Submitted:
22 August 2024
Posted:
26 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
- The presence of genes coding for microcystin, nodularin, cylindrospermopsin and saxitoxin.
- The presence of the anatoxin-a/homoanatoxin-a gene cluster by bioinformatic tools.
- The presence of BMAA.
2. Results
2.1. The Cyanotoxins Microcystin, Nodularin, Saxitoxin, Cylindrospermopsin and Anatoxin-a
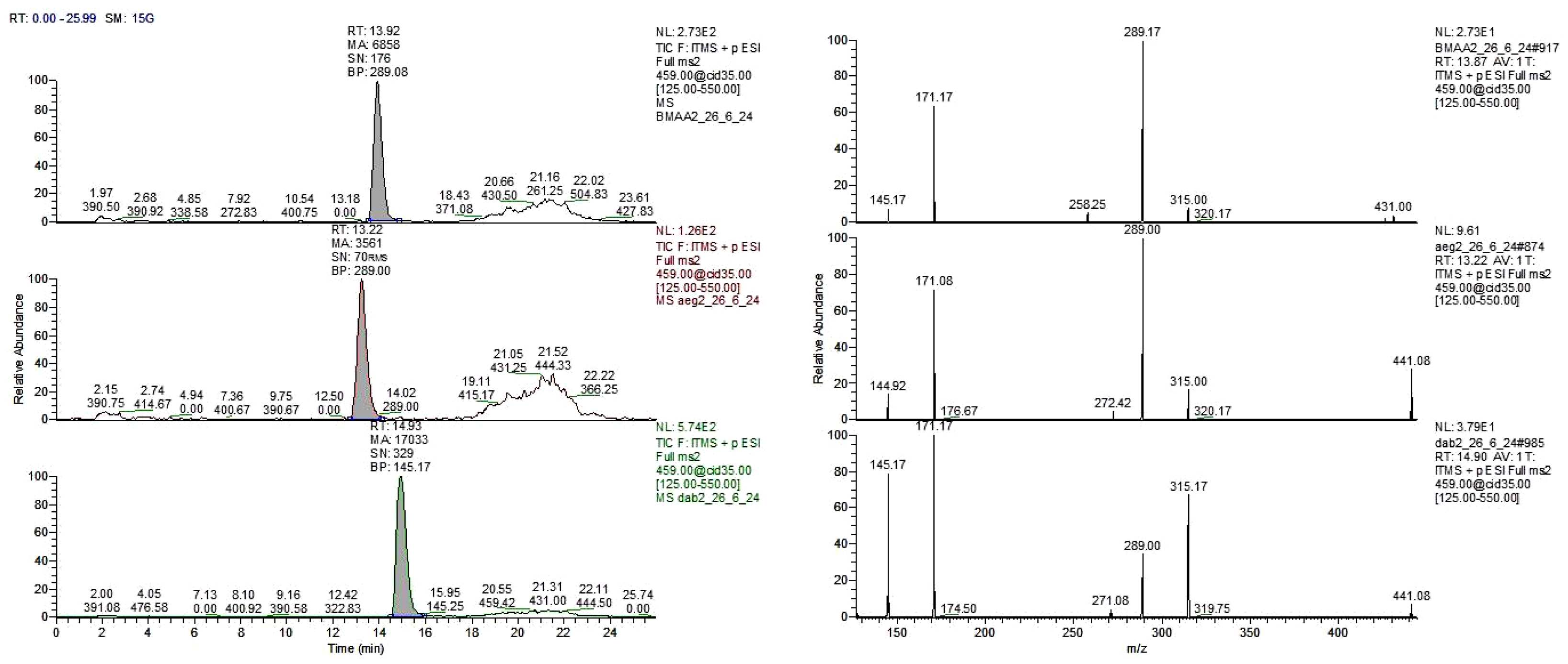
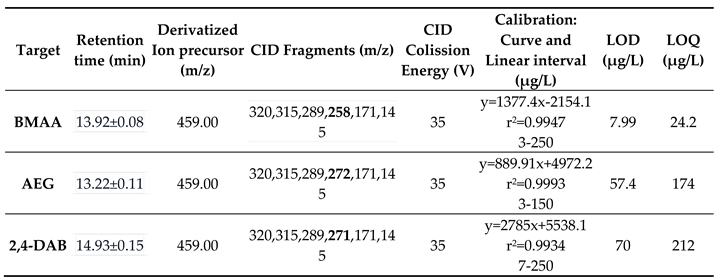
2.2. Detection of BMAA (β-N-methylamino-L-alanine)
3. Discussion
4. Materials and Methods
4.1. Azolla Accessions and Culturing
4.2. Isolation of Nostoc azollae from Azolla accessions
4.3. Detection and Analysis of BMAA (β-N-Methylamino-L-Alanine)
4.3.1. Method 1
4.3.2. Method 2
4.4. Cyanotoxin Genes at Azolla Accessions and Nostoc Azollae
4.4.1. DNA Extraction
4.4.3. BLAST of Anatoxin-a Genes against Nostoc azollae
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Carrapiço, F. Azolla as a Superorganism. Its Implication in Symbiotic Studies. In Symbioses and Stress: Joint Ventures in Biology; Seckbach, J., Grube, M., Eds.; Springer Netherlands: Dordrecht, 2010; pp. 225–241 ISBN 978-90-481-9449-0.
- Singh, P.; Khan, A.; Srivastava, A. Chapter 16 - Heterocyst and Akinete Differentiation in Cyanobacteria: A View toward Cyanobacterial Symbiosis. In Advances in Cyanobacterial Biology; Singh, P.K., Kumar, A., Singh, V.K., Shrivastava, A.K., Eds.; Academic Press, 2020; pp. 235–248 ISBN 978-0-12-819311-2.
- Bujak, J.P.; Bujak, A.A. Origin and Evolution of the Azolla Superorganism. Plants 2024, 13, Article number 2106 (online publication with no page numbers). [CrossRef]
- Ekman, M.; Tollbäck, P.; Klint, J.; Bergman, B. Protein Expression Profiles in an Endosymbiotic Cyanobacterium Revealed by a Proteomic Approach. Mol. Plant-Microbe Interactions® 2006, 19, 1251–1261. [CrossRef]
- Ekman, M.; Tollbäck, P.; Bergman, B. Proteomic Analysis of the Cyanobacterium of the Azolla Symbiosis: Identity, Adaptation, and NifH Modification. J. Exp. Bot. 2008, 59, 1023–1034. [CrossRef]
- Larsson, J.; Nylander, J.A.; Bergman, B. Genome Fluctuations in Cyanobacteria Reflect Evolutionary, Developmental and Adaptive Traits. BMC Evol. Biol. 2011, 11, 187. [CrossRef]
- Ran, L.; Larsson, J.; Vigil-Stenman, T.; Nylander, J.A.A.; Ininbergs, K.; Zheng, W.-W.; Lapidus, A.; Lowry, S.; Haselkorn, R.; Bergman, B. Genome Erosion in a Nitrogen-Fixing Vertically Transmitted Endosymbiotic Multicellular Cyanobacterium. PLoS ONE 2010, 5, e11486. [CrossRef]
- Bujak, J.; Bujak, A. The Azolla Story: A Message from the Future.; The Azolla Foundation, 2020; ISBN 1-5272-8335-6.
- Bujak, A.; Bujak, J. Azolla’s Use as a Biofertilizer and Livestock Feed. In Ferns; Marimuthu, J., Fernández, H., Kumar, A., Thangaiah, S., Eds.; Springer Nature Singapore: Singapore, 2022; pp. 671–695 ISBN 9789811661693.
- Watanabe, I.; Berja, N.S. The Growth of Four Species of Azolla as Affected by Temperature. Aquat. Bot. 1983, 15, 175–185. [CrossRef]
- Ansari, M.A.; Sharma, V.P. Role of Azolla in Controlling Mosquito Breeding in Ghaziabad District Villages (U.P.). Indian J. Malariol. 1991, 28, 51–54.
- Mwingira, V.; Mayala, B.; Senkoro, K.; Rumisha, S.; Shayo, H., Elizabeth; Mlozi, P.; Mboera, L. Mosquito Larval Productivity in Rice-Fields Infested with Azolla in Mvomero District, Tanzania. Tanzan. J. Health Res. 2009, 11, 17–22. [CrossRef]
- Rajendran, R.; Reuben, R. Evaluation of the Water Fern Azolla Microphylla for Mosquito Population Management in the Rice-Land Agro-Ecosystem of South India. Med. Vet. Entomol. 1991, 5, 299–310. [CrossRef]
- Bharati, K. Influence of Incorporation or Dual Cropping of Azolla on Methane Emission from a Flooded Alluvial Soil Planted to Rice in Eastern India. Agric. Ecosyst. Amp Environ. 2000.
- Mujiyo; Sunarminto, B.; Hanudin, E.; Widada, J.; Syamsiyah, J. Methane Emission on Organic Rice Experiment Using Azolla. Int. J. Appl. Environ. Sci. 2016, 11, 295–308.
- Xu, H.; Zhu, B.; Liu, J.; Li, D.; Yang, Y.; Zhang, K.; Jiang, Y.; Hu, Y.; Zeng, Z. Azolla Planting Reduces Methane Emission and Nitrogen Fertilizer Application in Double Rice Cropping System in Southern China. Agron. Sustain. Dev. 2017, 37, 29. [CrossRef]
- Winstead, D.; Di Gioia, F.; Jauregui, M.; Jacobson, M. Nutritional Properties of Raw and Cooked Azolla Caroliniana Willd., an Aquatic Wild Edible Plant. Food Sci. Nutr. 2024, 12, 2050–2060. [CrossRef]
- Bláha, L.; Babica, P.; Maršálek, B. Toxins Produced in Cyanobacterial Water Blooms - Toxicity and Risks. Interdiscip. Toxicol. 2009, 2, 36–41. [CrossRef]
- Burton, B.; Collins, K.; Brooks, J.; Marx, K.; Renner, A.; Wilcox, K.; Moore, E.; Osowski, K.; Riley, J.; Rowe, J.; et al. The Biotoxin BMAA Promotes Dysfunction via Distinct Mechanisms in Neuroblastoma and Glioblastoma Cells. PLOS ONE 2023, 18, e0278793. [CrossRef]
- Chorus, I.; Bartram, J. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management.; CRC Press, 1999;
- Chorus, I.; Welker, M. Toxic Cyanobacteria in Water - Second Edition; CRC Press, 2021; ISBN 978-1-00-308144-9.
- Merel, S.; D, W.; R, C.; S, S.; E, B.; O, T. State of Knowledge and Concerns on Cyanobacterial Blooms and Cyanotoxins. Environ. Int. 2013, 59. [CrossRef]
- Funari, E.; Testai, E. Human Health Risk Assessment Related to Cyanotoxins Exposure. Crit. Rev. Toxicol. 2008, 38, 97–125. [CrossRef]
- Cox, P.A.; Banack, S.A.; Murch, S.J.; Rasmussen, U.; Tien, G.; Bidigare, R.R.; Metcalf, J.S.; Morrison, L.F.; Codd, G.A.; Bergman, B. Diverse Taxa of Cyanobacteria Produce β-N-Methylamino-l-Alanine, a Neurotoxic Amino Acid. Proc. Natl. Acad. Sci. 2005, 102, 5074–5078. [CrossRef]
- Esterhuizen, M.; Downing, T.G. Beta-N-Methylamino-L-Alanine (BMAA) in Novel South African Cyanobacterial Isolates. Ecotoxicol. Environ. Saf. 2008, 71, 309–313. [CrossRef]
- Vega, A.; Bell, E.A. α-Amino-β-Methylaminopropionic Acid, a New Amino Acid from Seeds of Cycas Circinalis. Phytochemistry 1967, 6, 759–762. [CrossRef]
- Al-Sammak, M.A.; Hoagland, K.D.; Cassada, D.; Snow, D.D. Co-Occurrence of the Cyanotoxins BMAA, DABA and Anatoxin-a in Nebraska Reservoirs, Fish, and Aquatic Plants. Toxins 2014, 6, 488–508. [CrossRef]
- Hammerschlag, N.; Davis, D.A.; Mondo, K.; Seely, M.S.; Murch, S.J.; Glover, W.B.; Divoll, T.; Evers, D.C.; Mash, D.C. Cyanobacterial Neurotoxin BMAA and Mercury in Sharks. Toxins 2016, 8, 238. [CrossRef]
- Holtcamp, W. The Emerging Science of BMAA: Do Cyanobacteria Contribute to Neurodegenerative Disease? Environ. Health Perspect. 2012, 120, a110–a116. [CrossRef]
- Masseret, E.; Banack, S.; Boumédiène, F.; Abadie, E.; Brient, L.; Pernet, F.; Juntas-Morales, R.; Pageot, N.; Metcalf, J.; Cox, P.; et al. Dietary BMAA Exposure in an Amyotrophic Lateral Sclerosis Cluster from Southern France. PloS One 2013, 8, e83406. [CrossRef]
- Jiang, L.; Eriksson, J.; Lage, S.; Jonasson, S.; Shams, S.; Mehine, M.; Ilag, L.L.; Rasmussen, U. Diatoms: A Novel Source for the Neurotoxin BMAA in Aquatic Environments. PloS One 2014, 9, e84578. [CrossRef]
- Jiang, L.; Ilag, L. Detection of Endogenous BMAA in Dinoflagellate (Heterocapsa Triquetra) Hints at Evolutionary Conservation and Environmental Concern. PubRaw Sci. 2014, 1, 1–8.
- Lage, S.; Costa, P.R.; Moita, T.; Eriksson, J.; Rasmussen, U.; Rydberg, S.J. BMAA in Shellfish from Two Portuguese Transitional Water Bodies Suggests the Marine Dinoflagellate Gymnodinium Catenatum as a Potential BMAA Source. Aquat. Toxicol. Amst. Neth. 2014, 152, 131–138. [CrossRef]
- Kaasalainen, U.; Fewer, D.P.; Jokela, J.; Wahlsten, M.; Sivonen, K.; Rikkinen, J. Cyanobacteria Produce a High Variety of Hepatotoxic Peptides in Lichen Symbiosis. Proc. Natl. Acad. Sci. 2012, 109, 5886–5891. [CrossRef]
- Kaasalainen, U.; Fewer, D.P.; Jokela, J.; Wahlsten, M.; Sivonen, K.; Rikkinen, J. Lichen Species Identity and Diversity of Cyanobacterial Toxins in Symbiosis. New Phytol. 2013, 198, 647–651.
- Gehringer, M.M.; Adler, L.; Roberts, A.A.; Moffitt, M.C.; Mihali, T.K.; Mills, T.J.; Fieker, C.; Neilan, B.A. Nodularin, a Cyanobacterial Toxin, Is Synthesized in Planta by Symbiotic Nostoc Sp. ISME J. 2012, 6, 1834–1847.
- Koksharova, O.A.; Safronova, N.A. Non-Proteinogenic Amino Acid β-N-Methylamino-L-Alanine (BMAA): Bioactivity and Ecological Significance. Toxins 2022, 14, 539.
- Rouhiainen, L.; Vakkilainen, T.; Siemer, B.L.; Buikema, W.; Haselkorn, R.; Sivonen, K. Genes Coding for Hepatotoxic Heptapeptides (Microcystins) in the Cyanobacterium Anabaena Strain 90. Appl. Environ. Microbiol. 2004, 70, 686–692. [CrossRef]
- Méjean, A.; Paci, G.; Gautier, V.; Ploux, O. Biosynthesis of Anatoxin-a and Analogues (Anatoxins) in Cyanobacteria. Toxicon 2014, 91, 15–22.
- Kurland, L.T.; Mulder, D.W. Epidemiologic Investigations of Amyotrophic Lateral Sclerosis. 2. Familial Aggregations Indicative of Dominant Inheritance. I. Neurology 1955, 5, 182–196. [CrossRef]
- Kurland, L.T.; Mulder, D.W. Epidemiologic Investigations of Amyotrophic Lateral Sclerosis. 2. Familial Aggregations Indicative of Dominant Inheritance. II. Neurology 1955, 5, 249–268. [CrossRef]
- Cox, P.A.; Banack, S.A.; Murch, S.J. Biomagnification of Cyanobacterial Neurotoxins and Neurodegenerative Disease among the Chamorro People of Guam. Proc. Natl. Acad. Sci. U. S. A. 2003, 100, 13380–13383. [CrossRef]
- Murch, S.J.; Cox, P.A.; Banack, S.A. A Mechanism for Slow Release of Biomagnified Cyanobacterial Neurotoxins and Neurodegenerative Disease in Guam. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 12228–12231. [CrossRef]
- Xie, X.; Basile, M.; Mash, D.C. Cerebral Uptake and Protein Incorporation of Cyanobacterial Toxin β-N-Methylamino-L-Alanine. NeuroReport 2013, 24, 779. [CrossRef]
- Lobner, D.; Piana, P.M.T.; Salous, A.K.; Peoples, R.W. Beta-N-Methylamino-L-Alanine Enhances Neurotoxicity through Multiple Mechanisms. Neurobiol. Dis. 2007, 25, 360–366. [CrossRef]
- Rush, T.; Liu, X.; Lobner, D. Synergistic Toxicity of the Environmental Neurotoxins Methylmercury and β-N-Methylamino-L-Alanine. Neuroreport 2012, 23, 216–219. [CrossRef]
- Weiss, J.H.; Koh, J.Y.; Choi, D.W. Neurotoxicity of Beta-N-Methylamino-L-Alanine (BMAA) and Beta-N-Oxalylamino-L-Alanine (BOAA) on Cultured Cortical Neurons. Brain Res. 1989, 497, 64–71. [CrossRef]
- Dunlop, R.A.; Cox, P.A.; Banack, S.A.; Rodgers, K.J. The Non-Protein Amino Acid BMAA Is Misincorporated into Human Proteins in Place of L-Serine Causing Protein Misfolding and Aggregation. PloS One 2013, 8, e75376. [CrossRef]
- Pravadali-Cekic, S.; Vojvodic, A.; Violi, J.P.; Mitrovic, S.M.; Rodgers, K.J.; Bishop, D.P. Simultaneous Analysis of Cyanotoxins β-N-Methylamino-L-Alanine (BMAA) and Microcystins-RR, -LR, and -YR Using Liquid Chromatography–Tandem Mass Spectrometry (LC-MS/MS). Molecules 2023, 28, 6733. [CrossRef]
- Faassen, E.J.; Gillissen, F.; Lürling, M. A Comparative Study on Three Analytical Methods for the Determination of the Neurotoxin BMAA in Cyanobacteria. PLoS One 2012, 7, e36667.
- Pereira, A.L.; Monteiro, B.; Azevedo, J.; Campos, A.; Osório, H.; Vasconcelos, V. Effects of the Naturally-Occurring Contaminant Microcystins on the Azolla Filiculoides–Anabaena Azollae Symbiosis. Ecotoxicol. Environ. Saf. 2015, 118, 11–20.
- Santos, C.; Azevedo, J.; Campos, A.; Vasconcelos, V.; Pereira, A.L. Biochemical and Growth Performance of the Aquatic Macrophyte Azolla Filiculoides to Sub-Chronic Exposure to Cylindrospermopsin. Ecotoxicology 2015, 24, 1848–1857. [CrossRef]
- Pereira, A.; Carrapico, F. Culture of Azolla Filiculoides in Artificial Conditions. Plant Biosyst. 2009, 2009, 431–434. [CrossRef]
- Peters, G.A.; Mayne, B.C. The Azolla, Anabaena Azollae Relationship: I. Initial Characterization of the Association. Plant Physiol. 1974, 53, 813–819.
- Rai, A.K.; Rai, V. Effect of NaCl on Growth, Nitrate Uptake and Reduction and Nitrogenase Activity of Azolla Pinnata–Anabaena Azollae. Plant Sci. 2003, 164, 61–69.
- Baptista, M.S.; Vasconcelos, R.G.W.; Ferreira, P.C.; Almeida, C.M.R.; Vasconcelos, V.M. Assessment of the Non-Protein Amino Acid BMAA in Mediterranean Mussel Mytilus Galloprovincialis after Feeding with Estuarine Cyanobacteria. Environ. Sci. Pollut. Res. 2015, 22, 12501–12510. [CrossRef]
- Méjean, A.; Mann, S.; Maldiney, T.; Vassiliadis, G.; Lequin, O.; Ploux, O. Evidence That Biosynthesis of the Neurotoxic Alkaloids Anatoxin-a and Homoanatoxin-a in the Cyanobacterium Oscillatoria PCC 6506 Occurs on a Modular Polyketide Synthase Initiated by l -Proline. J. Am. Chem. Soc. 2009, 131, 7512–7513. [CrossRef]

| Accessiona | Species name | Origin and harvest year | Sourceb/collector |
|---|---|---|---|
| PI1*,$ | A. pinnata subsp. imbricata | Philippines, Sto Domingo, Albay, 1975 | IRRI |
| PI2 | Malaysia, Bumbong Lima, Butterworth, 1977 | IRRI | |
| PI23 | India, Cuttack, Orissa, 1978 | CRRI | |
| PI68 | Sri Lanka, Tissa, 1984 | S. Kulasooriya | |
| PI102 | Japan, Okinawa, 1987 | O. Mochida | |
| PI503 | Australia, Murdoch, 1978 | M. Dilworth | |
| PI531 | Indonesia, Bali, 1983 | - | |
| PI540 | China, Putian, 1989 | C. van Hove | |
| FI1001* | A. filiculoides | East Germany (ex- GDR), 1979 | IB China |
| FI1008 | USA, Cranmore Road, Sutter Co., California, 1981 | D. Rains | |
| FI1010 | Peru, PUFFI, Lima, 1982 | CIAT | |
| FI1042 | Brazil, Parana, 1987 | I. Watanabe | |
| FI1052 | South of France, North of Lyon, 1989 | P. Roger | |
| FI1090 | Japan, Tanabe-cho, 1992 | S. Kitoh | |
| FI1501 | Belgian, Harchies, 1987 | A. Lawalree | |
| FI1505 | South Africa, Verwoerd dam, 1987 | D. Toerien | |
| FI1507$ | Colombia, Zipaquira, 1987 | Y. Lopez | |
| FI1522 | Switzerland, Zurich Botanical Garden, 1987 | - | |
| FI-BGLU | Botanical Garden of Lisbon University, 2009 | A.L. Pereira | |
| FI-BGM | Botanical Garden of Madeira, Funchal, 2010 | C. Lobo | |
| ME2001* | A. mexicana | USA, Graylodge, California, 1978 | D. Rains |
| ME2008 | Colombia, CIAT, Cali, 1982 | CIAT | |
| ME2011 | Japan, Osaka, 1984 | T. Lumpkin | |
| ME2026$ | Brazil, Solimoes river, Pacencia Island, Iranduba, Amazonas (BLCC 18), 1984 | T. Lumpkin | |
| CA3001*,$ | A. caroliniana | USA, Ohio, 1978 | D. Rains |
| CA3017 | Brazil, Rio Grande do Sul, 1987 | I. Watanabe | |
| CA3502 | Egypt, Moshtohr University, 1987 | C. Myttenaere | |
| CA3507 | Suriname, Boxel, 1987 | H. Lardinois | |
| CA3513 | Zimbabwe, Causeway Botanical Garden, 1987 | T. Muller | |
| CA3524 | Holland, 1987 | E. Ohoto | |
| CA3525 | Ruanda, Cyili Rice Research Center, 1987 | C. van Hove | |
| MI4018* | A. microphylla | Paraguay, 1981 | D. Rains |
| MI4021$ | Equator, Santa Cruz Island, Galapagos, 1982 | T. Lumpkin | |
| MI4028 | Philippines, hybrid (MI4018xFI1001), 1985 | Do Van Cat | |
| MI4054 | Brazil, Baía, 1987 | I. Watanabe | |
| MI4510 | Philippines, Los Baños, IRRI, 1987 | C. van Hove | |
| NI5001*,$ | A. nilotica | Sudan, Kosti, 1982 | T. Lumpkin |
| NI5002# | Sudan, Kosti, 1989 | T. Lumpkin | |
| NI5501 | Burundi, Bujumbura, 1987 | J. Bouharmont | |
| RU6010* | A. rubra | New Zealand, Nouville, 1986 | C. van Hove |
| RU6502 | Australia, Victoria (37.40S-144.40E), 1985 | - | |
| RU6503 | New Zealand, between Lumdsen and Kingston, 1986 | C. van Hove | |
| PP7001*,$ | A. pinnata subsp. pinnata | Australia, Kakadu Northern Park Northern Territory, 1982 | Yatazawa |
| PP7506 | Sierra Leone, 1982 | C Dixon | |
| PP7509 | Nigeria, Moor plantation, 987 | C. van Hove | |
| PP7511 | Guinea-Bissau, Contuboel, 1987 | H. Diara | |
| PP7512 | Zaire, Kisantu, 1987 | B. Bruyneel | |
| PP7546 | Madagascar, Antsahavory, East zone, 1991 | C. van Hove |
| Gene | Primer | Sequence primer (5’→3´) | Size (bp) | Reference |
|---|---|---|---|---|
| Saxitoxin (sxt) | SXT683F | GGATCTCAAACATGATCCCA | 195 | Lopes et al. 2012 |
| SXT877R | GCCAAACGCAGTACCACTT | |||
| Cylindrospermopsin (cyl) (poliketide synthase) | K18F | CCTCGCACATAGCCATTTGC | 422 | Schembri et al. 2001 |
| M4R | GAAGCTCTGGAATCCGGTAA | |||
| Cylindrospermopsin (cyl) (peptide synthase) | M13 | GGCAAATTGTGATAGCCACGAGC | 597 | Fergusson 2003 |
| M14 | GATGGAACATCGCTCACTGGTG | Schembri et al. 2001 | ||
| Microcystin/Nodularin synthetase (mcyE/ndaF) | HepF | TTTGGGGTTAACTTTTTTGGCCATAGTC | 472 | Jungblut 2006 |
| HepR | AATTCTTGAGGCTGTAAATCGGGTTT | |||
| Microcystin synthetase (mcy A) | mcyA-Cd1F | AAAATTAAAAGCCGTATCAAA | 297 | Hisbergues et al. 2003 |
| mcyA-Cd1R | AAAAGTGTTTTATTAGCGGCTCAT | |||
| Microcystin synthetase (mcy B) | 2959F | TGGGAAGATGTTCTTCAGGTATCCAA | 350 | Nonneman & Zimba 2002 |
| 3278R | AGAGTGGAAACAATATGATAAGCTAC | |||
| Microcystin (mcy C) | FAA | CTATGTTATTTATACATCAGG | 758 | Neilan 1999 |
| RAA | CTCAGCTTAACTTGATTATC | |||
| Microcystin (mcy B, domain A) | 2156F | ATCACTTCAATCTAACGACT | 955 | Mikalsen 2003 |
| 3111R | GTTGCTGCTGTAAGAAA | |||
| Microcystin (mcy C, domain A) | PSCF1 | GCAACATCCCAAGAGCAAAG | 674 | Ouahid 2005 |
| PSCR1 | CCGACAACATCACAAAGGC | |||
| Microcystin (mcy D, domain ACP) | PKDF1 | GACGCTCAAATGATGAAACT | 647 | Ouahid 2005 |
| PKDR1 | GCAACCGATAAAAACTCCC | |||
| Microcystin (mcy D, domain KS) | PKDF2 | AGTTATTCTCCTCAAGCC | 859 | Ouahid 2005 |
| PKDR2 | CATTCGTTCCACTAAATCC | |||
| Microcystin (mcy E, domain GSA-AMT) | PKEF1 | CGCAAACCCGATTTACAG | 755 | Ouahid 2005 |
| PKER1 | CCCCTACCATCTTCATCTTC | |||
| Microcystin (mcy G, domain CM) | PKGF1 | ACTCTCAAGTTATCCTCCCTC | 425 | Ouahid 2005 |
| PKGR1 | AATCGCTAAAACGCCACC |
| Gene | Initial denaturation | Denaturation | Annealing | Extension | Final extension | Reference |
|---|---|---|---|---|---|---|
| sxt | 94°C; 3 min | 35 cycles | 72°C; 7 min | Lopes et al. 2012 | ||
| 94°C; 10 s | 52°C; 20 s | 72°C; 1 min | ||||
| cyl | 94°C; 10 min | 30 cycles | 72°C; 7 min | Fergusson 2003 | ||
| 94°C; 30 s | 55°C; 30 s | 72°C; 7 min | ||||
| mcyE/ndaF | 92°C; 2 min | 35 cycles | 72°C; 5 min | Jungblut 2006 | ||
| 92°C; 20 s | 56°C; 30 s | 72°C; 1 min | ||||
| mcy A | 95°C; 2 min | 35 cycles | 72°C; 7 min | Hisbergues et al. 2003 | ||
| 95°C; 90 s | 56°C; 30 s | 72°C; 50 s | ||||
| mcy B | 94°C; 2 min | 35 cycles | 72°C; 5 min | Nonneman 2002 | ||
| 94°C; 30 s | 59°C; 45 s | 72°C; 1 min | ||||
| mcy C | 94°C; 2 min | 35 cycles | 72°C; 7 min | Neilan 1999 | ||
| 94°C; 10 s | 50°C; 20 s | 72°C; 1 min | ||||
| mcy B, domain A | 94°C; 4 min | 30 cycles | 72°C; 7 min | Mikalsen 2003 | ||
| 95°C; 30 s | 52°C; 30 s | 72°C; 1 min | ||||
| mcy C, domain A | 94°C; 5 min | 35 cycles | 72°C; 7 min | Ouahid 2005 | ||
| 95°C; 1 min | 52°C; 30 s | 72°C; 1 min | ||||
| mcy D, domain ACP | 94°C; 5 min | 35 cycles | 72°C; 7 min | Ouahid 2005 | ||
| 95°C; 1 min | 52°C; 30 s | 72°C; 1 min | ||||
| mcy D, domain KS | 94°C; 5 min | 35 cycles | 72°C; 7 min | Ouahid 2005 | ||
| 95°C; 1 min | 52°C; 30 s | 72°C; 1 min | ||||
| mcy E, domain GST-AMT | 94°C; 5 min | 35 cycles | 72°C; 7 min | Ouahid 2005 | ||
| 95°C; 1 min | 52°C; 30 s | 72°C; 1 min | ||||
| mcy G, domain CM | 94°C; 5 min | 35 cycles | 72°C; 7 min | Ouahid 2005 | ||
| 95°C; 1 min | 52°C; 30 s | 72°C; 1 min | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





