (A) and dorsal (B) views of the same middle divider, solid arrow marks the anlage V of proter develops into new cirri posteriad, hollow arrow denotes new cirri from the anlage Ⅳ of the proter, double-arrowhead indicates the newly formed parabuccal cirri of the opisthe, ellipse encircles the newly formed frontal cirri of the opisthe, arrowheads in (A) and (B) show the conspicuous gap in the middle part of inner dorsal kinety and the parental caudal cirri, respectively, in this stage, the macronuclear nodules fuse into a single mass. C, D Ventral (C) and dorsal (D) views of the same middle-late divider, arrowhead in (C) indicates the buccal cirrus of the opisthe, arrowheads in (D) demonstrate the newly formed caudal cirri of the proter and opisthe, arrow indicates the fuse macronuclear mass begins to divide, ellipses encircle the cirri developed from the anlage Ⅳ in (C) and the parental caudal cirri in (D). E, F Ventral (E) and dorsal (F) views of the same late divider, arrow and arrowhead in (E) indicate the newly formed buccal cirrus of the opisthe and the parabuccal cirrus of the proter, ellipse encircles the cirri developed from the anlage Ⅳ, arrows and arrowheads in (F) point to the new caudal cirri and micronuclei, respectively, in this stage, the macronucleus complete its first division. G, H Ventral (G) and dorsal (H) views of the same late divider, arrowheads in (G) indicate frontoventral row formed from cirri that developed from the anlagen Ⅳ and V in proter, and from anlage Ⅳ in opisthe, arrows and arrowheads in (H) denote macronuclear nodules and caudal cirri, respectively, ellipse encircles the old caudal cirri, in this stage, the macronucleus complete its second division. 1–3, dorsal kineties anlagen 1–3; AZM, adoral zone of membranelles; EM, endoral membrane; L1–3, the inner, middle and outer left marginal rows; Ma, fused macronuclear mass; PM, paroral membrane; R1, 2, the inner and outer right marginal rows. Scale bars=30 μm.
Figure 5.
Middle to late morphogenetic stages of Pseudsincirra binaria sp. nov. after protargol staining. A, B Ventral (A) and dorsal (B) views of the same middle divider, solid arrow marks the anlage V of proter develops into new cirri posteriad, hollow arrow denotes new cirri from the anlage Ⅳ of the proter, double-arrowhead indicates the newly formed parabuccal cirri of the opisthe, ellipse encircles the newly formed frontal cirri of the opisthe, arrowheads in (A) and (B) show the conspicuous gap in the middle part of inner dorsal kinety and the parental caudal cirri, respectively, in this stage, the macronuclear nodules fuse into a single mass. C, D Ventral (C) and dorsal (D) views of the same middle-late divider, arrowhead in (C) indicates the buccal cirrus of the opisthe, arrowheads in (D) demonstrate the newly formed caudal cirri of the proter and opisthe, arrow indicates the fuse macronuclear mass begins to divide, ellipses encircle the cirri developed from the anlage Ⅳ in (C) and the parental caudal cirri in (D). E, F Ventral (E) and dorsal (F) views of the same late divider, arrow and arrowhead in (E) indicate the newly formed buccal cirrus of the opisthe and the parabuccal cirrus of the proter, ellipse encircles the cirri developed from the anlage Ⅳ, arrows and arrowheads in (F) point to the new caudal cirri and micronuclei, respectively, in this stage, the macronucleus complete its first division. G, H Ventral (G) and dorsal (H) views of the same late divider, arrowheads in (G) indicate frontoventral row formed from cirri that developed from the anlagen Ⅳ and V in proter, and from anlage Ⅳ in opisthe, arrows and arrowheads in (H) denote macronuclear nodules and caudal cirri, respectively, ellipse encircles the old caudal cirri, in this stage, the macronucleus complete its second division. 1–3, dorsal kineties anlagen 1–3; AZM, adoral zone of membranelles; EM, endoral membrane; L1–3, the inner, middle and outer left marginal rows; Ma, fused macronuclear mass; PM, paroral membrane; R1, 2, the inner and outer right marginal rows. Scale bars=30 μm.
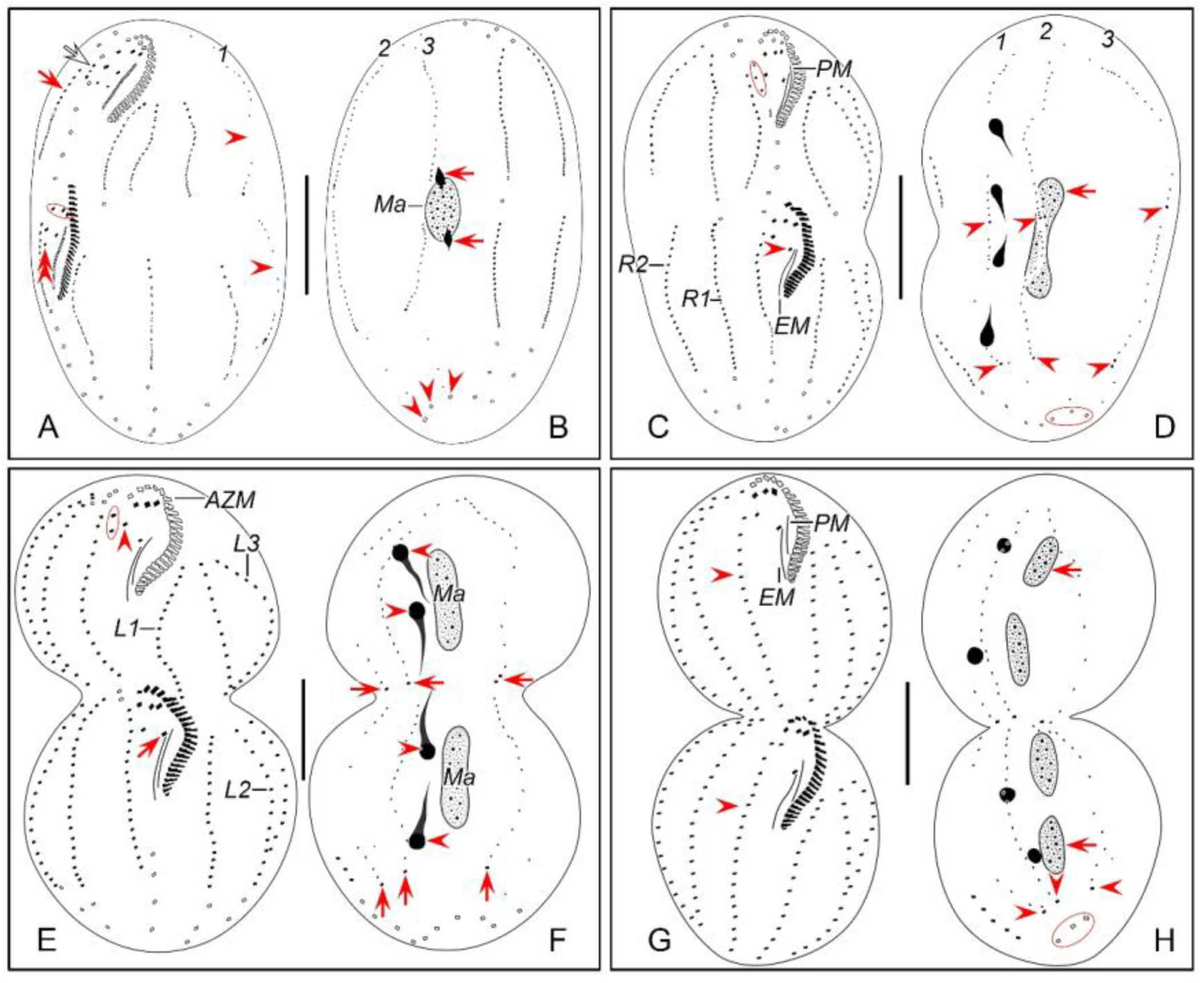
Figure 6.
Morphology of Perisincirra paucicirrata from life (A, F–M) and after protargol staining (B–E). A Ventral view of a representative individual, arrow indicates contractile vacuole. B, C Ventral (B) and dorsal (C) views of the same cell, showing infraciliature and nuclear apparatus, arrowhead and arrow in (B) mark buccal cirrus and pharyngeal fibers, respectively, arrow and arrowheads in (C) denote micronucleus and caudal cirri, separately. D, E Ventral (D) and dorsal (E) views of the same specimen as in B and C, to show the general morphology, arrow and arrowhead in (D) separately indicate endoral membrane and the buccal cirrus, arrow and arrowheads in (E) mark micronucleus and caudal cirri, respectively. F, G Ventral views of well-nourished cultured individuals showing shape variation. H Dorsal view, showing contractile vacuole (arrowhead). I Detail of the anterior portion of body, arrow marks adoral membranelles. J Detail of the middle part of cell, arrowheads indicate marginal cirri. K Detail of the anterior part of body, showing frontal cirri, parabuccal cirri, buccal lip (arrow) and buccal cirrus (arrowhead). L Detail of the posterior end of cell, arrowheads point to caudal cirri. M Showing the cytoplasm and nuclear apparatus, arrow indicates micronucleus (close to the anterior macronuclear nodule). AZM, adoral zone of membranelles; DK1–3, dorsal kineties 1–3; EM, endoral membrane; FC, frontal cirri; L1–3, inner, middle and outer left marginal row; Ma, macronuclear nodules; PBC, parabuccal cirri; PF, pharyngeal fibers; PM, paroral membrane; R1, 2, inner and outer right marginal row. Scale bars=30 μm.
Figure 6.
Morphology of Perisincirra paucicirrata from life (A, F–M) and after protargol staining (B–E). A Ventral view of a representative individual, arrow indicates contractile vacuole. B, C Ventral (B) and dorsal (C) views of the same cell, showing infraciliature and nuclear apparatus, arrowhead and arrow in (B) mark buccal cirrus and pharyngeal fibers, respectively, arrow and arrowheads in (C) denote micronucleus and caudal cirri, separately. D, E Ventral (D) and dorsal (E) views of the same specimen as in B and C, to show the general morphology, arrow and arrowhead in (D) separately indicate endoral membrane and the buccal cirrus, arrow and arrowheads in (E) mark micronucleus and caudal cirri, respectively. F, G Ventral views of well-nourished cultured individuals showing shape variation. H Dorsal view, showing contractile vacuole (arrowhead). I Detail of the anterior portion of body, arrow marks adoral membranelles. J Detail of the middle part of cell, arrowheads indicate marginal cirri. K Detail of the anterior part of body, showing frontal cirri, parabuccal cirri, buccal lip (arrow) and buccal cirrus (arrowhead). L Detail of the posterior end of cell, arrowheads point to caudal cirri. M Showing the cytoplasm and nuclear apparatus, arrow indicates micronucleus (close to the anterior macronuclear nodule). AZM, adoral zone of membranelles; DK1–3, dorsal kineties 1–3; EM, endoral membrane; FC, frontal cirri; L1–3, inner, middle and outer left marginal row; Ma, macronuclear nodules; PBC, parabuccal cirri; PF, pharyngeal fibers; PM, paroral membrane; R1, 2, inner and outer right marginal row. Scale bars=30 μm.
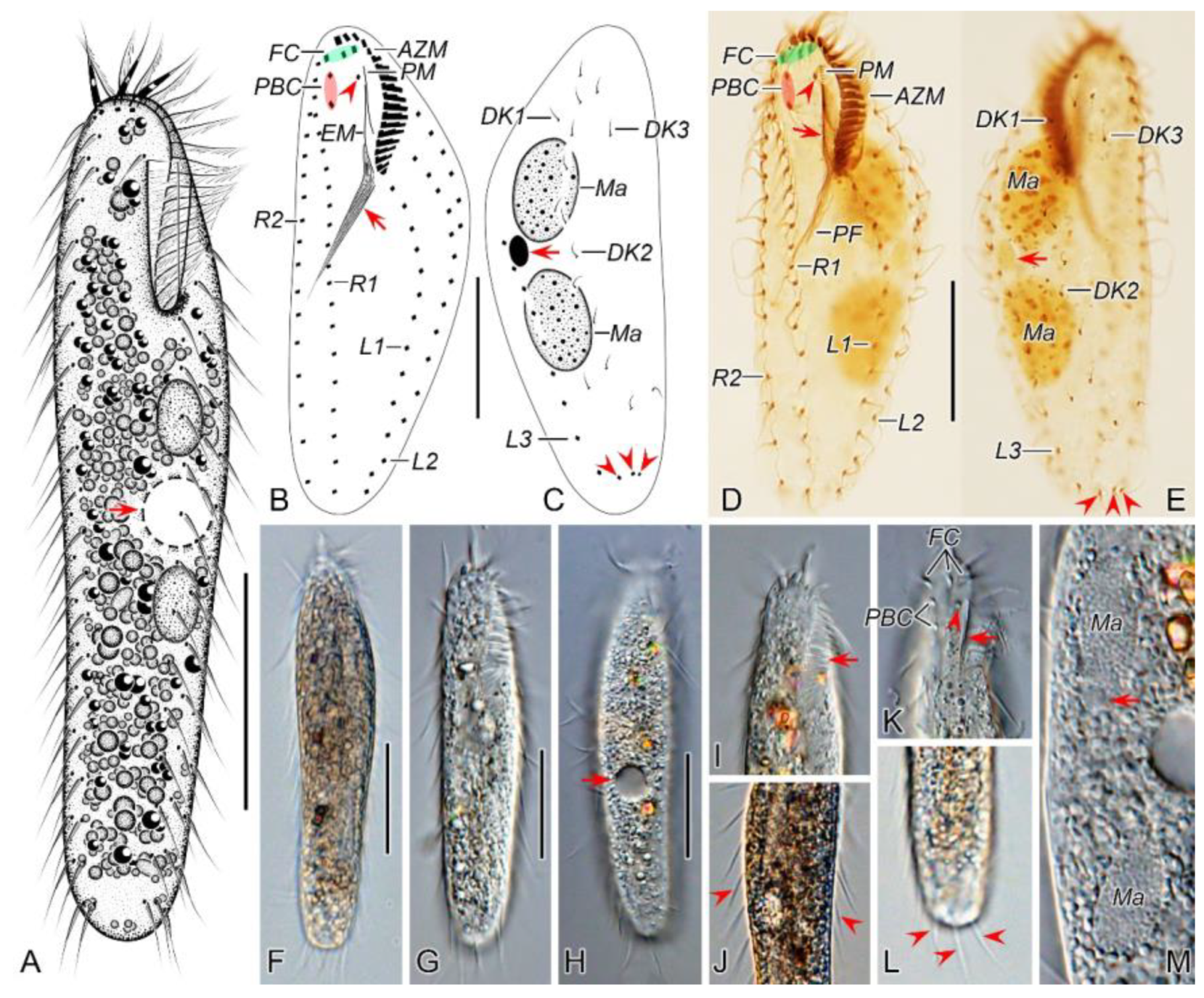
Figure 7.
Morphogenesis photomicrographs of Perisincirra paucicirrata after protargol staining. A Ventral view of a very early divider to show the newly formed oral primordium between right and left cirral rows in opisthe. B, C With the proliferation of basal bodies, the oral primordium of the opisthe lengthens. D, E Ventral views of early dividers, arrow in (D) denotes the newly formed adoral membranes at the anterior end of OP and arrowheads in (D, E) point to the anlage II fosrmed to the right anterior of the OP. F, G Ventral views of early dividers, arrowheads in (F) indicate replication bands and arrows in (F, G) denote undulating membranes anlage (anlage I). H, I Ventral views of early dividers, arrowheads mark incompletely formed adoral zone of membranes, in this stage, anlagen Ⅰ–Ⅲ of the opisthe are formed from the OP. J Ventral view of an early divider, arrow and arrowhead mark the dedifferentiation of buccal cirrus and anterior parabuccal cirrus. K–M Ventral views of early dividers, double-arrowhead indicates the anterior part of paroral disorganise and develop into anlage I of the proter, arrow and arrowhead denote anlagen Ⅱ and Ⅲ of the proter, respectively, hollow arrow demonstrates anlage Ⅲ begins to split into anlagen Ⅲ and Ⅳ. N Ventral view of early divider, showing anlage Ⅳ of the opisthe migrates rightward, arrowhead in (N) marks the dedifferentiation of posterior parabuccal cirrus, arrows point to marginal rows anlagen, in this stage cirral anlagen begin to develop into new cirri. O Ventral view of an early divider, arrowhead denotes the new left frontal cirrus and arrows indicate marginal rows anlagen, in this stage, anlagen Ⅰ–Ⅳ appear in both proter and opisthe. P Dorsal view of an early divider, numbers mark dorsal kineties anlagen 1–3 forming within each dorsal kinety and arrowheads demonstrate the parental caudal cirri. Q Dorsal view of an early to middle divider, numbers mark dorsal kineties anlagen 1–3 formed within old structures, arrow denotes the dividing micronucleus and arrowheads point to the old caudal cirri. R Ventral view of an early to middle divider, arrows and arrowhead indicate marginal rows anlagen developing into new cirri posteriad in both proter and opisthe and the dividing micronucleus, respectively, ellipses encircle the newly formed frontal cirri of the proter and opisthe. 1–3, dorsal kineties anlagen 1–3; L1, 3, inner and outer left marginal rows; OP, oral primordium; PM, paroral membrane; R1, 2, inner and outer right marginal row 1, 2; Ⅰ–Ⅳ, frontal ventral cirral anlagen Ⅰ–Ⅳ. Scale bars=30 μm.
Figure 7.
Morphogenesis photomicrographs of Perisincirra paucicirrata after protargol staining. A Ventral view of a very early divider to show the newly formed oral primordium between right and left cirral rows in opisthe. B, C With the proliferation of basal bodies, the oral primordium of the opisthe lengthens. D, E Ventral views of early dividers, arrow in (D) denotes the newly formed adoral membranes at the anterior end of OP and arrowheads in (D, E) point to the anlage II fosrmed to the right anterior of the OP. F, G Ventral views of early dividers, arrowheads in (F) indicate replication bands and arrows in (F, G) denote undulating membranes anlage (anlage I). H, I Ventral views of early dividers, arrowheads mark incompletely formed adoral zone of membranes, in this stage, anlagen Ⅰ–Ⅲ of the opisthe are formed from the OP. J Ventral view of an early divider, arrow and arrowhead mark the dedifferentiation of buccal cirrus and anterior parabuccal cirrus. K–M Ventral views of early dividers, double-arrowhead indicates the anterior part of paroral disorganise and develop into anlage I of the proter, arrow and arrowhead denote anlagen Ⅱ and Ⅲ of the proter, respectively, hollow arrow demonstrates anlage Ⅲ begins to split into anlagen Ⅲ and Ⅳ. N Ventral view of early divider, showing anlage Ⅳ of the opisthe migrates rightward, arrowhead in (N) marks the dedifferentiation of posterior parabuccal cirrus, arrows point to marginal rows anlagen, in this stage cirral anlagen begin to develop into new cirri. O Ventral view of an early divider, arrowhead denotes the new left frontal cirrus and arrows indicate marginal rows anlagen, in this stage, anlagen Ⅰ–Ⅳ appear in both proter and opisthe. P Dorsal view of an early divider, numbers mark dorsal kineties anlagen 1–3 forming within each dorsal kinety and arrowheads demonstrate the parental caudal cirri. Q Dorsal view of an early to middle divider, numbers mark dorsal kineties anlagen 1–3 formed within old structures, arrow denotes the dividing micronucleus and arrowheads point to the old caudal cirri. R Ventral view of an early to middle divider, arrows and arrowhead indicate marginal rows anlagen developing into new cirri posteriad in both proter and opisthe and the dividing micronucleus, respectively, ellipses encircle the newly formed frontal cirri of the proter and opisthe. 1–3, dorsal kineties anlagen 1–3; L1, 3, inner and outer left marginal rows; OP, oral primordium; PM, paroral membrane; R1, 2, inner and outer right marginal row 1, 2; Ⅰ–Ⅳ, frontal ventral cirral anlagen Ⅰ–Ⅳ. Scale bars=30 μm.
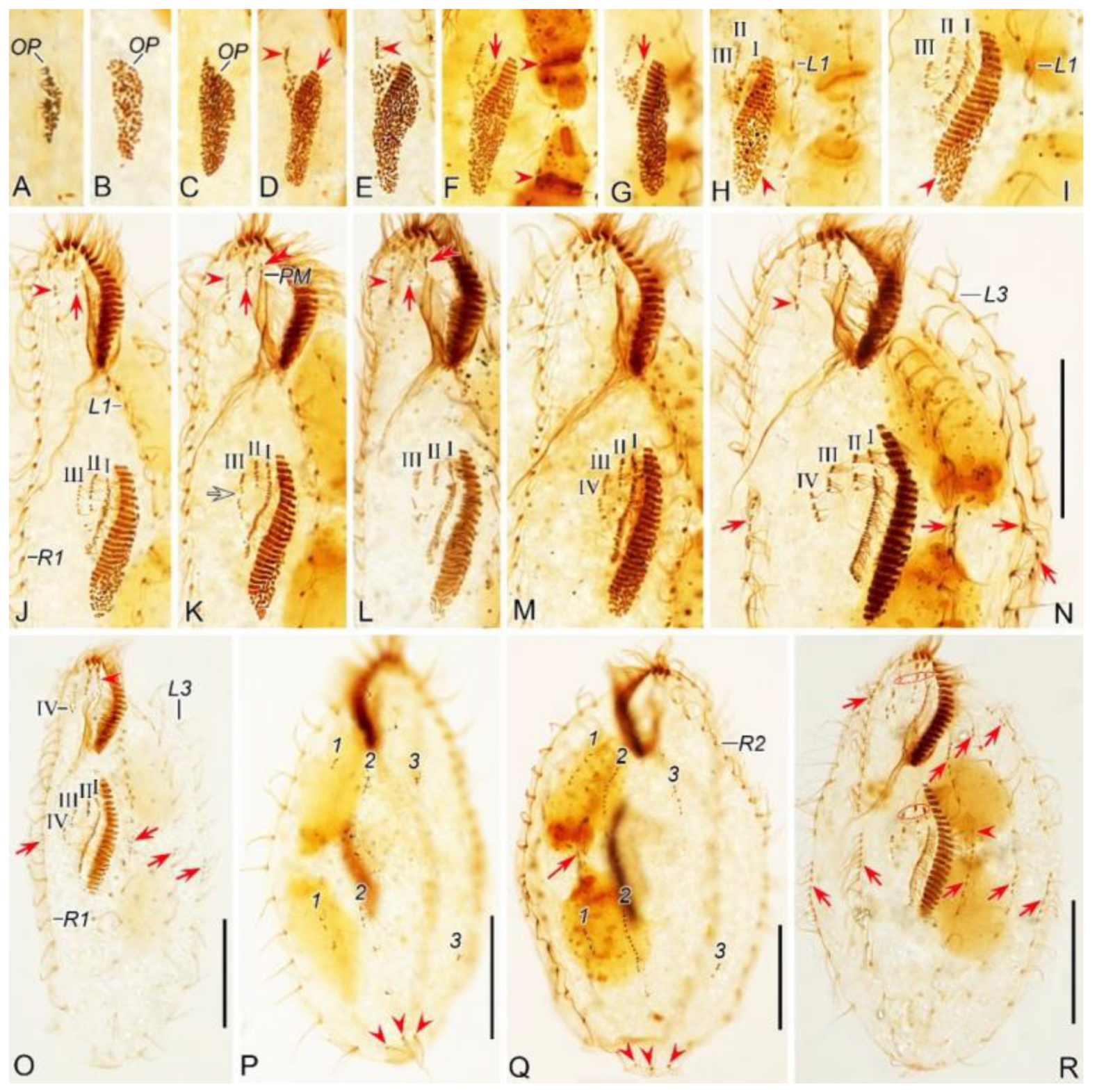
Figure 8.
Morphogenesis of Perisincirra paucicirrata after protargol staining. A Ventral view of a very early divider to show the newly formed oral primordium between right and left cirral rows in opisthe. B, C With the proliferation of basal bodies, the oral primordium of the opisthe lengthens. D, E Ventral views of early dividers, arrow in (D) denotes the newly formed adoral membranes at the anterior end of OP and arrowheads in (D, E) point to anlage II formed to the right anterior of the OP. F, G Ventral views of early dividers, arrowheads in (F) indicate replication bands and arrows in (F, G) denote undulating membranes anlage (anlage I). H, I Ventral views of early dividers, arrowheads mark incompletely formed adoral zone of membranes, in this stage, anlagen Ⅰ–Ⅲ of the opisthe are formed from the OP. J Ventral view of an early divider, arrow and arrowhead mark the dedifferentiation of buccal cirrus and anterior parabuccal cirrus. K–M Ventral views of early dividers, double-arrowhead indicates the anterior part of paroral disorganise and develop into anlage I of the proter, arrow and arrowhead denote anlagen Ⅱ and Ⅲ of the proter, respectively, hollow arrow demonstrates anlage Ⅲ begins to split into anlagen Ⅲ and Ⅳ. N Ventral views of early divider, showing anlage Ⅳ of the opisthe migrates rightward, arrowhead in (N) marks the dedifferentiation of posterior parabuccal cirrus, arrows point to marginal rows anlagen, in this stage, cirral anlagen begin to develop into new cirri. O Ventral view of an early divider, arrowhead denotes the new left frontal cirrus and arrows indicate marginal rows anlagen, in this stage, anlagen Ⅰ–Ⅳ appear in both proter and opisthe. P Dorsal view of an early divider, numbers mark dorsal kineties anlagen 1–3 forming within each dorsal kinety and arrowheads demonstrate the parental caudal cirri. Q Dorsal view of an early to middle divider, numbers mark dorsal kineties anlagen 1–3 formed within old structures, arrowheads point to the old caudal cirri. R Ventral view of an early to middle divider, arrows indicate marginal rows anlagen developing into new cirri posteriad in both proter and opisthe, ellipses encircle the newly formed frontal cirri of the proter and opisthe. 1–3, dorsal kineties anlagen 1–3; L1–3, inner, middle and outer left marginal rows; OP, oral primordium; PM, paroral membrane; R1, 2, inner and outer right marginal row 1, 2; Ⅰ–Ⅳ, frontal ventral cirral anlagen Ⅰ–Ⅳ. Scale bars=30 μm.
Figure 8.
Morphogenesis of Perisincirra paucicirrata after protargol staining. A Ventral view of a very early divider to show the newly formed oral primordium between right and left cirral rows in opisthe. B, C With the proliferation of basal bodies, the oral primordium of the opisthe lengthens. D, E Ventral views of early dividers, arrow in (D) denotes the newly formed adoral membranes at the anterior end of OP and arrowheads in (D, E) point to anlage II formed to the right anterior of the OP. F, G Ventral views of early dividers, arrowheads in (F) indicate replication bands and arrows in (F, G) denote undulating membranes anlage (anlage I). H, I Ventral views of early dividers, arrowheads mark incompletely formed adoral zone of membranes, in this stage, anlagen Ⅰ–Ⅲ of the opisthe are formed from the OP. J Ventral view of an early divider, arrow and arrowhead mark the dedifferentiation of buccal cirrus and anterior parabuccal cirrus. K–M Ventral views of early dividers, double-arrowhead indicates the anterior part of paroral disorganise and develop into anlage I of the proter, arrow and arrowhead denote anlagen Ⅱ and Ⅲ of the proter, respectively, hollow arrow demonstrates anlage Ⅲ begins to split into anlagen Ⅲ and Ⅳ. N Ventral views of early divider, showing anlage Ⅳ of the opisthe migrates rightward, arrowhead in (N) marks the dedifferentiation of posterior parabuccal cirrus, arrows point to marginal rows anlagen, in this stage, cirral anlagen begin to develop into new cirri. O Ventral view of an early divider, arrowhead denotes the new left frontal cirrus and arrows indicate marginal rows anlagen, in this stage, anlagen Ⅰ–Ⅳ appear in both proter and opisthe. P Dorsal view of an early divider, numbers mark dorsal kineties anlagen 1–3 forming within each dorsal kinety and arrowheads demonstrate the parental caudal cirri. Q Dorsal view of an early to middle divider, numbers mark dorsal kineties anlagen 1–3 formed within old structures, arrowheads point to the old caudal cirri. R Ventral view of an early to middle divider, arrows indicate marginal rows anlagen developing into new cirri posteriad in both proter and opisthe, ellipses encircle the newly formed frontal cirri of the proter and opisthe. 1–3, dorsal kineties anlagen 1–3; L1–3, inner, middle and outer left marginal rows; OP, oral primordium; PM, paroral membrane; R1, 2, inner and outer right marginal row 1, 2; Ⅰ–Ⅳ, frontal ventral cirral anlagen Ⅰ–Ⅳ. Scale bars=30 μm.
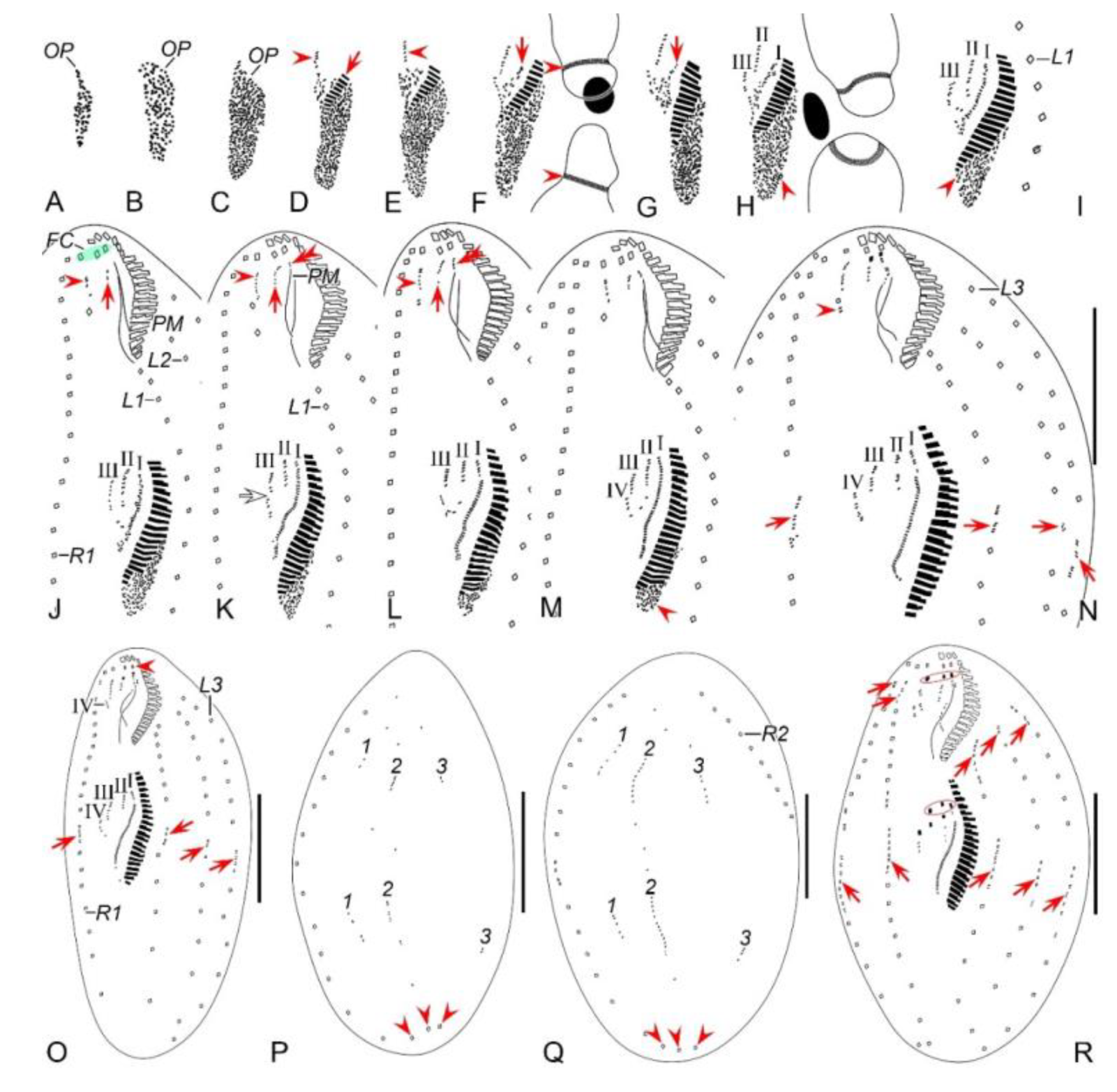
Figure 9.
Morphogenetic photomicrographs of Perisincirra paucicirrata after protargol staining. A Ventral view of an early-middle divider, arrows mark buccal cirrus of the proter and opisthe, arrowhead indicates the dividing micronucleus, in this stage, the macronuclear nodules fuse into a single mass. B Ventral view of a middle divider, showing anlagen I–IV differentiating into cirri, arrows point to the completion of development of marginal rows anlagen and arrowhead indicates the dividing micronucleus. C, D Ventral views of middle dividers, arrowheads markbuccal cirrus, arrows indicate anlage IV and the cirrus from it, which will be resorbed later, ellipses encircle parabuccal cirri formed from anlagen Ⅲ and IV. E, F Dorsal (E) and ventral (F) views of the same late-middle divider, arrowheads in (E) showing t the new caudal cirri formed at the rear end of dorsal kineties anlagen, ellipse encircles the parental caudal cirri, arrows in (F) indicate the parabuccal cirri formed from the anlage IV, arrowheads in (F) mark the parental frontal cirri, in this stage, the parental left frontal cirrus resorbed. G Ventral view of a late-middle divider, arrows indicate the parabuccal cirri formed from the anlage IV,arrowhead demonstrates the right parental frontal cirrus, up to this stage, the left and middle parental frontal cirri resorbed. H Ventral view of a late divider, showing the posterior parabuccal cirrus of the proter and opisthe migrate leftward and appear below the anterior parabuccal cirrus (arrowhead), arrows denote micronuclei, in this stage, the micronucleus divides into two mitotically and distributes to the proter and the opisthe; the macronuclear mass is ready for the first round of division. I, J Ventral (I) and dorsal (J) views of the same late divider, arrowheads in (I) mark the posterior parabuccal cirrus of the proter and opisthe; arrowheads in (J) indicate the caudal cirri for the proter and opisthe, ellipse encircles the parental caudal cirri, in this stage, the macronuclear nodule completes its first division. 1–3, dorsal kineties anlagen 1–3; FC, frontal cirri; LA3, outer left marginal row anlage; Ma, macronuclear nodules; RA2, outer right marginal row anlage. Scale bars=30 μm.
Figure 9.
Morphogenetic photomicrographs of Perisincirra paucicirrata after protargol staining. A Ventral view of an early-middle divider, arrows mark buccal cirrus of the proter and opisthe, arrowhead indicates the dividing micronucleus, in this stage, the macronuclear nodules fuse into a single mass. B Ventral view of a middle divider, showing anlagen I–IV differentiating into cirri, arrows point to the completion of development of marginal rows anlagen and arrowhead indicates the dividing micronucleus. C, D Ventral views of middle dividers, arrowheads markbuccal cirrus, arrows indicate anlage IV and the cirrus from it, which will be resorbed later, ellipses encircle parabuccal cirri formed from anlagen Ⅲ and IV. E, F Dorsal (E) and ventral (F) views of the same late-middle divider, arrowheads in (E) showing t the new caudal cirri formed at the rear end of dorsal kineties anlagen, ellipse encircles the parental caudal cirri, arrows in (F) indicate the parabuccal cirri formed from the anlage IV, arrowheads in (F) mark the parental frontal cirri, in this stage, the parental left frontal cirrus resorbed. G Ventral view of a late-middle divider, arrows indicate the parabuccal cirri formed from the anlage IV,arrowhead demonstrates the right parental frontal cirrus, up to this stage, the left and middle parental frontal cirri resorbed. H Ventral view of a late divider, showing the posterior parabuccal cirrus of the proter and opisthe migrate leftward and appear below the anterior parabuccal cirrus (arrowhead), arrows denote micronuclei, in this stage, the micronucleus divides into two mitotically and distributes to the proter and the opisthe; the macronuclear mass is ready for the first round of division. I, J Ventral (I) and dorsal (J) views of the same late divider, arrowheads in (I) mark the posterior parabuccal cirrus of the proter and opisthe; arrowheads in (J) indicate the caudal cirri for the proter and opisthe, ellipse encircles the parental caudal cirri, in this stage, the macronuclear nodule completes its first division. 1–3, dorsal kineties anlagen 1–3; FC, frontal cirri; LA3, outer left marginal row anlage; Ma, macronuclear nodules; RA2, outer right marginal row anlage. Scale bars=30 μm.
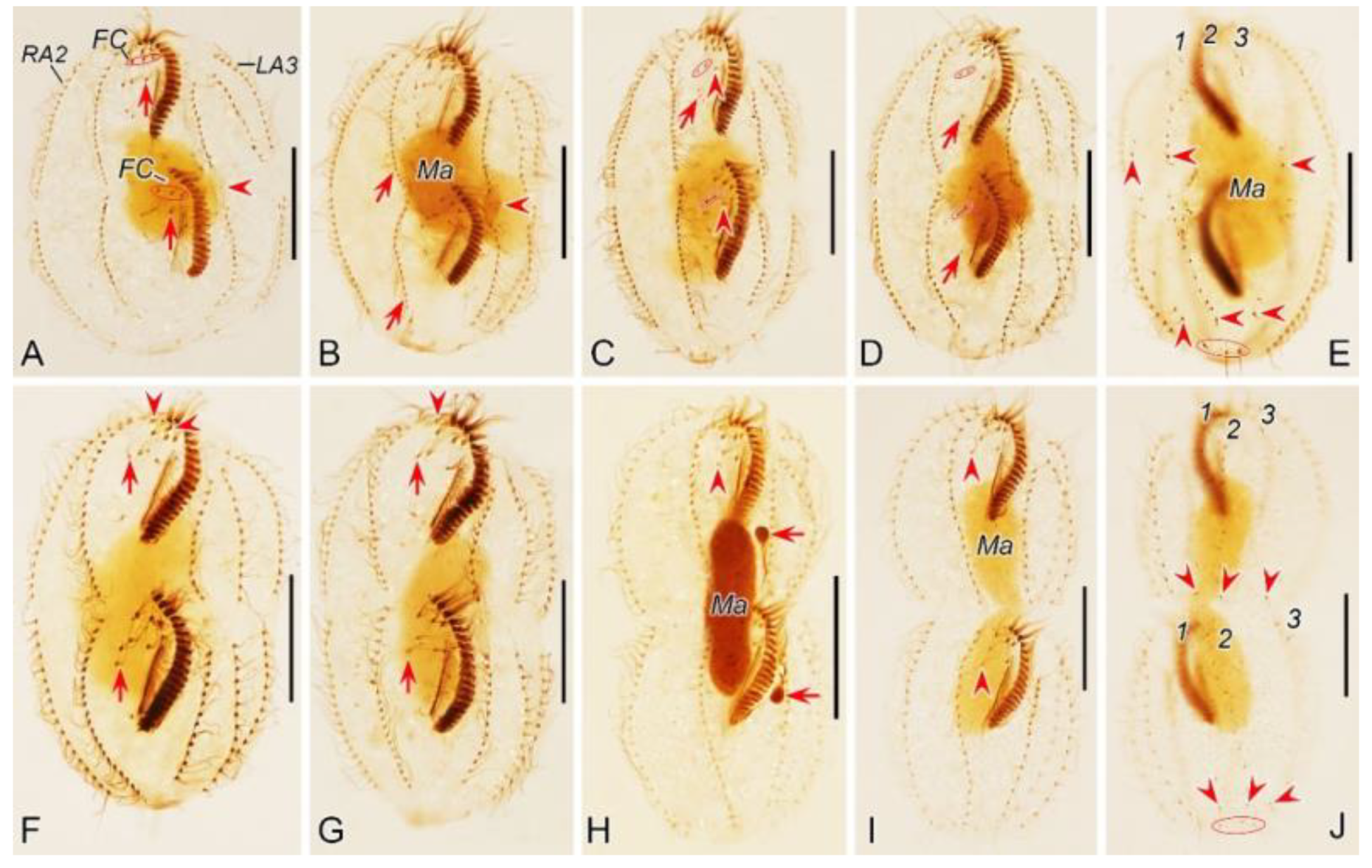
Figure 10.
Middle to late morphogenetic stages of Perisincirra paucicirrata after protargol staining. A Ventral view of an early-middle divider, arrows mark buccal cirrus of the proter and opisthe, arrowhead indicates the dividing micronucleus, in this stage, the macronuclear nodules fuse into a single mass. B Ventral view of a middle divider, showing anlagen I–IV differentiating into cirri, arrows point to completion of development of marginal rows anlagen and arrowhead indicates the dividing micronucleus. C, D Ventral views of middle dividers, arrowheads mark buccal cirrus, arrows indicate anlage IV and the cirrusfrom it, which will be resorbed later, ellipses encircle parabuccal cirri formed from anlagen Ⅲ and IV. E, F Dorsal (E) and ventral (F) views of the same late-middle divider, arrowheads in (E) showing the new caudal cirri formed at the rear end of dorsal kineties anlagen, ellipse encircles the parental caudal cirri, arrows in (F) indicate the parabuccal cirri formed from the anlage IV, arrowheads in (F) mark the parental frontal cirri, in this stage, the parental left frontal cirrus resorbed. G Ventral view of a late-middle divider, arrows indicate the parabuccal cirri formed from the anlage IV, arrowhead demonstrates the right parental cirrus, up to this stage, the left and middle parental frontal cirri resorbed. H Ventral view of a late divider, showing the posterior parabuccal cirrus of the proter and opisthe migrate leftward and appear below the anterior parabuccal cirrus (arrowhead), arrows denote micronuclei, in this stage, the micronucleus divides into two mitotically and distributes to the proter and the opisthe; the macronuclear mass is ready for the first round of division. I, J Ventral (I) and dorsal (J) views of the same late divider, arrowheads in (I) mark the posterior parabuccal cirrus of the proter and opisthe, arrows point to micronuclei; arrowheads in (J) indicate the caudal cirri for the proter and opisthe, ellipse encircles the parental caudal cirri, in this stage, the macronuclear nodule completes its first division. 1–3, dorsal kineties anlagen 1–3; FC, frontal cirri; LA3, outer left marginal row anlage; Ma, macronuclear nodules; RA2, outer right marginal row anlage. Scale bars=30 μm.
Figure 10.
Middle to late morphogenetic stages of Perisincirra paucicirrata after protargol staining. A Ventral view of an early-middle divider, arrows mark buccal cirrus of the proter and opisthe, arrowhead indicates the dividing micronucleus, in this stage, the macronuclear nodules fuse into a single mass. B Ventral view of a middle divider, showing anlagen I–IV differentiating into cirri, arrows point to completion of development of marginal rows anlagen and arrowhead indicates the dividing micronucleus. C, D Ventral views of middle dividers, arrowheads mark buccal cirrus, arrows indicate anlage IV and the cirrusfrom it, which will be resorbed later, ellipses encircle parabuccal cirri formed from anlagen Ⅲ and IV. E, F Dorsal (E) and ventral (F) views of the same late-middle divider, arrowheads in (E) showing the new caudal cirri formed at the rear end of dorsal kineties anlagen, ellipse encircles the parental caudal cirri, arrows in (F) indicate the parabuccal cirri formed from the anlage IV, arrowheads in (F) mark the parental frontal cirri, in this stage, the parental left frontal cirrus resorbed. G Ventral view of a late-middle divider, arrows indicate the parabuccal cirri formed from the anlage IV, arrowhead demonstrates the right parental cirrus, up to this stage, the left and middle parental frontal cirri resorbed. H Ventral view of a late divider, showing the posterior parabuccal cirrus of the proter and opisthe migrate leftward and appear below the anterior parabuccal cirrus (arrowhead), arrows denote micronuclei, in this stage, the micronucleus divides into two mitotically and distributes to the proter and the opisthe; the macronuclear mass is ready for the first round of division. I, J Ventral (I) and dorsal (J) views of the same late divider, arrowheads in (I) mark the posterior parabuccal cirrus of the proter and opisthe, arrows point to micronuclei; arrowheads in (J) indicate the caudal cirri for the proter and opisthe, ellipse encircles the parental caudal cirri, in this stage, the macronuclear nodule completes its first division. 1–3, dorsal kineties anlagen 1–3; FC, frontal cirri; LA3, outer left marginal row anlage; Ma, macronuclear nodules; RA2, outer right marginal row anlage. Scale bars=30 μm.
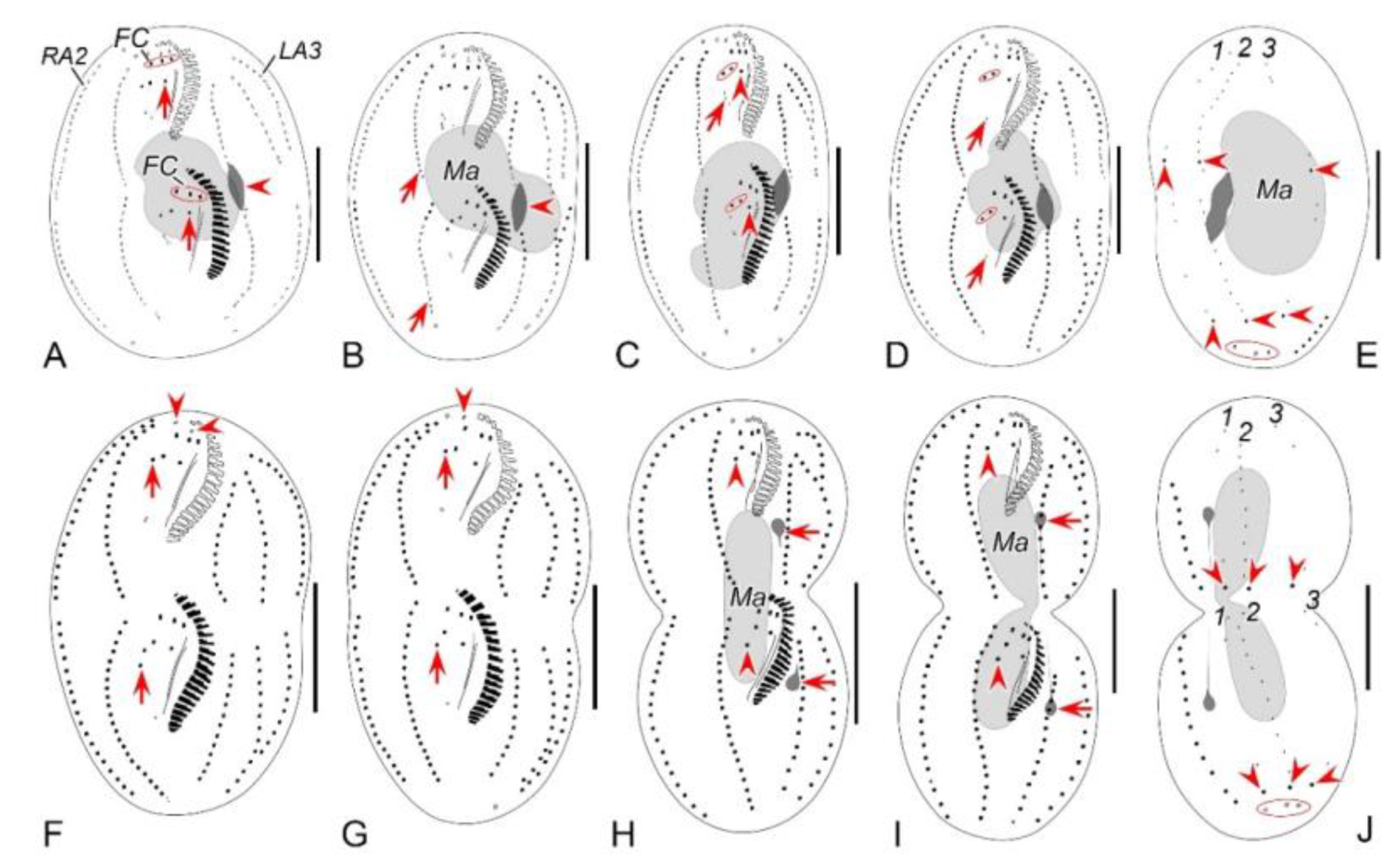
Figure 11.
Maximum likelihood (ML) tree based on the SSU rRNA gene sequences showing the positions of the two newly sequenced species (in red). Numbers at the nodes represent the ML bootstrap support and BI posterior probability values. Fully supported (100/1.00) branches are marked with filled circles. Symbol “Asterisks” indicate disagreement between the BI tree and the reference ML tree. All branches are drawn to scale. The scale bar corresponds to one substitution per 100 nucleotide positions.
Figure 11.
Maximum likelihood (ML) tree based on the SSU rRNA gene sequences showing the positions of the two newly sequenced species (in red). Numbers at the nodes represent the ML bootstrap support and BI posterior probability values. Fully supported (100/1.00) branches are marked with filled circles. Symbol “Asterisks” indicate disagreement between the BI tree and the reference ML tree. All branches are drawn to scale. The scale bar corresponds to one substitution per 100 nucleotide positions.
Table 1.
Morphometric characterization of Pseudosincirra binaria sp. nov.
Table 1.
Morphometric characterization of Pseudosincirra binaria sp. nov.
| Character |
Min |
Max |
Mean |
M |
SD |
CV |
n |
| Body length |
145 |
211 |
174 |
170 |
19.7 |
11.3 |
25 |
| Body width |
42 |
131 |
78 |
75 |
20.6 |
26.3 |
25 |
| Body length:body width, ratio |
1.5 |
3.8 |
2.3 |
2.3 |
0.5 |
21.8 |
25 |
| Adoral zone length |
35 |
48 |
41.4 |
41.3 |
3.2 |
7.7 |
25 |
| Adoral zone length: body length, ratio (%) |
19 |
32 |
24 |
24.3 |
3.0 |
12.4 |
25 |
| Adoral membranelles, number |
20 |
25 |
23 |
23 |
1.2 |
5.1 |
25 |
| Macronuclear nodules, number |
4 |
4 |
4.0 |
4 |
0 |
0 |
25 |
| Macronuclear nodules, length |
12 |
18 |
14.1 |
14.0 |
1.6 |
11.7 |
25 |
| Macronuclear nodules, width |
5 |
9 |
6.8 |
6.4 |
1.1 |
16.3 |
25 |
| Micronuclei, number |
1 |
2 |
2.0 |
2 |
0.2 |
10.2 |
25 |
| Micronuclei, diameter |
4 |
6 |
4.7 |
4.7 |
0.5 |
9.7 |
25 |
| Frontal cirri, number |
3 |
3 |
3 |
3 |
0 |
0 |
25 |
| Buccal cirrus, number |
1 |
1 |
1 |
1 |
0 |
0 |
25 |
| Parabuccal cirri, number |
1 |
1 |
1 |
1 |
0 |
0 |
25 |
| Frontoventral row, number |
1 |
1 |
1 |
1 |
0 |
0 |
25 |
| Frontoventral cirri, number |
15 |
21 |
18 |
18 |
1.6 |
8.6 |
25 |
| Left marginal rows, number |
3 |
3 |
3 |
3 |
0 |
0 |
25 |
| Cirri in L1, number |
14 |
19 |
16.7 |
17 |
1.5 |
8.8 |
25 |
| Cirri in L2, number |
14 |
18 |
16.2 |
16 |
1.1 |
7.0 |
25 |
| Cirri in L3, number |
14 |
19 |
16.5 |
17 |
1.3 |
8.0 |
25 |
| Right marginal rows, number |
2 |
2 |
2 |
2 |
0 |
0 |
25 |
| Cirri in R1, number |
20 |
26 |
23.2 |
23 |
1.7 |
7.4 |
25 |
| Cirri in R2, number |
21 |
26 |
23 |
23 |
1.5 |
6.6 |
25 |
| Dorsal kineties, number |
3 |
3 |
3 |
3 |
0 |
0 |
25 |
| Dikinetids in anterior DK1, number |
4 |
6 |
5.2 |
5 |
0.7 |
13.3 |
25 |
| Dikinetids in posterior DK1, number |
3 |
5 |
3.8 |
4 |
0.5 |
13.9 |
25 |
| Dikinetids in DK1, number |
7 |
10 |
8.9 |
9 |
1.0 |
10.7 |
25 |
| Dikinetids in DK2, number |
16 |
20 |
17.7 |
18 |
1.4 |
7.8 |
25 |
| Dikinetids in DK3, number |
12 |
16 |
13.6 |
13 |
1.2 |
8.8 |
25 |
| Caudal cirri, number |
3 |
3 |
3 |
3 |
0 |
0 |
25 |
Table 2.
Morphometric characterization of Perisincirra paucicirrata.
Table 2.
Morphometric characterization of Perisincirra paucicirrata.
| Character |
Min |
Max |
Mean |
M |
SD |
CV |
n |
| Body length |
75 |
135 |
105 |
108 |
16.2 |
15.4 |
21 |
| Body width |
32 |
58 |
42 |
42 |
7.6 |
18.3 |
21 |
| Body length:both width, ratio |
2.2 |
3 |
2.5 |
2.5 |
0.2 |
8 |
21 |
| Length of adoral zone |
22 |
35 |
27.7 |
28.7 |
3.3 |
11.8 |
21 |
| AZM: body length, ratio |
21 |
33 |
26.6 |
25.7 |
3.1 |
11.7 |
21 |
| Adoral membranelles, number |
18 |
20 |
19 |
19 |
0.7 |
3.5 |
21 |
| Macronuclear nodules, number |
2 |
2 |
2 |
2 |
0 |
0 |
21 |
| Macronuclear nodules, length |
19 |
36 |
27.5 |
28.1 |
5.8 |
21.2 |
21 |
| Macronuclear nodules, width |
8 |
18 |
12.4 |
11.9 |
2.9 |
23.3 |
21 |
| Micronuclei, number |
1 |
1 |
1 |
1 |
0 |
0 |
21 |
| Micronuclei, diameter |
4 |
7 |
5 |
5.2 |
1.0 |
20.2 |
21 |
| Frontal cirri, number |
3 |
3 |
3 |
3 |
0 |
0 |
21 |
| Buccal cirrus, number |
1 |
1 |
1 |
1 |
0 |
0 |
21 |
| Parabuccal cirri, number |
2 |
2 |
2 |
2 |
0 |
0 |
21 |
| Left marginal rows, number |
3 |
3 |
3 |
3 |
0 |
0 |
21 |
| Cirri in L1, number |
12 |
16 |
13.5 |
13 |
1.0 |
7.6 |
21 |
| Cirri in L2, number |
11 |
16 |
12.9 |
13 |
1.2 |
9.3 |
21 |
| Cirri in L3, number |
11 |
18 |
14 |
14 |
1.7 |
12.0 |
21 |
| Right marginal rows, number |
2 |
2 |
2 |
2 |
0 |
0 |
21 |
| Cirri in R1, number |
18 |
23 |
20.3 |
20 |
1.4 |
7.0 |
21 |
| Cirri in R2, number |
17 |
22 |
19.2 |
19 |
1.5 |
8 |
21 |
| Dorsal kineties, number |
3 |
3 |
3 |
3 |
0 |
0 |
21 |
| Dikinetids in DK 1, number |
2 |
4 |
3.3 |
3 |
0.6 |
17.1 |
21 |
| Dikinetids in DK 2, number |
8 |
11 |
9.8 |
10 |
0.9 |
9.1 |
21 |
| Dikinetids in DK 3, number |
3 |
4 |
3.3 |
3 |
0.5 |
14.1 |
21 |
| Caudal cirri, number |
3 |
3 |
3 |
3 |
0 |
0 |
21 |
Table 3.
Comparison of Qingdao population of Perisincirra paucicirrata with other populations and congener.
Table 3.
Comparison of Qingdao population of Perisincirra paucicirrata with other populations and congener.
| Characters |
P. paucicirrata |
P. paucicirrata |
P. paucicirrata |
P. kahli |
| Body length, in vivo |
75–125 |
60–110 |
70–130 |
85–160 |
| Body length:width, ratio |
4.7:1 |
4:1 |
5:1 |
10–15:1 |
| Frontal cirri, number |
3 |
3 |
3 |
3 |
| Ma, number |
2 |
2 |
2 |
2 |
| Mi, number |
1 |
1 |
1 |
2 |
| AM, number |
18–20 |
16–19 |
13–17 |
18–20* |
| Parabuccal cirri, number |
2 |
1–3 |
1–3 |
– |
Right marginal rows, number
Cirri in RMR 1, number |
2
18–23 |
2
15–24 |
2
5–12 |
2
– |
| Cirri in RMR 2, number |
17–22 |
14–23 |
4–8 |
18–20 |
| Left marginal rows, number |
3 |
3–4 |
2 |
2 |
| Cirri in LMR 1, number |
12–16 |
11–15 |
3–9 |
|
| Cirri in LMR 2, number |
11–16 |
10–16 |
5–8 |
16–20 |
| Cirri in LMR 3, number |
11–18 |
11–17 |
– |
– |
| Dorsal kineties, number |
3 |
3 |
3 |
3 |
| Caudal cirri, number |
3 |
3 |
3 |
– |
| Data source |
present study |
Li et al. (2013) |
Foissner et al.
(2002); Berger (2011) |
Berger
(2011) |