Submitted:
26 August 2024
Posted:
26 August 2024
You are already at the latest version
Abstract
Keywords:
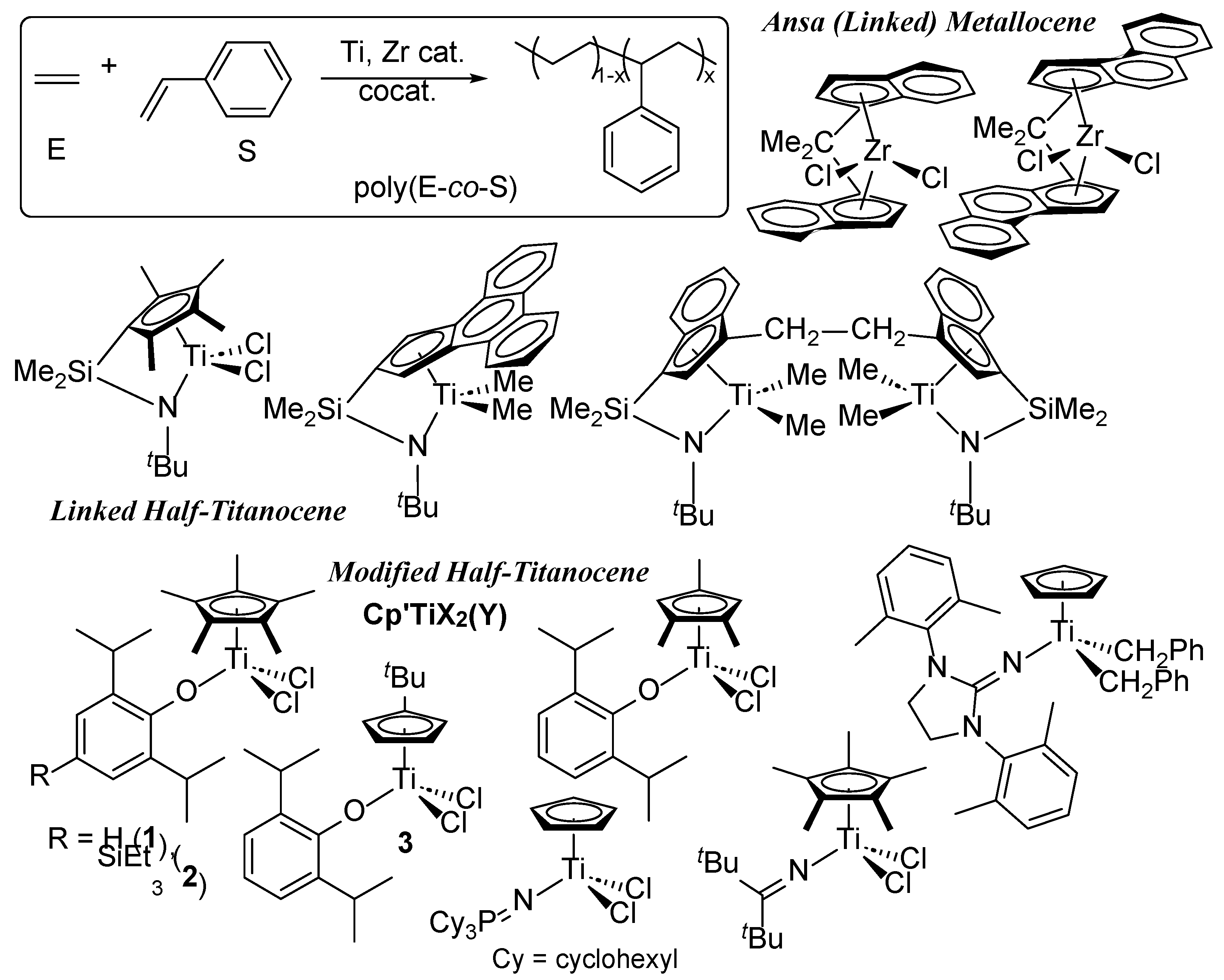
1. Introduction
2. Results and Discussion
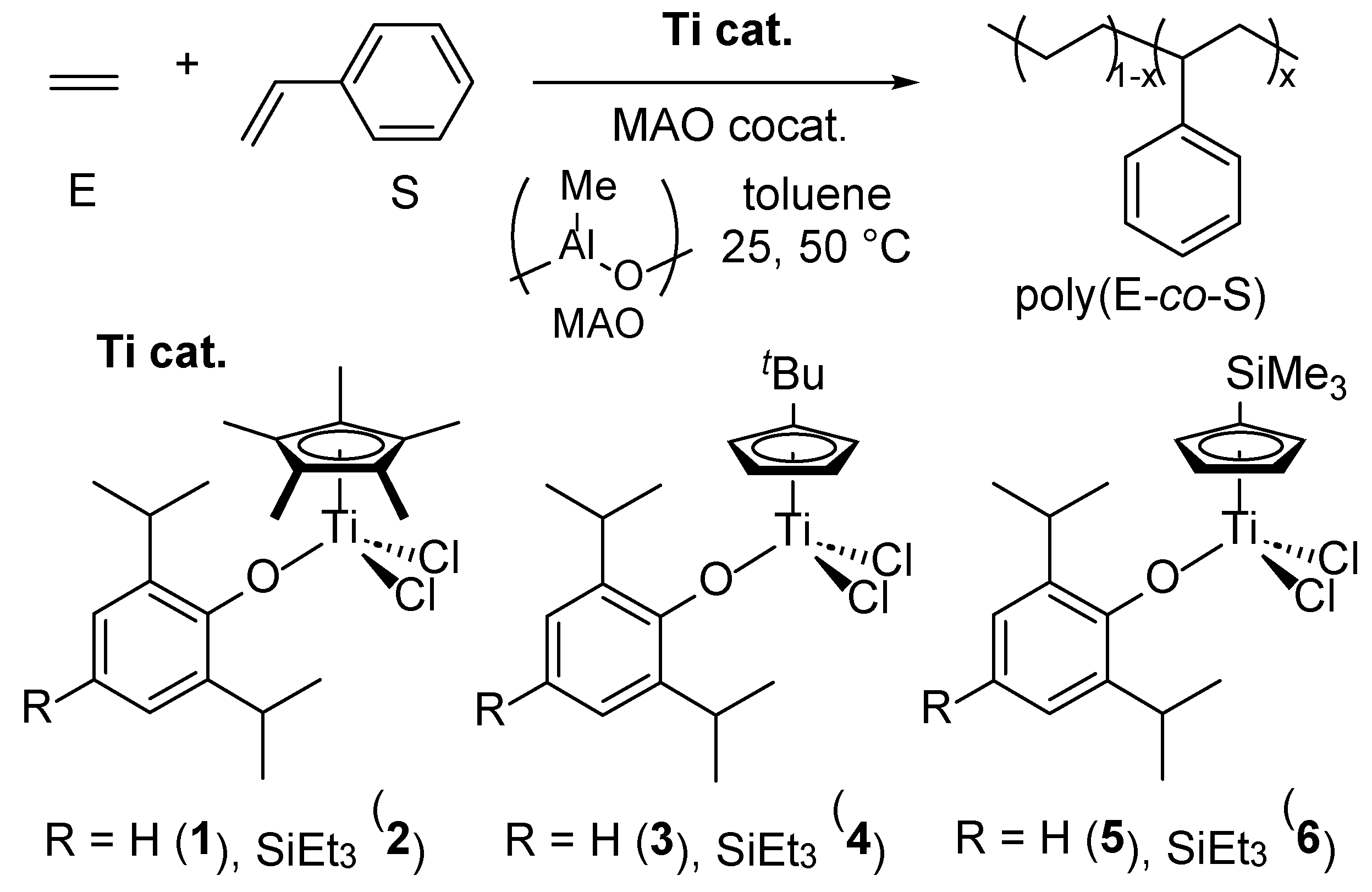
2.1. Synthesis and Structural Analysis of (Me3SiC5H4)TiCl2(O-2,6-iPr2-4-RC6H2) (R = H, SiEt3).
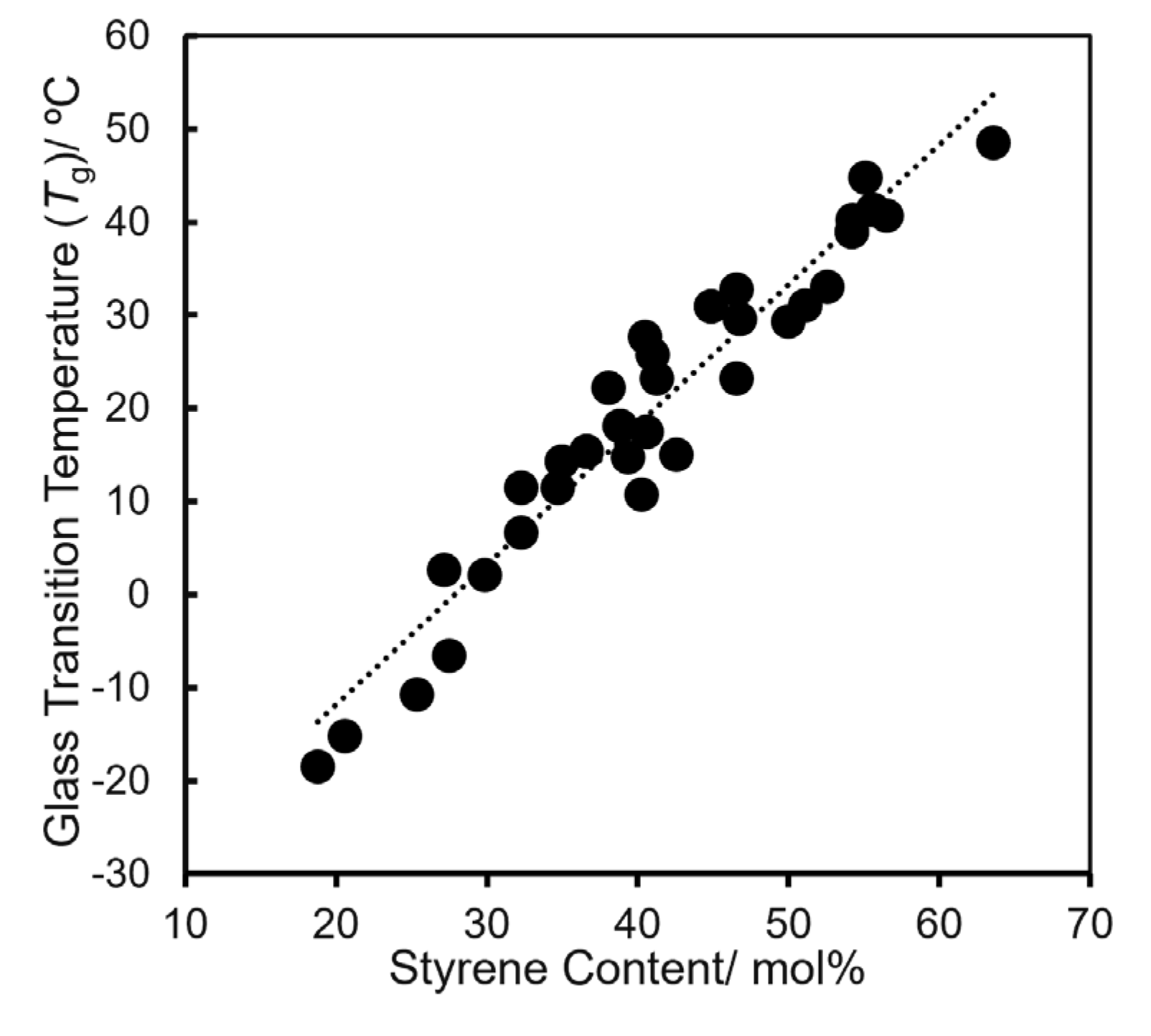
2.2. Ethylene/Styrene Copolymerization by Cp′TiCl2(O-2,6-iPr2-4-RC6H2) [Cp’ = Cp*, tBuC5H4, Me3SiC5H4, R = H, SiEt3]–MAO Catalyst Systems
3. Conclusions
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- For example (selected books, and reviews in early transition metal catalysts), see references 1-4: Organometallic Reactions and Polymerization; Osakada, K., Ed.; The Lecture Notes in Chemistry 85; Springer-Verlag: Berlin, Germany, 2014. [Google Scholar]
- Handbook of Transition Metal Polymerization Catalysts, 2nd ed.; Hoff, R., Ed.; Wiley: Hoboken, NJ, 2018. [Google Scholar]
- Nomura, K.; Kitphaitun, S. In Catalysis for a Sustainable Environment: Reactions, Processes and Applied Technologies; Pombeiro, A. J. L., Sutradhar, M., Alegria, E. C. B. A., Eds.; John Wiley & Sons, Ltd.: Chichester, West Sussex, UK, 2024; pp. 323–338. [Google Scholar]
- Baier, M.S.M.C.; Zuideveld, M.A.; Mecking, S. Post-Metallocenes in the Industrial Production of Polyolefins. Angew. Chem. Int. Ed. 2014, 53, 9722–9744. [Google Scholar] [CrossRef]
- Selected reviews, accounts in bimetallic catalyst systems, see references 5-7: Li H.; Marks, T. J. Nuclearity and cooperativity effects in binuclear catalysts and cocatalysts for olefin polymerization. Proc. Nat. Acad. Sci. USA 2006, 103, 15295–15302. [CrossRef]
- Delferro, M.; Marks, T.J. Multinuclear Olefin Polymerization Catalysts. Chem. Rev. 2011, 111, 2450–2485. [Google Scholar] [CrossRef]
- McInnis, J.P.; Delferro, M.; Marks, T.J. Multinuclear Group 4 Catalysis: Olefin Polymerization Pathways Modified by Strong Metal–Metal Cooperative Effects. Accounts Chem. Res. 2014, 47, 2545–2557. [Google Scholar] [CrossRef] [PubMed]
- Selected reviews in half-titanocenes, see references 8-10: Nomura, K. Half-titanocenes containing anionic ancillary donor ligands as promising new catalysts for precise olefin polymerization. Dalton Trans. 2009, 8811–8823.
- Nomura, K.; Liu, J. Half-titanocenes for precise olefin polymerisation: effects of ligand substituents and some mechanistic aspects. Dalton Trans. 2011, 40, 7666–7682. [Google Scholar] [CrossRef] [PubMed]
- van Doremaele, G.; van Duin, M.; Valla, M.; Berthoud, A. On the development of titanium κ1-amidinate complexes, commercialized as Keltan ACETM technology, enabling the production of an unprecedented large variety of EPDM polymer structures. J. Poly. Sci. Part A: Polym. Chem. 2017, 55, 2877–2891. [Google Scholar] [CrossRef]
- Nomura, K. Syndiotactic Polystyrene - Synthesis, Characterization, Processing, and Applications; Schellenberg, J., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, 2010; pp. 60–91. [Google Scholar]
- Selected reports in ethylene/styrene copolymerization using linked half-titanocenes (constrained geometry type) [5-7,11],see references 12-19: Stevens, J. C.; Timmers, F. J.; Wilson, D. R.; Schmidt, G. F.; Nickias, P. N.; Rosen, R. K.; Knight, G. W.; Lai, S. Y. Dow Chemical Co. EP 0416815 A2, 1991. Constrained geometry addition polymerization catalysts, processes for their preparation, precursors therefor, methods of use, and novel polymers formed therewith.
- Sernetz, F. G.; Mülhaupt, R.; Waymouth, R. M. Influence of polymerization conditions on the copolymerization of styrene with ethylene using Me2Si(Me4Cp)(N-tert-butyl)TiCl2/methylaluminoxane Ziegler-Natta catalysts. Macromol. Chem. Phys. 1996, 197, 1071–1083. [Google Scholar] [CrossRef]
- Sernetz, F.G.; Mülhaupt, R.; Amor, F.; Eberle, T.; Okuda, J. Copolymerization of ethene with styrene using different methylalumoxane activated half-sandwich complexes. J. Polym. Sci. Part A: Polym. Chem. 1997, 35, 1571–1578. [Google Scholar] [CrossRef]
- Timmers, F. J. Dow Chemical Co. US 6670432 B1, 2003. Olefin polymers formed by use of constrained geometry addition polymerization catalysts.
- Nomura, K.; Okumura, H.; Komatsu, T.; Naga, N.; Imanishi, Y. Effect of ligand in ethylene/styrene copolymerization by [Me2Si(C5Me4)(NR)]TiCl2 (R = tert-Bu, cyclohexyl) and (1,3-Me2C5H3)TiCl2(O-2,6- Pr2C6H3)-MAO catalyst system. J. Mol. Catal. A: Chem. 2002, 190, 225–234. [Google Scholar] [CrossRef]
- Guo, N.; Li, L.; Marks, T. J. Bimetallic catalysis for styrene homopolymerization and ethylene-styrene copolymerization. Exceptional comonomer selectivity and insertion regiochemistry. J. Am. Chem. Soc. 2004, 126, 6542–6543. [Google Scholar]
- Arriola, D. J.; Bokota, M.; Campbell, R. E., Jr.; Klosin, J.; LaPointe, R. E.; Redwine, O. D.; Shankar, R. B.; Timmers, F. J.; Abboud, K. A. Penultimate effect in ethylene-styrene copolymerization and the discovery of highly active Ethylene-styrene catalysts with increased styrene reactivity. J. Am. Chem. Soc. 2007, 129, 7065–7076. [Google Scholar] [CrossRef]
- Guo, N.; Stern, C. L.; Marks, T. J. Bimetallic effects in homopolymerization of styrene and copolymerization of ethylene and styrenic comonomers: Scope, kinetics, and mechanism. J. Am. Chem. Soc. 2008, 130, 2246–2261. [Google Scholar] [CrossRef] [PubMed]
- Selected reports in ethylene/styrene copolymerization using half-titanocenes [8,9,11], see references 20-29: Nomura, K.; Komatsu, T.; Imanishi, Y. Syndiospecific styrene polymerization and efficient ethylene/styrene copolymerization catalyzed by (cyclopentadienyl)(aryloxy)titanium(IV) complexes – MAO system. Macromolecules 2000, 33, 8122–8124.
- Nomura, K.; Okumura, H.; Komatsu, T.; Naga, N. Ethylene/Styrene Copolymerization by Various (Cyclopentadienyl)(aryloxy)titanium(IV) Complexes−MAO Catalyst Systems. Macromolecules 2002, 35, 5388–5395. [Google Scholar] [CrossRef]
- Kretschmer, W. P.; Dijkhuis, C.; Meetsma, A.; Hessen, B.; Teuben, J. H. A highly efficient titanium-based olefin polymerisation catalyst with a monoanionic iminoimidazolidide π-donor ancillary ligand. Chem. Commun. 2002, 608–609. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Lam, P.; Zhang, Z.; Yamashita, G.; Fan, L. Ethylene styrene interpolymers with double reverse styrene incorporation. US 657 9961 B1, 2003.
- Zhang, H.; Nomura, K. Living Copolymerization of Ethylene with Styrene Catalyzed by (Cyclopentadienyl)(ketimide)titanium(IV) Complex−MAO Catalyst System. J. Am. Chem. Soc. 2005, 127, 9364–9365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Nomura, K. Living Copolymerization of Ethylene with Styrene Catalyzed by (Cyclopentadienyl)(ketimide)titanium(IV) Complex−MAO Catalyst System: Effect of Anionic Ancillary Donor Ligand. Macromolecules 2006, 39, 5266–5274. [Google Scholar] [CrossRef]
- Nomura, K.; Pracha, S.; Phomphrai, K.; Katao, S.; Kim, D.-H.; Kim, H.J.; Suzuki, N. Synthesis and structural analysis of phenoxy-substituted half-titanocenes with different anionic ligands, Cp*TiX(Y)(O-2,6-iPr2C6H3): Effect of anionic ligands (X,Y) in ethylene/styrene copolymerization. J. Mol. Catal. A: Chem. 2012, 365, 136–145. [Google Scholar] [CrossRef]
- Son, K.-S.; Waymouth, R.M. Stereospecific styrene polymerization and ethylene-styrene copolymerization with titanocenes containing a pendant amine donor. J. Polym. Sci. Part A: Polym. Chem. 2010, 48, 1579–1585. [Google Scholar] [CrossRef]
- Aoki, H.; Nomura, K. Synthesis of Amorphous Ethylene Copolymers with 2-Vinylnaphthalene, 4-Vinylbiphenyl and 1-(4-Vinylphenyl)naphthalene. Macromolecules 2020, 54, 83–93. [Google Scholar] [CrossRef]
- Selected reports in ethylene/styrene copolymerization using metallocenes,11 see references 25-2629,30: Arai, T.; Ohtsu, T.; Suzuki, S. Stereoregular and Bernoullian copolymerization of styrene and ethylene by bridged metallocene catalysts. Macromol. Rapid Commun. 1998, 19, 327–331.
- Arai, T.; Suzuki, S.; Ohtsu, T. Olefin Polymerization: Emerging Frontiers; Arjunan, P., Ed.; ACS Symposium Series 749; American Chemical Society: Washington, D. C, 2000; pp. 66–80. [Google Scholar]
- Byun, D.J.; Fudo, A.; Tanaka, A.; Fujiki, M.; Nomura, K. Effect of Cyclopentadienyl and Anionic Ancillary Ligand in Syndiospecific Styrene Polymerization Catalyzed by Nonbridged Half-Titanocenes Containing Aryloxo, Amide, and Anilide Ligands: Cocatalyst Systems. Macromolecules 2004, 37, 5520–5530. [Google Scholar] [CrossRef]
- Kitphaitun, S.; Yan, Q.; Nomura, K. Effect of SiMe3, SiEt3 para-substituents for exhibiting high activity, introduction of hydroxy group in ethylene copolymerization catalyzed by phenoxide-modified half-titanocenes. Angew. Chem. Int. Ed. 2020, 59, 23072–23076. [Google Scholar] [CrossRef]
- Miyazawa, A.; Kase, T.; Soga, K. Cis-Specific Living Polymerization of 1,3-Butadiene Catalyzed by Alkyl and Alkylsilyl Substituted Cyclopentadienyltitanium Trichlorides with MAO. Macromolecules 2000, 33, 2796–2800. [Google Scholar] [CrossRef]
- Losio, S.; Bertini, F.; Vignali, A.; Fujioka, T.; Nomura, K.; Tritto, I. Amorphous Elastomeric Ultra-High Molar Mass Polypropylene in High Yield by Half-Titanocene Catalysts. Polymers 2024, 16, 512. [Google Scholar] [CrossRef]
- Nomura, K.; Naga, N.; Miki, M.; Yanagi, K. Olefin Polymerization by (Cyclopentadienyl)(aryloxy)titanium(IV) Complexes−Cocatalyst Systems. Macromolecules 1998, 31, 7588–7597. [Google Scholar] [CrossRef]
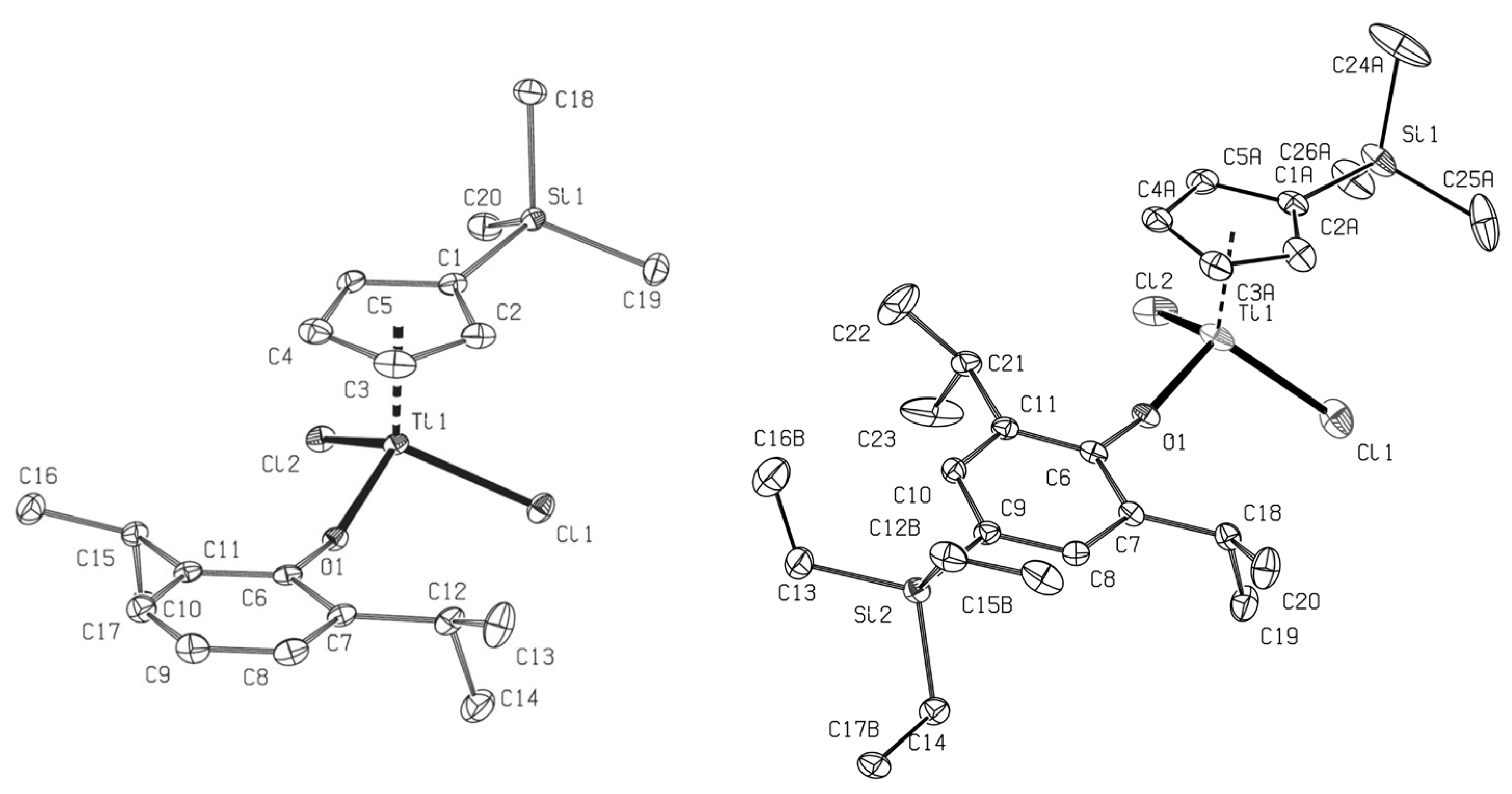
| 1b | 2c | 5 | 6 | 7b | |
|---|---|---|---|---|---|
| Bond Distances (Å) | |||||
| Ti(1)‒Cl(1) | 2.305(2) | 2.2723(9) | 2.2660(5) | 2.2721(15) | 2.262(1) |
| Ti(1)‒Cl(2) | 2.239(2) | 2.2677(8) | 2.2730(5) | 2.2534(14) | 2.262(1) |
| Ti(1)‒O(1) | 1.772(3) | 1.7965(19) | 1.7829(13) | 1.773(2) | 1.760(4) |
| Si(1)‒C(1) | -- | 1.875(3) | 1.876(2) | 1.864(7) | -- |
| O(1)‒C(6) | 1.367(5) | 1.363(3) | 1.373(2) | 1.375(4) | 1.368(4) |
| Ti(1)‒C(1) | 2.365(7) | 2.403(3) | 2.3678(17) | 2.373(13) | 2.282(8) |
| Ti(1)‒C(2) | 2.438(7) | 2.411(3) | 2.3854(19) | 2.323(9) | 2.299(5) |
| Ti(1)‒C(3) | 2.365(7) | 2.359(3) | 2.348(2) | 2.325(8) | 2.235(5) |
| Ti(1)‒C(4) | 2.311(7) | 2.345(3) | 2.335(2) | 2.390(6) | 2.235(5) |
| Ti(1)‒C(5) | 2.317(4) | 2.357(3) | 2.330(2) | 2.398(8) | 2.299(5) |
| Bond Angles (º) | |||||
| Cl(1)‒Ti(1)‒Cl(2) | 103.45(5) | 102.33(3) | 105.11(2) | 103.74(5) | 104.23(7) |
| Cl(1)‒Ti(1)‒O(1) | 99.1(2) | 100.97(7) | 102.92(4) | 102.31(10) | 102.53(9) |
| Cl(2)‒Ti(1)‒O(1) | 104.1(2) | 103.24(7) | 102.52(4) | 103.04(9) | 102.53(9) |
| Ti(1)‒O(1)‒C(6) | 173.0(3) | 174.62(19) | 151.41(12) | 157.0(2) | 163.0(4) |
| run | cat. | St | yieldb | activityb | THF soluble fraction | ||||
| / mL | / mg | cont.c/ wt.% | Mnd×10-4 | Mw/Mnd | Tge/ ºC | Stf/ mol% | |||
| 1 | 1 | 3 | 60.0 | 720 | 97 | 16.7 | 1.66 | -18.5 | 18.8 |
| 2 | 1 | 5 | 50.6 | 610 | 90 | 13.5 | 1.32 | -6.6 | 27.5 |
| 3 | 1 | 10 | 39.5 | 470 | 86 | 6.86 | 1.26 | 15.0 | 42.6 |
| 4 | 2 | 3 | 32.4 | 390 | 96 | 9.23 | 1.65 | -15.3 | 20.6 |
| 5 | 2 | 5 | 23.5 | 280 | 95 | 9.91 | 1.41 | -10.8 | 25.4 |
| 6 | 2 | 10 | 19.2 | 230 | 93 | 7.38 | 1.14 | 10.7 | 40.3 |
| 7 | 3 | 3 | 429 | 5150 | >99 | 4.65 | 1.51 | 6.6 | 32.3 |
| 8 | 3 | 5 | 406 | 4870 | >99 | 3.98 | 1.48 | 27.6 | 40.5 |
| 9 | 3 | 10 | 371 | 4450 | >99 | 3.34 | 1.87 | 31.0 | 51.1 |
| 10 | 4 | 3 | 197 | 2360 | >99 | 14.7 | 1.81 | 11.4 | 32.3 |
| 11 | 4 | 5 | 180 | 2160 | >99 | 6.76 | 1.51 | 22.2 | 38.1 |
| 12 | 4 | 10 | 154 | 1850 | >99 | 5.28 | 2.05 | 29.5 | 46.8 |
| 13 | 5 | 3 | 323 | 3880 | >99 | 5.18 | 1.58 | 14.2 | 35.0 |
| 14 | 5 | 5 | 450 | 5400 | >99 | 4.65 | 1.76 | 23.2 | 41.3 |
| 15 | 5 | 10 | 723 | 8680 | >99 | 3.82 | 1.70 | 38.9 | 54.2 |
| 16 | 6 | 3 | 361 | 4330 | >99 | 10.9 | 1.80 | 15.3 | 36.6 |
| 17 | 6 | 5 | 411 | 4930 | 98 | 12.8 | 1.79 | 30.9 | 44.9 |
| 18 | 6 | 10 | 506 | 6070 | 96 | 8.78 | 1.71 | 41.3 | 55.6 |
| run | cat. | E | St | temp. | yieldb | activityb | THF soluble fraction | ||||
| (μmol) | / atm | /mL | / ºC | /mg | cont.c/ wt.% | Mnd×10-4 | Mw/Mnd | Tge/ ºC | Stf/ mol% | ||
| 19 | 5 (0.50) | 6 | 3 | 20 | 556 | 6670 | 97 | 9.12 | 1.88 | 2.0 | 29.9 |
| 13 | 5 (0.50) | 4 | 3 | 20 | 323 | 3880 | 99 | 5.18 | 1.58 | 14.2 | 35.0 |
| 14 | 5 (0.50) | 4 | 5 | 20 | 450 | 5400 | 99 | 4.65 | 1.76 | 23.2 | 46.6 |
| 15 | 5 (0.50) | 4 | 10 | 20 | 723 | 8680 | >99 | 3.82 | 1.70 | 38.9 | 54.2 |
| 20 | 5 (0.30) | 4 | 3 | 20 | 192 | 3840 | >99 | 5.35 | 1.76 | 11.4 | 34.7 |
| 19 | 5 (0.50) | 6 | 3 | 20 | 556 | 6670 | 97 | 9.12 | 1.88 | 2.0 | 29.9 |
| 21 | 5 (0.30) | 4 | 5 | 20 | 223 | 4460 | 99 | 4.78 | 1.68 | 25.7 | 41.0 |
| 22 | 5 (0.30) | 4 | 10 | 20 | 264 | 5280 | >99 | 4.04 | 1.80 | 40.2 | 54.3 |
| 23 | 5 (0.50) | 4 | 3 | 50 | 162 | 1940 | 97 | 2.24 | 1.61 | 18.1 | 38.9 |
| 24g | 5 (0.50) | 4 | 3 | 50 | 110 | 1320 | 95 | 1.96 | 1.43 | 14.7 | 39.4 |
| 25h | 5 (0.50) | 4 | 3 | 50 | 185 | 2220 | 97 | 2.48 | 1.43 | 17.4 | 40.6 |
| 26 | 5 (0.50) | 4 | 5 | 50 | 199 | 2390 | 90 | 1.46 | 1.70 | 29.3 | 50.0 |
| 27 | 5 (0.50) | 4 | 10 | 50 | 229 | 2750 | 86 | 1.08 | 1.63 | 40.6 | 56.5 |
| 28 | 5 (0.50) | 2 | 3 | 20 | 177 | 2120 | >99 | 4.45 | 1.47 | 33.0 | 52.6 |
| 29 | 5 (0.50) | 2 | 10 | 20 | 243 | 2920 | 98 | 1.17 | 1.90 | 48.5 | 63.6 |
| 30 | 6 (0.50) | 6 | 3 | 20 | 349 | 7000 | 99 | 20.2 | 1.99 | 2.6 | 27.2 |
| 16 | 6 (0.50) | 4 | 3 | 20 | 361 | 4330 | >99 | 10.9 | 1.80 | 15.3 | 36.6 |
| 17 | 6 (0.50) | 4 | 5 | 20 | 411 | 4930 | 98 | 12.8 | 1.79 | 30.9 | 44.9 |
| 18 | 6 (0.50) | 4 | 10 | 20 | 506 | 6070 | 96 | 8.78 | 1.71 | 41.3 | 55.6 |
| 31 | 6 (0.50) | 4 | 3 | 50 | 217 | 2610 | 96 | 3.84 | 1.64 | 18.1 | 38.8 |
| 32 | 6 (0.50) | 4 | 5 | 50 | 291 | 3490 | 96 | 3.18 | 2.04 | 32.7 | 46.6 |
| 33 | 6 (0.50) | 4 | 10 | 50 | 359 | 4310 | 83 | 2.95 | 2.04 | 44.7 | 55.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





