Submitted:
23 August 2024
Posted:
26 August 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Methodology
Vascular Anatomy of the Cornea and Ocular Surface
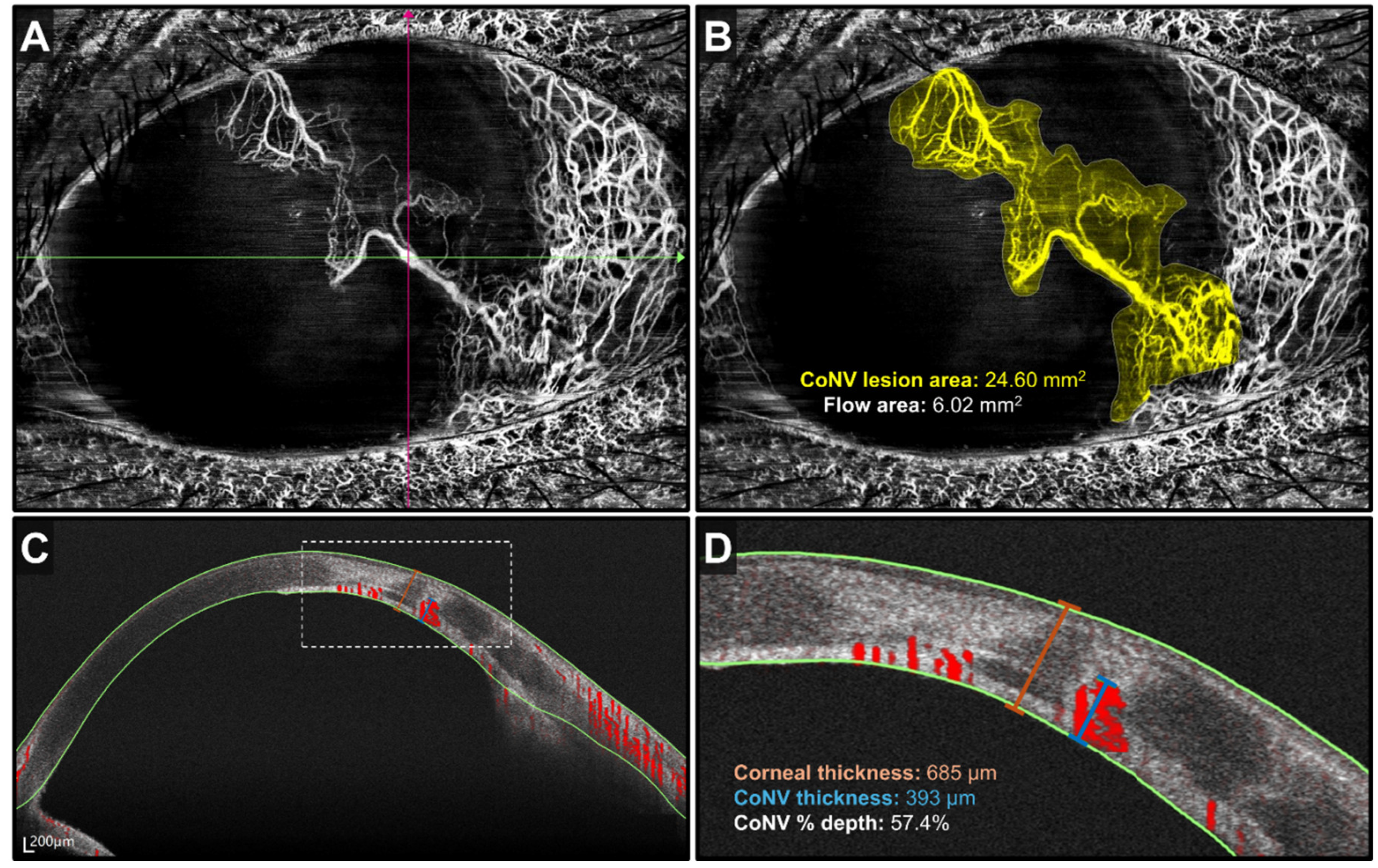
Optical Coherence Tomography Angiography of the Cornea
Optical Coherence Tomography Angiography of the Ocular Surface
Optical Coherence Tomography Angiography of the Conjunctiva, Episclera and Sclera
Limitations and Future Development
Conclusion
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, W.D.; Devarajan, K.; Chua, J.; Schmetterer, L.; Mehta, J.S.; Ang, M. Optical Coherence Tomography Angiography for the Anterior Segment. Eye and Vision 2019, 6, 1–9. [Google Scholar]
- Ang, M.; Baskaran, M.; Werkmeister, R.M.; Chua, J.; Schmidl, D.; Aranha Dos Santos, V.; Garhöfer, G.; Mehta, J.S.; Schmetterer, L. Anterior Segment Optical Coherence Tomography. Progress in Retinal and Eye Research 2018, 66, 132–156. [Google Scholar] [CrossRef]
- Grulkowski, I.; Liu, J.J.; Potsaid, B.; Jayaraman, V.; Lu, C.D.; Jiang, J.; Cable, A.E.; Duker, J.S.; Fujimoto, J.G. Retinal, Anterior Segment and Full Eye Imaging Using Ultrahigh Speed Swept Source OCT with Vertical-Cavity Surface Emitting Lasers. Biomedical optics express 2012, 3, 2733–2751. [Google Scholar] [CrossRef]
- Stanzel, T.P.; Devarajan, K.; Lwin, N.C.; Yam, G.H.; Schmetterer, L.; Mehta, J.S.; Ang, M. Comparison of Optical Coherence Tomography Angiography to Indocyanine Green Angiography and Slit Lamp Photography for Corneal Vascularization in an Animal Model. Scientific reports 2018, 8, 11493. [Google Scholar] [CrossRef]
- Kirwan, R.P.; Zheng, Y.; Tey, A.; Anijeet, D.; Sueke, H.; Kaye, S.B. Quantifying Changes in Corneal Neovascularization Using Fluorescein and Indocyanine Green Angiography. American journal of ophthalmology 2012, 154, 850–858. [Google Scholar] [CrossRef]
- Brunner, M.; Romano, V.; Steger, B.; Vinciguerra, R.; Lawman, S.; Williams, B.; Hicks, N.; Czanner, G.; Zheng, Y.; Willoughby, C.E. Imaging of Corneal Neovascularization: Optical Coherence Tomography Angiography and Fluorescence Angiography. Investigative Ophthalmology & Visual Science 2018, 59, 1263–1269. [Google Scholar]
- Ang, M.; Cai, Y.; MacPhee, B.; Sim, D.A.; Keane, P.A.; Sng, C.C.; Egan, C.A.; Tufail, A.; Larkin, D.F.; Wilkins, M.R. Optical Coherence Tomography Angiography and Indocyanine Green Angiography for Corneal Vascularisation. British Journal of Ophthalmology 2016, 100, 1557–1563. [Google Scholar] [CrossRef]
- Yannuzzi, L.A.; Rohrer, K.T.; Tindel, L.J.; Sobel, R.S.; Costanza, M.A.; Shields, W.; Zang, E. Fluorescein Angiography Complication Survey. Ophthalmology 1986, 93, 611–617. [Google Scholar] [CrossRef]
- de Carlo, T.E.; Romano, A.; Waheed, N.K.; Duker, J.S. A Review of Optical Coherence Tomography Angiography (OCTA). Int J Retin Vitr 2015, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Akagi, T.; Uji, A.; Huang, A.S.; Weinreb, R.N.; Yamada, T.; Miyata, M.; Kameda, T.; Ikeda, H.O.; Tsujikawa, A. Conjunctival and Intrascleral Vasculatures Assessed Using Anterior Segment Optical Coherence Tomography Angiography in Normal Eyes. American Journal of Ophthalmology 2018, 196, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Guduru, A.; Gupta, A.; Tyagi, M.; Jalali, S.; Chhablani, J. Optical Coherence Tomography Angiography Characterisation of Best Disease and Associated Choroidal Neovascularisation. Br J Ophthalmol 2018, 102, 444–447. [Google Scholar] [CrossRef]
- Sharma, S.; Ang, M.; Najjar, R.P.; Sng, C.; Cheung, C.Y.; Rukmini, A.V.; Schmetterer, L.; Milea, D. Optical Coherence Tomography Angiography in Acute Non-Arteritic Anterior Ischaemic Optic Neuropathy. British Journal of Ophthalmology 2017, 101, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Ang, M.; Devarajan, K.; Das, S.; Stanzel, T.; Tan, A.; Girard, M.; Schmetterer, L.; Mehta, J.S. Comparison of Anterior Segment Optical Coherence Tomography Angiography Systems for Corneal Vascularisation. British Journal of Ophthalmology 2018, 102, 873–877. [Google Scholar] [CrossRef]
- Tan, A.C.; Tan, G.S.; Denniston, A.K.; Keane, P.A.; Ang, M.; Milea, D.; Chakravarthy, U.; Cheung, C.M.G. An Overview of the Clinical Applications of Optical Coherence Tomography Angiography. Eye 2018, 32, 262–286. [Google Scholar] [CrossRef]
- Parrulli, S.; Corvi, F.; Cozzi, M.; Monteduro, D.; Zicarelli, F.; Staurenghi, G. Microaneurysms Visualisation Using Five Different Optical Coherence Tomography Angiography Devices Compared to Fluorescein Angiography. Br J Ophthalmol 2021, 105, 526–530. [Google Scholar] [CrossRef]
- Sridhar, M.S. Anatomy of Cornea and Ocular Surface. Indian J Ophthalmol 2018, 66, 190–194. [Google Scholar] [CrossRef] [PubMed]
- DelMonte, D.W.; Kim, T. Anatomy and Physiology of the Cornea. Journal of Cataract & Refractive Surgery 2011, 37, 588–598. [Google Scholar]
- Qazi, Y.; Wong, G.; Monson, B.; Stringham, J.; Ambati, B.K. Corneal Transparency: Genesis, Maintenance and Dysfunction. Brain Research Bulletin 2010, 81, 198–210. [Google Scholar] [CrossRef]
- Azar, D.T. Corneal Angiogenic Privilege: Angiogenic and Antiangiogenic Factors in Corneal Avascularity, Vasculogenesis, and Wound Healing (an American Ophthalmological Society Thesis). Transactions of the American Ophthalmological Society 2006, 104, 264. [Google Scholar]
- Chang, J.-H.; Gabison, E.E.; Kato, T.; Azar, D.T. Corneal Neovascularization. Current Opinion in Ophthalmology 2001, 12, 242. [Google Scholar] [CrossRef]
- Feizi, S.; Azari, A.A.; Safapour, S. Therapeutic Approaches for Corneal Neovascularization. Eye and vision 2017, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sharif, Z.; Sharif, W. Corneal Neovascularization: Updates on Pathophysiology, Investigations & Management. Romanian journal of ophthalmology 2019, 63, 15. [Google Scholar] [PubMed]
- Ang, M.; Cai, Y.; Shahipasand, S.; Sim, D.A.; Keane, P.A.; Sng, C.C.; Egan, C.A.; Tufail, A.; Wilkins, M.R. En Face Optical Coherence Tomography Angiography for Corneal Neovascularisation. British Journal of Ophthalmology 2016, 100, 616–621. [Google Scholar] [CrossRef]
- Roshandel, D.; Eslani, M.; Baradaran-Rafii, A.; Cheung, A.Y.; Kurji, K.; Jabbehdari, S.; Maiz, A.; Jalali, S.; Djalilian, A.R.; Holland, E.J. Current and Emerging Therapies for Corneal Neovascularization. The ocular surface 2018, 16, 398–414. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Shields, J.A. Tumors of the Conjunctiva and Cornea. Survey of Ophthalmology 2004, 49, 3–24. [Google Scholar] [CrossRef]
- Watson, P.G.; Young, R.D. Scleral Structure, Organisation and Disease. A Review. Experimental Eye Research 2004, 78, 609–623. [Google Scholar] [CrossRef]
- Singh, R.B.; Liu, L.; Anchouche, S.; Yung, A.; Mittal, S.K.; Blanco, T.; Dohlman, T.H.; Yin, J.; Dana, R. Ocular Redness – I: Etiology, Pathogenesis, and Assessment of Conjunctival Hyperemia. The Ocular Surface 2021, 21, 134–144. [Google Scholar] [CrossRef]
- Liu, Z.; Karp, C.L.; Galor, A.; Al Bayyat, G.J.; Jiang, H.; Wang, J. Role of Optical Coherence Tomography Angiography in the Characterization of Vascular Network Patterns of Ocular Surface Squamous Neoplasia. The ocular surface 2020, 18, 926–935. [Google Scholar] [CrossRef]
- Kiritoshi, S.; Oie, Y.; Nampei, K.; Sato, S.; Morota, M.; Nishida, K. Anterior Segment Optical Coherence Tomography Angiography in Patients Following Cultivated Oral Mucosal Epithelial Transplantation. American Journal of Ophthalmology 2019, 208, 242–250. [Google Scholar] [CrossRef]
- Aschauer, J.; Klimek, M.; Donner, R.; Lammer, J.; Roberts, P.; Schranz, M.; Schmidinger, G. Non-Invasive Quantification of Corneal Vascularization Using Anterior Segment Optical Coherence Tomography Angiography. Scientific Reports 2024, 14, 2124. [Google Scholar] [CrossRef]
- Almeida, I.; Dias, L.; Jesus, J.; Fonseca, I.; Matias, M.J.; Pedro, J.C. Optical Coherence Tomography Angiography in Herpetic Leucoma. BMC Medical Imaging 2022, 22, 17. [Google Scholar] [CrossRef]
- Nanji, A.; Redd, T.; Chamberlain, W.; Schallhorn, J.M.; Chen, S.; Ploner, S.; Maier, A.; Fujimoto, J.G.; Jia, Y.; Huang, D. Application of Corneal Optical Coherence Tomography Angiography for Assessment of Vessel Depth in Corneal Neovascularization. Cornea 2020, 39, 598–604. [Google Scholar] [CrossRef]
- Ong, H.S.; Tey, K.Y.; Ke, M.; Tan, B.; Chua, J.; Schmetterer, L.; Mehta, J.S.; Ang, M. A Pilot Study Investigating Anterior Segment Optical Coherence Tomography Angiography as a Non-Invasive Tool in Evaluating Corneal Vascularisation. Scientific Reports 2021, 11, 1212. [Google Scholar] [CrossRef] [PubMed]
- Binotti, W.W.; Koseoglu, N.D.; Nosé, R.M.; Kenyon, K.R.; Hamrah, P. Novel Parameters to Assess the Severity of Corneal Neovascularization Using Anterior Segment Optical Coherence Tomography Angiography. American Journal of Ophthalmology 2021, 222, 206–217. [Google Scholar] [CrossRef]
- Armitage, W.J.; Goodchild, C.; Griffin, M.D.; Gunn, D.J.; Hjortdal, J.; Lohan, P.; Murphy, C.C.; Pleyer, U.; Ritter, T.; Tole, D.M. High-Risk Corneal Transplantation: Recent Developments and Future Possibilities. Transplantation 2019, 103, 2468–2478. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, B.; Taylor, R.S.; Cursiefen, C. Corneal Neovascularization as a Risk Factor for Graft Failure and Rejection after Keratoplasty: An Evidence-Based Meta-Analysis. Ophthalmology 2010, 117, 1300–1305. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.Y.; Pan, C.T.; Feng, Y. Localization of Corneal Neovascularization Using Optical Coherence Tomography Angiography. Cornea 2019, 38, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Koenig, Y.; Bock, F.; Kruse, F.E.; Stock, K.; Cursiefen, C. Angioregressive Pretreatment of Mature Corneal Blood Vessels before Keratoplasty: Fine-Needle Vessel Coagulation Combined with Anti-VEGFs. Cornea 2012, 31, 887–892. [Google Scholar] [CrossRef]
- Hos, D.; Saban, D.R.; Bock, F.; Regenfuss, B.; Onderka, J.; Masli, S.; Cursiefen, C. Suppression of Inflammatory Corneal Lymphangiogenesis by Application of Topical Corticosteroids. Archives of Ophthalmology 2011, 129, 445–452. [Google Scholar] [CrossRef]
- Lipman, R.M.; Epstein, R.J.; Hendricks, R.L. Suppression of Corneal Neovascularization With Cyclosporine. Archives of Ophthalmology 1992, 110, 405–407. [Google Scholar] [CrossRef]
- Park, S.-C.; Kim, J.-H. Effects of Laser Photocoagulation on Corneal Neovascularization in Rabbits. Journal of Refractive Surgery 1994, 10, 631–639. [Google Scholar] [CrossRef]
- Faraj, L.A.; Elalfy, M.S.; Said, D.G.; Dua, H.S. Fine Needle Diathermy Occlusion of Corneal Vessels. British Journal of Ophthalmology 2014, 98, 1287–1290. [Google Scholar] [CrossRef] [PubMed]
- Devarajan, K.; Ong, H.S.; Lwin, N.C.; Chua, J.; Schmetterer, L.; Mehta, J.S.; Ang, M. Optical Coherence Tomography Angiography Imaging to Monitor Anti-VEGF Treatment of Corneal Vascularization in a Rabbit Model. Scientific Reports 2019, 9, 17576. [Google Scholar] [CrossRef]
- Foo, V.H.X.; Ke, M.; Tan, C.Q.L.; Schmetterer, L.; Mehta, J.S.; Ang, M. Anterior Segment Optical Coherence Tomography Angiography Assessment of Corneal Vascularisation after Combined Fine-Needle Diathermy with Subconjunctival Ranibizumab: A Pilot Study. Advances in Therapy 2021, 38, 4333–4343. [Google Scholar] [CrossRef] [PubMed]
- Ang, M.; Foo, V.; Ke, M.; Tan, B.; Tong, L.; Schmetterer, L.; Mehta, J.S. Role of Anterior Segment Optical Coherence Tomography Angiography in Assessing Limbal Vasculature in Acute Chemical Injury of the Eye. British Journal of Ophthalmology 2022, 106, 1212–1216. [Google Scholar] [CrossRef] [PubMed]
- Binotti, W.W.; Nosé, R.M.; Koseoglu, N.D.; Dieckmann, G.M.; Kenyon, K.; Hamrah, P. The Utility of Anterior Segment Optical Coherence Tomography Angiography for the Assessment of Limbal Stem Cell Deficiency. Ocul Surf 2021, 19, 94–103. [Google Scholar] [CrossRef]
- Fatima, A.; Iftekhar, G.; Sangwan, V.S.; Vemuganti, G.K. Ocular Surface Changes in Limbal Stem Cell Deficiency Caused by Chemical Injury: A Histologic Study of Excised Pannus from Recipients of Cultured Corneal Epithelium. Eye (Lond) 2008, 22, 1161–1167. [Google Scholar] [CrossRef]
- Sauder, G.; Jonas, J.B. Limbal Stem Cell Deficiency after Subconjunctival Mitomycin C Injection for Trabeculectomy. Am J Ophthalmol 2006, 141, 1129–1130. [Google Scholar] [CrossRef]
- Arafat, S.N.; Suelves, A.M.; Spurr-Michaud, S.; Chodosh, J.; Foster, C.S.; Dohlman, C.H.; Gipson, I.K. Neutrophil Collagenase, Gelatinase and Myeloperoxidase in Tears of Stevens-Johnson Syndrome and Ocular Cicatricial Pemphigoid Patients. Ophthalmology 2014, 121, 79–87. [Google Scholar] [CrossRef]
- Steger, B. Ocular Surface Angiography: From Neovessels to Neoplasia. BMJ open ophthalmology 2021, 6, e000829. [Google Scholar] [CrossRef]
- Clare, G.; Suleman, H.; Bunce, C.; Dua, H. Amniotic Membrane Transplantation for Acute Ocular Burns. Cochrane database of systematic reviews 2012. [Google Scholar] [CrossRef] [PubMed]
- Cozzi, M.; Staurenghi, G.; Invernizzi, A. Anterior Segment and Ocular Adnexa OCT Angiography. Ophthalmology 2020, 127, 220. [Google Scholar] [CrossRef] [PubMed]
- Dua, H.S.; King, A.J.; Joseph, A. A New Classification of Ocular Surface Burns. British Journal of Ophthalmology 2001, 85, 1379–1383. [Google Scholar] [CrossRef] [PubMed]
- Fung, S.S.M.; Stewart, R.M.K.; Dhallu, S.K.; Sim, D.A.; Keane, P.A.; Wilkins, M.R.; Tuft, S.J. Anterior Segment Optical Coherence Tomographic Angiography Assessment of Acute Chemical Injury. Am J Ophthalmol 2019, 205, 165–174. [Google Scholar] [CrossRef]
- Yang, Q.-C.; Yao, F.; Li, Q.-Y.; Chen, M.-J.; Zhang, L.-J.; Shu, H.-Y.; Liang, R.-B.; Pan, Y.-C.; Ge, Q.-M.; Shao, Y. Ocular Microvascular Alteration in Sjögren Syndrome. Quantitative Imaging in Medicine and Surgery 2022, 12, 1324. [Google Scholar] [CrossRef] [PubMed]
- Cui, T.; Sun, H.; Hu, Z.; Shi, Y.; Zhu, J.; Jin, M.; Qin, B. Optical Coherence Tomography Angiography for Evaluation of Conjunctival Vessels in Dry Eyes. Journal of Ophthalmology 2023, 2023. [Google Scholar] [CrossRef]
- Lee, W.H.; Lim, H.-B.; Kim, J.; Ryu, C.K.; Shin, Y.-I.; Kim, J.-Y. REPEATABILITY OF MACULAR MICROVASCULATURE MEASUREMENTS USING OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY ACCORDING TO TEAR BREAKUP TIME IN DRY EYE DISEASE. Retina 2021, 41, 2301–2309. [Google Scholar] [CrossRef]
- Cai, S.; Zhao, F.; Du, C. Repeatability of Ocular Surface Vessel Density Measurements with Optical Coherence Tomography Angiography. BMC Ophthalmol 2019, 19, 248. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, N.J.; Marinkovic, M.; Bleeker, J.C.; Luyten, G.P.; Jager, M.J. Anterior Segment OCTA of Melanocytic Lesions of the Conjunctiva and Iris. American Journal of Ophthalmology 2021, 222, 137–147. [Google Scholar] [CrossRef]
- Mittal, R.; Rath, S.; Vemuganti, G.K. Ocular Surface Squamous Neoplasia – Review of Etio-Pathogenesis and an Update on Clinico-Pathological Diagnosis. Saudi Journal of Ophthalmology 2013, 27, 177–186. [Google Scholar] [CrossRef]
- Nampei, K.; Oie, Y.; Kiritoshi, S.; Morota, M.; Satoh, S.; Kawasaki, S.; Nishida, K. Comparison of Ocular Surface Squamous Neoplasia and Pterygium Using Anterior Segment Optical Coherence Tomography Angiography. American Journal of Ophthalmology Case Reports 2020, 20, 100902. [Google Scholar] [CrossRef] [PubMed]
- Binotti, W.W.; Mills, H.; Nosé, R.M.; Wu, H.K.; Duker, J.S.; Hamrah, P. Anterior Segment Optical Coherence Tomography Angiography in the Assessment of Ocular Surface Lesions. The Ocular Surface 2021, 22, 86–93. [Google Scholar] [CrossRef]
- Theotoka, D.; Liu, Z.; Wall, S.; Galor, A.; Al Bayyat, G.J.; Feuer, W.; Jianhua, W.; Karp, C.L. Optical Coherence Tomography Angiography in the Evaluation of Vascular Patterns of Ocular Surface Squamous Neoplasia during Topical Medical Treatment. The ocular surface 2022, 25, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Cai, S.; Huang, Z.; Ding, P.; Du, C. Optical Coherence Tomography Angiography in Pinguecula and Pterygium. Cornea 2020, 39, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Kiseleva, T.N.; Saakyan, S.V.; Makukhina, V.V.; Lugovkina, K.V.; Milash, S.V.; Musova, N.F.; Zharov, A.A. The Use of Optical Coherence Tomography Angiography in Differential Diagnosis of Conjunctival Melanocytic Tumors. Ophthalmology Reports 2023, 16, 27–37. [Google Scholar] [CrossRef]
- Zhao, Z.; Yue, Y.; Zhang, S.; Zhang, J.; Zhu, X.; Aragno, V.; Labbe, A.; Fan, X.; Yao, F. Optical Coherence Tomography Angiography for Marginal Corneal Vascular Remodelling after Pterygium Surgery with Limbal-Conjunctival Autograft. Eye (Lond) 2020, 34, 2054–2062. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Devarajan, K.; Tan, T.-E.; Ang, M.; Mehta, J.S. Optical Coherence Tomography Angiography for Evaluation of Reperfusion After Pterygium Surgery. Am J Ophthalmol 2019, 207, 151–158. [Google Scholar] [CrossRef]
- Hau, S.C.; Devarajan, K.; Ang, M. Anterior Segment Optical Coherence Tomography Angiography and Optical Coherence Tomography in the Evaluation of Episcleritis and Scleritis. Ocular Immunology and Inflammation 2021, 29, 362–369. [Google Scholar] [CrossRef]
- Okhravi, N.; Odufuwa, B.; McCluskey, P.; Lightman, S. Scleritis. Survey of ophthalmology 2005, 50, 351–363. [Google Scholar] [CrossRef]
- Salama, A.; Elsheikh, A.; Alweis, R. Is This a Worrisome Red Eye? Episcleritis in the Primary Care Setting. Journal of community hospital internal medicine perspectives 2018, 8, 46–48. [Google Scholar] [CrossRef]
- Chen, L.; Meng, L.; Sun, L.; Chen, Y. Scleral Changes in Systemic Lupus Erythematosus Patients Using Swept Source Optical Coherence Tomography. Front. Immunol. 2023, 14. [Google Scholar] [CrossRef]
- Carreño, E.; Olivas-Vergara, O.M. Systemic Vasculitis and Its Association with the Eye. Ophthalmologica 2023, 246, 174–180. [Google Scholar] [CrossRef]
- Diaz, J.D.; Sobol, E.K.; Gritz, D.C. Treatment and Management of Scleral Disorders. Survey of ophthalmology 2016, 61, 702–717. [Google Scholar] [CrossRef]
- Guex-Crosier, Y.; Durig, J. Anterior Segment Indocyanine Green Angiography in Anterior Scleritis and Episcleritis. Ophthalmology 2003, 110, 1756–1763. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.; Bovey, E. Anterior Segment Fluorescein Angiography in the Diagnosis of Scleral Inflammation. Ophthalmology 1985, 92, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Akhavanrezayat, A.; Halim, M.S.; Onghanseng, N.L.; Hassan, M.; Mahajan, S.; Uludag, G.; Ormaechea, M.S.; Tran, A.N.T.; Chea, S.; Park, J.H. Longitudinal Assessment of Patients with Anterior Scleritis Using Scleral Area Vessel Density. Investigative Ophthalmology & Visual Science 2020, 61, 4825–4825. [Google Scholar]
- Akagi, T.; Uji, A.; Okamoto, Y.; Suda, K.; Kameda, T.; Nakanishi, H.; Ikeda, H.O.; Miyake, M.; Nakano, E.; Motozawa, N.; et al. Anterior Segment Optical Coherence Tomography Angiography Imaging of Conjunctiva and Intrasclera in Treated Primary Open-Angle Glaucoma. American Journal of Ophthalmology 2019, 208, 313–322. [Google Scholar] [CrossRef]
- Gan, J.; Sng, C.C.A.; Ke, M.; Chieh, C.S.; Tan, B.; Schmetterer, L.; Ang, M. Anterior Segment Optical Coherence Tomography Angiography Following Trabecular Bypass Minimally Invasive Glaucoma Surgery. Front Med (Lausanne) 2022, 9, 830678. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Akagi, T.; Kameda, T.; Suda, K.; Miyake, M.; Ikeda, H.O.; Numa, S.; Kadomoto, S.; Uji, A.; Tsujikawa, A. Prediction of Trabecular Meshwork-Targeted Micro-Invasive Glaucoma Surgery Outcomes Using Anterior Segment OCT Angiography. Sci Rep 2021, 11, 17850. [Google Scholar] [CrossRef]
- Tey, K.Y.; Teo, K.; Tan, A.C.; Devarajan, K.; Tan, B.; Tan, J.; Schmetterer, L.; Ang, M. Optical Coherence Tomography Angiography in Diabetic Retinopathy: A Review of Current Applications. Eye and Vision 2019, 6, 1–10. [Google Scholar] [CrossRef]
- Ang, M.; Tan, A.C.; Cheung, C.M.G.; Keane, P.A.; Dolz-Marco, R.; Sng, C.C.; Schmetterer, L. Optical Coherence Tomography Angiography: A Review of Current and Future Clinical Applications. Graefe’s Archive for Clinical and Experimental Ophthalmology 2018, 256, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Ang, M.; Sim, D.A.; Keane, P.A.; Sng, C.C.A.; Egan, C.A.; Tufail, A.; Wilkins, M.R. Optical Coherence Tomography Angiography for Anterior Segment Vasculature Imaging. Ophthalmology 2015, 122, 1740–1747. [Google Scholar] [CrossRef] [PubMed]
- Tey, K.Y.; Cheong, E.Z.K.; Ang, M. Potential Applications of Artificial Intelligence in Image Analysis in Cornea Diseases: A Review. Eye and Vision 2024, 11, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Alio Del Barrio, J.L.; Wilkins, M.R.; Ang, M. Serial Optical Coherence Tomography Angiography for Corneal Vascularization. Graefes Arch Clin Exp Ophthalmol 2017, 255, 135–139. [Google Scholar] [CrossRef]
| Advantages | Disadvantages | |
| AS-OCT angiography | 1. Non-invasive and relatively time-efficient 2. Zero risks of contrast-related adverse effects 3. Does not require clinician to operate |
1. Image quality can be affected due to image, motion and projection artefacts 2. Operator-dependent |
| Traditional dye-based angiography (Fluorescein angiography and indocyanine green angiography) | 1. Ability to differentiate normal and abnormal vessels even in corneal scarring 2. Contrast leakage helps differentiate afferent and efferent vessels while providing details about vessel maturity and pathology |
1. Contrast leakage can obscure visualization of deeper vessels 2. Invasive and time-consuming 3. Risks of contrast-related adverse effects 4. Limited use in hepatic and renal impairments 5. Requires clinician to perform 6. Operator-dependent |
| Anatomical location | Pathologies | Potential Applications |
| Cornea | Corneal neovascularization (CoNV) | Diagnosis: Superficial or mid-stromal CoNV in interstitial keratitis is suggestive of varicella-zoster virus while deep CoNV suggests herpes simplex virus [31,32] Assessment: Correlation of vessel density to CoNV severity and visual acuity[33,34] Assessment/Treatment response: Pre-operative selection of vessels for FND as well as post-treatment monitoring response to FND with anti-VEGF in human eyes with CoNV and corneal scarring[44] Treatment response: Depth of corneal vascularity post PK and DALK[37] |
| Ocular Surface | Limbal stem cell deficiency | Assessment: Objective staging of limbal ischemia[46,52] |
| Dry eye disease | Assessment: Positive correlation between number of conjunctival vessels and severity of disease[55] | |
| Conjunctiva, Episclera and Sclera | Ocular surface squamous neoplasia (OSSN) | Diagnosis: Greater diameter and peri-lesional vessel depth and diameter in malignant lesions compared to benign lesions[62] Diagnosis: Differentiation from other lesions such as pterygium (“zigzag vessels” in both the superficial and deep layers in OSSN and “straight vessels” in the superficial layer in pterygium)[61] Treatment response: Decreased subepithelial vessel area density post-treatment with topical immunotherapy or chemotherapy[63] |
| Pterygium and conjunctival autografts | Treatment response: Inverse correlation between postoperative thickness and revascularization of autograft[66,67] | |
| Episcleritis and Scleritis | Diagnosis: Greater vessel density index in scleritis compared to episcleritis[68] Assessment: Positive correlation between vessel density and severity of scleritis[76] |
|
| Glaucoma | Treatment response: Decreased episcleral vessel density post-MIGS[79] Treatment response: Lower IOP in hypovascularized conjunctival blebs compared to hypervascularized blebs post-trabeculectomy[76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





