Submitted:
23 August 2024
Posted:
26 August 2024
Read the latest preprint version here
Abstract
Keywords:
1. A Different Kind of Evolutionary Transition
2. Tissues, Organization, and Metabolism
2.1. Metabolism: Constraints and Complementation
3. A Model for the Origin of Tissues and Organs
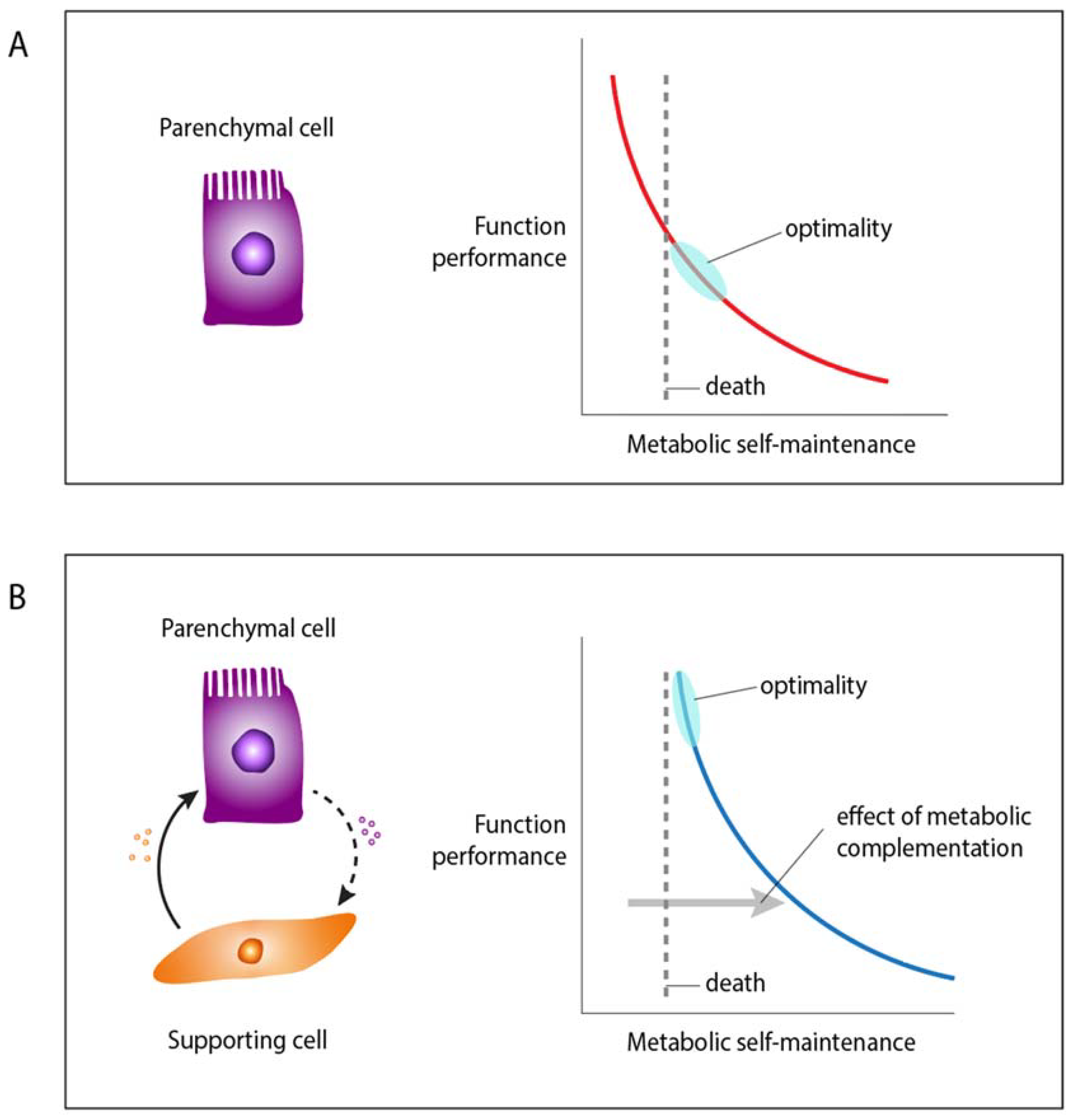
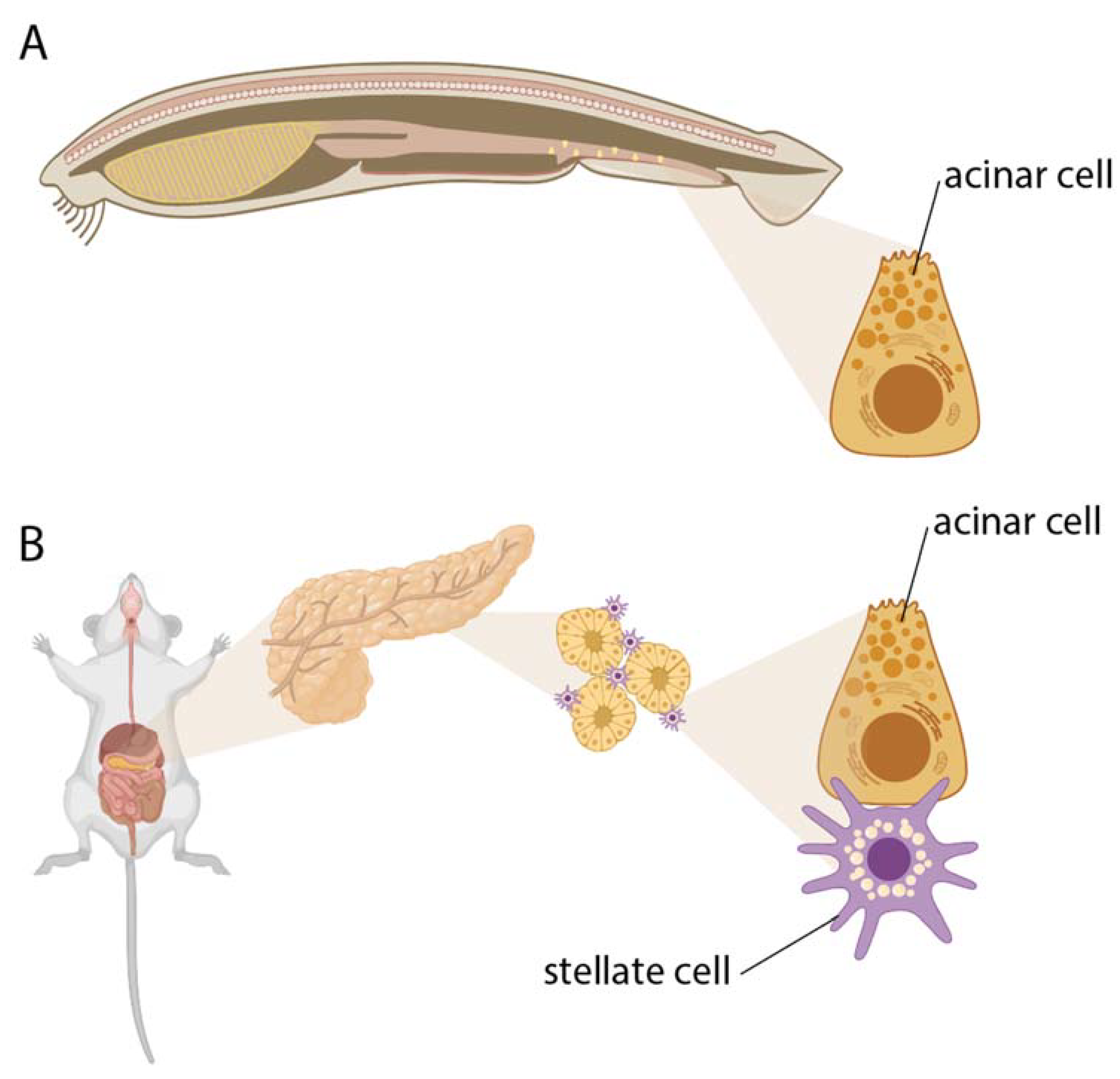
3.1. Examples of Tissue-Sustaining Metabolic Interactions
3.2. Why Was Metabolism Overlooked or Neglected in Evolutionary Biology?
3.3. Implications of the Model
4. Discussion: On the Origins of Within-Organism Levels of Organization
Author Contributions
Funding
Data Accessibility
Competing Interests
Ethics statement
References
- Mayr, E. The Emergence of Evolutionary Novelties.; Chicago University Press: Chicago, 1960. [Google Scholar]
- Chavan, A.R.; Griffith, O.W.; Stadtmauer, D.; Maziarz, J.; et al. Evolution of embryo implantation was enabled by the origin of decidual stromal cells in eutherian mammals. Mol Biol Evol. 2020. [Google Scholar] [CrossRef]
- DeMIGUEL, D.; Azanza, B.; Morales, J. Key innovations in ruminant evolution: a paleontological perspective. Integr Zool 2014, 9, 412–433. [Google Scholar] [CrossRef] [PubMed]
- Ronco, F.; Salzburger, W. Tracing evolutionary decoupling of oral and pharyngeal jaws in cichlid fishes. Evol Lett 2021, 5, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Leake, L.D. Comparative Histology. An introduction to the Microscopic Structure of Animals.; Academic Press: New York, 1975. [Google Scholar]
- Falkmer, S. Comparative morphology of pancreatic islets in animals. In The diabetic Pancreas; Volk, B.W., Arquille, E.R., Eds.; Plenum medical Book coMPANY: New York, 1985. [Google Scholar]
- Falkmer, S.; Dafgård, E.; el-Salhy, M.; Engström, W.; Grimelius, L.; Zetterberg, A. Phylogenetical aspects on islet hormone families: a minireview with particular reference to insulin as a growth factor and to the phylogeny of PYY and NPY immunoreactive cells and nerves in the endocrine and exocrine pancreas. Peptides 1985, 6 (Suppl. S3), 315–320. [Google Scholar] [CrossRef] [PubMed]
- Schlosser, G. Origin of Sensory and Neurosecretory Cell Types: Vertebrate Cranial Placodes; CRC Press: 2021.
- Maynard Smith, J.; Szathmary, E. The Major Transitions in Evolution; Oxford University Press: Oxford, 1995. [Google Scholar]
- McShea, D.W. PERSPECTIVE METAZOAN COMPLEXITY AND EVOLUTION: IS THERE A TREND? Evolution 1996, 50, 477–492. [Google Scholar] [CrossRef]
- Michod, R. Darwinian Dynamics: evolutionary transitions in fitness and individuality.; Princeton University Press: Princeton, NJ, 2000. [Google Scholar]
- Michod, R.E. Evolution of individuality during the transition from unicellular to multicellular life. Proc Natl Acad Sci U S A 2007, 104 (Suppl. S1), 8613–8618. [Google Scholar] [CrossRef]
- Okasha S. 2006. Evolution and the levels of selection Oxford.
- Oxford; New York: Clarendon Press.
- Oxford University Press.
- West, S.A.; Fisher, R.M.; Gardner, A.; Kiers, E.T. Major evolutionary transitions in individuality. Proc Natl Acad Sci U S A 2015, 112, 10112–10119. [Google Scholar] [CrossRef]
- Buss, L.W. The Evolution of Individuality; Princeton University Press: 1987.
- Brunet, T.; King, N. The Origin of Animal Multicellularity and Cell Differentiation. Dev Cell 2017, 43, 124–140. [Google Scholar] [CrossRef]
- Herron, M.; Conlin, P.; Ratcliff, W. The Evolution of Multicellularity. 2021, 1.
- Niklas, K.J.; Newman, S. Multicellularity : origins and evolution; The MIT Press: Cambridge, MA, 2016. [Google Scholar]
- Wagner, G.P. Homology, Genes and Evolutionary Innovation; Princeton University Press: Princeton, 2014. [Google Scholar]
- Nejad Kourki, A. The evolution of complex multicellularity in animals. Biology & Philosophy 2022, 37, 43. [Google Scholar] [CrossRef]
- Adler, M.; Moriel, N.; Goeva, A.; Avraham-Davidi, I.; Mages, S.; Adams, T.S.; Kaminski, N.; Macosko, E.Z.; Regev, A.; Medzhitov, R.; et al. Emergence of division of labor in tissues through cell interactions and spatial cues. Cell Rep 2023, 42, 112412. [Google Scholar] [CrossRef]
- Adler, M.; Chavan, A.R.; Medzhitov, R. Tissue Biology: In Search of a New Paradigm. Annu Rev Cell Dev Biol 2023. [Google Scholar] [CrossRef]
- Zhou, X.; Franklin, R.A.; Adler, M.; Jacox, J.B.; Bailis, W.; Shyer, J.A.; Flavell, R.A.; Mayo, A.; Alon, U.; Medzhitov, R. Circuit Design Features of a Stable Two-Cell System. Cell 2018, 172, 744–757. [Google Scholar] [CrossRef]
- Hall, B.K. The embryonic development of bone. American Scientist 1988, 76, 174–181. [Google Scholar]
- DiFrisco, J.; Love, A.; Wagner, G.P. Character identity mechanisms: a conceptual model.
- for comparative-mechanistic biology. Biology & Philosophy 2020, 1-32.
- Weiße, A.Y.; Oyarzún, D.A.; Danos, V.; Swain, P.S. Mechanistic links between cellular trade-offs, gene expression, and growth. Proc Natl Acad Sci U S A 2015, 112, E1038–E1047. [Google Scholar] [CrossRef] [PubMed]
- Molenaar, D.; van Berlo, R.; de Ridder, D.; Teusink, B. Shifts in growth strategies reflect tradeoffs in cellular economics. Mol Syst Biol 2009, 5, 323. [Google Scholar] [CrossRef]
- Kempes, C.P.; Dutkiewicz, S.; Follows, M.J. Growth, metabolic partitioning, and the size of microorganisms. Proc Natl Acad Sci U S A 2012, 109, 495–500. [Google Scholar] [CrossRef]
- Gladden, L.B. Lactate metabolism: a new paradigm for the third millennium. J Physiol 2004, 558, 5–30. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A. Cell-cell and intracellular lactate shuttles. J Physiol 2009, 587, 5591–5600. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C. Regulation of glutathione synthesis. Mol Aspects Med 2009, 30, 42–59. [Google Scholar] [CrossRef]
- DeLong, J.P.; Okie, J.G.; Moses, M.E.; Sibly, R.M.; Brown, J.H. Shifts in metabolic scaling, production, and efficiency across major evolutionary transitions of life. Proc Natl Acad Sci U S A 2010, 107, 12941–12945. [Google Scholar] [CrossRef]
- Pavlicev, M.; Wagner, G.P. Reading the palimpsest of cell interactions: What questions may we ask of the data? iScience 2024, 27, 109670. [Google Scholar] [CrossRef] [PubMed]
- Force, A.; Lynch, M.; Pickett, F.B.; Amores, A.; Yan, Y.L.; Postlethwait, J. Preservation of duplicate genes by complementary, degenerative mutations. Genetics 1999, 151, 1531–1545. [Google Scholar] [CrossRef] [PubMed]
- Love, A.C.; Wagner, G.P. Co-option of stress mechanisms in the origin of evolutionary novelties. Evolution 2022, 76, 394–413. [Google Scholar] [CrossRef] [PubMed]
- Hermann, H. Direct metabolic interactions between animal cells. Their role in tissue function and development is reconsidered. Science 1960, 132, 529–532. [Google Scholar] [CrossRef]
- Gasbarrini, A.; Borle, A.B.; Farghali, H.; Bender, C.; Francavilla, A.; Van Thiel, D. Effect of anoxia on intracellular ATP, Na+i, Ca2+i, Mg2+i, and cytotoxicity in rat hepatocytes. J Biol Chem 1992, 267, 6654–6663. [Google Scholar] [CrossRef]
- Brezis, M.; Epstein, F.H. Cellular mechanisms of acute ischemic injury in the kidney. Annu Rev Med 1993, 44, 27–37. [Google Scholar] [CrossRef]
- Gibbons, H.M.; Dragunow, M. Adult human brain cell culture for neuroscience research. Int J Biochem Cell Biol 2010, 42, 844–856. [Google Scholar] [CrossRef]
- Turner, D.A.; Adamson, D.C. Neuronal-astrocyte metabolic interactions: understanding the transition into abnormal astrocytoma metabolism. J Neuropathol Exp Neurol 2011, 70, 167–176. [Google Scholar] [CrossRef]
- Almeida, A.; Jimenez-Blasco, D.; Bolaños, J.P. Cross-talk between energy and redox metabolism in astrocyte-neuron functional cooperation. Essays Biochem 2023, 67, 17–26. [Google Scholar] [CrossRef]
- Bolaños, J.P. Bioenergetics and redox adaptations of astrocytes to neuronal activity. J Neurochem 2016, 139 (Suppl. S2), 115–125. [Google Scholar] [CrossRef]
- Bonvento, G.; Bolaños, J.P. Astrocyte-neuron metabolic cooperation shapes brain activity. Cell Metab 2021, 33, 1546–1564. [Google Scholar] [CrossRef]
- Kastriti, M.E.; Adameyko, I. Specification, plasticity and evolutionary origin of peripheral glial cells. Curr Opin Neurobiol 2017, 47, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Rey, S.; Zalc, B.; Klämbt, C. Evolution of glial wrapping: A new hypothesis. Dev Neurobiol 2021, 81, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Song, B.; Lee, I.S. Glia: Models for Human Neurodevelopmental and Neurodegenerative Disorders. Int J Mol Sci 2020, 21. [Google Scholar] [CrossRef]
- Van Venrooij, W.J.; Poort, C.; Kramer, M.F.; Jansen, M.T. Relationship between extracellular amino acids and protein synthesis in vitro in the rat pancreas. Eur J Biochem 1972, 30, 427–433. [Google Scholar] [CrossRef]
- Mann, G.E.; Peran, S. Basolateral amino acid transport systems in the perfused exocrine pancreas: sodium-dependency and kinetic interactions between influx and efflux mechanisms. Biochim Biophys Acta 1986, 858, 263–274. [Google Scholar] [CrossRef]
- Kosowski, H.; Schild, L.; Kunz, D.; Halangk, W. Energy metabolism in rat pancreatic acinar cells during anoxia and reoxygenation. Biochimica et Biophysica Acta 1989, 118–126. [Google Scholar] [CrossRef]
- Masamune, A.; Shimosegawa, T. Pancreatic stellate cells--multi-functional cells in the pancreas. Pancreatology 2013, 13, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Bläuer, M.; Nordback, I.; Sand, J.; Laukkarinen, J. A novel explant outgrowth culture model for mouse pancreatic acinar cells with long-term maintenance of secretory phenotype. Eur J Cell Biol 2011, 90, 1052–1060. [Google Scholar] [CrossRef]
- Petersen, O.H. The 2022 George E Palade Medal Lecture: Toxic Ca. Pancreatology 2023, 23, 1–8. [Google Scholar] [CrossRef]
- Criddle, D.N.; Tepkin, A.V. Bioenergetics of the Exocrine Pancreas: Physiology to Pathophysiology. In Pancreapedia: Exocrine Pancreas knowledge base, 2020.
- Pan, Z.; Van den Bossche, J.L.; Rodriguez-Aznar, E.; Janssen, P.; Lara, O.; Ates, G.; Massie, A.; De Paep, D.L.; Houbracken, I.; Mambretti, M.; et al. Pancreatic acinar cell fate relies on system x. Cell Death Dis 2023, 14, 536. [Google Scholar] [CrossRef]
- Phillips, P.A.; McCarroll, J.A.; Park, S.; Wu, M.J.; Pirola, R.; Korsten, M.; Wilson, J.S.; Apte, M.V. Rat pancreatic stellate cells secrete matrix metalloproteinases: implications for extracellular matrix turnover. Gut 2003, 52, 275–282. [Google Scholar] [CrossRef]
- Riopel, M.M.; Li, J.; Liu, S.; Leask, A.; Wang, R. β1 integrin-extracellular matrix interactions are essential for maintaining exocrine pancreas architecture and function. Lab Invest 2013, 93, 31–40. [Google Scholar] [CrossRef]
- Phillips, P.A.; Yang, L.; Shulkes, A.; Vonlaufen, A.; Poljak, A.; Bustamante, S.; Warren, A.; Xu, Z.; Guilhaus, M.; Pirola, R.; et al. Pancreatic stellate cells produce acetylcholine and may play a role in pancreatic exocrine secretion. Proc Natl Acad Sci U S A 2010, 107, 17397–17402. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.S.; Cui, Z.J. Pancreatic Stellate Cells Serve as a Brake Mechanism on Pancreatic Acinar Cell Calcium Signaling Modulated by Methionine Sulfoxide Reductase Expression. Cells 2019, 8. [Google Scholar] [CrossRef]
- Sousa, C.M.; Biancur, D.E.; Wang, X.; Halbrook, C.J.; Sherman, M.H.; Zhang, L.; Kremer, D.; Hwang, R.F.; Witkiewicz, A.K.; Ying, H.; et al. Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion. Nature 2016, 536, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Vaziri-Gohar, A.; Zarei, M.; Brody, J.R.; Winter, J.M. Metabolic Dependencies in Pancreatic Cancer. Front Oncol 2018, 8, 617. [Google Scholar] [CrossRef]
- Hamada, S.; Matsumoto, R.; Masamune, A. Pancreatic Stellate Cells and Metabolic Alteration: Physiology and Pathophysiology. Front Physiol 2022, 13, 865105. [Google Scholar] [CrossRef]
- Wu, B.; Xu, W.; Wu, K.; Li, Y.; Hu, M.; Feng, C.; Zhu, C.; Zheng, J.; Cui, X.; Li, J.; et al. Single-cell analysis of the amphioxus hepatic caecum and vertebrate liver reveals genetic mechanisms of vertebrate liver evolution. Nat Ecol Evol 2024. [Google Scholar] [CrossRef] [PubMed]
- Krejci, A.; Tennessen, J.M. Metabolism in time and space - exploring the frontier of developmental biology. Development 2017, 144, 3193–3198. [Google Scholar] [CrossRef]
- Juliano, C.E. Metabolites play an underappreciated role in development. Proc Natl Acad Sci U S A 2024, 121, e2410810121. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J. Amino acid auxotrophy as a system of immunological control nodes. Nat Immunol 2016, 17, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Ganeshan, K.; Chawla, A. Metabolic regulation of immune responses. Annu Rev Immunol 2014, 32, 609–634. [Google Scholar] [CrossRef]
- Chi, H. Immunometabolism at the intersection of metabolic signaling, cell fate, and systems immunology. Cell Mol Immunol 2022, 19, 299–302. [Google Scholar] [CrossRef]
- Schmidt-Rhaesa, A. The evolution of organ systems; Oxford University Press: Oxford ; New York, 2007.
- Jiang, Y.; Xiong, X.; Danska, J.; Parkinson, J. Metatranscriptomic analysis of diverse microbial communities reveals core metabolic pathways and microbiome-specific functionality. Microbiome 2016, 4, 2. [Google Scholar] [CrossRef]
- Damiani, C.; Maspero, D.; Di Filippo, M.; Colombo, R.; Pescini, D.; Graudenzi, A.; Westerhoff, H.V.; Alberghina, L.; Vanoni, M.; Mauri, G. Integration of single-cell RNA-seq data into population models to characterize cancer metabolism. PLoS Comput Biol 2019, 15, e1006733. [Google Scholar] [CrossRef]
- Alghamdi, N.; Chang, W.; Dang, P.; Lu, X.; Wan, C.; Gampala, S.; Huang, Z.; Wang, J.; Ma, Q.; Zang, Y.; et al. A graph neural network model to estimate cell-wise metabolic flux using single-cell RNA-seq data. Genome Res 2021, 31, 1867–1884. [Google Scholar] [CrossRef]
- Jia, Z.; Wei, Y.; Zhang, Y.; Song, K.; Yuan, J. Metabolic reprogramming and heterogeneity during the decidualization process of endometrial stromal cells. Cell Commun Signal 2024, 22, 385. [Google Scholar] [CrossRef]
- McShea, D.; Venit, E.P. What is a part? In The Character Concept in Evolutionary Biology., Wagner, G.P., Ed. Academic Press: San Diego, 2001.
- Wagner, G.P.; Altenberg, L. Perspective: Complex Adaptations and the Evolution of Evolvability. Evolution 1996, 50, 967–976. [Google Scholar] [CrossRef]
- Raff, R.A. The shape of life : genes, development, and the evolution of animal form; University of Chicago Press: Chicago, 1996. [Google Scholar]
- Wagner, G.P.; Pavlicev, M.; Cheverud, J.M. The road to modularity. Nat Rev Genet 2007, 8, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Dighe, A.; Maziarz, J.; Ibrahim-Hashim, A.; Gatenby, R.A.; Kshitiz; Levchenko, A.; Wagner, G.P. Experimental and phylogenetic evidence for correlated gene expression evolution in endometrial and skin fibroblasts. iScience 2024, 27, 108593. [CrossRef]
- Pavlicev, M.; Wagner, G.P. A model of developmental evolution: selection, pleiotropy and compensation. Trends Ecol Evol 2012, 27, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Cairns-Smith, A. The Complexity ratchet. In Progress in Search for Extraterrestrial Life., Shostak, G., Ed. Astronomical Society of the Pacific.: San Francisco., 1995; pp. 31–36.
- Liard, V.; Parsons, D.P.; Rouzaud-Cornabas, J.; Beslon, G. The Complexity Ratchet: Stronger than Selection, Stronger than Evolvability, Weaker than Robustness. Artif Life 2020, 26, 38–57. [Google Scholar] [CrossRef]
- Lukeš, J.; Archibald, J.M.; Keeling, P.J.; Doolittle, W.F.; Gray, M.W. How a neutral evolutionary ratchet can build cellular complexity. IUBMB Life 2011, 63, 528–537. [Google Scholar] [CrossRef]
- Parker, J. Organ Evolution: Emergence of Multicellular Function. Annu Rev Cell Dev Biol 2024. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




