Introduction
Patients with conductive or mixed hearing loss can typically benefit from ossiculoplasty or stapedotomy procedures, or from bone conduction (BC) or air conduction (AC) behind-the-ear hearing aids. However, surgical rehabilitation can be challenging in patients with active chronic otitis media or after radical mastoidectomy; AC hearing aids are contraindicated in case of persistent purulent otorrhea, external ear canal atresia or chronic dermatosis/dermatitis. Revision stapedotomy in otosclerosis patients carries an increased risk of sensorineural damage and elderly patients with comorbidities may not be fit for surgery [
1]. Additionally, some patients refuse to use AC hearing aids for aesthetic reasons. BC hearing aids (BCHA), usually mounted on eyeglasses, were the conventional solution for this type of hearing loss. Despite their ease of use, BCHA have shortcomings, including inefficient coupling with the bone, often unsatisfactory results, and poor aesthetic appeal. To address these clinical challenges and achieve better functional outcomes, bone conduction hearing implants (BCHI) have progressively gained success as reliable and effective rehabilitation tools [
1]. The first passive BCHI, a bone anchored hearing aid (BAHA), was implanted in 1977. It required the surgical implantation of a titanium screw (fixture) that would be osteo-integrated in the temporal squama, with an abutment across the skin (percutaneous), after elevation of a cutaneous flap, soft tissue reduction and periosteal incision [
2]. Over decades, substantial clinical evidence has supported the audiological efficacy of BCHI, but also highlighted the need for continued care at the implantation site and the risks of recurrent local infections, soft tissue complications, and implant extrusion [
3,
4,
5]. In response to these issues, several transcutaneous systems have been developed, such as the BAHA Attract® (Cochlear Inc., Molnlycke, Sweden) [
6] and the Sophono™ system (Medtronic, Inc., Fridley, Minnesota, USA) [
7,
8]. Transcutaneous BCHI are designed to reduce adverse side effects, costs and the risk of an invasive surgical procedure while also improving visual appeal [
9,
10,
11,
12]. However, these devices provide slightly lower audiological benefits compared to percutaneous devices due to the damping effect of soft tissues, as conventional BCHA. Even though implant-related soft tissue problems can be resolved with trans-cutaneous devices, the requirement for surgical positioning still discourages many patients and parents of children from opting for a BCHI. Recently
, “active” middle-ear implants and transcutaneous BC devices with an implanted actuator (“active” BCHI), such as the Bonebridge® (Medel, Innsbruck) and the OSIA® (Cochlear co, Lane Cove., Australia)
have been developed to amplify bone vibration and
improve the speech perception abilities [
8,
13]
. When surgery
is not possible or contraindicated and amplification by AC hearing aids
is not feasible, then implantable hearing devices are the logical next option [
1]
. However, some patients with conductive or mixed hearing loss still refuse both active or passive BCHI due to
concerns about the invasiveness of the surgery, potential medical complications, and the devices' actual effectiveness, or because they
are unable to receive a BCHI for medical reasons [
1,
5,
15,
17]
. In such instances, non-implanted wearable applications of BCHIs, such as soft-bands or adhesive BCHA (e.g.,Adhear
®) [
14],
have been proposed as viable alternatives to surgery [
1,
14,
16]
.
In our clinical practice, a few patients who were familiar with BCHI but not candidable for surgery were willing to try wearable solutions such as headbands or adhesive BCHI, despite their innovative and powerful design, but rejected these options for aesthetic reasons or because they founf them less comfortable to use. Recently, a non-implantable wearable configuration for the BCHI, SoundArc
®, has been recently added to the non-surgical options [
18]. Its rigid bow, similar to the BC headphones used for leisure, aimed to improve the aesthetic appeal for adults while still maintaining a good BC coupling. However, this wearable option was not mentioned in the recent Consensus Statement on Bone Conduction Devices [
1] In the present study, we compared the audiological outcomes, the hearing loss-related fatigue and residual disability using this SoundArc in experienced BCHA mounted on eye-glasses users.
Methods
A prospective observational single-subject repeated-measures study was conducted at a Tertiary Referral University Hospital, with each subject serving as their own control. The study included 14 patients (8 females, 6 males) with bilateral conductive and mixed hearing loss, who were consecutively admitted for consultation. Patients were adult (> 18 years) who were experienced BCHA users (having used a BCHA for at least 3 years) and who refused the surgical implantation of BCHI, despite repeated in depth counselling in accordance to the guidelines. Exclusion criteria were age<18 years; retro-cochlear or central auditory disorders; cognitive impairment or psychiatric conditions; external, middle and/or inner ear malformations and unwillingness to participate to the study.
Patients details are listed in
Table 1. The median age was: 77.8 ± 5.1 years (ranging from 68 to 88 years). Diagnoses included chronic otitis media (12/14 cases, 86%), including 2 patients who had undergone a radical mastoidectomy and otosclerosis (2/14 cases, 14%). This cohort was enrolled during a period of 9 months. Medical history and otoscopy were recorded.
The study protocol included two baseline measurements: 1) unaided pure-tone audiometry [UNI EN ISO 1_tonale]; 2) unaided speech audiometry; subsequently, three aided free-field thresholds were obtained: 3) aided pure-tone audiometry; 4) aided speech perception tests in quiet; and 5) aided speech perception tests in noise. The speech perception test consisted in the repetition of 10 words presented in open set (word recognition score, WRS) both in quiet and noise; four lists were randomly selected, one for each task, among 20 lists of phonetically balanced words of common use and meaningful for the Italian language [
19]. Speech perception in noise (cocktail party) was tested with a signal-to-noise ratio (SNR) of +10, 0 dB and -10 dB HL. All tests were performed in a free-field sound-treated room according to UNI EN ISO 8253-3 at 60 dB HL in both quiet and noise (speech signal at 0 ̊ azimuth and the noise at 180° azimuth, S0N90) [
20]; the loudspeakers were placed at 1 meter from the centre of the participant’s head [
21].
All aided tests were performed with the personal BCHA to which each patient was familiar. In a second round of testing, aided pure-tone and speech audiometry were repeated in a free field using BCHI SoundArc®. Seven patients wore a unilateral Baha 5® sound processor, while the more powerful processor (BAHA 5 Power®) was needed in 9 out of 14 (64%). All subjects were evaluated after a period of a one-month free trial of SoundArc use. Audiological measurements were performed by the same expert audiologist (GF) to reduce any measurement bias.
All patients were also asked to fill the following questionnaires regarding the use of their BCHA: Hearing Handicap Inventory for Adult or Elderly [HHIA / HHIE] [
22,
23,
24,
25] ; International Inventory for Hearing Aids [IOI – HA] [
26]; Speech, Spatial, Qualities of Hearing Scale [SSQ] and Fatigue impact scale [FIS] [
27,
28]. These questionnaires were also collected after the aided trial with SoundArc, together with the collection of a visuo-analogic scale [VAS] graded between 0 (minimum) to 10 (maximum) about wearability (comfort, weight, and aesthetics).
Statistical analysis. The Shapiro-Wilk normality test was first applied to the dataset to assess the normality of continuous variables. A Wilcoxon rank-signed exact test (2-tailed) was used to assess differences between the results obtained with BCHA and SoundArc. Values were considered statistically significant when p < .05 All statistics were calculated with the Statistical Package for the Social Sciences 26 for Windows software package (SPSS Inc, Chicago, Illinois).
Thy study received approval by the Ethical Committee of Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico of Milan, Italy. (IRB 2018_468) Informed consent was obtained from all subjects involved in the study. Patients’ anonymity has been guaranteed.
Results
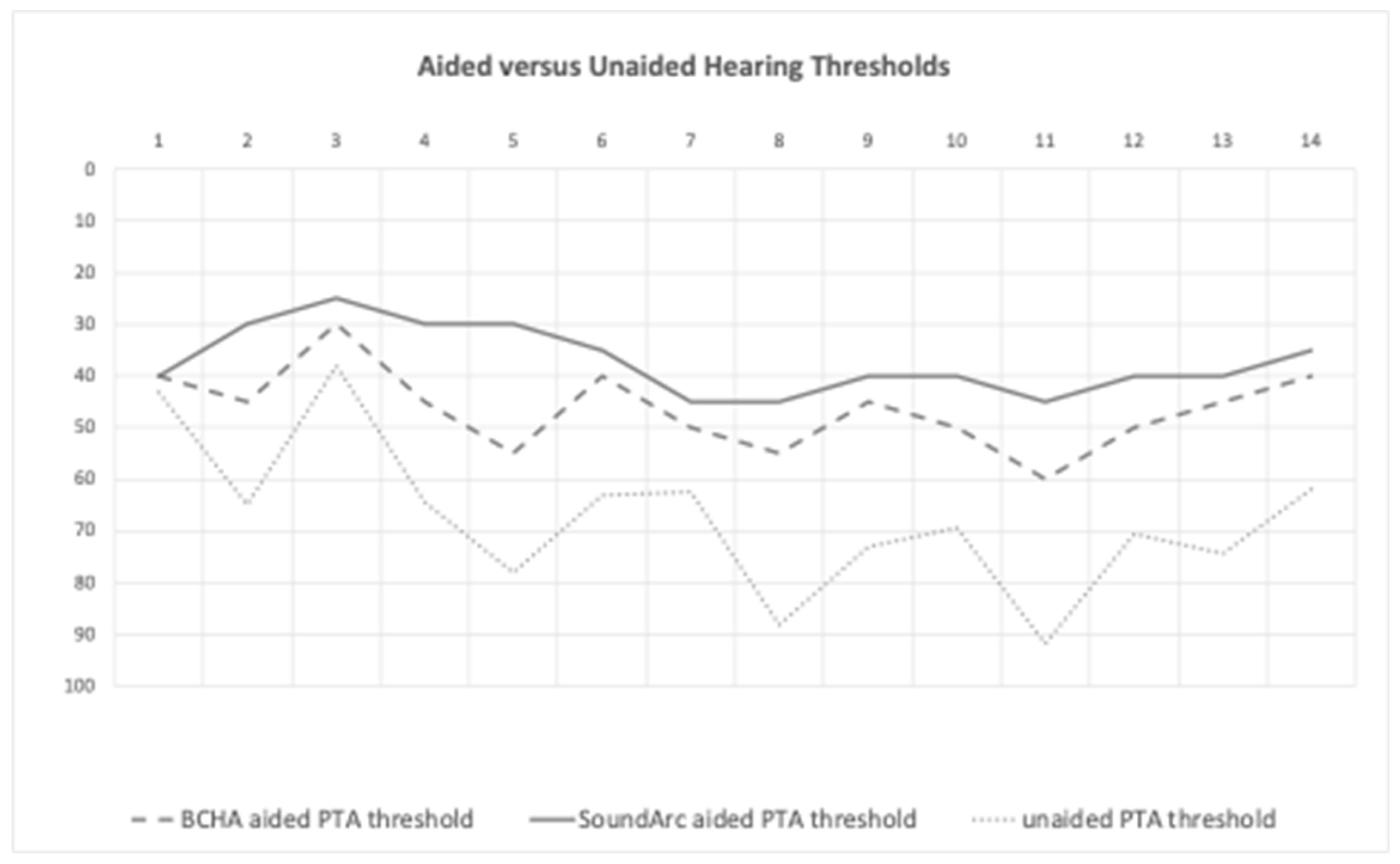
Aided versus Unaided Hearing Thresholds
The patients’ AC pure tone average (PTA) across 0.5, 1, 2 and 4 KHz was 66.9 dB HL (±16 dB HL) for the right ear and 66.7 dB HL (±10,4 dB) for the left ear. The air bone gap (ABG) average at 0.25, 1 and 2 KHz was 37.1 dB HL (±9.7 dB HL) for the right ear and 37.3 dB HL (±10.4 dB HL) for the left ear.
The mean BCHA-aided thresholds were 46.4 ± 7.7 dB HL, and the mean SoundArc-aided thresholds were 37.1 ± 6.4 dB HL, resulting in a significative functional gain for both the aided conditions versus the unaided condition, (p=.0001) (
Figure 1) Only two patients (#1 and #3) did not show a significant improvement; they were the only two patients with a moderate conductive hearing loss and preserved BC thresholds. (
Table 1)
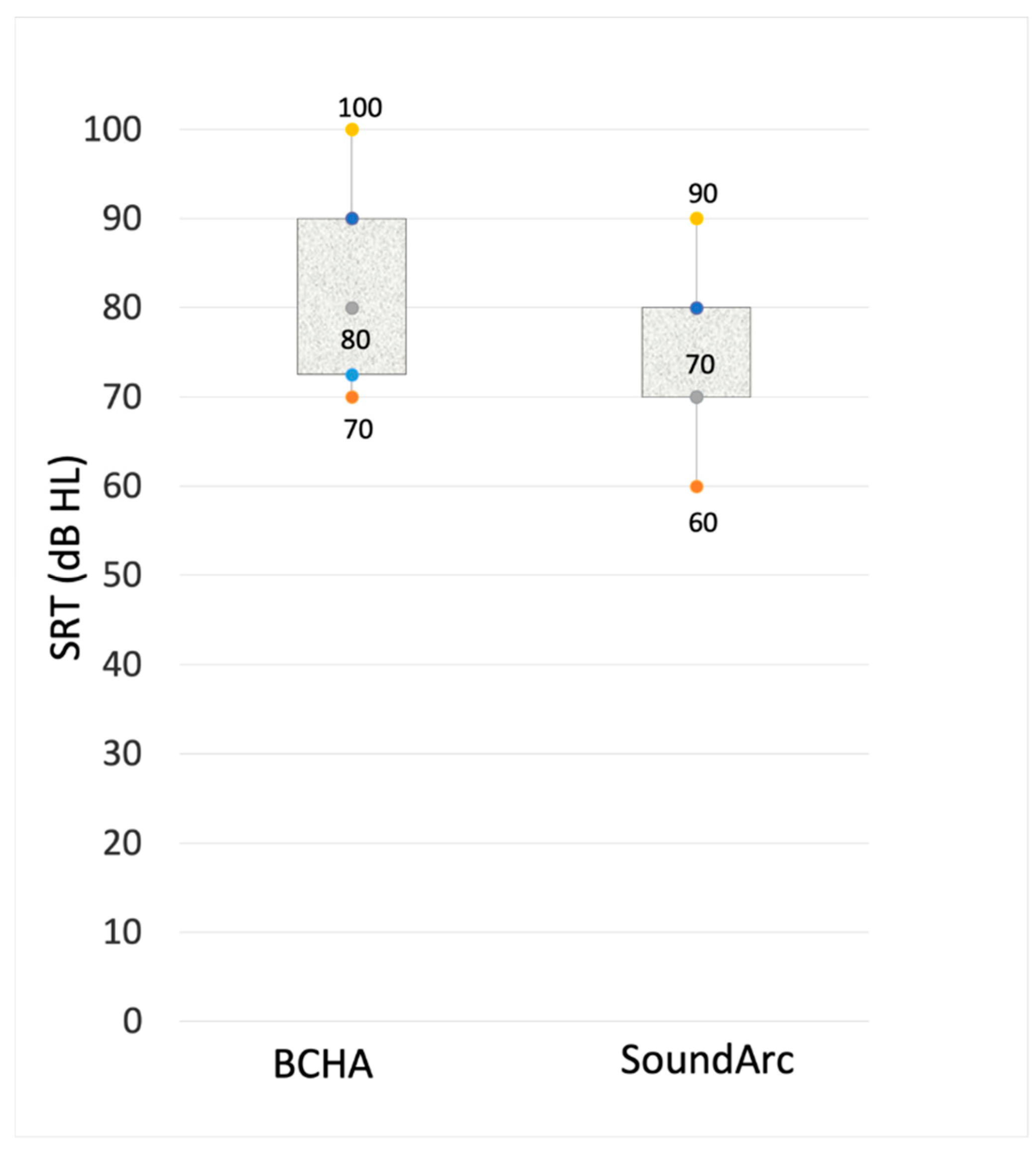
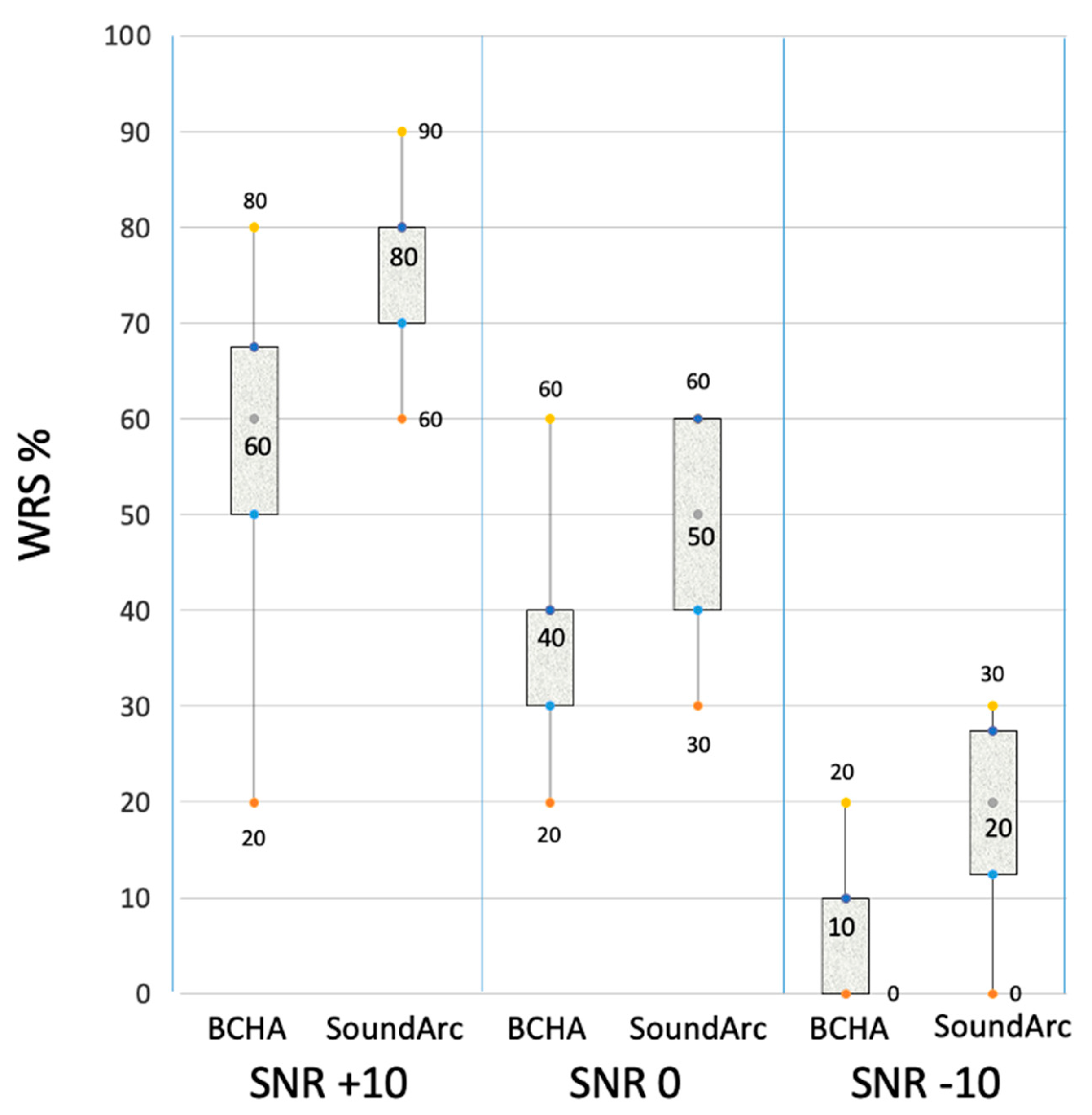
Speech tests in quiet and noise. Raw data for the single patients are reported in
Table 2. Speech perception outcomes expressed as speech reception thresholds (SRT) are reported in
Figure 2. With SoundArc patients achieved slightly better speech perception in quiet. As reported in
Figure 3, more consistent differences between BCHA and SoundArc were found in noise: WRS in noise was improved with SoundArc in all the tested conditions: +19.3 at SNR +10 dB, p=.002; + 12.1 at SNR 0 dB, p=.006; and +11.4 at SNR -10 dB, p=.002.
Subjective Outcomes/Questionnaires
The SSQ scores were remarkably better for SoundArc, at a significant level for all three sub-domains, especially for “
speech”. (
Table 3) Similarly, all subjects reported a significative improvement of VAS scores for 2 of the 3 items (+7.6 ± 1 for the comfort, +3,0 ± 1 for the weight) while the subjective judgment for the aesthetic appeal was substantially unchanged (+1.1 ± 1.3).
Conversely, the patients did not report any significative subjective benefits at IOI-HA (4.0 ± 1.0 for BCHA; 4.0 ± 1.0 for SoundArc, p= n.s.).
The HHIA / HHIE scores disclosed a substantial absence of reduced participation restriction, with no statistically significant difference between SoundArc and BCHA in any of the measures. In agreement with the latter, all patients reported low levels of listening fatigue, as assessed by FIS scores (ranging from 0 to 3), for both aided conditions.
Discussion
The first description of a sound conduction tool was reported by Girolamo Cardano, which simply explained the possibility to “…
hear distant sounds, voices and words that he could not hear under normal circumstances…” using a rod or the shaft of a spear held between one’s teeth [
29]. In the beginning of the 20th century, the development of the carbon microphone speaker allowed the construction of a BC vibrator placed on the mastoid area, notably supported by eyeglasses since the 1950s. BCHA, usually mounted on specially designed eyeglasses, have been the traditional and easiest solution for conductive hearing loss. However, their appreciation and diffusion has been limited by inefficient coupling with the temporal bone, by partially unsatisfactory results and also for aesthetic reasons. The literature of the last 40 years is redundant in the indications to BCHI, ranging from external and middle ear congenital malformations to failures of middle ear surgery for COM or otosclerosis [
1]. In response to the demand for a non-surgical BC solution, two different options were developed in order to benefit for BCHI sound processors: headbands/softbands and adhesive fixation. These solutions are often used for temporary preoperative trials, and occasionally also for permanent use, especially in young children with congenital conductive hearing loss, such as external ear atresia and ossicular fixation, or until the skull growth allows the implantation of the fixture [
30]. A comparison between SoundArc and softband had been already reported in a previous study by Gawliczek et al. in 2018 [
18]. The authors reported a considerable improvement in hearing and speech understanding in subjects with a simulated, purely conductive, bilateral hearing loss, but no significant difference between the two wearing options was found, probably due to the use of the same device and to the similar force measurements in the two coupling methods. As a matter of fact, so far all these solutions have gained limited success especially in older children and in adults, mainly due to their low aesthetic appeal [
31,
32,
33], but also for the significant sound attenuation produced by the skin, which can reach 15 dB HL at 3 KHz [
30,
34,
35].
As far as we know, this is the first report comparing the functional gain with both conventional BCHA on eye-glasses and SoundArc in patients with chronic conductive/mixed hearing loss, which are used to the old traditional system in order to understand if it is reasonable to propose SoundArc as an alternative or not. The present study demonstrates that SoundArc, after a short trial period, provided better speech perception performance in a noisy background, and improved SSQ scores, despite patients were experienced users of their BCHA for many years. In addition, all patients reported their appreciation in terms of comfort of use with the SoundArc. However, the results of other subjective reports of benefit (HHIA/HHIE, FIS) were inconsistent. This study has two main limitations: first, the paucity of the patients enrolled does not allow to draw definitive conclusions; second, the short duration of the trial period with SoundArc compared to the long term acquaintance to BCHA (>3 years), might flaw the statistical analysis. The lack of a difference in subjective reports against a significative evidenced benefit allows to think that some results might be different after a longer use of SoundArc. Therefore, the outcomes of the present study must be considered preliminary. Furthermore, the major cost of the not wearable solutions of passive BCHI compared to BCHA might have influenced the subjective report. The main benefit derived from SoundArc, in the selected sample of patients of the present study, appears to be an advantage in speech perception under unfavourable listening conditions, especially for those with greater hearing loss. A better coupling effect of SoundArc compared to the head band has been already reported [
18]. A possible explanation is probably related with a better coupling of SoundArc also in comparison to the BCHA mounted on eyeglasses. These mechanical aspects will be object of a further study.
In conclusion, Sound Arc represents a viable alternative to the BCHA mounted on eyeglasses. It should be included in the list of the non-surgical wearable options for patients not suitable or refusing the implantable devices (BCHI), as its clinical functional outcomes surpass those of the traditional BCHA.
Author Contributions
Conceptualization, (G.C. and F.D.B.), Methodology (C.A. and G.C.), Validation (G.C.), Formal Analysis (F.D.B.), Investigation (G.F., C.A.) Writing – Review & Editing, (F.D.B.).; Visualization, (F.D.B.).; Supervision, (V.V., E.P. and D.Z.); Project Administration (C.A.), Resources, (C.A.).; Data Curation, (G.F.).; Writing – Original Draft Preparation, (G.C.).
Funding
this research received no external funding.
Data Availability Statement
Data supporting reported results are available on request.
Acknowledgements
The authors are grateful to all the patients for their patience and cooperation, in particular to P.O. who, due to his personal experience, motivated us to collect this data and write this study. We also acknowledge the staff of Amplifon Monza, Italy for providing the free trial to all patients.
Conflict of Interest
The authors declare no conflict of interest.
References
- Maier, H.; Lenarz, T.; Agha-Mir-Salim, P.; Agterberg, M.J.H.; Anagiotos, A.; Arndt, S.; Ball, G.; Bance, M.; Barbara, M.; Baumann, U.; et al. Consensus Statement on Bone Conduction Devices and Active Middle Ear Implants in Conductive and Mixed Hearing Loss. Otol. Neurotol. 2022, 43, 513–529. [Google Scholar] [CrossRef]
- Tjellström, A. Dimensions of Importance in Reconstructive Middle Ear Surgery. Acta Oto-Laryngologica 1977, 83, 488–490. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.P.; Symms, J.T.; Beasley, K.; Coffman, H.M. Bone conduction implants. Curr. Opin. Otolaryngol. Head Neck Surg. 2020, 28, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Wazen JJ, Wycherly B, Daugherty J. Complications of bone-anchored hearing devices. Adv Otorhinolaryngol. 2011;71:63-72. [CrossRef] [PubMed]
- Candreia, C.; Birrer, R.; Fistarol, S.; Kompis, M.; Caversaccio, M.; Arnold, A.; Stieger, C. Predisposing factors for adverse skin reactions with percutaneous bone anchored hearing devices implanted with skin reduction techniques. 2016, 273, 4185–4192. [CrossRef]
- Kurz, A.; Flynn, M.; Caversaccio, M.; Kompis, M. Speech Understanding with a New Implant Technology: A Comparative Study with a New Nonskin Penetrating Baha System. BioMed Res. Int. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mclean, T.; Pai, I.; Philipatos, A.; Gordon, M. The Sophono Bone-Conduction System: Surgical, Audiologic, and Quality-of-Life Outcomes. Ear, Nose Throat J. 2017, 96, E28–E33. [Google Scholar] [CrossRef] [PubMed]
- Riss, D.; Arnoldner, C.; Baumgartner, W.-D.; Blineder, M.; Flak, S.; Bachner, A.; Gstoettner, W.; Hamzavi, J.-S. Indication criteria and outcomes with the Bonebridge transcutaneous bone-conduction implant. Laryngoscope 2014, 124, 2802–2806. [Google Scholar] [CrossRef]
- Sylvester, D.C.; Gardner, R.; Reilly, P.G.; Rankin, K.; Raine, C.H. Audiologic and Surgical Outcomes of a Novel, Nonpercutaneous, Bone Conducting Hearing Implant. Otol. Neurotol. 2013, 34, 922–926. [Google Scholar] [CrossRef]
- Powell, H.R.; Rolfe, A.M.; Birman, C.S. A Comparative Study of Audiologic Outcomes for Two Transcutaneous Bone-Anchored Hearing Devices. Otol. Neurotol. 2015, 36, 1525–1531. [Google Scholar] [CrossRef]
- Gerdes, T.; Salcher, R.B.; Schwab, B.; Lenarz, T.; Maier, H. Comparison of Audiological Results Between a Transcutaneous and a Percutaneous Bone Conduction Instrument in Conductive Hearing Loss. Otol. Neurotol. 2016, 37, 685–691. [Google Scholar] [CrossRef]
- Magele, A.; Schoerg, P.; Stanek, B.; Gradl, B.; Sprinzl, G.M. Active transcutaneous bone conduction hearing implants: Systematic review and meta-analysis. PLOS ONE 2019, 14, e0221484. [Google Scholar] [CrossRef]
- Rauch, A.-K.; Wesarg, T.; Aschendorff, A.; Speck, I.; Arndt, S. Long-term data of the new transcutaneous partially implantable bone conduction hearing system Osia®. Eur. Arch. Oto-Rhino-Laryngology 2022, 279, 4279–4288. [Google Scholar] [CrossRef]
- Almuhawas, F.; Alzhrani, F.; Saleh, S.; Alsanosi, A.; Yousef, M. Auditory Performance and Subjective Satisfaction with the ADHEAR System. Audiol. Neurotol. 2020, 26, 1–10. [Google Scholar] [CrossRef]
- Larsson, A.; Tjellström, A.; Stalfors, J. Implant Losses for the Bone-Anchored Hearing Devices Are More Frequent in Some Patients. Otol. Neurotol. 2015, 36, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Skarzynski, P.H.; Ratuszniak, A.; Osinska, K.; Koziel, M.; Krol, B.; Cywka, K.B.; Skarzynski, H. A Comparative Study of a Novel Adhesive Bone Conduction Device and Conventional Treatment Options for Conductive Hearing Loss. Otol. Neurotol. 2019, 40, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Verheij, E.; Bezdjian, A.; Grolman, W.; Thomeer, H.G.X.M. A Systematic Review on Complications of Tissue Preservation Surgical Techniques in Percutaneous Bone Conduction Hearing Devices. Otol. Neurotol. 2016, 37, 829–837. [Google Scholar] [CrossRef]
- Gawliczek, T.; Wimmer, W.; Munzinger, F.; Caversaccio, M.; Kompis, M. Speech Understanding and Sound Localization with a New Nonimplantable Wearing Option for Baha. BioMed Res. Int. 2018, 2018, 1–8. [Google Scholar] [CrossRef] [PubMed]
- BOCCA, E. L'audiometria vocale [Vocal audiometry]. Otorinolaringol Ital. 1951;19(6):461-500. Undetermined Language. [PubMed]
- UNI EN ISO 8253-3 Acoustics — Audiometric test methods — Part 1: Pure-tone air and bone conduction audiometry. https://www.iso.org/standard/43601.html.
- UNI EN ISO 389-7 Acoustics — Reference zero for the calibration of audiometric equipment — Part 7: Reference threshold of hearing under free-field and diffuse-field listening conditions. 2019 (https://www.iso.org/standard/77365.html ).
- Newman, C.W.; Weinstein, B.E.; Jacobson, G.P.; Hug, G.A. The Hearing Handicap Inventory for Adults. Ear Hear. 1990, 11, 430–433. [Google Scholar] [CrossRef] [PubMed]
- Monzani, D.; Genovese, E.; Palma, S.; Rovatti, V.; Borgonzoni, M.; Martini, A. Measuring the psychosocial consequences of hearing loss in a working adult population: focus on validity and reliability of the Italian translation of the hearing handicap inventory. . 2007, 27, 186–91. [Google Scholar]
- Ventry, I.M.; Weinstein, B.E. The Hearing Handicap Inventory for the Elderly. Ear Hear. 1982, 3, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Di Berardino, F.; Nocini, R.; Ariotti, B.; Apa, E.; Gherpelli, C.; Monzani, D. Validity of the Italian adaptation of the Hearing Handicap Inventory for the Elderly (HHIE-It). Acta Otorhinolaryngol. Ital. 2023, 43, 74–81. [Google Scholar] [CrossRef]
- Cox, R.M.; Stephens, D.; Kramer, S.E. Translations of the International Outcome Inventory for Hearing Aids (IOI-HA): Traducciones del Inventario Internacional de Resultados para Auxiliares Auditivos (IOI-HA). Int. J. Audiol. 2002, 41, 3–26. [Google Scholar] [CrossRef]
- Gatehouse, S.; Noble, W. The Speech, Spatial and Qualities of Hearing Scale (SSQ). Int. J. Audiol. 2004, 43, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Fisk, J.D.; Ritvo, P.G.; Ross, L.; Haase, D.A.; Marrie, T.J.; Schlech, W.F. Measuring the functional impact of fatigue: Initial validation of the Fatigue Impact Scale. Clin. Infect. Dis. 1994, 18 (Suppl 1), S79–S83. [Google Scholar] [CrossRef]
- Cardano G: De subtilitate libri XXI. Parisiis, Fezandat & Roberri, 1550, liber XIII, p 234.
- Zarowski AJ, Verstraeten N, Somers T, Riff D, Offeciers EF. Headbands, testbands and softbands in preoperative testing and application of bone-anchored devices in adults and children. Adv Otorhinolaryngol. 2011;71:124-131. [CrossRef] [PubMed]
- Kompis, M.; Wimmer, W.; Caversaccio, M. Long term benefit of bone anchored hearing systems in single sided deafness. Acta Oto-Laryngologica 2016, 137, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Siau, R.T.K.; Dhillon, B.; Siau, D.; Green, K.M.J. Bone-anchored hearing aids in conductive and mixed hearing losses: why do patients reject them? . 2016, 273, 3117–3122. [Google Scholar] [CrossRef]
- Powell, R.; Wearden, A.; Pardesi, S.M.; Green, K. Understanding the low uptake of bone-anchored hearing aids: a review. J. Laryngol. Otol. 2017, 131, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Kurz, A.; Flynn, M.; Caversaccio, M.; Kompis, M. Speech Understanding with a New Implant Technology: A Comparative Study with a New Nonskin Penetrating Baha System. BioMed Res. Int. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Mandavia, R.; Carter, A.; Haram, N.; Mossialos, E.; Schilder, A. An evaluation of the quality of evidence available to inform current bone conducting hearing device national policy. Clin. Otolaryngol. 2017, 42, 1000–1024. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).