Submitted:
23 August 2024
Posted:
26 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Cohorts
Clinical Data and Definitions
Metabolomic Profiling and Processing
Software, Statistical and Bioinformatics Analysis
Pathway Analysis
Univariate Associations
3. Results
Demographic Characteristics
| never/former smokers without COPD or emphysema | current smokers without COPD or emphysema | former/current smokers with COPD or emphysema | ||||||
| COPDGene (N=1,346) |
SPIROMICS (N=413) |
COPDGene (N=818) |
SPIROMICS (N=323) |
COPDGene (N=3,540) |
SPIROMICS (N=1,681) | P-value | ||
| Age (years), mean (SD) | 65.4 (9.25) | 62.9 (9.51) | 59.1 (6.33) | 55.2 (8.82) | 66.2 (8.56) | 65.0 (8.05) | < 0.001 | |
| Race White/Black/Other % | 87.4/12.6/0 | 81.8/12.3/5.9 | 42.7/57.3/0 | 55.7/38.7/5.6 | 71.3/28.7/0 | 80.5/15.2/4.3 | < 0.001 | |
| Gender, Male, n (%) | 582 (43.2%) | 181 (43.8%) | 387 (47.3%) | 154 (47.7%) | 1888 (53.3%) | 945 (56.2%) | < 0.001 | |
| Smoking Status Never/Former/Current % | 29.3/70.7/0 | 40/60/0 | 0/0/100 | 0/0/100 | 0/65.2/34.8 | 0/67.3/32.7 | NA | |
| Num. recent exacerbations | 0 (0, 1.00) | 0 (0, 1.00) | 0 (0, 1.00) | 0 (0, 2.00) | 0 (0, 2.00) | 0 (0, 2.00) | < 0.001 | |
| GOLD stage, n (%) | NA | |||||||
| GOLD 0 | 951 (70.7%) | 248 (60.0%) | 818 (100%) | 323 (100%) | 514 (14.5%) | 130 (7.7%) | ||
| GOLD 1 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 507 (14.3%) | 334 (19.9%) | ||
| GOLD 2 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1063 (30.0%) | 667 (39.7%) | ||
| GOLD 3 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 565 (16.0%) | 347 (20.6%) | ||
| GOLD 4 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 235 (6.6%) | 142 (8.4%) | ||
| PRISm | 12 (0.9%) | 0 (0%) | 0 (0%) | 0 (0%) | 656 (18.5%) | 61 (3.6%) | ||
| Never smoker | 383 (28.5%) | 165 (40.0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| FEV1 (Liters) | 2.71 (0.700) | 2.85 (0.697) | 2.69 (0.671) | 2.89 (0.704) | 1.86 (0.792) | 1.86 (0.823) | NA | |
| Emphysema% | 1.59 (1.85) | 1.64 (1.39) | 0.883 (1.06) | 0.997 (0.903) | 8.01 (10.6) | 10.4 (11.2) | NA | |
| FVC (Liters) | 3.45 (0.876) | 3.62 (0.882) | 3.45 (0.884) | 3.71 (0.908) | 2.98 (0.967) | 3.38 (1.06) | NA | |
| FEV1/FVC | 0.787 (0.0493) | 0.788 (0.0505) | 0.784 (0.0487) | 0.782 (0.0487) | 0.616 (0.151) | 0.542 (0.147) | NA | |
| History of diabetes, n (%) | 169 (12.6%) | 54 (13.1%) | 125 (15.3%) | 25 (7.7%) | 670 (18.9%) | 231 (13.7%) | < 0.001 | |
| History of stroke, n (%) | 20 (1.5%) | 14 (3.4%) | 28 (3.4%) | 10 (3.1%) | 130 (3.7%) | 66 (3.9%) | 0.00323 | |
| History of heart attack, n (%) | 56 (4.2%) | 17 (4.1%) | 32 (3.9%) | 5 (1.5%) | 244 (6.9%) | 123 (7.3%) | < 0.001 | |
| History of coronary artery disease, n (%) | 86 (6.4%) | 24 (5.8%) | 32 (3.9%) | 7 (2.2%) | 346 (9.8%) | 172 (10.2%) | < 0.001 | |
| Chronic Bronchitis, n (%) | 46 (3.4%) | 30 (7.3%) | 116 (14.2%) | 74 (22.9%) | 643 (18.2%) | 367 (21.8%) | < 0.001 | |
| Exacerbations included those treated with antibiotics and/or corticosteroids in the 12 months prior to the visit; shown are n (percentage), mean (standard deviation), or median (5th, 95th percentiles); spirometry volumes are in post-bronchodilator therapy and in liters; GOLD 0 (FEV1 >= 80% & FEV1/FVC >= 0.7) | GOLD 1 (FEV1 >= 80% & FEV1 /FVC < 0.7) | GOLD 2 (50% <= FEV1 < 80% & FEV1 /FVC < 0.7) | GOLD 3 (30% <= FEV1 < 50% & FEV1/FVC < 0.7) | GOLD 4 (FEV1 < 30% & FEV1 /FVC < 0.7) | PRISm (Preserved Ratio, Impaired Spirometry) (FEV1/FVC >= 0.7 but FEV1 < 80%); history of diabetes, stroke, heart attack, and coronary artery disease based on subject self-report; chronic Bronchitis defined by answers to questions about both cough and phlegm. | ||||||||
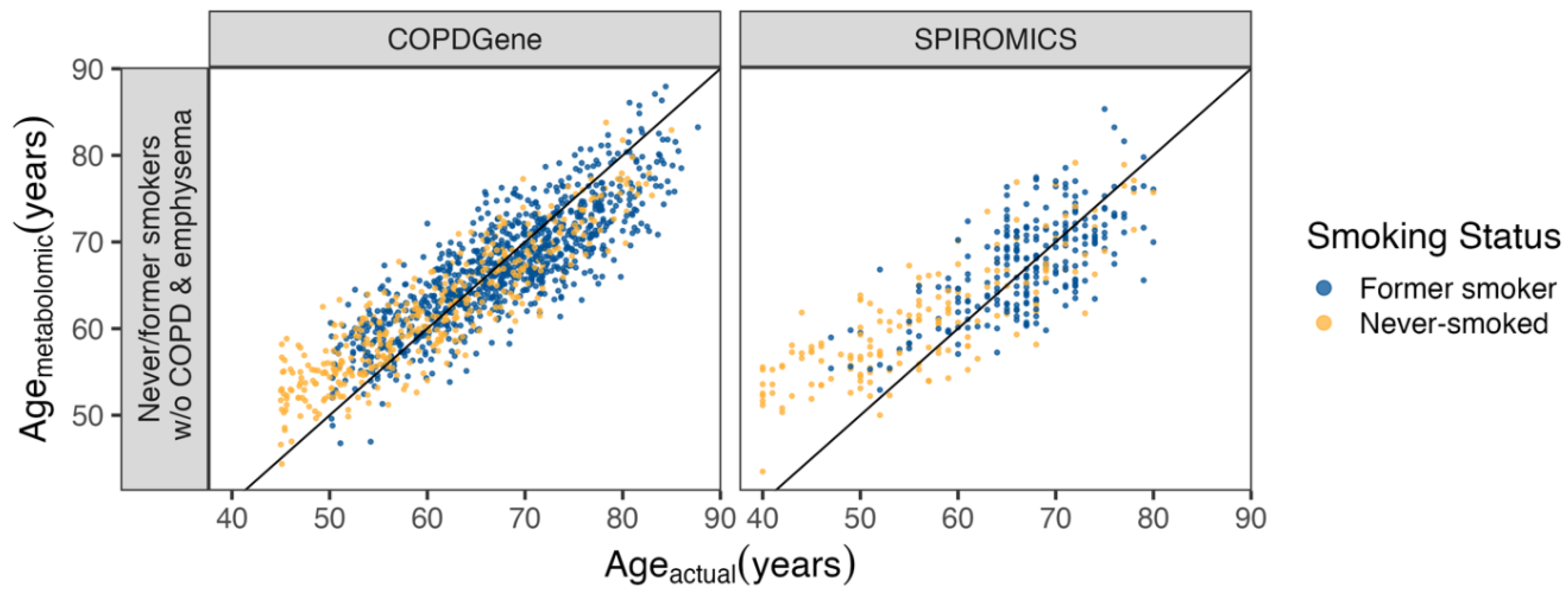
Metabolomic Age Score
Differences between COPD Subjects with Accelerated and Decelerated Metabolomic Age
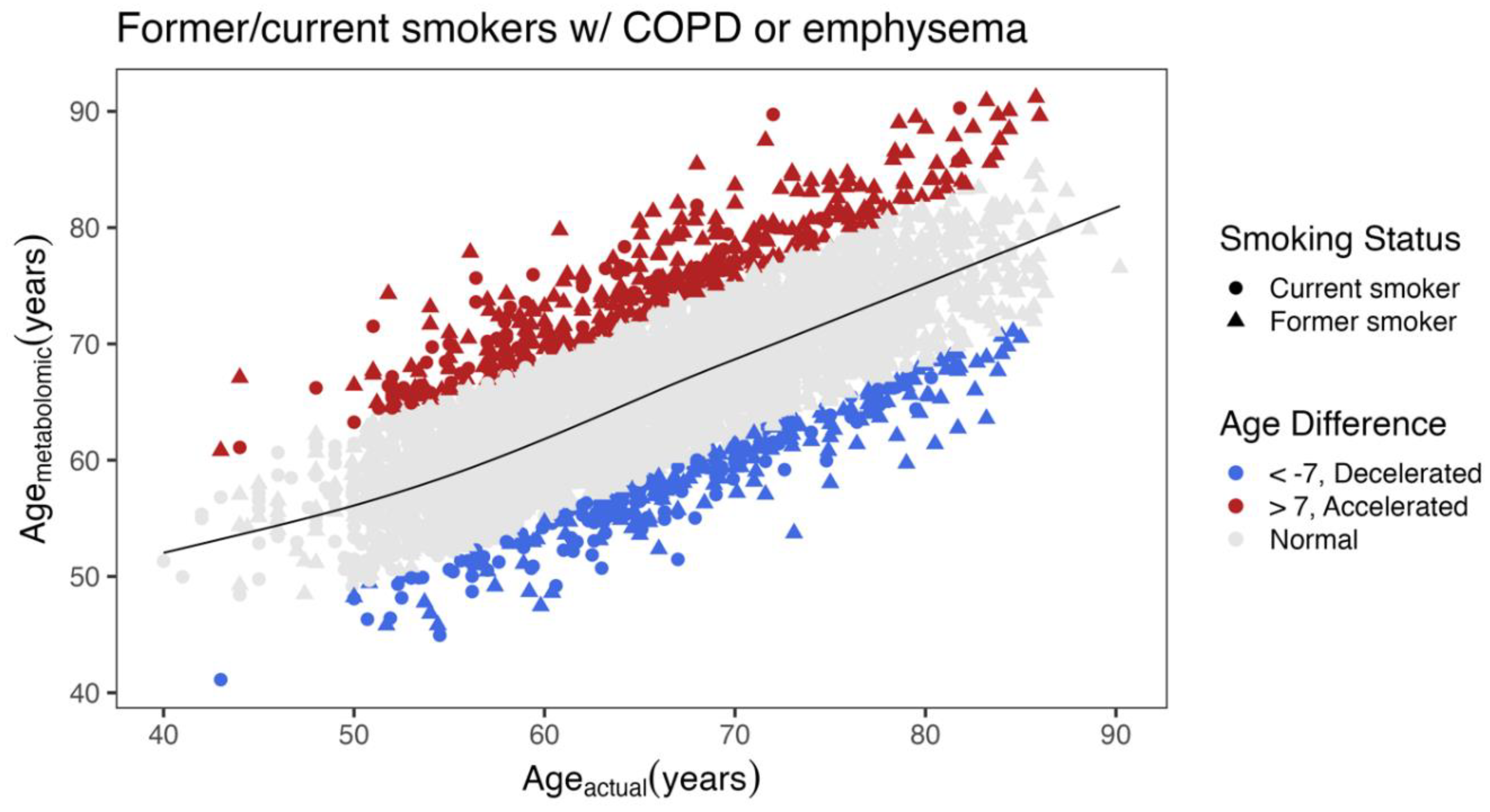
| Decelerated (N=277) |
Accelerated (N=400) |
P-value | |||
| Age (years), mean (SD) | 67.9 (8.32) | 65.3 (8.56) | < 0.001 | ||
| Metabolomic age (years) | 58.3 (5.79) | 75.2 (6.18) | NA | ||
| Race White/Black/Other % | 45.1/53.4/1.5 | 88.3/10.5/1.4 | < 0.001 | ||
| Gender, Male, n (%) | 197 (71.1%) | 172 (43.0%) | < 0.001 | ||
| Smoking Status Former/Current % | 57.8/42.2 | 75.0/25.0 | < 0.001 | ||
| Exacerbations | 0 (0, 2.00) | 0 (0, 2.00) | 0.0178 | ||
| GOLD stage, n (%) | |||||
| GOLD 0 | 38 (13.7%) | 27 (6.8%) | < 0.001 | ||
| GOLD 1 | 65 (23.5%) | 45 (11.3%) | |||
| GOLD 2 | 90 (32.5%) | 131 (32.8%) | |||
| GOLD 3 | 41 (14.8%) | 93 (23.3%) | |||
| GOLD 4 | 10 (3.6%) | 50 (12.5%) | |||
| PRISm | 33 (11.9%) | 54 (13.5%) | |||
| FEV1 (liters) | 2.00 (0.801) | 1.60 (0.724) | < 0.001 | ||
| Emphysema% | 7.16 (9.45) | 10.8 (12.4) | < 0.001 | ||
| FVC | 3.21 (0.967) | 2.87 (0.938) | < 0.001 | ||
| FEV1/FVC | 0.613 (0.136) | 0.554 (0.160) | < 0.001 | ||
| History of diabetes, n (%) | 43 (15.5%) | 96 (24.0%) | 0.00879 | ||
| History of stroke, n (%) | 12 (4.3%) | 29 (7.3%) | 0.157 | ||
| History of heart attack, n (%) | 7 (2.5%) | 59 (14.8%) | < 0.001 | ||
| History of coronary artery disease, n (%) | 11 (4.0%) | 79 (19.8%) | < 0.001 | ||
| Chronic Bronchitis, n (%) | 52 (18.8%) | 87 (21.8%) | 0.396 | ||
| Exacerbations included those treated with antibiotics and/or corticosteroids in the 12 months prior to the visit; shown are n (percentage), mean (standard deviation), or median (5th, 95th percentiles); spirometry volumes are in post-bronchodilator therapy and in liters; GOLD 0 (FEV1 >= 80% & FEV1/FVC >= 0.7) | GOLD 1 (FEV1 >= 80% & FEV1 /FVC < 0.7) | GOLD 2 (50% <= FEV1 < 80% & FEV1 /FVC < 0.7) | GOLD 3 (30% <= FEV1 < 50% & FEV1/FVC < 0.7) | GOLD 4 (FEV1 < 30% & FEV1 /FVC < 0.7) | PRISm (Preserved Ratio, Impaired Spirometry) (FEV1/FVC >= 0.7 but FEV1 < 80%); history of diabetes, stroke, heart attack, and coronary artery disease based on subject self-report; chronic Bronchitis defined by answers to questions about both cough and phlegm. | |||||
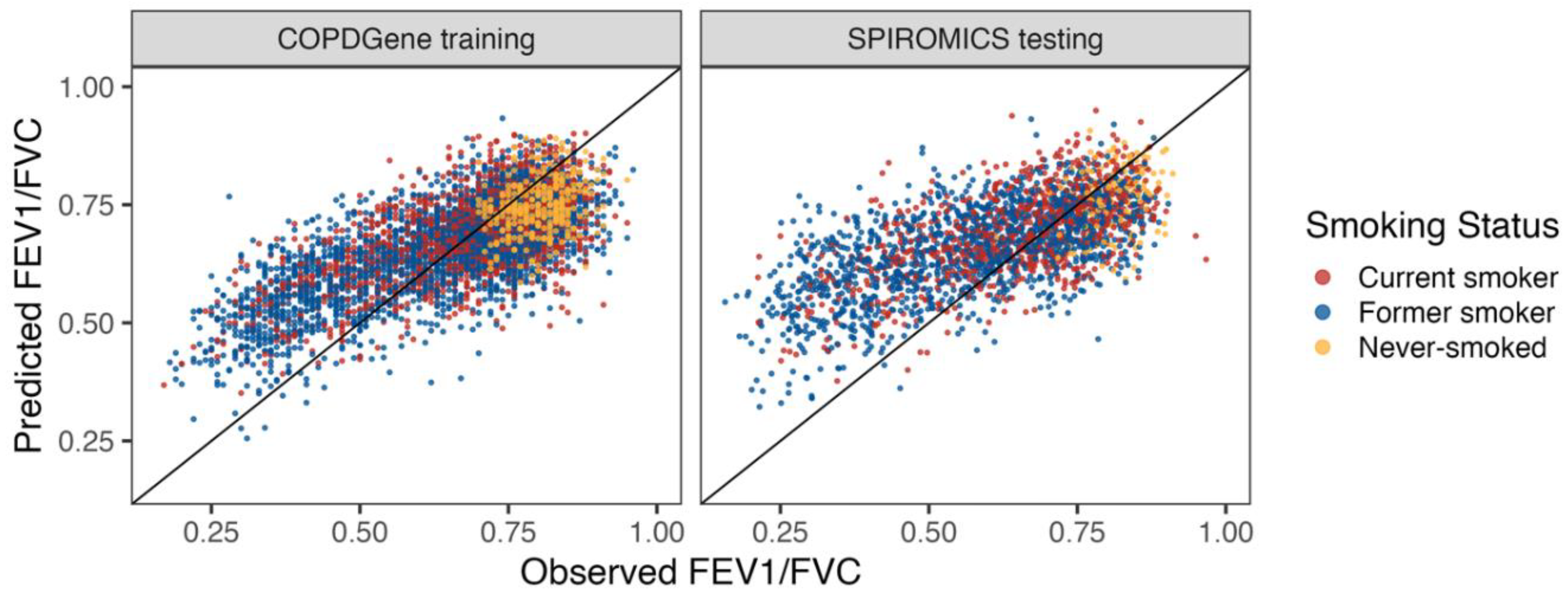
A metabolomic Lung Obstruction Score
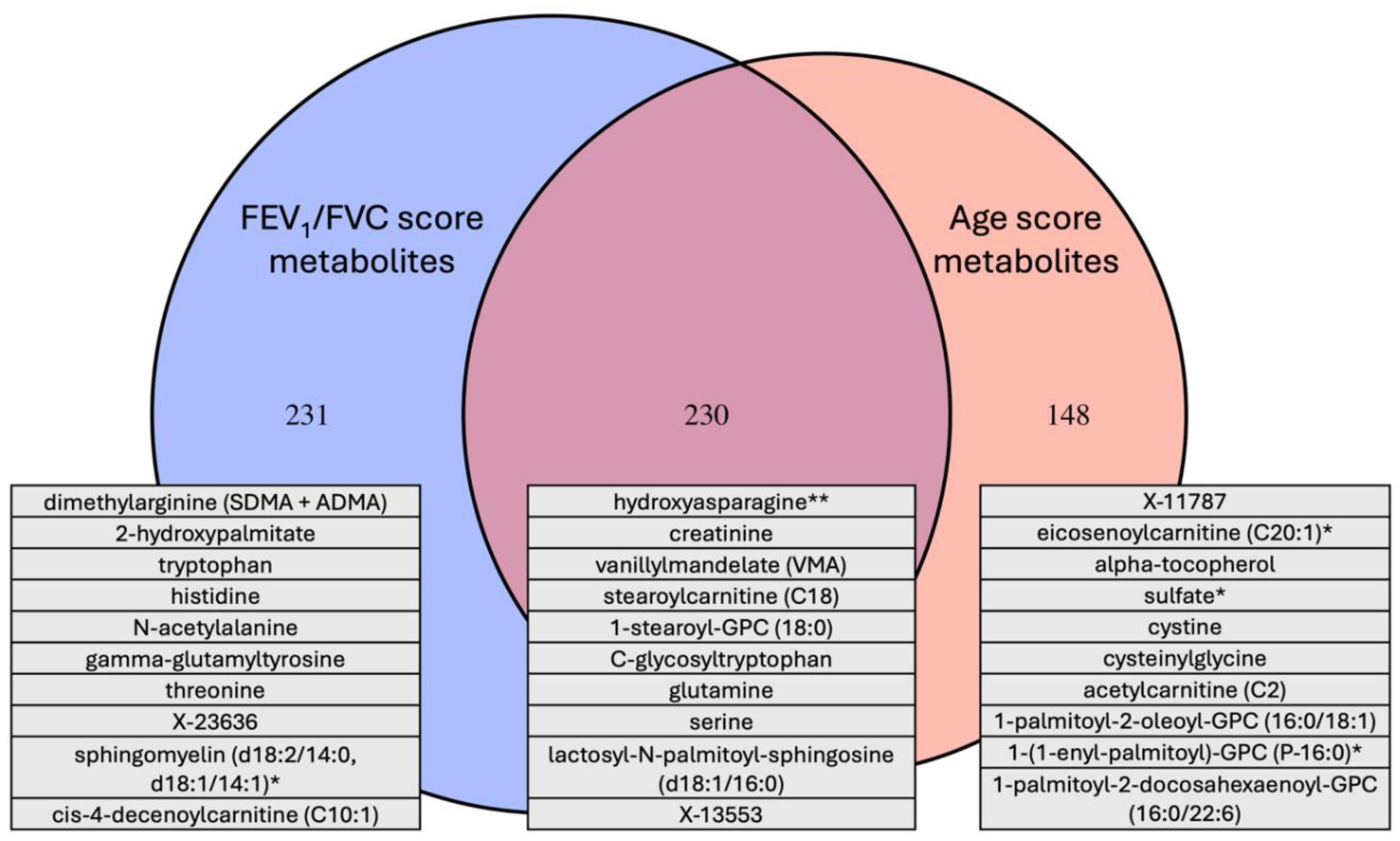
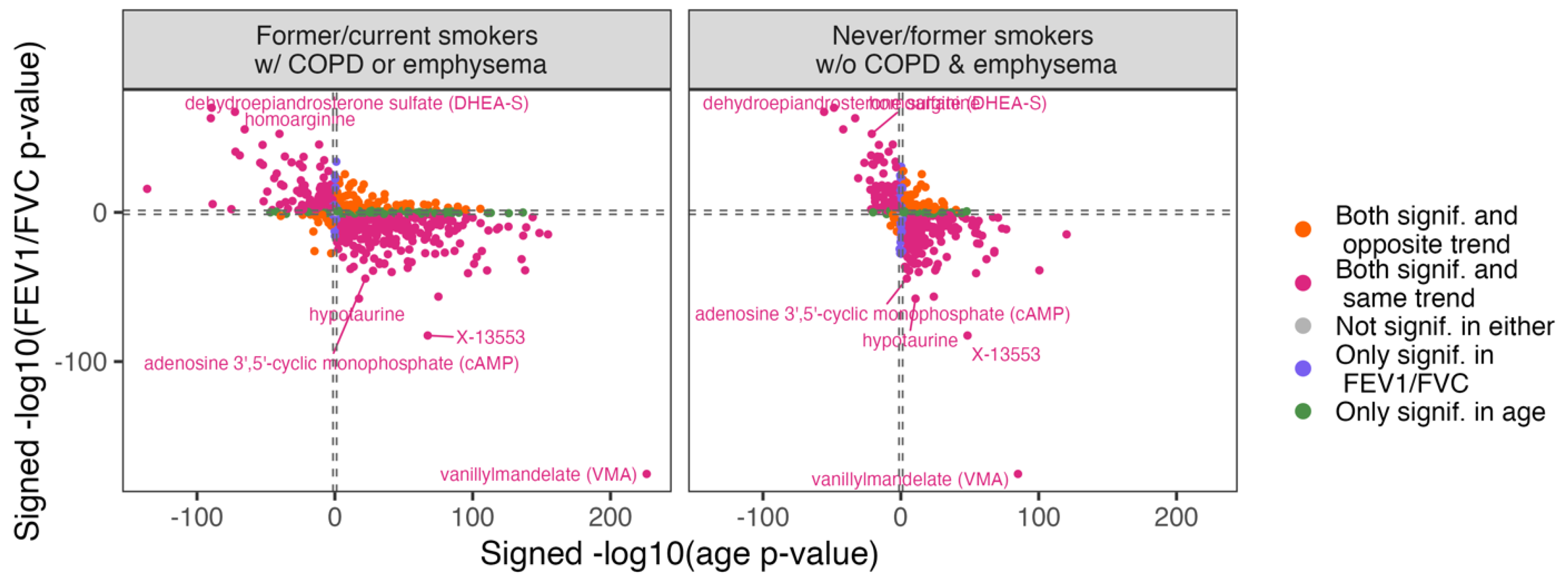
Overlap between the Age and COPD Metabolome Scores
Overlap between Metabolite Univariate Associations with Age and COPD
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zamzam, M.A.; Azab, N.Y.; El Wahsh, R.A.; Ragab, A.Z.; Allam, E.M. Quality of life in COPD patients. Egyptian Journal of Chest Diseases and Tuberculosis. 2012, 61, 281–289. [Google Scholar] [CrossRef]
- Syamlal, G.; Kurth, L.M.; Dodd, K.E.; Blackley, D.J.; Hall, N.B.; Mazurek, J.M. Chronic Obstructive Pulmonary Disease Mortality by Industry and Occupation - United States, 2020. MMWR Morb Mortal Wkly Rep. 2022, 71, 1550–1554. [Google Scholar] [CrossRef]
- Agusti, A.; Celli, B.R.; Criner, G.J.; et al. Global Initiative for Chronic Obstructive Lung Disease 2023 Report: GOLD Executive Summary. Am J Respir Crit Care Med. 2023, 207, 819–837. [Google Scholar] [CrossRef] [PubMed]
- Agusti, A.; Soriano, J.B. COPD as a systemic disease. COPD. 2008, 5, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Divo, M.J.; Celli, B.R.; Poblador-Plou, B.; et al. Chronic Obstructive Pulmonary Disease (COPD) as a disease of early aging: Evidence from the EpiChron Cohort. PLoS One. 2018, 13, e0193143. [Google Scholar] [CrossRef] [PubMed]
- Adav, S.S.; Wang, Y. Metabolomics Signatures of Aging: Recent Advances. Aging Dis. 2021, 12, 646–661. [Google Scholar] [CrossRef] [PubMed]
- Auro, K.; Joensuu, A.; Fischer, K.; et al. A metabolic view on menopause and ageing. Nat Commun. 2014, 5, 4708. [Google Scholar] [CrossRef]
- Chaleckis, R.; Murakami, I.; Takada, J.; Kondoh, H.; Yanagida, M. Individual variability in human blood metabolites identifies age-related differences. Proc Natl Acad Sci U S A. 2016, 113, 4252–4259. [Google Scholar] [CrossRef]
- Jove, M.; Mate, I.; Naudi, A.; et al. Human Aging Is a Metabolome-related Matter of Gender. J Gerontol A Biol Sci Med Sci. 2016, 71, 578–585. [Google Scholar] [CrossRef]
- Godbole, S.; Bowler, R.P. Metabolome Features of COPD: A Scoping Review. Metabolites. 2022, 12. [Google Scholar] [CrossRef]
- Pinto-Plata, V.; Casanova, C.; Divo, M.; et al. Plasma metabolomics and clinical predictors of survival differences in COPD patients. Respir Res. 2019, 20, 219. [Google Scholar] [CrossRef] [PubMed]
- Godbole, S.; Labaki, W.W.; Pratte, K.A.; et al. A Metabolomic Severity Score for Airflow Obstruction and Emphysema. Metabolites. 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; You, H.; Xu, M.Y.; et al. A Novel Metabolic Score for Predicting the Acute Exacerbation in Patients with Chronic Obstructive Pulmonary Disease. Int J Chron Obstruct Pulmon Dis. 2023, 18, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Vaarhorst, A.A.; Verhoeven, A.; Weller, C.M.; et al. A metabolomic profile is associated with the risk of incident coronary heart disease. Am Heart J. 2014, 168, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhu, C.; Nambi, V.; et al. Metabolomic Pattern Predicts Incident Coronary Heart Disease. Arterioscler Thromb Vasc Biol. 2019, 39, 1475–1482. [Google Scholar] [CrossRef]
- Floegel, A.; Stefan, N.; Yu, Z.; et al. Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes. 2013, 62, 639–648. [Google Scholar] [CrossRef]
- Oexner, R.R.; Ahn, H.; Theofilatos, K.; et al. Serum metabolomics improves risk stratification for incident heart failure. Eur J Heart Fail. 2024, 26, 829–840. [Google Scholar] [CrossRef]
- Alotaibi, M.; Liu, Y.; Magalang, G.A.; et al. Deriving Convergent and Divergent Metabolomic Correlates of Pulmonary Arterial Hypertension. Metabolites. 2023, 13. [Google Scholar] [CrossRef]
- Horvath, S. DNA methylation age of human tissues and cell types. Genome Biol. 2013, 14, R115. [Google Scholar] [CrossRef]
- Hannum, G.; Guinney, J.; Zhao, L.; et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cell. 2013, 49, 359–367. [Google Scholar] [CrossRef]
- Rutledge, J.; Oh, H.; Wyss-Coray, T. Measuring biological age using omics data. Nat Rev Genet. 2022, 23, 715–727. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, W.; Duan, Y.; et al. Progress in biological age research. Front Public Health. 2023, 11, 1074274. [Google Scholar] [CrossRef] [PubMed]
- Regan, E.A.; Hokanson, J.E.; Murphy, J.R.; et al. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010, 7, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Couper, D.; LaVange, L.M.; Han, M.; et al. Design of the Subpopulations and Intermediate Outcomes in COPD Study (SPIROMICS). Thorax. 2014, 69, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Gillenwater, L.A.; Pratte, K.A.; Hobbs, B.D.; et al. Plasma Metabolomic Signatures of Chronic Obstructive Pulmonary Disease and the Impact of Genetic Variants on Phenotype-Driven Modules. Netw Syst Med. 2020, 3, 159–181. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, Y.; Benjamini, Y. More powerful procedures for multiple significance testing. Stat Med. 1990, 9, 811–818. [Google Scholar] [CrossRef]
- Li, S.; Kim, H.E. Implications of Sphingolipids on Aging and Age-Related Diseases. Front Aging. 2021, 2, 797320. [Google Scholar] [CrossRef]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell. 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Houben, J.M.; Mercken, E.M.; Ketelslegers, H.B.; et al. Telomere shortening in chronic obstructive pulmonary disease. Respir Med. 2009, 103, 230–236. [Google Scholar] [CrossRef]
- Aoshiba, K.; Zhou, F.; Tsuji, T.; Nagai, A. DNA damage as a molecular link in the pathogenesis of COPD in smokers. European Respiratory Journal. 2012, 39, 1368–1376. [Google Scholar] [CrossRef]
- Caramori, G.; Adcock, I.M.; Casolari, P.; et al. Unbalanced oxidant-induced DNA damage and repair in COPD: a link towards lung cancer. Thorax. 2011, 66, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.C.; Rabinovitch, P.S.; Kaeberlein, M. mTOR is a key modulator of ageing and age-related disease. Nature. 2013, 493, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Gu, Y.; Yang, M.; Cao, D.; Wu, F. The Gene Expression Biomarkers for Chronic Obstructive Pulmonary Disease and Interstitial Lung Disease. Front Genet. 2019, 10, 1154. [Google Scholar] [CrossRef]
- Fukuda, M.; Hata, A.; Niwa, S.; et al. Plasma vanillylmandelic acid level as an index of psychological stress response in normal subjects. Psychiatry Res. 1996, 63, 7–16. [Google Scholar] [CrossRef]
- Aydin, M.; Altintas, N.; Cem Mutlu, L.; et al. Asymmetric dimethylarginine contributes to airway nitric oxide deficiency in patients with COPD. Clin Respir J. 2017, 11, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.A.; Duongh, M.; Young, A.W.; Subbarao, P.; Gauvreau, G.M.; Grasemann, H. Asymmetric dimethylarginine in chronic obstructive pulmonary disease (ADMA in COPD). Int J Mol Sci. 2014, 15, 6062–6071. [Google Scholar] [CrossRef]
- Ruzsics, I.; Nagy, L.; Keki, S.; et al. L-Arginine Pathway in COPD Patients with Acute Exacerbation: A New Potential Biomarker. COPD. 2016, 13, 139–145. [Google Scholar] [CrossRef]
- Jonker, R.; Deutz, N.E.; Erbland, M.L.; Anderson, P.J.; Engelen, M.P. Alterations in whole-body arginine metabolism in chronic obstructive pulmonary disease. Am J Clin Nutr. 2016, 103, 1458–1464. [Google Scholar] [CrossRef]
- Valenca, S.S.; Rueff-Barroso, C.R.; Pimenta, W.A.; et al. L-NAME and L-arginine differentially ameliorate cigarette smoke-induced emphysema in mice. Pulm Pharmacol Ther. 2011, 24, 587–594. [Google Scholar] [CrossRef]
- Ubhi, B.K.; Riley, J.H.; Shaw, P.A.; et al. Metabolic profiling detects biomarkers of protein degradation in COPD patients. Eur Respir J. 2012, 40, 345–355. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





