Submitted:
26 August 2024
Posted:
27 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
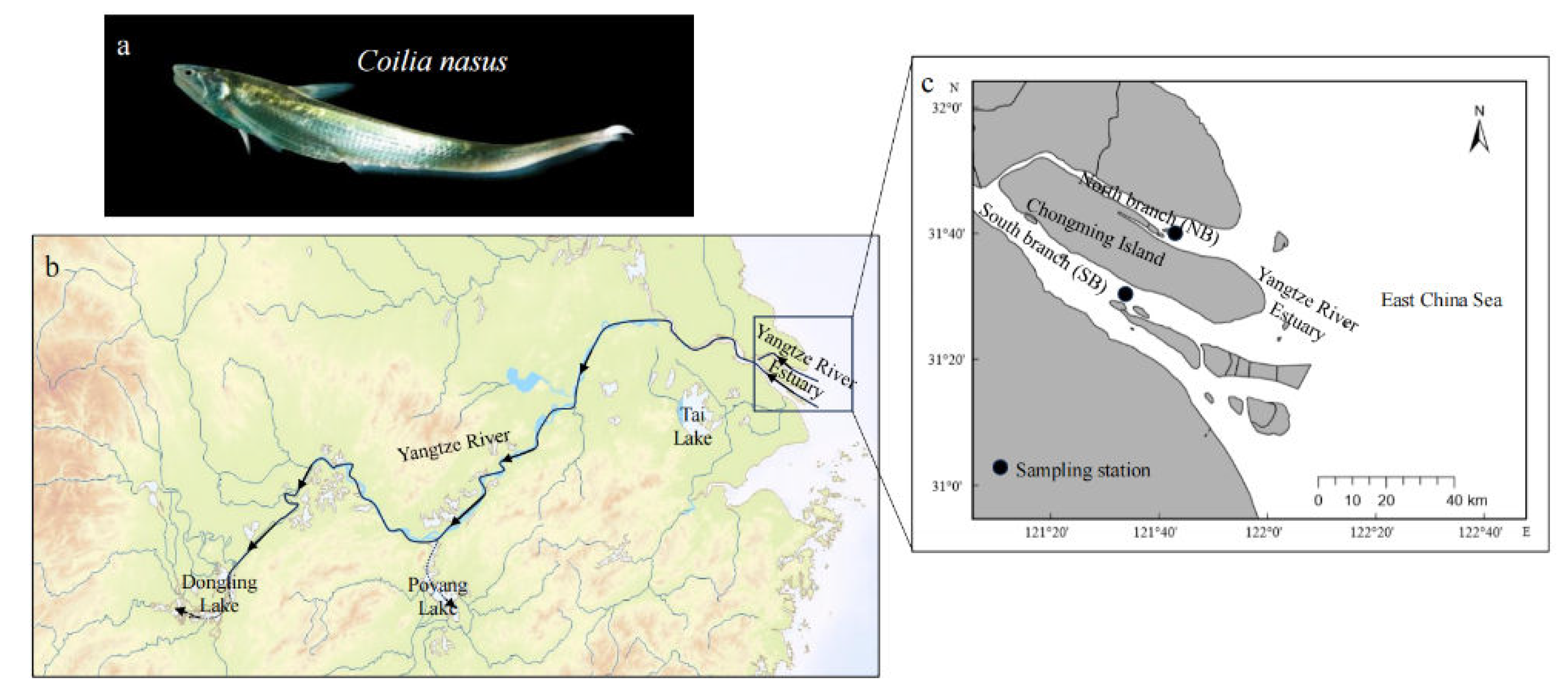
2.1. Sampling Sites, Sample Collection, and Processing
2.2. Otolith Treatment and Microchemical Analysis
2.3. Data analysis
3. Results
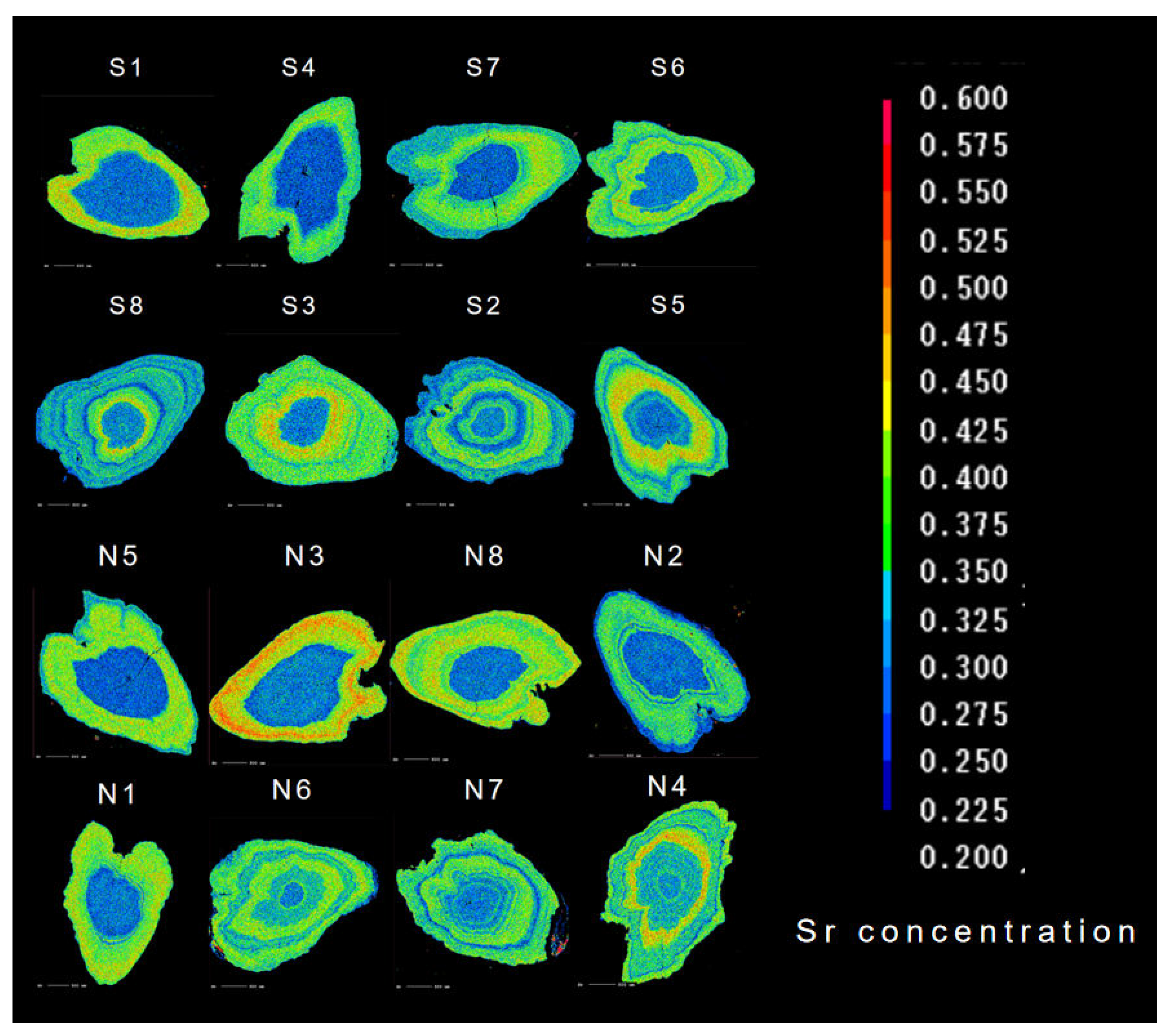
3.1. Sr/Ca Ratios Quantitative Line and Sr Content Analysis
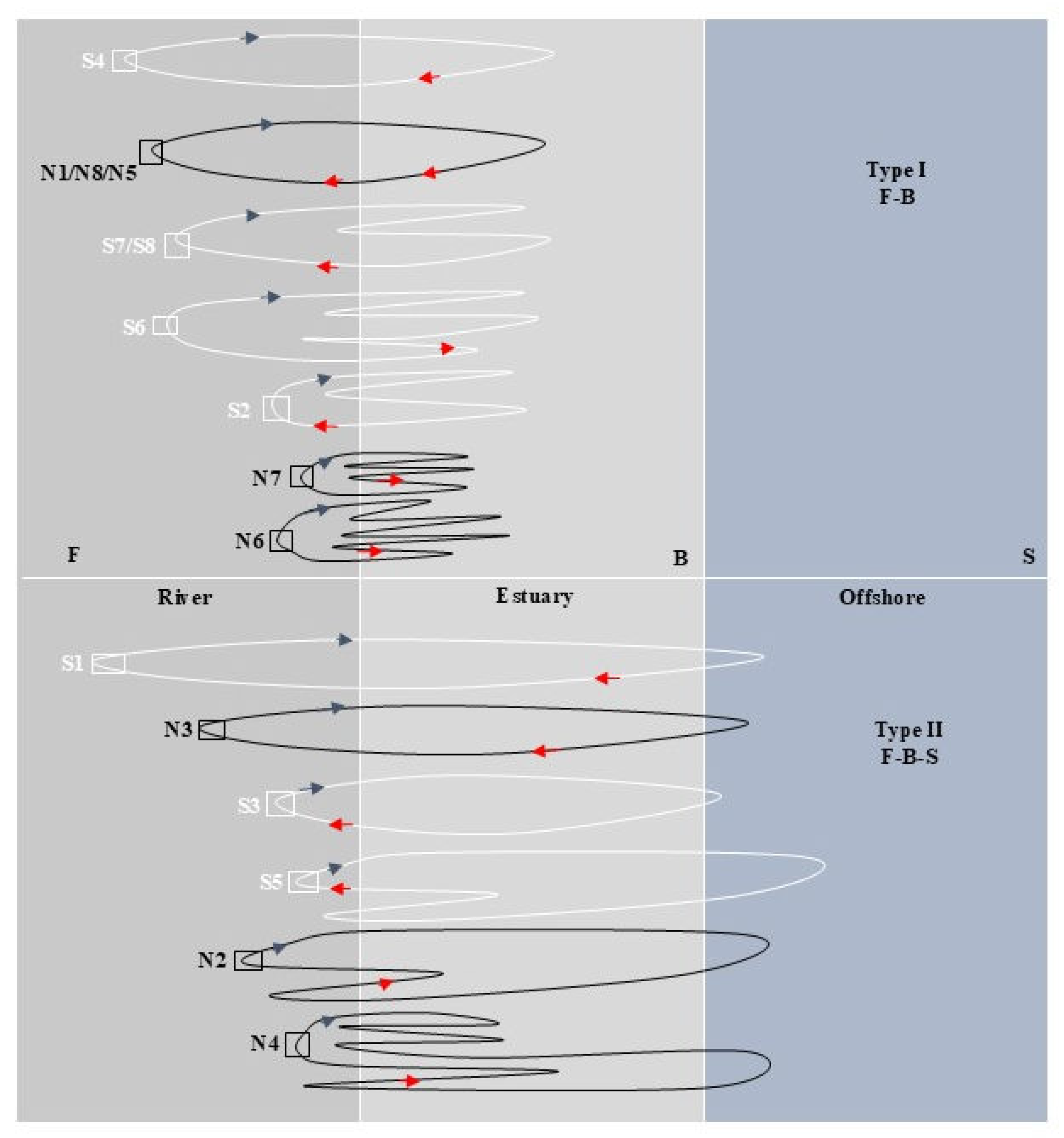
3.2. Habitat History Decomposition and Migration Pattern Analysis
3.3. Freshwater Dependence Analysis and Spawning Grounds Distribution Inference
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhuang, P.; Zhang, T.; Li, S.F.; Ni, Y.; Wang, Y.H.; Deng, S.M.; Zhang, L.Z.; Ling, J.Z.; Hu, F.; Yang, G.; et al. Fishes of the Yangtze Estuary; China Agriculture Press: Beijing, China, 2018; pp. 124–127. [Google Scholar]
- Yang, Y.F.; Jiang, T.; Gao, X.P.; Xuan, Z.Y.; Chen, X.B.; Li, L.K.; Liu, H.B.; Yang, J. Discovery of anadromous Coilia nasus in the Ganjiang River, Lake Poyang Basin, China. J. Lake Sci. 2021, 33, 1595–1606. [Google Scholar]
- Yuan, C.M. Spawning migration of Coilia nasus. Bull. Biol. 1987, 12, 1–3. [Google Scholar]
- Zhu, D.L. Natural reproduction and observation of embryonic development of Coilia nasus from the Yangtze River. Fish. Sci. Technol. Inf. 1992, 19, 49–51. [Google Scholar]
- Xuan, Z.Y.; Jiang, T.; Liu, H.B.; Qiu, C.; Chen, X.B.; Yang, J. Are there still anadromous the estuarine tapertail anchovies Coilia nasus in Dongting lake? Acta Hydrob. Sin. 2020, 44, 838–843. [Google Scholar]
- Zhang, M.Y.; Xu, D.P.; Liu, K.; Shi, W.G. Studies on biological characteristics and change of resource of Coilia nasus schlegel in the lower research of the Yangtze River. Resour. Env. Yangtze Basin 2005, 14, 694–698. [Google Scholar]
- Li, Y.X.; He, W.P.; Liu, J.S.; Li, Z.J.; Xie, S.G. Annulus validation and age and growth estimation of anadromous Coilia ectenes in the Yangtze Estuary. Acta Hydrob. Sin. 2010, 34, 787–793. [Google Scholar] [CrossRef]
- Jiang, T.; Zhou, X.Q.; Liu, H.B.; Liu, H.Z.; Yang, J. Two microchemistry patterns in otoliths of Coilia nasus from Poyang Lake, China. J. Fish. China 2013, 37, 239–244. [Google Scholar] [CrossRef]
- Jiang, T.; Liu, H.B.; Xuan, Z.Y.; Chen, X.B.; Yang, J. Classification of ecomorphotypes of Coilia nasus from the middle and lower reaches of the Yangtze River Basin. J. Lake Sci. 2020, 32, 518–527. [Google Scholar]
- Hu, Y.H.; Jiang, T.; Liu, H.B.; Chen, X.B.; Yang, J. Habitat histories of different ecomorphotypes of Coilia nasus from the Yalu River in Dandong City of Liaoning Province based on otolith microchemical analysis. Mar. Fish. 2023, 45, 278–290. [Google Scholar]
- Xu, Q.; Ren, Q.Q.; Jiang, T.; Lin, B.A.; Jiang, X.B.; Yang, J.; Liu, M. Otolith microchemistry reveals diverse habitat uses and migratory patterns of two Coilia species (Engraulidae) in the Min River Estuary, southern China. Mar. Environ. Res. 2024, 193, 106296. [Google Scholar] [CrossRef]
- Zhao, Z.; Slypko, I.; Demianenko, K.; Zhu, G.P. Otolith chemistry reveals ontogenetic movement of the Antarctic toothfish (Dissostichus Mawsoni) in the Amundsen Sea Polynya, Antarctica. Fish. Res. 2024, 276, 107046. [Google Scholar] [CrossRef]
- Doerr, L.R.; Houghton, C.J.; Hansen, S.P.; Pangle, K.L.; Ransom, A.L.; Forsythe, P.S. Can otolith microchemistry identify the natal origin of larval lake whitefish Coregonus clupeaformis in the waters of Green Bay? J. Great Lakes Res. 2021, 47, 1771–1780. [Google Scholar] [CrossRef]
- Xu, Q.; Ren, Q.Q.; Jiang, T.; Jiang, C.R.; Fang, L.P.; Zhang, M.Z.; Yang, J.; Liu, M. Otolith microchemistry reveals various habitat uses and life histories of Chinese gizzard shad Clupanodon thrissa in the Min River and the estuary, Fujian Province, China. Fish. Res. 2023, 264, 106723. [Google Scholar] [CrossRef]
- Teichert, N.; Lizé, A.; Tabouret, H.; Roussel, J.M.; Bareille, G.; Trancart, T.; Acou, A.; Virag, L.S.; Pécheyran, C.; Carpentier, A.; et al. European flounder foraging movements in an estuarine nursery seascape inferred from otolith microchemistry and stable isotopes. Mar. Environ. Res. 2022, 182, 105797. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Guo, H.; Shen, H.; Li, X.; Tang, W.; Liu, J.; Jin, J.; Mi, Y. Preliminary results of Sr:Ca ratios of Coilia nasus in otoliths by Micro-PIXE. Nucl. Instrum. Meth. B. 2007, 260, 349–352. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.H.; Feng, G.P.; Yang, J.; Zhao, F.; Shen, C.C.; Song, C.; Jiang, T. Otolith microchemistry assessment: Evidence of migratory Coilia nasus of Yangtze River living in the Shengsi sea area. Fishes 2022, 7, 172. [Google Scholar] [CrossRef]
- Jiang, T.; Liu, H.B.; Shen, X.Q.; Shimasaki, Y.; Oshima, Y.; Yang, J. Life history variations among different population of Coilia nasus along the Chinese coast inferred from otolith microchemistry. J. Fac. Agr. Kyushu Univ. 2014, 59, 383–389. [Google Scholar] [CrossRef]
- Stirnimann, L.; Conversi, A.; Marini, S. Detection of regime shifts in the environment: testing “STARS” using synthetic and observed time series. ICES J. Mar. Sci. 2019, 76, 2286–2296. [Google Scholar] [CrossRef]
- Yang, J.; Arai, T.; Liu, H.; Miyazaki, N.; Tsukamoto, K. Reconstructing habitat use of Coilia mystus and Coilia ectenes of the Yangtze River estuary, and of Coilia ectenes of Taihu Lake, based on otolith strontium and calcium. J. Fish. Biol. 2006, 69, 1120–1135. [Google Scholar] [CrossRef]
- Kotake, A.; Arai, T.; Ozawa, T.; Nojima, S.; Miller, M.J.; Tsukamoto, K. Variation in migratory history of Japanese eels, Anguilla japonica, collected in coastal waters of the Amakusa Islands, Japan, inferred from otolith Sr/Ca ratios. Mar. Biol. 2003, 142, 849–854. [Google Scholar] [CrossRef]
- Sokta, L.; Jiang, T.; Liu, H.B.; Xuan, Z.Y.; Qiu, C.; Chen, X.B.; Yang, J. Loss of Coilia nasus habitats in Chinese freshwater lakes: An otolith microchemistry assessment. Heliyon 2020, 6, e04571. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.M.; Lin, J.B.; Qin, A.Z.; Liu, R.H. On the history and present situation of the taxonomy of Coilia in China——Some experiences on reforming the old taxonomy of fishes. J. Nanjing Univ. 1976, 2, 1–13. [Google Scholar]
- Li, W.X.; Wang, G.T. Helminth communities in Coilia nasus from anadromous freshwater and landlocked stocks. Chinese J. Zool. 2014, 49, 233–243. [Google Scholar]
- Wang, L.; Tang, W.Q.; Dong, W.X. The signatures of stable isotopes δ15N and δ13C in anadromous and non-anadromous Coilia nasus living in the Yangtze River, and the adjacent sea waters. J. Ocean Univ. China 2015, 14, 1053–1058. [Google Scholar] [CrossRef]
- Cheng, F.Y.; Wang, Q.; Delser, P.M.; Li, C.H. Multiple freshwater invasions of the tapertail anchovy (Clupeiformes: Engraulidae) of the Yangtze River. Ecol. Evol. 2019, 9, 12202–12215. [Google Scholar] [CrossRef]
- Xuan, Z.Y.; Jiang, T.; Liu, H.B.; Yang, J. Otolith microchemistry and microsatellite DNA provide evidence for divergence between estuarine tapertail anchovy (Coilia nasus) populations from the Poyang Lake and the Yangtze River Estuary of China. Reg. Stud. Mar. Sci. 2022, 56, 102649. [Google Scholar] [CrossRef]
- Xuan, Z.Y.; Jiang, T.; Liu, H.B.; Chen, X.B.; Yang, J. Otolith microchemical evidence revealing multiple spawning site origination of the anadromous tapertail anchovy (Coilia nasus) in the Changjiang (Yangtze) River Estuary. Acta Oceanol. Sin. 2023, 42, 120–130. [Google Scholar] [CrossRef]
- Li, M.M.; Jiang, T.; Chen, T.T.; Liu, H.B.; Yang, J. Otolith microchemistry of the estuarine tapertail anchovy Coilia nasus from the Anqing section of the Yangtze River and its significance for migration ecology. Acta Ecol. Sin. 2017, 37, 2788–2795. [Google Scholar]
- Li, M.M.; Jiang, T.; Khumbanyiwa, D.D.; Liu, H.B. , Yang, J. Reconstructing habitat history of Coilia nasus from the Hexian section of the Yangtze River in Anhui province by otolith microchemistry. Acta Hydrob. Sin. 2017, 41, 1054–1061. [Google Scholar]
- Chen, T.T.; Jiang, T.; Li, M.M.; Liu, H.B.; Yang, J. Inversion of habitat history for the long-jaw ecotype Coilia nasus collected from Nanjing section of the Yangtze River. J. Fish. China 2016, 40, 882–892. [Google Scholar]
- Hu, Y.H.; Jiang, T.; Liu, H.B.; Chen, X.B.; Yang, J. Otolith microchemical fingerprints of Coilia nasus from the Taizhou section of Changjiang River in Jiangsu Province. Chinese J. Ecol. 2024, 43, 967–974. [Google Scholar]
- Chen, T.T.; Jiang, T.; Lu, M.J.; Liu, H.B.; Yang, J. Microchemistry analysis of otoliths of Coilia nasus and Coilia brachygnathus from the Jingjiang section of the Yangtze River. J. Lake Sci. 2016, 28, 149–155. [Google Scholar]
- Liu, H.B.; Jiang, T.; Xuan, Z.Y.; Qiu, C.; Yang, J. Otolith microchemical analysis of Tapertail Anchovy Coilia nasus from Ariake sea and its adjacent tributaries in Japan. Fish. Sci. 2020, 39, 500–508. [Google Scholar]
- Jiang, T.; Yang, J.; Lu, M.J.; Liu, H.B.; Chen, T.T.; Gao, Y.W. Discovery of a spawning area for anadromous Coilia nasus Temminck et Schlegel, 1846 in Poyang Lake, China. J. Appl. Ichthyol. 2017, 33, 189–192. [Google Scholar] [CrossRef]
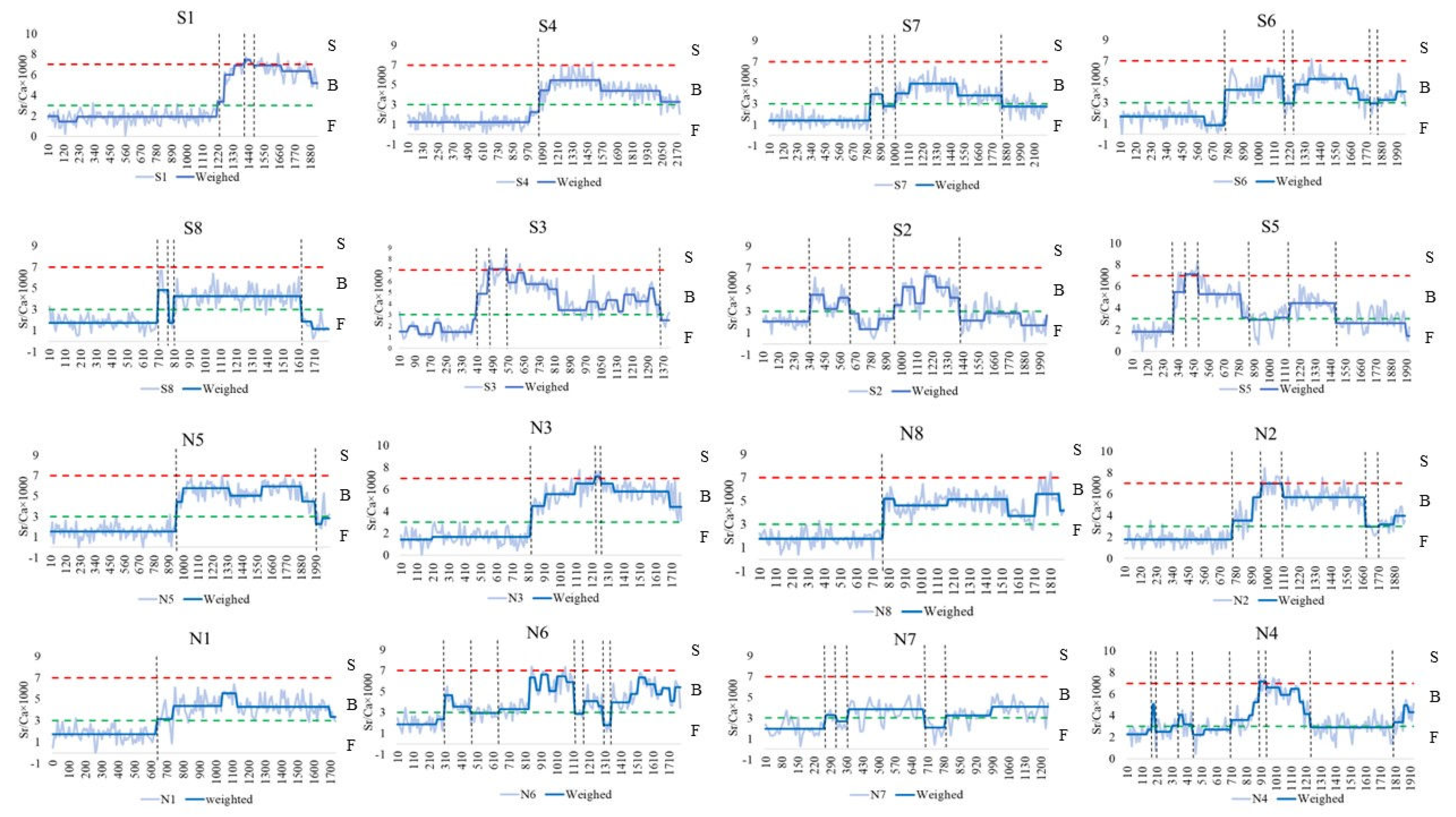


| Water Area | Sample Number | Change Stages | Spectrum | Length from Otolith Core Radius/µm | Number of Element Points (N) | Sr/Ca Ratios(Mean ± SD) |
|---|---|---|---|---|---|---|
| SB | S4 | 1(F) | Blue | 0-1060 | 107 | 1.35±0.60a |
| 2(B) | Green-Yellow | 1070-2190 | 113 | 4.62±1.06b | ||
| S2 | 1(F) | Blue | 0-340 | 35 | 2.05±0.45a | |
| 2(B) | Green-Yellow | 350-650 | 31 | 3.92±0.89b | ||
| 3(F) | Blue | 660-940 | 29 | 1.89±0.73c | ||
| 4(B) | Green-Yellow | 950-1400 | 46 | 4.85±1.12d | ||
| 5(F) | Blue | 1410-2040 | 64 | 2.37±0.95a | ||
| S7 | 1(F) | Blue | 0-800 | 81 | 1.41±0.52a | |
| 2(B) | Green-Yellow | 810-900 | 10 | 3.88±0.53b | ||
| 3(F) | Blue | 910-990 | 9 | 2.73±0.35c | ||
| 4(B) | Green-Yellow | 1000-1840 | 85 | 4.31±0.89b | ||
| 5(F) | Blue | 1850-2200 | 36 | 2.70±0.67c | ||
| S8 | 1(F) | Blue | 0-700 | 71 | 1.72±0.60ac | |
| 2(B) | Green-Yellow | 710-770 | 7 | 5.11±1.09b | ||
| 3(F) | Blue | 780-820 | 5 | 2.32±0.95c | ||
| 4(B) | Green-Yellow | 830-1620 | 80 | 4.31±0.80d | ||
| 5(F) | Blue | 1630-1800 | 18 | 1.48±0.71a | ||
| S6 | 1(F) | Blue | 0-780 | 78 | 1.53±0.65a | |
| 2(B) | Green-Yellow | 790-1170 | 39 | 4.71±0.96b | ||
| 3(F) | Blue | 1180-1250 | 8 | 2.66±0.63c | ||
| 4(B) | Green-Yellow | 1260-1790 | 54 | 4.76±1.00b | ||
| 5(F) | Blue | 1800-1850 | 6 | 2.67±0.52c | ||
| 6(B) | Green-Yellow | 1860-2050 | 20 | 3.71±0.79d | ||
| S1 | 1(F) | Blue | 0-1210 | 122 | 1.85±0.59a | |
| 2(B) | Green-Yellow | 1220-1400 | 19 | 5.57±1.45b | ||
| 3(S) | Red | 1410-1470 | 7 | 7.38±0.21c | ||
| 4(B) | Green-Yellow | 1480-1930 | 46 | 6.43±0.75d | ||
| S3 | 1(F) | Blue | 0-400 | 41 | 1.63±0.62a | |
| 2(B) | Green-Yellow | 410-470 | 7 | 5.67±1.31b | ||
| 3(S) | Red | 480-560 | 9 | 7.03±0.80c | ||
| 4(B) | Green-Yellow | 570-1350 | 79 | 4.62±1.21d | ||
| 5(F) | Blue | 1360-1400 | 5 | 2.64±0.50e | ||
| S5 | 1(F) | Blue | 0-300 | 30 | 1.87±0.60a | |
| 2(B) | Green-Yellow | 310-410 | 11 | 5.93±1.32b | ||
| 3(S) | Red | 420-480 | 7 | 7.45±0.39c | ||
| 4(B) | Green-Yellow | 490-840 | 36 | 5.26±0.95d | ||
| 5(F) | Blue | 850-1030 | 19 | 2.53±0.95e | ||
| 6(B) | Green-Yellow | 1040-1490 | 46 | 4.18±0.84f | ||
| 7(F) | Blue | 1500-2010 | 52 | 2.88±0.86e | ||
| NB | N1 | 1(F) | Blue | 0-650 | 65 | 1.78±0.64a |
| 2(B) | Green-Yellow | 660-1730 | 108 | 4.34±0.93b | ||
| N8 | 1(F) | Blue | 0-770 | 78 | 1.77±0.61a | |
| 2(B) | Green-Yellow | 780-1890 | 112 | 4.80±0.94b | ||
| N5 | 1(F) | Blue | 0-940 | 93 | 1.57±0.57a | |
| 2(B) | Green-Yellow | 950-1980 | 104 | 5.43±0.80b | ||
| 3(F) | Blue | 1990-2090 | 11 | 2.99±1.05c | ||
| N7 | 1(F) | Blue | 0-260 | 27 | 2.02±0.75a | |
| 2(B) | Green-Yellow | 270-310 | 5 | 3.26±0.40b | ||
| 3(F) | Blue | 320-360 | 5 | 2.12±0.30a | ||
| 4(B) | Green-Yellow | 370-690 | 33 | 3.75±0.91b | ||
| 5(F) | Blue | 700-780 | 9 | 1.99±0.80a | ||
| 6(B) | Green-Yellow | 790-1230 | 45 | 3.63±0.86b | ||
| N6 | 1(F) | Blue | 0-300 | 31 | 1.99±0.62a | |
| 2(B) | Green-Yellow | 310-460 | 16 | 3.91±0.70b | ||
| 3(F) | Blue | 470-650 | 19 | 2.83±0.52a | ||
| 4(B) | Green-Yellow | 660-1110 | 46 | 4.94±1.50b | ||
| 5(F) | Blue | 1120-1170 | 6 | 2.88±0.24a | ||
| 6(B) | Green-Yellow | 1180-1270 | 10 | 4.08±0.72b | ||
| 7(F) | Blue | 1280-1340 | 7 | 2.36±1.21a | ||
| 8(B) | Green-Yellow | 1350-1780 | 44 | 4.92±1.01b | ||
| N3 | 1(F) | Blue | 0-820 | 82 | 1.66±0.62a | |
| 2(B) | Green-Yellow | 830-1220 | 40 | 5.55±1.04b | ||
| 3(S) | Red | 1230-1260 | 4 | 7.37±0.48c | ||
| 4(B) | Green-Yellow | 1270-1770 | 51 | 5.72±0.93b | ||
| N2 | 1(F) | Blue | 0-750 | 76 | 1.80±0.62a | |
| 2(B) | Green-Yellow | 760-970 | 22 | 4.41±1.44b | ||
| 3(S) | Red | 980-1080 | 11 | 7.05±0.81c | ||
| 4(B) | Green-Yellow | 1090-1680 | 60 | 5.75±0.79d | ||
| 5(F) | Blue | 1690-1770 | 9 | 2.62±0.37e | ||
| 6(B) | Green-Yellow | 1780-1950 | 18 | 3.55±0.70f | ||
| N4 | 1(F) | Blue | 0-170 | 18 | 2.32±0.53a | |
| 2(B) | Green-Yellow | 180-220 | 5 | 3.52±1.76bcf | ||
| 3(F) | Blue | 230-340 | 12 | 2.60±0.56ab | ||
| 4(B) | Green-Yellow | 350-440 | 10 | 3.46±0.86bcf | ||
| 5(F) | Blue | 450-700 | 26 | 2.66±0.63ab | ||
| 6(B) | Green-Yellow | 710-890 | 19 | 3.82±1.11cf | ||
| 7(S) | Red | 900-940 | 5 | 7.05±0.70d | ||
| 8(B) | Green-Yellow | 950-1260 | 32 | 5.68±1.31e | ||
| 9(F) | Blue | 1270-1820 | 56 | 2.92±0.82abc | ||
| 10(B) | Green-Yellow | 1830-1930 | 11 | 4.20±0.86f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





