1. Introduction
1.1. Context
Spiders play a significant role in the ecosystem, functioning as critical predators and maintaining the delicate balance in ecosystems by capturing prey ranging from insects to birds, consuming millions of pounds annually [
1]. One method they use to achieve this is by constructing intricate webs facilitated by the secretion of spider silk [
2]. Spider silk possesses exceptional strength and toughness, being pound for pound stronger than steel and Kevlar [
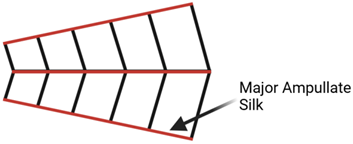
3]. A single spider can produce up to 7 distinct types of silk for differing uses, each serving different purposes within the spider’s ecological niche (
Table 1) [
4,
5].
1.2. Composition
All spider silks primarily comprise spider fibroins, or “spidroins” for short [
6]. These spidroins have three regions: an initial N-terminus region, a middle, and a final C-terminus region [
7].
These nonrepeating initial and terminal regions are around 100-200 amino acids in length and are theorized to be responsible for the initiation and assembly of spider proteins into spider silk. These initial and terminal regions are most likely conserved throughout the order
Araneae, as in other species’ spidroins, a high degree of similarity in the initial and terminal areas was identified [
7].
The middle section consists of repeating sequences of 10-50 amino acids that account for 90% of the spider silk protein [
7]. As identical initial and terminal regions are seen throughout most variations of spider silks, this suggests that the repeating subsections are responsible for the unique mechanical characteristics of the silk fiber, possibly contributing to variations in secondary structure [
8].
As a result, the secondary structure has a unique semi-crystalline composition, allowing for the combination of strength and flexibility. This means there is a fluid, ever-changing matrix composed of helices, β-turns, and random coils [
8].
Regarding tertiary structure, spidroins lack considerable tertiary structure because the silk proteins are in a liquid solution. However, a quaternary structure is present due to weak intramolecular and intermolecular interactions between other proteins and neighboring domains, assuming the spider silks are produced naturally through silk glands [
7]. The quaternary structure has yet to be well documented in the artificial production of spidroins.
1.3. Mechanical Properties
Spider silks have several useful inherent properties resulting from their protein structure, including vibration absorption, high toughness, and a unique ability to reassemble in liquids. A study done on Major Ampullate (MA) silk, the strongest type of silk, found that it has a density of 1.3 g/cm
3, a strength of 1.1 Gpa, and an elasticity of 27%. For comparison, Nylon 6.6 has a density of 1.1 g/cm
3, a strength of 0.95 Gpa, and an elasticity of 18%. Thus, MA silk has a higher density, elasticity strength, and toughness than Nylon 6.6, the superior choice for industrial-grade heavy-duty applications [
9].
Spidroins also possess a unique property: they can create structured fibers when exposed to external triggers. This process is done through self-assembly and allows structures to be made from the proteins when placed in liquid. However, this process only works if the functional domains in the protein are present and intact in their natural state [
10].
2. Current Method of Producing Spider Silk from Artificial Sources
2.1. Unicellular Host Production
With present technology, obtaining spider silk from natural sources is infeasible due to cost and inefficiency. Due to their aggressive nature, spiders cannot be kept in shared habitat [
11]. Therefore, the most feasible method to obtain recombinant spider silk is genetic editing and engineering of transgenic silks.
Unicellular organisms, especially E. coli, are potential candidates for the expression of recombinant spider silks. This bacterium’s genetic engineering capabilities through recombinant plasmid (DNA) introduction, a method already used for large-scale production of proteins such as insulin, make it a good candidate. This is due to its low cost, short replication time, and scalability. Thus, most recombinant spider silks have been expressed in
E. coli [
12].
The recombinant DNA approach involves creating the spider silk gene and inserting the genetic segment into a suitable DNA vector. This DNA vector is then transformed into a host organism such as E. coli. After this, the proteins are expressed and then purified [
13].
However, there are problems with utilizing
E. coli as a host organism. The first is that the yield is low per individual cell, and this yield decreases the larger the spidroin produced. In addition, the proteins were more likely to undergo a premature synthesis termination the longer the chosen gene length [
13]. Secondly,
E. coli produces endotoxins, which must be removed during the purification process via simple cell wash, adding more steps to the lengthy procedure [
14].
Spidroins have also been produced in yeast organisms, such as
Pichia pastoris, with better success. Synthetic genes were expressed at increased levels under the methanol-inducible AOX1 promoter. They could produce spidroins from genes of 3000 codons or more, with no sign of the truncation seen in
E. coli production, though genes longer than 1600 codons saw less efficient expression compared to shorter genes. These modifications were stable for at least 100 doublings without human intervention [
15]. This shows that yeast organisms such as
Pichia pastoris may be a better option than
E. coli, as larger spidroins can be produced even if the process is less efficient.
2.2. Multicellular Host Production
Another potential candidate for spidroin expression in multicellular organisms. In a lab setting, silk has been made by transgenic silkworms that expressed two spidroins codenamed PySp1 and ASG1, utilizing silkworms’ posterior silk glands. Though authentic spider silk was not created, the two spider genes significantly enhanced the properties of the silk created, as the toughness increased by up to 91.5% [
16]. The benefit of this method is that no endotoxin removal is needed as with bacteria such as E. coli, and silkworm silk collection is a feasible method for large-scale production of spider silk.
In other multicellular organisms such as transgenic plants like tobacco, recombinant spidroins MaSp1 and MaSp2 proteins have been produced. This process was done with two different promoters, in conjunction with a plant secretory signal, a translational enhancer, and an endoplasmic reticulum retention signal; MaSp1 and MaSp2 genes were expressed and successfully accumulated in the leaves of transgenic plants grown in greenhouse and field trials [
17]. However, this method requires additional post-processing to extract the proteins, increasing the complexity and cost of the process.
3. Spider Silk Use in Tissue Engineering
3.1. Function of Extracellular Matrices
Scaffolds, essential to tissue engineering, are crucial in recovering living tissue. They mimic the extracellular matrix or ECM, the material outside the cells, which is a non-cellular three-dimensional framework composed of various glycoproteins such as collagen, elastin, and fibronectin [
18]. They are bound together with cell adhesion receptors, creating a network that stretches through all tissues in organisms. These cell surface receptors take signals from the cells and send them to the ECM, which leads to various cellular functions such as growth, differentiation, and growth. As a result, the ECM is highly dynamic and can constantly be altered. However, its deregulation indicates pathological or harmful conditions in the sample organism [
19].
An ECM has 4 main functions, which a scaffold must replicate to aid recovery and avoid deregulation (
Table 2) [
20].
3.2. Requirements for Scaffolds
The most significant requirement for any scaffold is that it be biocompatible. This means cells should be able to be added, function, and proliferate to eventually lay down matrix, or the substance that fills the space between cells in tissue. During this time, the scaffold should elicit a minimal immune system response from the body [
20].
The next requirement is that the scaffold be degradable, as the scaffold’s role is to aid tissue regeneration. The scaffold needs to degrade at the perfect rate so the body can grow new tissue unconstricted and slow enough to support tissue regeneration [
21]. This ensures seamless regeneration and the absence of adverse effects.
Lastly, the scaffold architecture is of utmost importance, as for cellular penetration and diffusion of nutrients to happen, a high porosity must be achieved. By allowing cellular penetration and diffusion of nutrients, the surrounding tissue can receive the necessary nutrients, and the waste in the area occupied by the scaffold can be removed. In addition, mean pore size is essential as cells interact with scaffolds via ligands, and pores need to be large enough for cells to migrate into the scaffold and bind to the ligands. This pore size depends on the location of the scaffold, as separate locations use different ligands, which can be unique sizes [
22]. Thus, a scaffold must be able to manipulate its porosity to allow for proper cell interaction, development, and function.
3.3. Biocompatibility of Spider Silk
Spider silk has numerous properties that aid tissue regeneration, making it an excellent scaffold choice. It has displayed antimicrobial properties, as in an experiment using native spider silk as a wound dressing, minimal inflammatory response was observed. The spider silk treatment saw a minimal release of cytokines that promote inflammation and tissue growth in terms of greater migration of keratinocytes and fibroblasts. It also saw comparable wound closure compared to standard treatments macroscopically [
23]. This indicates that it can promote tissue regeneration.
Spider silk is also biodegradable. Spider egg sac silk’s biodegradation in vivo and in vitro have been studied and have been found to have little biodegradation compared to Vicryl, a popular suture used for short-term wound recovery that is a reliable and cost-effective option made from polyglactin 910[
24,
25]. One drawback, however, is that the enzymatic cleaning needed to be safe for human implantation/use requires it to be treated with chemicals. This reduces its tensile properties and enhances biocompatibility, making spider egg sac silk suitable for biomedical applications where slow biodegradability is desired [
24].
3.4. Current Spider Silk Scaffolds
Various methods exist to incorporate spider silk components or spidroins into scaffolds to develop effective and functional tissue engineering components. Scaffolds that use spidroins can be divided into two categories: those that use spidroins as the main component and those that use spidroins as a secondary component.
For the first category, scaffolds that use spidroins as the main component, the most common method is salt leaching. Salt leaching is a novel method for processing materials or solutions into porous materials, such as 3D foams. This method works by using a porogen with a polymer solution, and the porogen is then leached out, hence the method’s name. This leaves highly porous sponges as the inorganic salts used are not soluble in the solvents used to degrade the biodegradable polymers. After the mixture hardens, the porogen can be removed with another solvent, leaving a foam-like scaffold structure behind [
26].
One lab took the recombinant spider silk protein eADF4(C16) and a variant of this gene containing an RGD motif (essential for binding cells to the ECM). It was processed into 3D scaffolds for soft tissue engineering applications [
27]. They utilized salt leaching to manufacture these scaffolds, but unlike similar scaffolds, the new scaffold had low swelling. By changing the salt crystal size, the pores sizes and porosity of these 3D foams can be adjusted. In addition, fibroblasts, the cell type used, could adhere and reproduce on the modified gene variant with the RGD motif [
27].
Another lab developed a similar scaffold, albeit for a different purpose. They made a scaffold completely out of recombinant spidroins to develop fibroblasts. The structure was based on the known nucleotide sequence of the cDNA from
Nephila Clavipes and was then amplified in E. coli. This process also uses salt leaching, where the pore sizes depend on the NaCl particle size. When placed in cell culture, the fibroblasts properly proliferated within and provided adequate cell adhesion and support for their development. However, the team found that while the scaffold was stable in PBS, there was rapid degradation upon being placed in oxidizing agents such as Fenton’s reagent, miming conditions during an acute tissue response. In addition, there was a mild foreign body response, which indicates that the scaffold would require periodic monitoring to avoid excessive inflammation or tissue rejection [
28].
The secondary avenue, scaffolds that use spidroins as a secondary component, has seen similar success. In an experiment, nonwoven mesh tubes were made from recombinant spidroins and filled with collagen fibers. This enabled neuronal cell differentiation with neurites (projections that extend from the main cell body of a neuron and allow communication with other cells) capable of firing action potential and forming functional synapses. This tube can also prevent inflammatory cells from moving downstream, maintaining nutrients, gas, and waste flow via its porous structure [
29].
As an alternative to having the spidroins as an individual component like the mesh tubes described above, other groups have integrated two components: chimeric spidroin NTW
1-4CT and poly L-lactic-
co-ε-caprolactone or PLCL into functional nanofibrous using electrospinning, a method of producing fine fibers by using an electrical field. These scaffolds have smaller nanofibers, increased porosity, tensile strength, and wettability - the ability of liquids to keep contact with a given surface [
30,
31]
. The scaffolds created were compared to pure PLCL scaffolds.
Thus, regardless of the chosen avenue, current spidroins scaffolds have seen preliminary success [
30].
4. The Issues with Existing Spidroins Scaffolds
One glaring issue is that the integrity of the scaffold can be potentially compromised. In an experiment that used silk from major ampullate spidroin one from the species
Nephila Clavipes, in addition to a peptide to target cancer cells, a higher anti-silk antibody titer was observed. There was also a certain degree of immunological response to the treatment [
32]. This indicates that the scaffold can be targeted by the body, potentially leading to chronic inflammation of surrounding tissue or premature scaffold biodegradation. The premature degradation of the scaffold during implantation would have disastrous effects on tissue regrowth and may make it impossible for another scaffold to be reimplanted to replace it.
The second most significant issue is the unintentional addition of endotoxins to recombinant proteins during preparation. This is because endotoxins can be made by the host cell used to produce the biomaterial. For example,
E. coli can produce lipopolysaccharides as an endotoxin. Though endotoxins can be removed, this process is tedious and thus expensive [
33].
Lastly, a more viable commercial method for creating spidroins must be developed that allows researchers and labs to modify the chosen spider gene for application. Currently, labs are limited to shorter spidroins, as longer spidroins require more time for processing and may be prematurely truncated. This reduces the number of properties that can be enhanced and limits the post-processing addition of active ingredients/biomolecules, as the smaller proteins lead to weaker scaffolds overall [
14,
34].
Thus, if we want to see a viable scaffold used in humans during our lifetime, we must develop a cheaper and more scalable way of creating larger spidroins, and these spidroins would have to be edited such that they wouldn’t elicit a response from the human immunological system.
References
- Dockrill, P. (2017, March 15). Spiders eat more each year than all humans put together. ScienceAlert. https://www.sciencealert.com/spiders-eat-more-each-year-than-all-humans-put-together#:~:text=Taking%20into%20account%20the%20various%20density%20of%20spider,and%20800%20million%20tonnes%20of%20prey%20each%20year.
- Atwood, A. (2024, February 23). Spider methods of capturing prey. Spider Facts and Information. https://spidersworlds.com/spider-methods-of-capturing-prey/.
- Wu, K. J. (2020, November 4). Spider silk is stronger than steel. it also assembles itself. The New York Times. https://www.nytimes.com/2020/11/04/science/spider-silk-web-self-assembly.html.
- Morgan, E. (2016). Sticky Layers and Shimmering Weaves: A Study of Two Human Uses of Spider Silk. Journal of Design History, 29(1), 8-23. Retrieved 3 5, 2024, from https://repository.lboro.ac.uk/articles/sticky_layers_and_shimmering_weaves_a_study_of_two_human_uses_of_spider_silk/9335912.
- Orkin. (2021, August 3). What is spider silk?: Spider Silk Types & Facts. https://www.orkin.com/pests/spiders/spider-silk-facts-and-information.
- Malay, A. D., Arakawa, K., & Numata, K. (2017). Analysis of repetitive amino acid motifs reveals the essential features of spider dragline silk proteins. PloS one, 12(8), e0183397. [CrossRef]
- Römer, L., & Scheibel, T. (2008). The elaborate structure of spider silk: structure and function of a natural high-performance fiber. Prion, 2(4), 154–161. [CrossRef]
- Wang, Q., McArdle, P., Wang, S.L. et al. Protein secondary structure in spider silk nanofibrils. Nat Commun 13, 4329 (2022). [CrossRef]
- Ko, F. K., & Jovicic, J. (2004). Modeling of mechanical properties and structural design of Spider Web. Biomacromolecules, 5(3), 780–785. [CrossRef]
- Malay, A. D., Suzuki, T., Katashima, T., Kono, N., Arakawa, K., & Numata, K. (2020). Spider silk self-assembly via modular liquid-liquid phase separation and nanofibrillation. Science Advances, 6(45). [CrossRef]
- Lesté-Lasserre, C. (2021, May 5). Female black widow spider mates with and eats multiple males. New Scientist. https://www.newscientist.com/article/2276085-female-black-widow-spider-mates-with-and-eats-multiple-males/.
- Tokareva, O., Michalczechen-Lacerda, V. A., Rech, E. L., & Kaplan, D. L. (2013). Recombinant DNA production of spider silk proteins. Microbial biotechnology, 6(6), 651–663. [CrossRef]
- Debabov, V. G., & Bogush, V. G. (2020). Recombinant Spidroins as the Basis for New Materials. ACS biomaterials science & engineering, 6(7), 3745–3761. [CrossRef]
- Rising, A., Widhe, M., Johansson, J., & Hedhammar, M. (2011). Spider silk proteins: recent advances in recombinant production, structure-function relationships and biomedical applications. Cellular and molecular life sciences : CMLS, 68(2), 169–184. [CrossRef]
- Fahnestock, S. R., & Bedzyk, L. A. (1997). Production of synthetic spider dragline silk protein in Pichia pastoris. Applied Microbiology and Biotechnology, 47(1), 33–39. [CrossRef]
- Tang, X., Ye, X., Wang, X., Zhao, S., Wu, M., Ruan, J., & Zhong, B. (2021). High mechanical property silk produced by transgenic silkworms expressing the spidroins pysp1 and ASG1. Scientific Reports, 11(1). [CrossRef]
- Menassa, R., Zhu, H., Karatzas, C. N., Lazaris, A., Richman, A., & Brandle, J. (2004). Spider dragline silk proteins in transgenic tobacco leaves: accumulation and field production. Plant biotechnology journal, 2(5), 431–438. [CrossRef]
- Okamoto, M. (2019). The role of scaffolds in tissue engineering. Handbook of Tissue Engineering Scaffolds: Volume One, 23–49. [CrossRef]
- Theocharis, A. D., Skandalis, S. S., Gialeli, C., & Karamanos, N. K. (2016). Extracellular matrix structure. Advanced drug delivery reviews, 97, 4–27. [CrossRef]
- O’Brien, F. J. (2011). Biomaterials & Scaffolds for tissue engineering. Materials Today, 14(3), 88–95. [CrossRef]
- Tajvar, S., Hadjizadeh, A., & Samandari, S. S. (2023). Scaffold degradation in Bone Tissue Engineering: An overview. International Biodeterioration & Biodegradation, 180, 105599. [CrossRef]
- Loh, Q. L., & Choong, C. (2013). Three-dimensional scaffolds for tissue engineering applications: role of porosity and pore size. Tissue engineering. Part B, Reviews, 19(6), 485–502. [CrossRef]
- Liebsch, C., Bucan, V., Menger, B., Köhne, F., Waldmann, K. H., Vaslaitis, D., Vogt, P. M., Strauss, S., & Kuhbier, J. W. (2018). Preliminary investigations of spider silk in wounds in vivo - Implications for an innovative wound dressing. Burns : journal of the International Society for Burn Injuries, 44(7), 1829–1838. [CrossRef]
- Gellynck, K., Verdonk, P., Forsyth, R., Almqvist, K. F., Van Nimmen, E., Gheysens, T., Mertens, J., Van Langenhove, L., Kiekens, P., & Verbruggen, G. (2008). Biocompatibility and biodegradability of Spider Egg Sac Silk. Journal of Materials Science: Materials in Medicine, 19(8), 2963–2970. [CrossRef]
- Khdour, H. (2024, March 29). Vicryl suture: Materials, types, uses, effectiveness. Medicogenic. https://medicogenic.com/vicryl-suture-materials-types-uses-effectiveness.
- Tessmar, J., Holland, T., & Mikos, A. (2005). Salt leaching for polymer scaffolds. Scaffolding In Tissue Engineering, 111–124. [CrossRef]
- Schacht, K., Vogt, J., & Scheibel, T. (2016). Foams Made of Engineered Recombinant Spider Silk Proteins as 3D Scaffolds for Cell Growth. ACS biomaterials science & engineering, 2(4), 517–525. [CrossRef]
- Moisenovich, M. M., Pustovalova, O. L., Arhipova, A. Y., Vasiljeva, T. V., Sokolova, O. S., Bogush, V. G., Debabov, V. G., Sevastianov, V. I., Kirpichnikov, M. P., & Agapov, I. I. (2011). In vitro and in vivo biocompatibility studies of a recombinant analogue of spidroin 1 scaffolds. Journal of biomedical materials research. Part A, 96(1), 125–131. [CrossRef]
- Pawar, K., Welzel, G., Haynl, C., Schuster, S., & Scheibel, T. (2019). Recombinant spider silk and collagen-based nerve guidance conduits support neuronal cell differentiation and functionality in vitro. ACS Applied Bio Materials, 2(11), 4872–4880. [CrossRef]
- Zhou, Y., Shen, Q., Lin, Y., Xu, S., & Meng, Q. (2020). Evaluation of the potential of chimeric spidroins/poly(l-lactic-co-ε-caprolactone) (PLCL) nanofibrous scaffolds for tissue engineering. Materials Science and Engineering: C, 111. [CrossRef]
- Zhang, M., Chu, L., Chen, J., Qi, F., Li, X., Chen, X., & Yu, D.-G. (2024). Asymmetric wettability fibrous membranes: Preparation and biologic applications. Composites Part B: Engineering, 269. [CrossRef]
- Deptuch, T., Penderecka, K., Kaczmarek, M., Molenda, S., & Dams-Kozlowska, H. (2022). In vivo study of the immune response to bioengineered spider silk spheres. Scientific reports, 12(1), 13480. [CrossRef]
- Gorbet, M. B., & Sefton, M. V. (2005). Endotoxin: the uninvited guest. Biomaterials, 26(34), 6811–6817. [CrossRef]
- Branković, M., Zivic, F., Grujovic, N., Stojadinovic, I., Milenkovic, S., & Kotorcevic, N. (2024). Review of Spider Silk Applications in Biomedical and Tissue Engineering. Biomimetics, 9(3). [CrossRef]
Table 1.
All images are the creation of the author. Types of spider silk are identified by the gland type and differentiated by function.
Table 2.
The functions of an ECM and how a scaffold accomplishes the specific functions.
Table 2.
The functions of an ECM and how a scaffold accomplishes the specific functions.
| How do Scaffolds function as ECM? |
| |
Function of ECM |
Matching function of Scaffold |
| 1 |
Provide support for natural cells |
Provide support for artificial cells to mature and differentiate |
| 2 |
Provide mechanical properties |
Help stabilize injured tissue and engineered tissue |
| 3 |
Provide directions for cells in response to the environment |
Interact with other cells proactively to lead to proliferation and differentiation |
| 4 |
Contain growth factors and release them as needed |
Function as a delivery agent and storage for externally applied growth factors |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).