1. Introduction
High blood pressure is a significant risk factor for heart and brain diseases worldwide and a leading cause of premature death, necessitating systematic management as a public health issue [
1]. Hypertension is a complex metabolic disease caused by the intricate interplay between genetic and various environmental factors [
2]. Previous studies have suggested that air pollution could be a contributing factor to hypertension [
3].
Heavy metals including lead (Pb), mercury (Hg), and cadmium (Cd) are representative of harmful environmental factors commonly encountered in daily life, potentially causing several health issues [
4,
5]. Heavy metals can enter the human body through the respiratory or digestive systems, accumulate in various organs, and are not easily excreted [
6]. These characteristics suggest that heavy metal exposure can lead to various chronic diseases among residents living near environmentally polluted areas and serve as a good indicator for long-term exposure due to their long latency periods. Previous studies have shown significant differences in the body burden of heavy metals based on gender [
7], and the prevalence of hypertension varies between men and women [
8]. Therefore, the impact of heavy metal exposure on blood pressure is likely to differ by sex.
Typically, the health impacts of exposure to harmful pollutants have been assessed using multiple linear regression models after adjusting for known confounding variables. However, these methods estimate the individual effects while adjusting for other pollutants, thereby limiting their ability to evaluate the combined effects of related pollutants. Additionally, multiple linear regression assumes a linear relationship between heavy metal exposure and blood pressure, restricting the analysis of nonlinear relationships. Therefore, there is a need for alternative analytical methods that can overcome the limitations of traditional methods in environmental epidemiology studies, particularly for residents living in vulnerable areas with a high potential for combined exposure to various heavy metals.
One alternative analytical method is Bayesian kernel machine regression (BKMR), which has increasingly been used in recent environmental epidemiology studies to evaluate the health impacts of combined exposure to various heavy metals [
9,
10,
11]. This study aimed to evaluate the effect of combined exposure to blood Pb, Hg, and Cd on blood pressure using the BKMR method, based on health impact surveys of residents living near incinerators, a representative vulnerable area. The results were compared with those obtained from multiple linear regression analyses.
2. Materials and Methods
2.1. Study Subjects
This study is based on the findings from a health impact survey conducted on residents living in the Buki-myeon area of Cheongju, Korea, where three waste incineration facilities are located within a 3 km radius, and on residents based in a control area located 16–23 km away from the incineration facilities. All the study participants had lived in these areas for more than 10 years. Among 1,112 all the participants, 561 were included in the final analysis with both biomaterial samples and health examinations, no work experience at the incinerator, and no history of hypertension medication.
2.2. Measurement of Blood Heavy Metal Concentrations
The blood levels of Hg, Pb, and Cd were analyzed in the collected samples. Blood Hg was analyzed using a direct mercury analyzer (DMA 80 Milestone) and the gold amalgamation method with 100 µL of well-mixed blood placed in the sample container of the analyzer. The blood Pb levels were measured using a polarized Zeeman atomic absorption spectrophotometer (Model Z-2700, Hitachi, Tokyo, Japan), and blood Cd levels were measured using a flameless atomic absorption spectrophotometer with a Zeeman graphite furnace (Z-8270, Hitachi, Tokyo, Japan). The systolic and diastolic blood pressures were recorded with a digital blood pressure monitor (Omron HEM-7143, Japan).
2.3. Statistical Analysis
The concentrations of heavy metals in the blood showed a right-skewed distribution and were log-transformed for inclusion in the regression model. To adjust for confounding variables, the regression model included age, sex (analyses of the total group), alcohol consumption, smoking status, monthly household income, and diabetes status as covariates. Multiple linear regression models were employed to assess the relationship between heavy metal levels and systolic and diastolic blood pressure using the following equations:
Here, Y represents systolic or diastolic blood pressure, while Hg, Pb, and Cd denote the log-transformed concentrations of Hg, Pb, and Cd, respectively. Z = Z1,…, Zp stands for potential confounding variables.
BKMR was applied to estimate the combined and nonlinear effects of the three heavy metals on blood pressure. The equations for the BKMR model are as follows:
where h is the modeling function for the nonlinear effects. A Markov chain Monte Carlo algorithm with 10,000 iterations was employed to analyze potential interactions between metal exposures and response curves, while keeping other metal exposures fixed at the 25th, 50th, and 75th percentiles. Statistical analyses were conducted using R software (version 4.2.3; R Foundation for Statistical Computing), with a p-value of <0.05 considered statistically significant.
2.4. Ethical Considerations
The study protocol was reviewed and approved by the Institutional Review Board of Chungbuk National University (CBNU202012-HRBR-0207), and all participants provided written informed consent.
3. Results
Of the 561 participants, 253 were men and 308 were women. The proportion of smokers and drinkers, including former smokers, significantly differed between men and women. However, there were no significant differences in the average household income or average age between the sex. The mean body mass index (BMI) was significantly higher in women copmpared to men. Nevertheless, the average systolic and diastolic blood pressure did not differ significantly between the sexes. The concentrations of heavy metals in the blood were expressed as geometric means and geometric standard deviations, considering their distribution characteristics. Men had significantly higher blood concentrations of Hg and Pb, whereas women had significantly higher Cd levels (
Table 1).
Multiple linear regression analysis revealed no statistically significant associations between the systolic blood pressure and any of the heavy metals in the total group. However, an increase in blood Cd concentration was statistically significantly associated with an increase in diastolic blood pressure (β = 1.903, 95% confidence intervals [CI]: 0.165, 3.641). Stratified analyses demonstrated that an increase in blood Pb concentration was positively associated with diastolic blood pressure (β = 3.298, 95% CI: 0.362, 6.235) in men, while an increase in blood Cd concentration was positively associated with diastolic blood pressure (β = 2.628, 95% CI: 0.143, 5.113) in women. No heavy metals were significantly associated with changes in systolic blood pressure (
Table 2).
The BKMR model estimates the combined exposure effects by calculating the posterior mean and 95% CIs for blood pressure changes associated with variations in the levels of the three heavy metals. The model predicted variations in blood pressure as the levels of three heavy metals changed concurrently, comparing these variations to the median levels of the metal mixture. Additionally, it assessed the blood pressure changes linked to the interquartile range (IQR) for each heavy metal, while keeping the other two metals constant at the 25th, 50th, and 75th percentiles.
In the total group, the estimated joint effect of the three metals on systolic blood pressure was -0.12 at the 25th percentile and -0.17 at the 75th percentile, with no statistically significant associations observed (
Figure 1A). Blood Cd concentrations showed a positive correlation with systolic blood pressure, while Hg and Pb concentrations exhibited negative correlations; however, these associations were not statistically significant. The estimated changes in systolic blood pressure associated with IQR increases in blood Cd were 0.42 (95% CI: -1.07, 1.90), 0.39 (95% CI: -1.08, 1.86), and 0.36 (95% CI: -1.12, 1.85) when the concentrations of the other two metals were held constant at the 25th, 50th, and 75th percentiles, respectively (
Figure 1B). A parabolic nonlinear relationship was identified between blood Cd level and systolic blood pressure (
Figure 1C–D).
In the BKMR analysis for diastolic blood pressure, the joint effect of the blood Pb, Cd and Hg was significantly associated with diastolic blood pressure below the 45th percentile and above the 55th percentile. The joint effect ranged from -1.25 to 0.91 between the 25th and 75th percentiles (
Figure 2A). When the other two metals were fixed at specific percentiles, increases in blood Cd IQR were significantly associated with diastolic blood pressure. The effects of IQR increases in Pb, Cd, and Hg on diastolic blood pressure did not vary significantly depending on the levels of the other two metals (
Figure 2B). A nonlinear relationship was identified between blood Cd and diastolic blood pressure, whereas Pb and Hg exbited a positive linear association with diastolic blood pressure (
Figure 2C–D).
In men, the joint effect estimates of the three metals on systolic blood pressure were -1.07 at the 25th percentile and 0.64 at the 75th percentile, with no statistically significant associations observed at any point (
Figure 3A). Blood concentrations of Cd, Hg, and Pb were positively associated with systolic blood pressure, although these associations were not statistically significant. The effects of IQR increases in the three metals on systolic blood pressure were not significantly different depending on the levels of the other two metals (
Figure 3B). A parabolic nonlinear relationship was observed between blood Cd levels and systolic blood pressure (
Figure 3C–D).
The BKMR analysis on heavy metal exposure and diastolic blood pressure in male participants revealed that the combined effect of the three heavy metals was significantly associated with diastolic blood pressure below the 45th percentile and above the 55th percentile. The combined effect within the 25th to 75th percentiles ranged from -1.97 to 1.56 (
Figure 4A). When the concentrations of the other two heavy metals were held constant at the 25th, 50th, and 75th percentiles, a statistically significant relationship was found between the increase in the IQR of blood Pb concentration and diastolic blood pressure. The estimated changes in diastolic blood pressure for each IQR increase in blood Pb were 1.70 (95% CI: 0.10, 3.30), 1.71 (95% CI: 0.13, 3.29), and 1.71 (95% CI: 0.07, 3.35), respectively. The effect of the IQR increase in blood levels of Pb, Cd, and Hg on diastolic blood pressure did not significantly vary depending on the concentrations of the other two heavy metals (
Figure 4B). When the other two heavy metals were fixed at their median values, there was a non-linear relationship between blood Cd and Hg concentrations and diastolic blood pressure, whereas blood Pb concentration demonstrated a positive linear relationship with diastolic blood pressure (
Figure 4C–D).
In women, the joint effects of the three metals had a decreasing trend in systolic blood pressure as the levels of the metals increased, particularly when the levels were above the median. The joint effects at the 25th and 75th percentiles ranged from 0.97 to -1.42, with statistically significant decreases (
Figure 5A). No significant associations were observed between the effects of IQR changes of individual metals on systolic blood pressure (
Figure 5B). A nonlinear relationship was observed between blood Hg levels and systolic blood pressure (
Figure 5C–D).
For diastolic blood pressure in women, the joint effect estimates of the three metals were –0.83 at the 25th percentile and 0.52 at the 75th percentile, respectively, with no statistically significant associations at any point (
Figure 6A). Blood Cd and Pb concentrations were positively associated with diastolic blood pressure, with Cd levels showing a statistically significant association. The effects of IQR increases in Cd on diastolic blood pressure were 1.30 (95% CI: -0.01, 2.62), 1.31 (95% CI: 0.02, 2.60), and 1.32 (95% CI: -0.01, 2.65) when the other two metals were fixed at the 25th, 50th, and 75th percentiles, respectively (
Figure 6B). A nonlinear relationship was observed between blood Hg levels and diastolic blood pressure (
Figure 6C–D).
4. Discussion
In this study, the combined effects of exposure to Cd, Hg, and Pb on systolic and diastolic blood pressure were evaluated according to sex. The impact of exposure to these three heavy metals on blood pressure was analyzed using multiple linear regression analysis and the BKMR method. Multiple linear regression analysis for all participants, as well as for men and women separately, revealed no significant relationship between the blood concentrations of the three heavy metals and systolic blood pressure across all analysis models. However, there was a significant positive correlation between blood Cd levels in the total group and women in terms of diastolic blood pressure and between blood Pb levels in men. Additionally, these findings were corroborated by BKMR analysis. Although certain heavy metals demonstrated a nonlinear relationship with changes in blood pressure, the consistency of the results across both analytical methods demonstrated the robustness of this study’s findings. The Pb exposure was higher in men than in women, whereas the Cd exposure was higher in women. This differential exposure suggests that the heavy metals contributing to increased blood pressure differ between men and women.
One finding of this study is that the health impacts of heavy metal exposure vary by sex. This is generally known to differ based on sex [
12,
13]. Previous research has suggested these differences occur due to differences in body fat, hormone types, and concentrations of competitive elements such as calcium and iron, as well as differences in dietary habits and cosmetic use [
14]. Additionally, due to physiological differences between men and women, there is a possibility that the health effects of exposure to hazardous substances may differ between the sexes. Studies have shown that men are more vulnerable to cardiovascular diseases like hypertension compared to women, despite women generally being more affected by heavy metal exposure [
15,
16,
17]. Although the health effects of heavy metals are greater in women than in men, several studies have reported that men are more vulnerable to cardiovascular diseases, including high blood pressure, than women [
17]. The heavy metal most frequently associated with cardiovascular disease is Pb. Navas-Acien et al. [
18,
19] reported that Pb exposure is related to hypertension, arteriosclerosis, and coronary artery disease and that men have a greater increase in the incidence of high blood pressure due to Pb exposure than women. Additionally, a British study [
20] reported an association between blood Pb concentration and diastolic blood pressure in men but no association with systolic blood pressure and no association in women, aligning with the conclusions of this study.
Cd is a heavy metal that is well known for its association with cardiovascular diseases. Liu et al. [
21] reported that Cd exposure is related to endothelial dysfunction, a risk factor for cardiovascular disease, whereas Barregard et al. [
22] reported that Cd exposure increased the occurrence of cardiovascular disease in men but did not increase the risk of cardiovascular disease in women at the same concentration, indicating a difference in sensitivity between genders.
In the case of Hg, another heavy metal associated with cardiovascular disease, the incidence of high blood pressure increased in men as blood Hg concentration increased, while women did not show a significant increase in disease occurrence at the same Hg concentration [
23,
24]. These results are consistent with the findings of the current study, suggesting that men are relatively more vulnerable than women to developing cardiovascular diseases, such as high blood pressure, due to exposure to harmful heavy metals.
The precise mechanism underlying this sex difference has not yet been revealed. The most compelling hypothesis concerns the role of sex hormones. Estrogen, a female hormone, is known to have a protective effect on the occurrence of cardiovascular disease, whereas testosterone, the male hormone, has a negative effect on the cardiovascular system [
12]. Smith et al. [
15] suggest that men may be more vulnerable to cardiovascular disease caused by heavy metals due to the effects of testosterone.
In older adults, it is known that as vascular elasticity decreases, systolic blood pressure increases more than diastolic blood pressure, which increases pulse pressure (systolic blood pressure–diastolic blood pressure) and thereby increases the risk of cardiovascular disease [
24]. However, the current study showed results that were contradictory to these existing findings. BKMR analysis using pulse pressure as the dependent variable showed that the joint effects of heavy metals were negatively related to pulse pressure in both men and women (data not shown). The reasons for these differences could not be explained within the scope of this study. This limitation may be inherent to observational rather than experimental research.
Existing research results on the relationship between blood heavy metal concentrations and blood pressure are diverse. Although the exact cause of this diversity is unknown, most heavy metal exposures are often simultaneous exposures to multiple heavy metals rather than to a single heavy metal, and the interactions between heavy metals can affect health indicators. However, each study measured only a portion of the heavy metals to evaluate the relationship, or even if several heavy metals were measured, the individual relationship with a single heavy metal was identified at the analysis step; therefore, there is a possibility that various results may be derived depending on the study. From this viewpoint, assessing the combined effects of different types of heavy metals using methods like BKMR can be a crucial tool in environmental epidemiology, as it helps to reduce bias.
Understanding the toxicity mechanism of a single heavy metal and its relationship with health effects is vital from a toxicological perspective. However, from a public health perspective, it is more meaningful to evaluate the combined effects of multiple exposures to various heavy metals rather than the individual effects of a single heavy metal. Evaluating the joint effects of multiple exposures may be more important in the process of finding evidence to determine whether the health effects observed in residents of environmentally vulnerable areas are caused by air pollution sources such as incinerators.
This study had several limitations. First, several factors affect blood pressure changes. However, for this study, these factors were not sufficiently reflected. Second, blood pressure may change throughout the day; however, in this study, blood pressure was measured only once. There is, therefore, a possibility of individual variation depending on the measurement time. Finally, because this study excluded all participants taking blood pressure medication, the number of participants was relatively small, which may have resulted in a somewhat low statistical power. Further research with larger sample sizes is required.
Nevertheless, this study is significant in that it evaluated the effect of combined exposure to heavy metals on blood pressure by sex among residents of incinerator areas. The results suggest that men and women respond differently to heavy metal exposure and that environmental epidemiological surveys should consider these differences to effectively mitigate the health impacts of heavy metal exposure. Future studies should continue to explore the underlying mechanisms driving these sex-specific differences to inform better public health strategies.
Author Contributions
Conceptualization, Y.D.K. and H.K.; methodology, S.Y.E. and H.K; data curation, S.H.; formal analysis, I.G.K.; investigation, Y.S.H., Y.D.K. and H.K.; writing—original draft preparation, I.G.K.; writing—review and editing, S.Y., J.H.J., K,C., J.L. and Y.D.K.; supervision, Y.D.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant from the National Institute of Environmental Research (NIER), funded by the Ministry of Environment (MOE) of the Republic of Korea (NIER-2019-04-02-069).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Chungbuk National University (CBNU-202012-HRBR-0207).
Informed Consent Statement
Individuals were provided information about the purpose of this study, and those who wished to participate provided written consent.
Data Availability Statement
The data is unavailable due to the regulations of the Ministry of Environment, Korea.
Acknowledgments
We thank the relevant ministries, including the Ministry of Environment, National Institute of Environmental Research and Cheongju-si, Republic of Korea.
Conflicts of Interest
The authors wish to declare that there are no competing interests regarding the publication of this article.
References
- Mills, K.T.; Stefanescu, A.; He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, S.; Newton-Cheh, C.; Dominiczak, A.F. Genetic basis of blood pressure and hypertension. Trends Genet. 2012, 28, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhang, B.; Ke, W.; Feng, B.; Lin, H.; Xiao, J.; Zeng, W.; Li, X.; Tao, J.; Yang, Z.; et al. Associations of Short-Term and Long-Term Exposure to Ambient Air Pollutants With Hypertension. Hypertension 2016, 68, 62–70. [Google Scholar] [CrossRef]
- Joo, Y.; Kwon, Y.M.; Kim, S.Y.; Choi, K.; Lee, C.; Yu, S.D.; Yoo, J. A Study on Heavy Metals Exposure and Major Sociodemographic Influence Factors among Korean Adults - Korean National Environmental Health Survey (2009-2017). Journal of Environmental Health Sciences. 2019, 45, 541–555. [Google Scholar] [CrossRef]
- World Health Organization (WHO). 10 chemicals of public health concern. Available: https://www.who.int/news-room/photo-story/ photo-story-detail/10-chemicals-of-public-health-concern [Accessed 23 September 2023].
- Lee, J.-M.; Seok, K.-J.; Ryu, J.-Y.; Jung, W.-S.; Park, J.-B.; Shin, K.-H.; Jang, S.-J. Association between Heavy Metal Exposure and Prevalence of Metabolic Syndrome in Adults of South Korea. Korean J. Fam. Pr. 2017, 7, 172–178. [Google Scholar] [CrossRef]
- Park, Y.; Lee, S.-J. Association of Blood Heavy Metal Levels and Renal Function in Korean Adults. Int. J. Environ. Res. Public Heal. 2022, 19, 6646. [Google Scholar] [CrossRef]
- De Almeida Lopes, A.C.B.; Silbergeld, E.K.; Navas-Acien, A.; Zamoiski, R.; da Cunha Martins, A., Jr.; Camargo, A.E.I.; Urbano, M.; Mesas, A.; Paoliello, M.M.B. Association between blood lead and blood pressure: a population-based study in Brazilian adults. Environ. Heal. 2017, 16, 27. [Google Scholar] [CrossRef]
- Kim, M.; Park, C.; Sakong, J.; Ye, S.; Son, S.Y.; Baek, K. Association of heavy metal complex exposure and neurobehavioral function of children. Ann. Occup. Environ. Med. 2023, 35, e23. [Google Scholar] [CrossRef]
- Cai, J.; Li, Y.; Liu, S.; Liu, Q.; Xu, M.; Zhang, J.; Wei, Y.; Mo, X.; Lin, Y.; Tang, X.; et al. Associations between multiple heavy metals exposure and glycated hemoglobin in a Chinese population. Chemosphere 2021, 287, 132159. [Google Scholar] [CrossRef]
- Moon, S.-I.; Yim, D.-H.; Choi, K.; Eom, S.-Y.; Choi, B.-S.; Park, J.-D.; Kim, H.; Kim, Y.-D. Association Between Multiple Heavy Metal Exposures and Cholesterol Levels in Residents Living Near a Smelter Plant in Korea. J. Korean Med Sci. 2024, 39, e77. [Google Scholar] [CrossRef]
- Ali, I.; Haque, A.; Fatima, M. Gender Difference in Heavy Metal Toxicity and the Role of Sex Hormones. Ann. Clin. Lab. Sci. 2018, 48, 710–721. [Google Scholar]
- Nguyen, H.D. Cadmium, lead, and mercury interactions on obstructive lung function in pre- and postmenopausal women. Environ. Sci. Pollut. Res. 2023, 30, 73485–73496. [Google Scholar] [CrossRef]
- Vahter, M.; Åkesson, A.; Lidén, C.; Ceccatelli, S.; Berglund, M. Gender differences in the disposition and toxicity of metals. Environ. Res. 2007, 104, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.L.; Johnson, A.B.; Jones, K.L. Gender Differences in Susceptibility to Heavy Metal-Induced Cardiovascular Disease: The Role of Sex Hormones. Environ. Health Perspect. 2021, 129, 037001. [Google Scholar]
- Smith, J.; Johnson, A. Gender Differences in Heavy Metal Metabolism and Susceptibility to Oxidative Stress. Environ. Toxicol. Health. 2021, 45, 123–135. [Google Scholar]
- Idowu, O.; Oyedele, O.; Olaniyan, S.D.; Lawan, E.; Hannah, A. A REVIEW OF SEX DIFFERENCES IN VULNERABILITY TO HEAVY METALS. 2023, 11.
- Navas-Acien, A.; Selvin, E.; Sharrett, A.R.; Calderon-Aranda, E.; Silbergeld, E.; Guallar, E. Lead, Cadmium, Smoking, and Increased Risk of Peripheral Arterial Disease. Circulation 2004, 109, 3196–3201. [Google Scholar] [CrossRef]
- Navas-Acien, A.; Guallar, E.; Silbergeld, E.K.; Rothenberg, S.J. Lead Exposure and Cardiovascular Disease—A Systematic Review. Environ. Heal. Perspect. 2007, 115, 472–482. [Google Scholar] [CrossRef]
- Bost, L.; Primatesta, P.; Dong, W.; Poulter, N. Blood lead and blood pressure: evidence from the Health Survey for England 1995. J. Hum. Hypertens. 1999, 13, 123–128. [Google Scholar] [CrossRef]
- Liu, C.; Xu, X.; Bai, Y. Cadmium Exposure and Cardiovascular Disease: A Systematic Review. Int. J. Environ. Res. Public Health 2018, 15, 257. [Google Scholar]
- Barregard, L.; Bergström, G.; Fagerberg, B. Cadmium Exposure in Relation to Myocardial Infarction and Stroke: A Longitudinal Population-Based Study. Epidemiology 2001, 12, 431–437. [Google Scholar]
- Sun, C.; Wang, X.; Zheng, Y.; Dong, G. Sex-Specific Associations of Mercury Exposure with Risk of Hypertension: Results from NHANES 2011–2014. J. Hypertens. 2019, 37, 1749–1755. [Google Scholar]
- Panagiotakos, D.B.; Kromhout, D.; Menotti, A.; Chrysohoou, C.; Dontas, A.; Pitsavos, C.; Adachi, H.; Blackburn, H.; Nedeljkovic, S.; Nissinen, A. The Relation Between Pulse Pressure and Cardiovascular Mortality in 12 763 Middle-aged Men From Various Parts of the World. Arch. Intern. Med. 2005, 165, 2142–2147. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Joint effect of the heavy metals on systolic blood pressure (SBP) in the total population using the BKMR regression model. The model was adjusted for age, sex, drinking, smoking, diabetes mellitus status, economic status, and body mass index. (A) The overall effect of combined metal exposure: 95% CI of the SBP estimate at each quantile compared to when all heavy metals are at median concentration. (B) Single pollutant association: 95% CI of SBP estimates for the interquartile range change of each heavy metal concentration, with the other metals fixed at the 25th, 50th, and 75th percentiles (C) Univariate exposure-response functions and 95% confidence bands for each heavy metal, with other pollutants fixed at the 50th percentile. (D) Bivariate exposure-response functions: when the levels of other heavy metals are at the median, and the levels of the expos2 metal (row) are at the 25th, 50th, and 75th percentiles, respectively, illustrating the change in SBP level according to the variation in the expos1 (column) concentration. Cd = cadmium, Pb = lead, Hg = mercury
Figure 1.
Joint effect of the heavy metals on systolic blood pressure (SBP) in the total population using the BKMR regression model. The model was adjusted for age, sex, drinking, smoking, diabetes mellitus status, economic status, and body mass index. (A) The overall effect of combined metal exposure: 95% CI of the SBP estimate at each quantile compared to when all heavy metals are at median concentration. (B) Single pollutant association: 95% CI of SBP estimates for the interquartile range change of each heavy metal concentration, with the other metals fixed at the 25th, 50th, and 75th percentiles (C) Univariate exposure-response functions and 95% confidence bands for each heavy metal, with other pollutants fixed at the 50th percentile. (D) Bivariate exposure-response functions: when the levels of other heavy metals are at the median, and the levels of the expos2 metal (row) are at the 25th, 50th, and 75th percentiles, respectively, illustrating the change in SBP level according to the variation in the expos1 (column) concentration. Cd = cadmium, Pb = lead, Hg = mercury

Figure 2.
Joint effect of the heavy metals on diastolic blood pressure (DBP) in the total population using the BKMR regression model. The model was adjusted for age, sex, drinking, smoking, diabetes mellitus status, economic status, and body mass index. (A) The overall effect of combined metal exposure: 95% CI of the DBP estimate at each quantile compared to when all heavy metals are at median concentration. (B) Single pollutant association: 95% CI of DBP estimates for the interquartile range change of each heavy metal concentration, with the other metals fixed at the 25th, 50th, and 75th percentiles (C) Univariate exposure-response functions and 95% confidence bands for each heavy metal, with other pollutants fixed at the 50th percentile. (D) Bivariate exposure-response functions: when the levels of other heavy metals are at the median, and the levels of the expos2 metal (row) are at the 25th, 50th, and 75th percentiles, respectively, illustrating the change in DBP level according to the variation in the expos1 (column) concentration. Cd = cadmium, Pb = lead, Hg = mercury
Figure 2.
Joint effect of the heavy metals on diastolic blood pressure (DBP) in the total population using the BKMR regression model. The model was adjusted for age, sex, drinking, smoking, diabetes mellitus status, economic status, and body mass index. (A) The overall effect of combined metal exposure: 95% CI of the DBP estimate at each quantile compared to when all heavy metals are at median concentration. (B) Single pollutant association: 95% CI of DBP estimates for the interquartile range change of each heavy metal concentration, with the other metals fixed at the 25th, 50th, and 75th percentiles (C) Univariate exposure-response functions and 95% confidence bands for each heavy metal, with other pollutants fixed at the 50th percentile. (D) Bivariate exposure-response functions: when the levels of other heavy metals are at the median, and the levels of the expos2 metal (row) are at the 25th, 50th, and 75th percentiles, respectively, illustrating the change in DBP level according to the variation in the expos1 (column) concentration. Cd = cadmium, Pb = lead, Hg = mercury
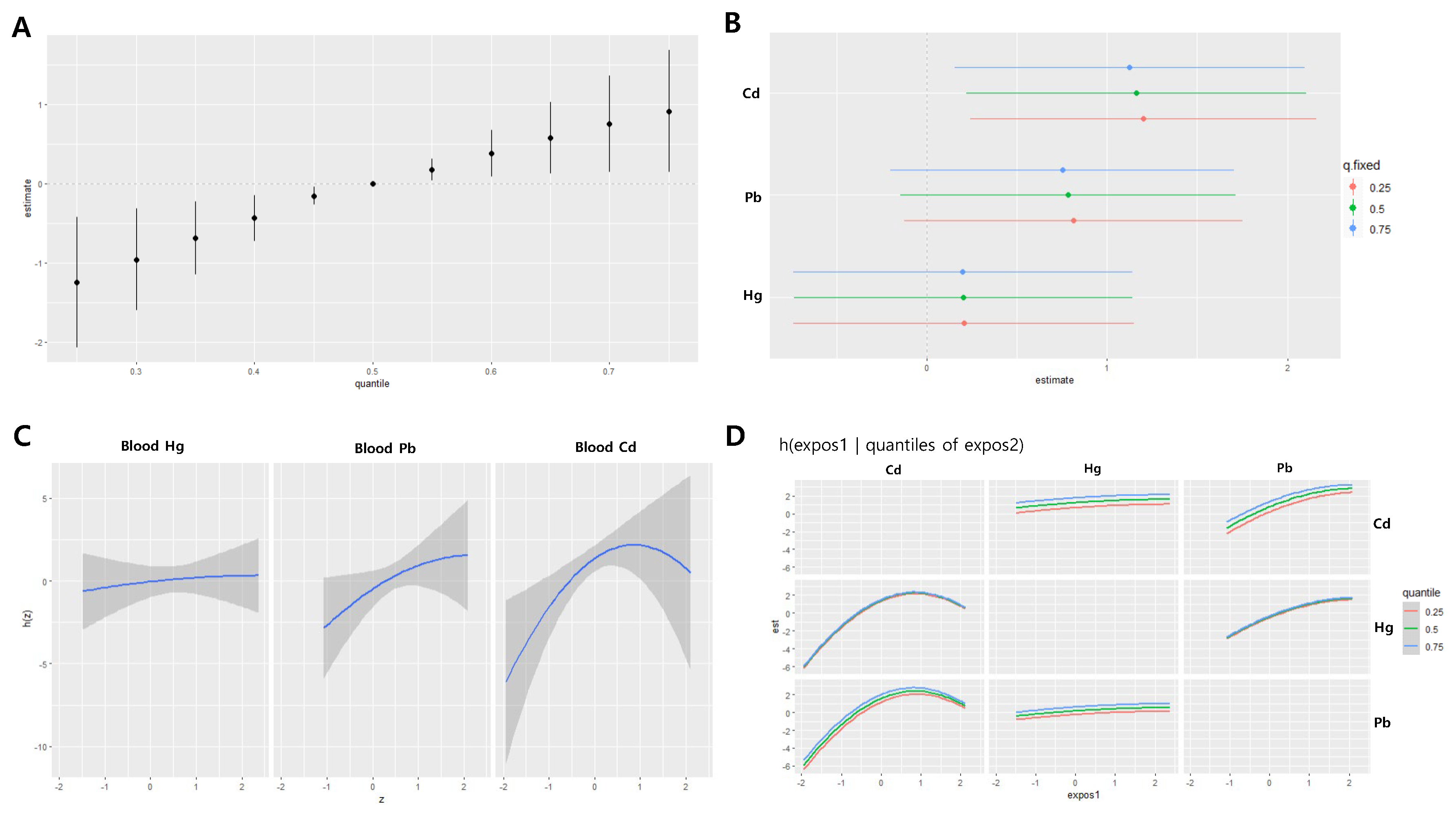
Figure 3.
Joint effect of the heavy metals on systolic blood pressure (SBP) in the men group using the BKMR regression model. The model was adjusted for age, sex, drinking, smoking, diabetes mellitus status, economic status, and body mass index. (A) The overall effect of combined metal exposure: 95% CI of the SBP estimate at each quantile compared to when all heavy metals are at median concentration. (B) Single pollutant association: 95% CI of SBP estimates for the interquartile range change of each heavy metal concentration, with the other metals fixed at the 25th, 50th, and 75th percentiles (C) Univariate exposure-response functions and 95% confidence bands for each heavy metal, with other pollutants fixed at the 50th percentile. (D) Bivariate exposure-response functions: when the levels of other heavy metals are at the median, and the levels of the expos2 metal (row) are at the 25th, 50th, and 75th percentiles, respectively, illustrating the change in SBP level according to the variation in the expos1 (column) concentration. Cd = cadmium, Pb = lead, Hg = mercury
Figure 3.
Joint effect of the heavy metals on systolic blood pressure (SBP) in the men group using the BKMR regression model. The model was adjusted for age, sex, drinking, smoking, diabetes mellitus status, economic status, and body mass index. (A) The overall effect of combined metal exposure: 95% CI of the SBP estimate at each quantile compared to when all heavy metals are at median concentration. (B) Single pollutant association: 95% CI of SBP estimates for the interquartile range change of each heavy metal concentration, with the other metals fixed at the 25th, 50th, and 75th percentiles (C) Univariate exposure-response functions and 95% confidence bands for each heavy metal, with other pollutants fixed at the 50th percentile. (D) Bivariate exposure-response functions: when the levels of other heavy metals are at the median, and the levels of the expos2 metal (row) are at the 25th, 50th, and 75th percentiles, respectively, illustrating the change in SBP level according to the variation in the expos1 (column) concentration. Cd = cadmium, Pb = lead, Hg = mercury
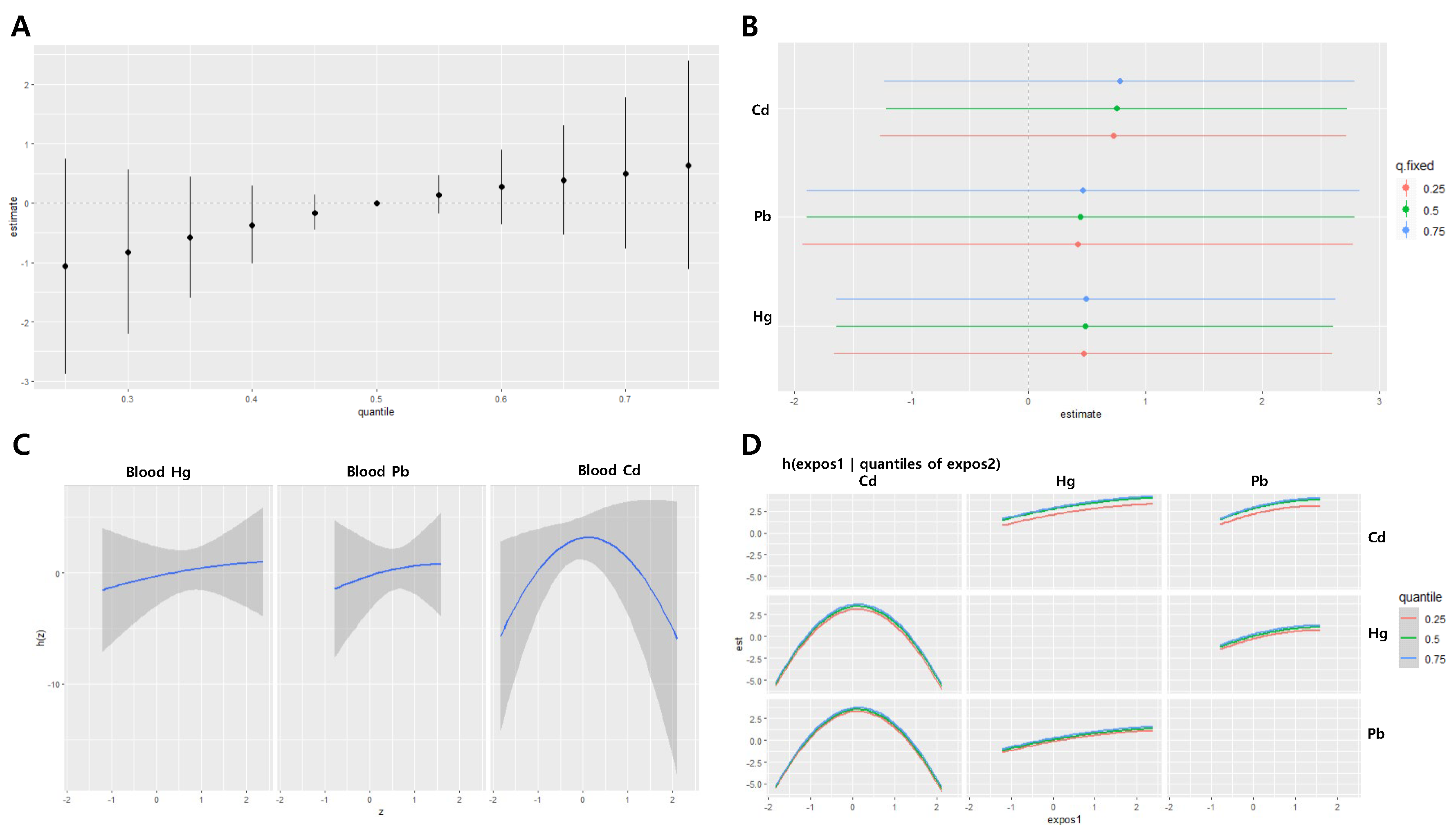
Figure 4.
Joint effect of the heavy metals on diastolic blood pressure (DBP) in the men group using the BKMR regression model. The model was adjusted for age, sex, drinking, smoking, diabetes mellitus status, economic status, and body mass index. (A) The overall effect of combined metal exposure: 95% CI of the DBP estimate at each quantile compared to when all heavy metals are at median concentration. (B) Single pollutant association: 95% CI of DBP estimates for the interquartile range change of each heavy metal concentration, with the other metals fixed at the 25th, 50th, and 75th percentiles (C) Univariate exposure-response functions and 95% confidence bands for each heavy metal, with other pollutants fixed at the 50th percentile. (D) Bivariate exposure-response functions: when the levels of other heavy metals are at the median, and the levels of the expos2 metal (row) are at the 25th, 50th, and 75th percentiles, respectively, illustrating the change in DBP level according to the variation in the expos1 (column) concentration. Cd = cadmium, Pb = lead, Hg = mercury
Figure 4.
Joint effect of the heavy metals on diastolic blood pressure (DBP) in the men group using the BKMR regression model. The model was adjusted for age, sex, drinking, smoking, diabetes mellitus status, economic status, and body mass index. (A) The overall effect of combined metal exposure: 95% CI of the DBP estimate at each quantile compared to when all heavy metals are at median concentration. (B) Single pollutant association: 95% CI of DBP estimates for the interquartile range change of each heavy metal concentration, with the other metals fixed at the 25th, 50th, and 75th percentiles (C) Univariate exposure-response functions and 95% confidence bands for each heavy metal, with other pollutants fixed at the 50th percentile. (D) Bivariate exposure-response functions: when the levels of other heavy metals are at the median, and the levels of the expos2 metal (row) are at the 25th, 50th, and 75th percentiles, respectively, illustrating the change in DBP level according to the variation in the expos1 (column) concentration. Cd = cadmium, Pb = lead, Hg = mercury
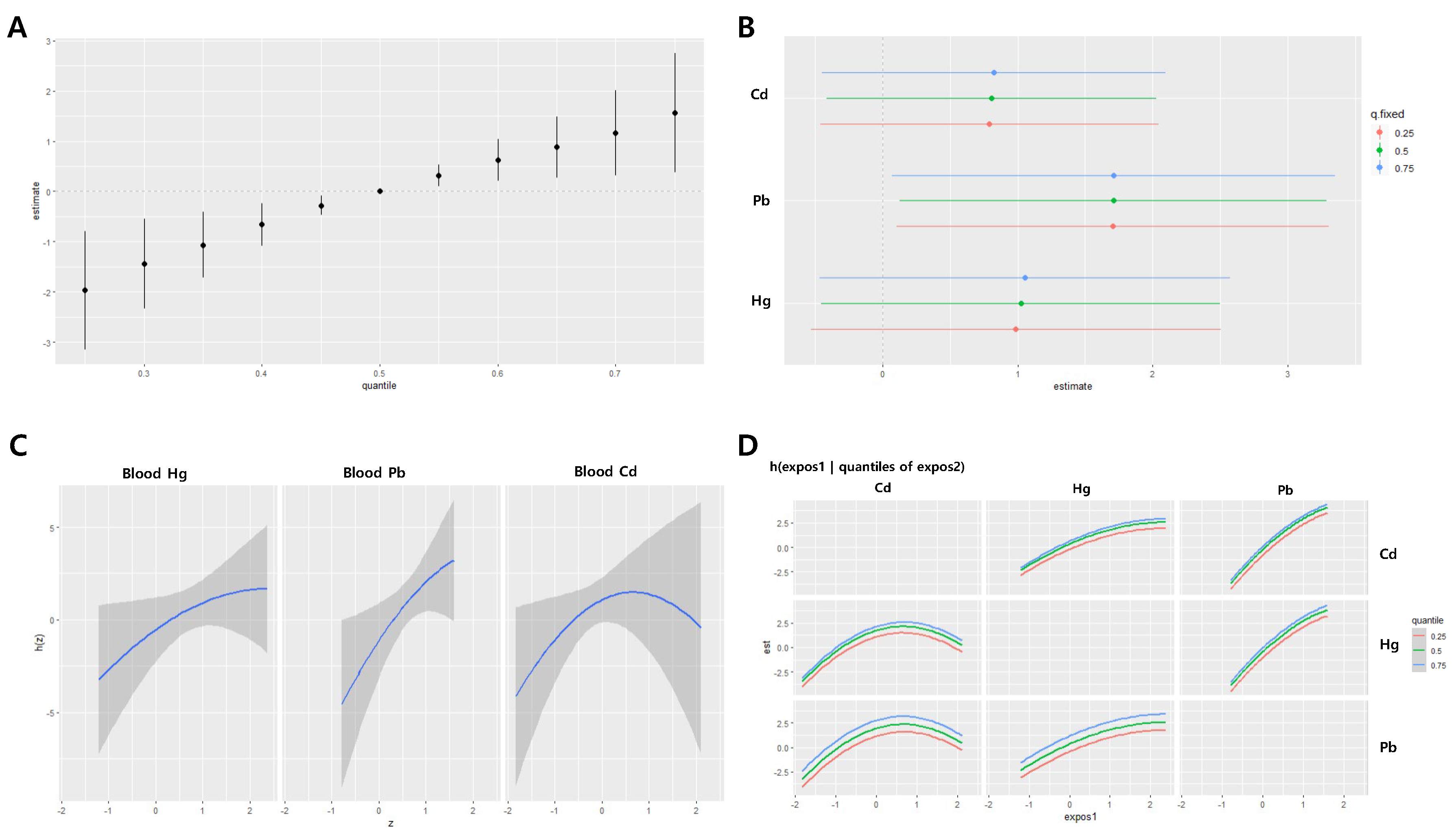
Figure 5.
Joint effect of the heavy metals on systolic blood pressure (SBP) in the women group using the BKMR regression model. The model was adjusted for age, sex, drinking, smoking, diabetes mellitus status, economic status, and body mass index. (A) The overall effect of combined metal exposure: 95% CI of the SBP estimate at each quantile compared to when all heavy metals are at median concentration. (B) Single pollutant association: 95% CI of SBP estimates for the interquartile range change of each heavy metal concentration, with the other metals fixed at the 25th, 50th, and 75th percentiles (C) Univariate exposure-response functions and 95% confidence bands for each heavy metal, with other pollutants fixed at the 50th percentile. (D) Bivariate exposure-response functions: when the levels of other heavy metals are at the median, and the levels of the expos2 metal (row) are at the 25th, 50th, and 75th percentiles, respectively, illustrating the change in SBP level according to the variation in the expos1 (column) concentration. Cd = cadmium, Pb = lead, Hg = mercury
Figure 5.
Joint effect of the heavy metals on systolic blood pressure (SBP) in the women group using the BKMR regression model. The model was adjusted for age, sex, drinking, smoking, diabetes mellitus status, economic status, and body mass index. (A) The overall effect of combined metal exposure: 95% CI of the SBP estimate at each quantile compared to when all heavy metals are at median concentration. (B) Single pollutant association: 95% CI of SBP estimates for the interquartile range change of each heavy metal concentration, with the other metals fixed at the 25th, 50th, and 75th percentiles (C) Univariate exposure-response functions and 95% confidence bands for each heavy metal, with other pollutants fixed at the 50th percentile. (D) Bivariate exposure-response functions: when the levels of other heavy metals are at the median, and the levels of the expos2 metal (row) are at the 25th, 50th, and 75th percentiles, respectively, illustrating the change in SBP level according to the variation in the expos1 (column) concentration. Cd = cadmium, Pb = lead, Hg = mercury
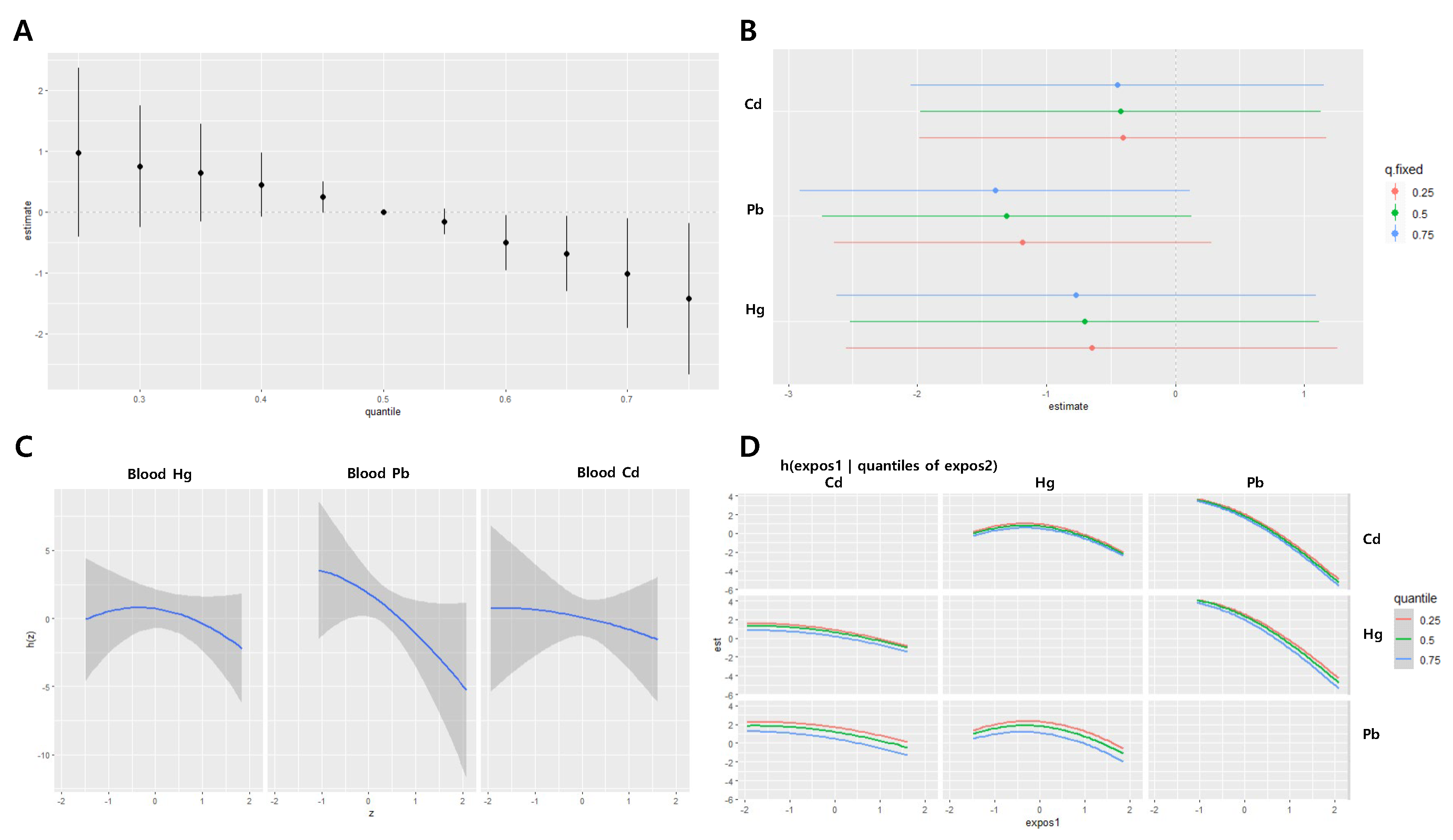
Figure 6.
Joint effect of the heavy metals on diastolic blood pressure (DBP) in the women group using the BKMR regression model. The model was adjusted for age, sex, drinking, smoking, diabetes mellitus status, economic status, and body mass index. (A) The overall effect of combined metal exposure: 95% CI of the DBP estimate at each quantile compared to when all heavy metals are at median concentration. (B) Single pollutant association: 95% CI of DBP estimates for the interquartile range change of each heavy metal concentration, with the other metals fixed at the 25th, 50th, and 75th percentiles (C) Univariate exposure-response functions and 95% confidence bands for each heavy metal, with other pollutants fixed at the 50th percentile. (D) Bivariate exposure-response functions: when the levels of other heavy metals are at the median, and the levels of the expos2 metal (row) are at the 25th, 50th, and 75th percentiles, respectively, illustrating the change in DBP level according to the variation in the expos1 (column) concentration. Cd = cadmium, Pb = lead, Hg = mercury
Figure 6.
Joint effect of the heavy metals on diastolic blood pressure (DBP) in the women group using the BKMR regression model. The model was adjusted for age, sex, drinking, smoking, diabetes mellitus status, economic status, and body mass index. (A) The overall effect of combined metal exposure: 95% CI of the DBP estimate at each quantile compared to when all heavy metals are at median concentration. (B) Single pollutant association: 95% CI of DBP estimates for the interquartile range change of each heavy metal concentration, with the other metals fixed at the 25th, 50th, and 75th percentiles (C) Univariate exposure-response functions and 95% confidence bands for each heavy metal, with other pollutants fixed at the 50th percentile. (D) Bivariate exposure-response functions: when the levels of other heavy metals are at the median, and the levels of the expos2 metal (row) are at the 25th, 50th, and 75th percentiles, respectively, illustrating the change in DBP level according to the variation in the expos1 (column) concentration. Cd = cadmium, Pb = lead, Hg = mercury
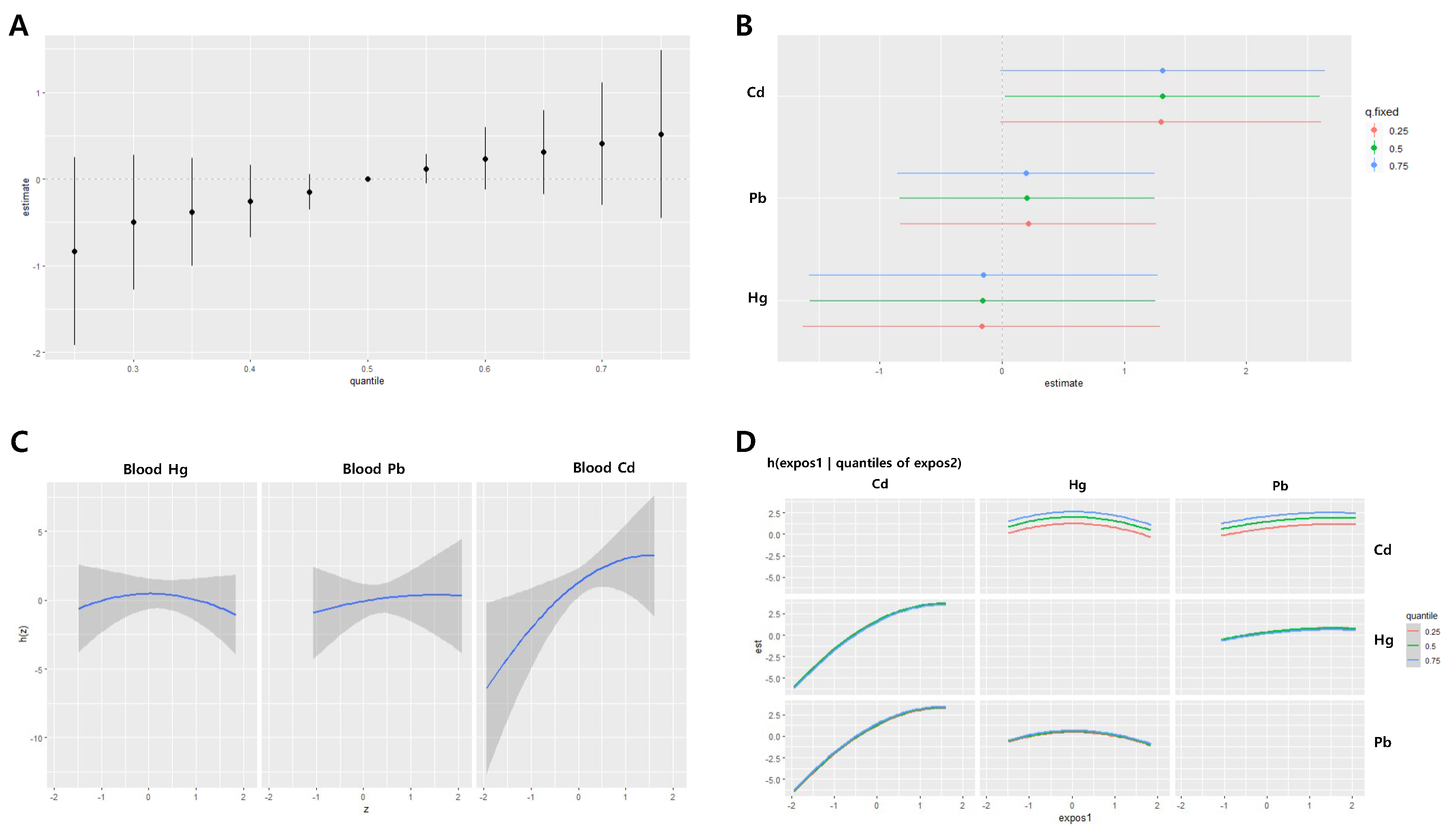
Table 1.
Demographic characteristics of the study participants.
Table 1.
Demographic characteristics of the study participants.
Characteristics |
Total
(n = 561) |
Men (n = 253) |
Women (n = 308) |
P-value |
| Smoking status |
|
|
|
|
| Non-smoker |
367 (65.42) |
67 (26.48) |
300 (97.40) |
<0.001 |
| Smoker |
194 (34.58) |
186 (73.52) |
8 (2.60) |
|
| Drinking status |
|
|
|
|
| Non-drinker |
249 (44.39) |
172 (67.98) |
77 (25.00) |
<0.001 |
| Drinker |
312 (55.61) |
81 (32.02) |
231 (75.00) |
|
| Monthly income |
|
|
|
|
| <2 million won |
312 (55.61) |
140 (55.34) |
172 (55.84) |
0.372 |
| 2–6 million won |
146 (26.02) |
72 (28.46) |
74 (24.03) |
|
| >6 million won |
7 (1.25) |
4 (1.58) |
3 (0.97) |
|
| Unknown |
96 (17.11) |
37 (14.62) |
59 (19.16) |
|
| Age (years) |
66.38 ± 12.13 |
65.83 ± 11.29 |
66.83 ± 12.78 |
0.295 |
| BMI (kg/m2) |
24.19 ± 3.59 |
23.73 ± 3.33 |
24.57 ± 3.76 |
<0.05 |
| SBP (mmHg) |
129.31 ± 14.58 |
130.02 ± 14.80 |
128.73 ± 14.40 |
0.313 |
| DBP (mmHg) |
76.47 ± 9.39 |
76.91 ± 9.73 |
76.11 ± 9.11 |
0.199 |
| Blood Hg (µg/L) |
1.59 (1.91) |
2.04 (1.81) |
1.30 (1.86) |
<0.001 |
| Blood Pb (µg/dL) |
1.56 (1.54) |
1.80 (1.48) |
1.38 (1.53) |
<0.001 |
| Blood Cd (µg/L) |
0.98 (1.61) |
0.84 (1.61) |
1.10 (1.55) |
<0.001 |
Table 2.
Associations of blood Hg, Pb, and Cd levels with blood pressure.
Table 2.
Associations of blood Hg, Pb, and Cd levels with blood pressure.
| |
β (95% CI) |
| |
SBP |
DBP |
|
| Total |
|
|
|
| Blood Hg |
–0.250 (–2.388, 1.887) |
0.425 (–0.922, 1.772) |
|
| Blood Pb |
–0.752 (–3.737, 2.233) |
1.720 (–0.161, 3.602) |
|
| Blood Cd |
0.917 (–1.842, 3.675) |
1.903 (0.165, 3.641) |
|
| |
|
|
|
| Men |
|
|
|
| Blood Hg |
0.899 (–2.329, 4.127) |
1.465 (–0.525, 3.456) |
|
| Blood Pb |
1.125 (–3.637, 5.887) |
3.298 (0.362, 6.235) |
|
| Blood Cd |
0.990 (–3.007, 4.988) |
1.457 (–1.008, 3.922) |
|
| |
|
|
|
| Women |
|
|
|
| Blood Hg |
–1.087 (–3.925, 1.750) |
–0.309 (–2.129, 1.510) |
|
| Blood Pb |
–2.231 (–6.075, 1.614) |
0.612 (–1.853, 3.077) |
|
| Blood Cd |
1.805 (–2.070, 5.679) |
2.628 (0.143, 5.113) |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).