Submitted:
07 August 2024
Posted:
27 August 2024
You are already at the latest version
Abstract

Keywords:
1. Introduction
- a)
- Are there secular variations in the abundance of diagenetic spheroids during the Proterozoic?
- b)
- Could Proterozoic diagenetic spheroids represent sedimentological signatures of abiotic carbon cycling favoured by environmental oxidation?
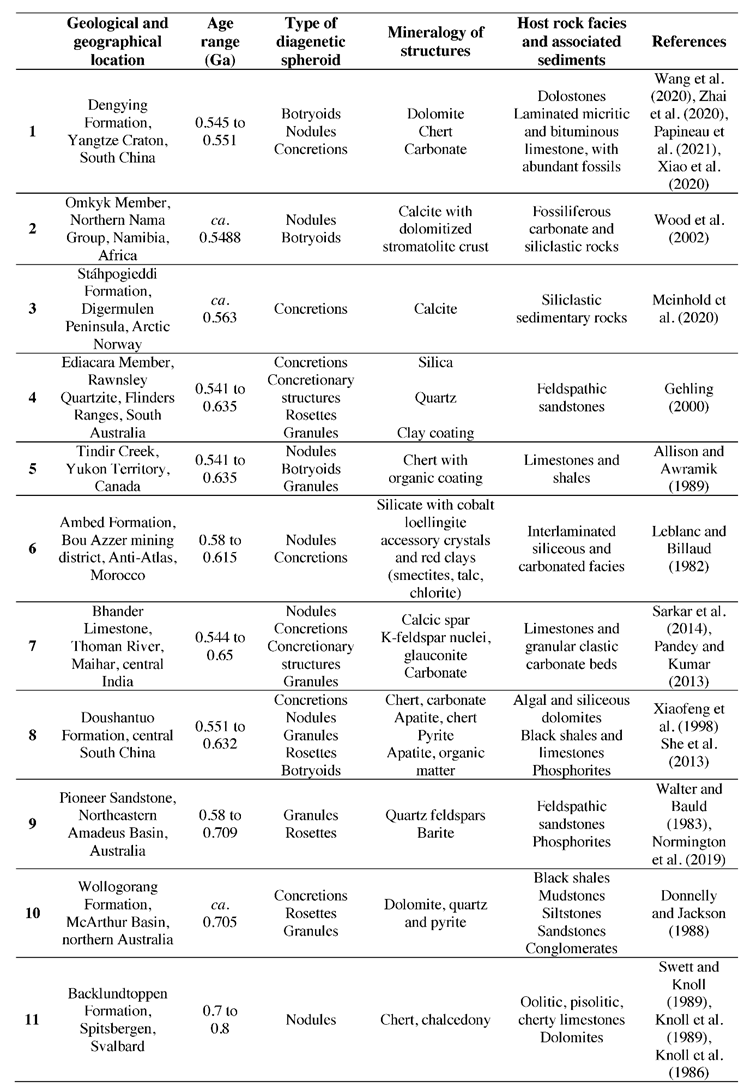
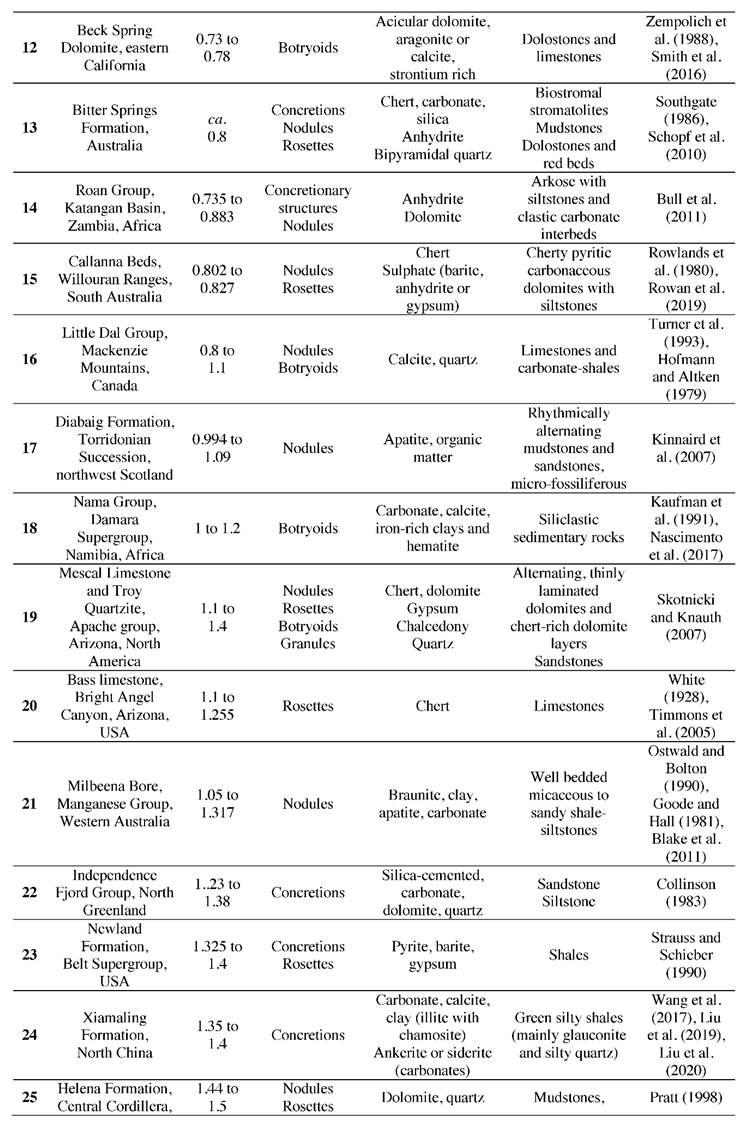
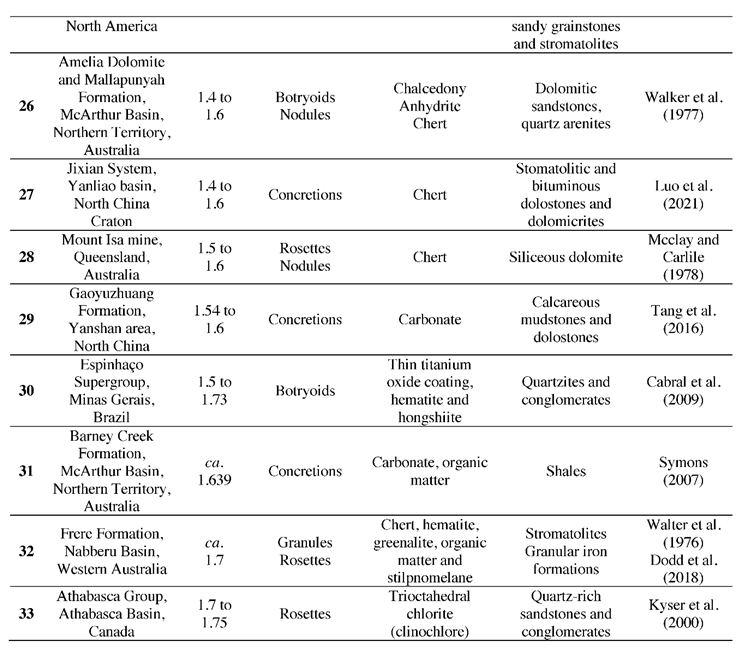
2. Approach for New Compilation
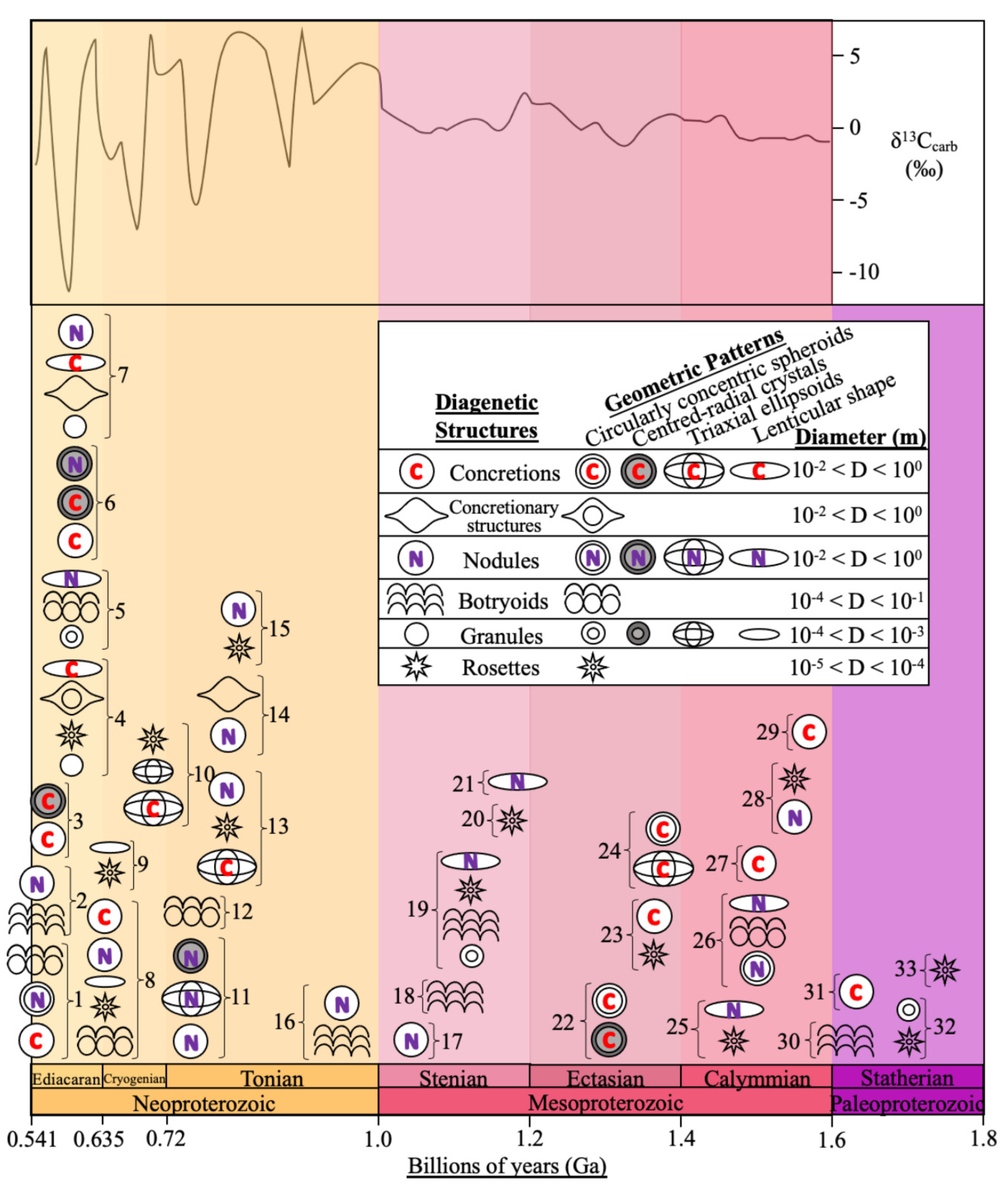
3. Observations from Compilation of Proterozoic Spheroids
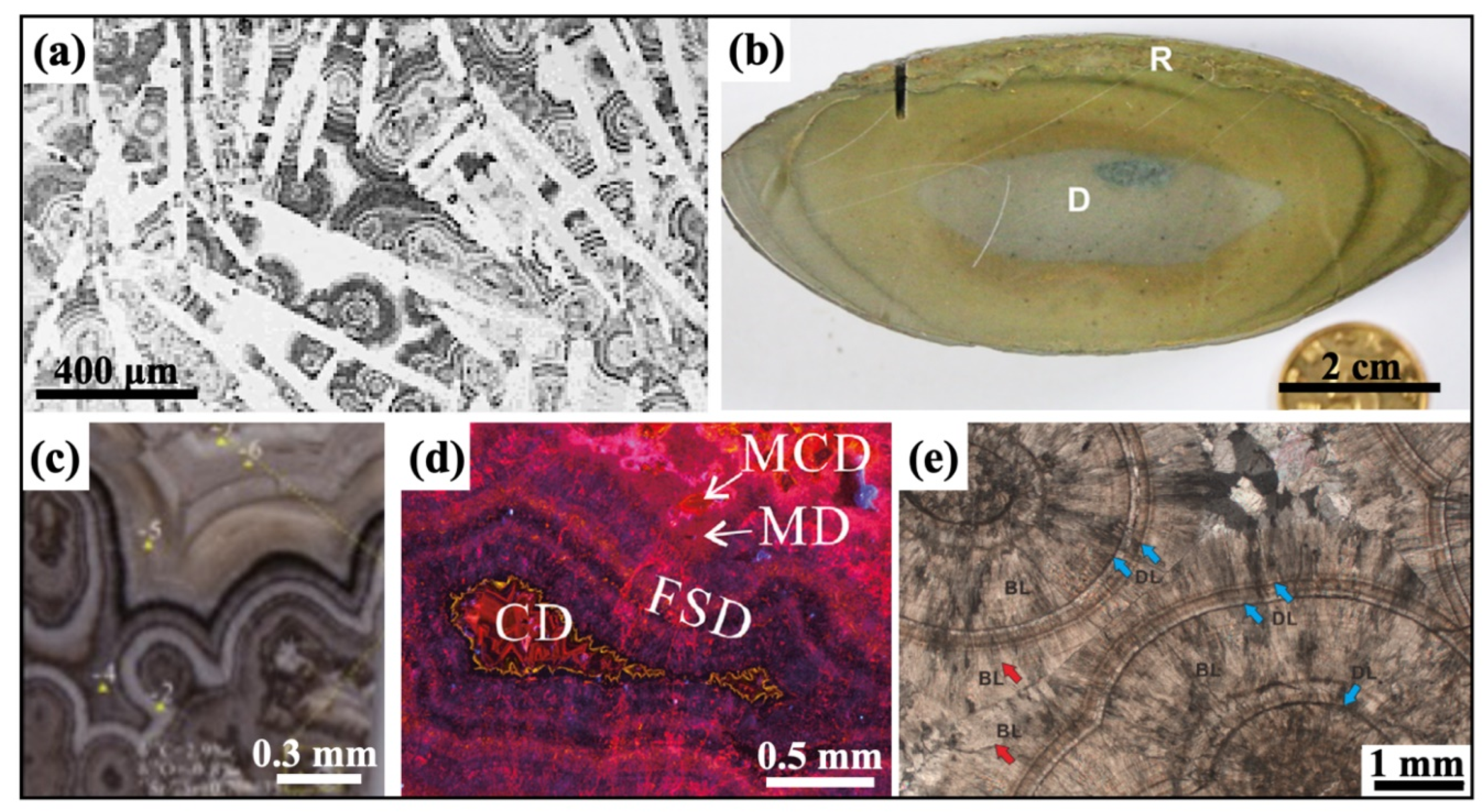
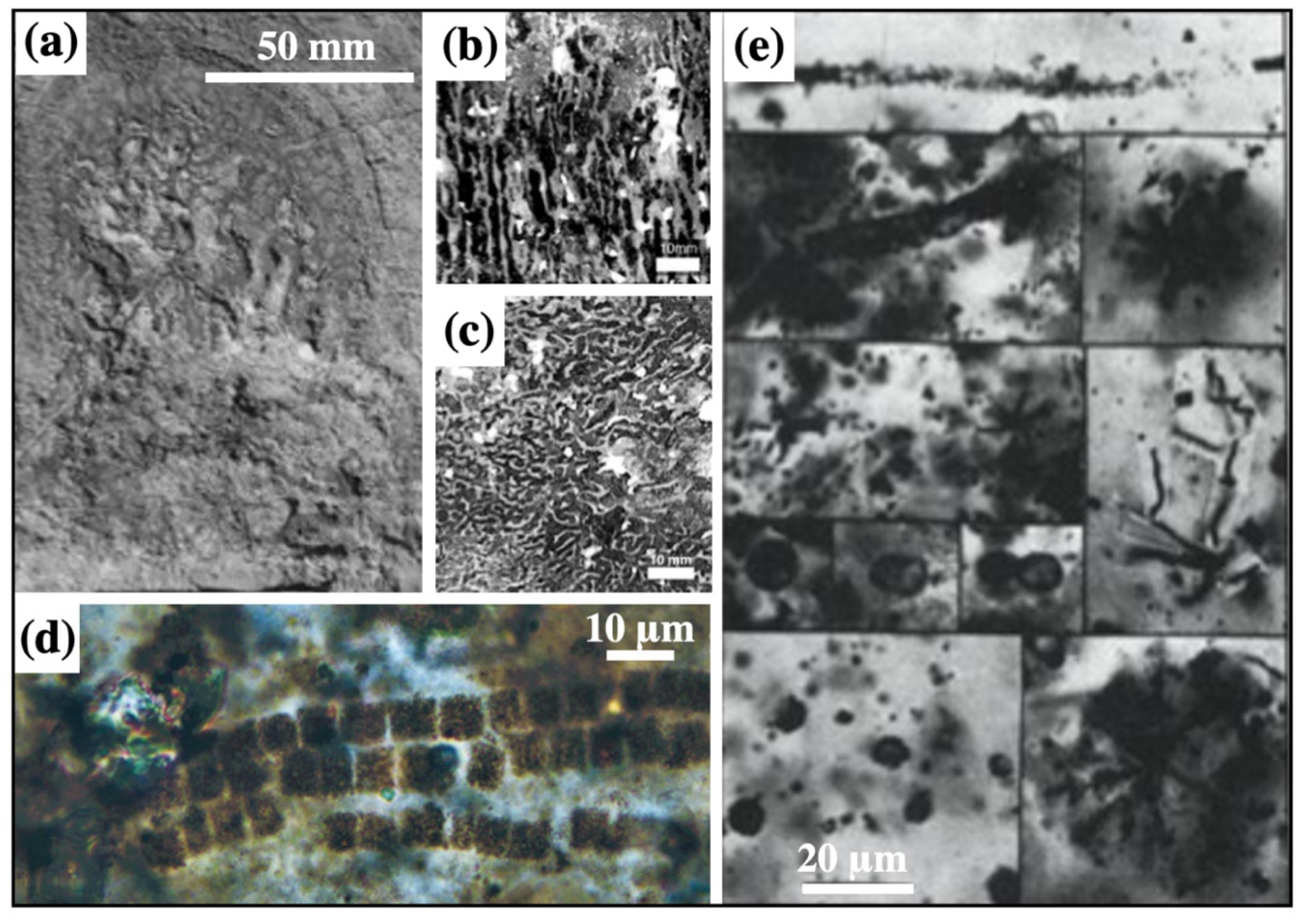
3.1. Geometric Patterns of Diagenetic Spheroids
3.2. Mineralogy and Palaeontology of Diagenetic Spheroids
3.3. Distribution Trend of Diagenetic Spheroids
4. Discussion
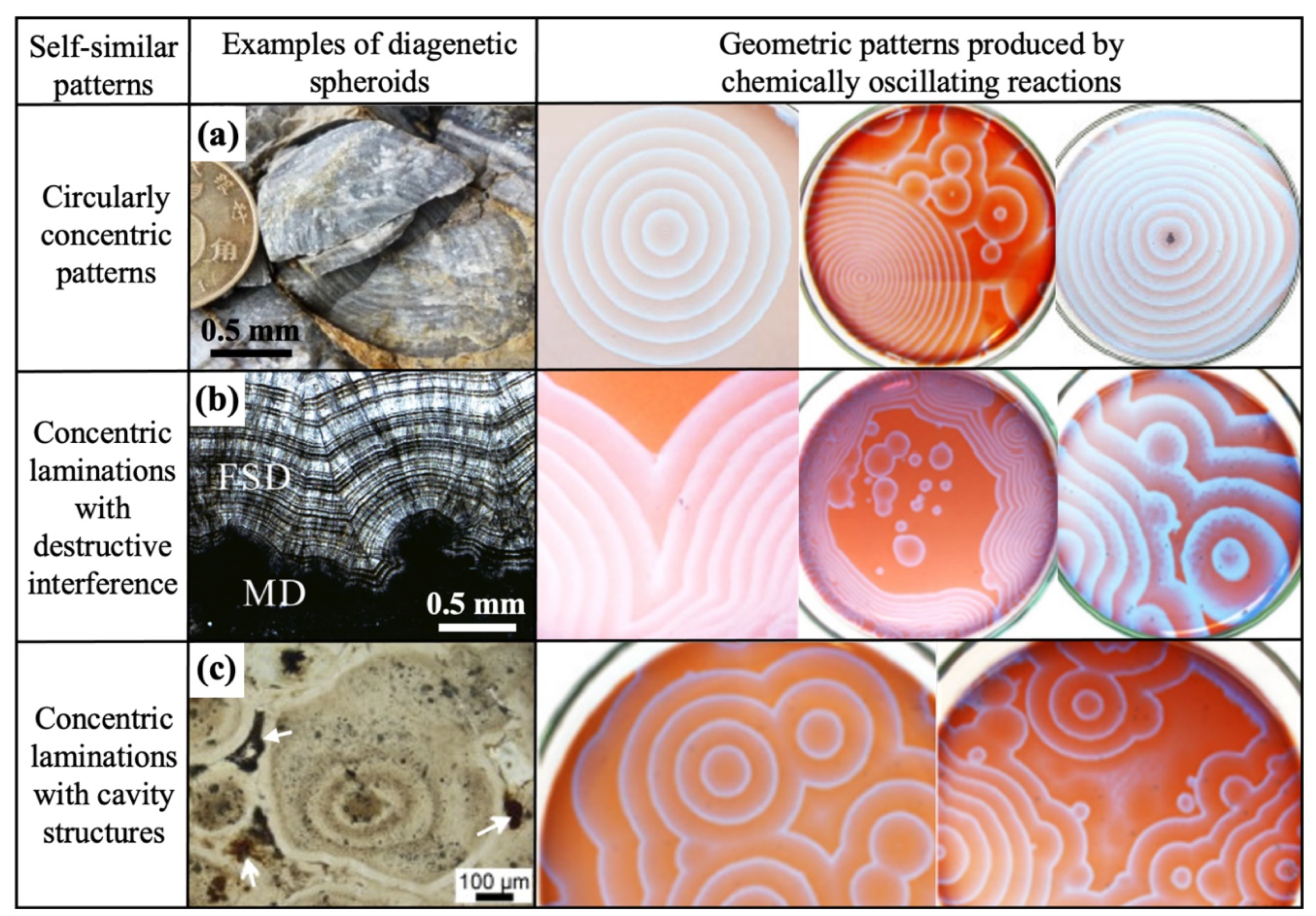
4.1. Geometric Patterns in Proterozoic Diagenetic Spheroids Compared to Those of COR
4.2. Substances of COR Compared to Those of Proterozoic Diagenetic Spheroids
4.3. Influences of Atmospheric Oxygenation on Occurrences of Diagenetic Spheroids
5. Conclusion
References
- Ader, M., Macouin, M., Trindade, R.I.F., Hadrien, M., Yang, Z.Y., Sun, Z.M. and Besse, J. (2009). A multilayered water column in the Ediacaran Yangtze platform? Insights from carbonate and organic matter paired δ13C. Earth and Planetary Science Letters, 288(1-2), 213-227. DOI: 10.1016/j.epsl.2009.09.024.
- Agladze, K.I., Krinsky, V.I. and Pertsov, A.M. (1984). Chaos in the non-stirred Belousov–Zhabotinsky reaction is induced by interaction of waves and stationary dissipative structures. Nature, 308, 834-835. DOI: 10.1038/308834a0.
- Akin, S.J., Pufahl, P.K., Hiatt, E.E. and Pirajno, F. (2013). Oxygenation of shallow marine environments and chemical sedimentation in Palaeoproterozoic peritidal settings: Frere Formation, Western Australia. Sedimentology, 60(7), 1559-1582. DOI: 10.1111/sed.12038.
- Allison, C.W. and Awramik, S.M. (1989). Organic-walled microfossils from earliest Cambrian or latest Proterozoic Tindir Group rocks, northwest Canada. Precambrian Research, 43(4), 253-294. DOI: 10.1016/0301-9268(89)90060-0.
- Belmonte, A.L., Ouyang, Q. and Flesselles, J.M. (1997). Experimental survey of spiral dynamics in the Belousov-Zhabotinsky reaction. Journal de Physique II, 7(10), 1425-1468. DOI: 10.1051/jp2:1997195.
- Bernard, S., Benzerara, K., Beyssac, O., Menguy, N., Guyot, F., Brown Jr, G.E. and Goffé, B. (2007). Exceptional preservation of fossil plant spores in high-pressure metamorphic rocks. Earth and Planetary Science Letters, 262(1-2), 257-272. DOI: 10.1016/j.epsl.2007.07.041.
- Blake, T.S., Rothery, E., Muhling, J.R., Drake-Brockman, J.A.P., Sprigg, L.C., Ho, S.E., Rasmussen, B. and Fletcher, I.R. (2011). Two episodes of regional-scale Precambrian hydrothermal alteration in the eastern Pilbara, Western Australia. Precambrian Research, 188(1-4), 73-103. DOI: 10.1016/j.precamres.2011.03.010.
- Bosak, T., Bush, J.W.M., Flynn, M.R., Liang, B., Ono, S., Petroff, A.P. and Sim, M.S. (2010). Formation and stability of oxygen-rich bubbles that shape photosynthetic mats. Geobiology, 8(1), 45-55. DOI: 10.1111/j.1472-4669.2009.00227.x.
- Briggs, T.S. and Rauscher, W.C. (1973). An oscillating iodine clock. Journal of Chemical Education, 50(7), 496. DOI: 10.1021/ed050p496.
- Bristow, T.F., Bonifacie, M., Derkowski, A., Eiler, J.M. and Grotzinger, J.P. (2011). A hydrothermal origin for isotopically anomalous cap dolostone cements from south China. Nature, 474(7349), 68-71. DOI: 10.1038/nature10096.
- Bull, S., Selley, D., Broughton, D., Hitzman, M., Cailteux, J., Large, R. and McGoldrick, P. (2011). Sequence and carbon isotopic stratigraphy of the Neoproterozoic Roan Group strata of the Zambian copperbelt. Precambrian Research, 190(1-4), 70-89. DOI: 10.1016/j.precamres.2011.07.021.
- Cabral, A.R., Lehmann, B., Tupinambá, M., Schlosser, S., Kwitko-Ribeiro, R. and de Abreu, F.R. (2009). The platiniferous Au-Pd belt of Minas Gerais, Brazil, and genesis of its botryoidal Pt-Pd aggregates. Economic Geology, 104(8), 1265-1276. DOI: 10.2113/gsecongeo.104.8.1265.
- Burley, S.D., Kantorowicz, J.D. and Waugh, B. (1985). Clastic diagenesis. Geological Society, London, Special Publications, 18(1), 189-226. DOI: 10.1144/GSL.SP.1985.018.01.10.
- Cañadas, F., Papineau, D., Leng, M.J. and Li, C. (2022). Extensive primary production promoted the recovery of the Ediacaran Shuram excursion. Nature communications, 13, 148. DOI: 10.1038/s41467-021-27812-5.
- Canfield, D.E. and Raiswell, R. (1999). The evolution of the sulfur cycle. American Journal of Science, 299(7-9), 697-723. DOI: 10.2475/ajs.299.7-9.697.
- Chen, I.C., Kuksenok, O., Yashin, V.V., Moslin, R.M., Balazs, A.C. and Van Vliet, K.J. (2011). Shape-and size-dependent patterns in self-oscillating polymer gels. Soft Matter, 7(7), 3141-3146. DOI: 10.1039/C0SM01007C.
- Cohen, K.M., Finney, S.C., Gibbard, P.L. and Fan, J.X. (2013). The ICS International Chronostratigraphic Chart. Episodes, 36(3), 199-204.
- Collinson, J.D. (1983). Sedimentology of unconformities within a fluvio-lacustrine sequence; Middle Proterozoic of Eastern North Greenland. Sedimentary Geology, 34(2-3), 145-166. DOI: 10.1016/0037-0738(83)90084-2.
- Curtis, C.D. (1977). Geochemistry: sedimentary geochemistry: environments and processes dominated by involvement of an aqueous phase. Philosophical Transactions of the Royal Society of London. Series A, Mathematical and Physical Sciences, 286(1336), 353-372. DOI: 10.1098/rsta.1977.0123.
- Dahanayake, K., Gerdes, G. and Krumbein, W.E. (1985). Stromatolites, oncolites and oolites biogenically formed in situ. Naturwissenschaften, 72(10), 513-518. DOI: 10.1007/BF00367596.
- Dahanayake, K. and Krumbein, W.E. (1986). Microbial structures in oolitic iron formations. Mineralium Deposita, 21(2), 85-94. DOI: 10.1007/BF00204266.
- Davies, P.J., Bubela, B. and Ferguson, J. (1978). The formation of ooids. Sedimentology, 25(5), 703-730. DOI: 10.1111/j.1365-3091.1978.tb00326.x.
- Diaz, M.R. and Eberli, G.P. (2019). Decoding the mechanism of formation in marine ooids: A review. Earth-Science Reviews, 190, 536-556. DOI: 10.1016/j.earscirev.2018.12.016.
- Ding, W., Dong, L., Sun, Y., Ma, H., Xu, Y., Yang, R., Peng, Y., Zhou, C. and Shen, B. (2019). Early animal evolution and highly oxygenated seafloor niches hosted by microbial mats. Scientific Reports, 9(1), 13628. DOI: 10.1038/s41598-019-49993-2.
- Dodd, M.S., Papineau, D., She, Z., Fogel, M.L., Nederbragt, S. and Pirajno, F. (2018). Organic remains in late Palaeoproterozoic granular iron formations and implications for the origin of granules. Precambrian Research, 310, 133-152. DOI: 10.1016/j.precamres.2018.02.016.
- Donnelly, T.H. and Jackson, M.J. (1988). Sedimentology and geochemistry of a mid-Proterozoic lacustrine unit from northern Australia. Sedimentary geology, 58(2-4), 145-169. DOI: 10.1016/0037-0738(88)90067-X.
- Epstein, I.R., Kustin, K., De Kepper, P. and Orbán, M. (1983). Oscillating chemical reactions. Scientific American, 248(3), 112-123.
- Field, R.J. and Schneider, F.W. (1989). Oscillating chemical reactions and nonlinear dynamics. Journal of Chemical Education 66(3), 195. DOI: 10.1021/ed066p195.
- Fike, D.A., Grotzinger, J.P., Pratt, L.M. and Summons, R.E. (2006). Oxidation of the Ediacaran Ocean. Nature, 444, 744-747. DOI: 10.1038/nature05345.
- Flannery, D.T., Allwood, A.C., Hodyss, R., Summons, R.E., Tuite, M., Walter, M.R. and Williford, K.H. (2019). Microbially influenced formation of Neoarchean ooids. Geobiology, 17(2), 151-160. DOI: 10.1111/gbi.12321.
- Fouke, B.W. (2011). Hot-spring Systems Geobiology: abiotic and biotic influences on travertine formation at Mammoth Hot Springs, Yellowstone National Park, USA. Sedimentology, 58, 170-219. DOI: 10.1111/j.1365-3091.2010.01209.x.
- Gabriel, N.W., Papineau, D., She, Z., Leider, A. and Fogel, M.L. (2021). Organic diagenesis in stromatolitic dolomite and chert from the late Palaeoproterozoic McLeary Formation. Precambrian Research, 354, 106052. DOI: 10.1016/j.precamres.2020.106052.
- Gehling, J.G. (2000). Environmental interpretation and a sequence stratigraphic framework for the terminal Proterozoic Ediacara Member within the Rawnsley Quartzite, South Australia. Precambrian Research, 100(1-3), 65-95. DOI: 10.1016/S0301-9268(99)00069-8.
- Glasauer, S., Mattes, A. and Gehring, A. (2013). Constraints on the Preservation of Ferriferous Microfossils. Geomicrobiology Journal, 30(6), 479-489. DOI: 10.1080/01490451.2012.718408.
- Goddéris, Y., Donnadieu, Y., Dessert, C., Dupré, B., Fluteau, F., François, L.M., Meert, J., Nédélec, A. and Ramstein, G. (2007). Coupled modeling of global carbon cycle and climate in the Neoproterozoic: links between Rodinia breakup and major glaciations. Comptes Rendus Geoscience, 339(3-4), 212-222. DOI: 10.1016/j.crte.2005.12.002.
- Goode, A.D.T. and Hall, W.D.M. (1981). The middle Proterozoic Eastern Bangemall Basin, Western Australia. Precambrian Research, 16(1-2), 11–29. DOI: 10.1016/0301-9268(81)90003-6.
- Gray, C. (2002). An analysis of the Belousov-Zhabotinskii reaction. Rose-Hulman Undergraduate Mathematics Journal, 3(1), 1.
- Griffin, J.D., Zeller, M.A. and Nicewicz, D.A. (2015). Hydrodecarboxylation of carboxylic and malonic acid derivatives via organic photoredox catalysis: substrate scope and mechanistic insight. Journal of the American Chemical Society, 137(35), 11340-11348. DOI: 10.1021/jacs.5b07770.
- Hardisty, D.S., Lu, Z., Bekker, A., Diamond, C.W., Gill, B.C., Jiang, G., Kah, L.C., Knoll, A.H., Loyd, S.J., Osburn, M.R. and Planavsky, N.J. (2017). Perspectives on Proterozoic surface ocean redox from iodine contents in ancient and recent carbonate. Earth and Planetary Science Letters, 463, pp.159-170. DOI: 10.1016/j.epsl.2017.01.032.
- Hastings, H.M., Field, R.J. and Sobel, S.G. (2003). Microscopic fluctuations and pattern formation in a supercritical oscillatory chemical system. The Journal of Chemical Physics, 119(6), 3291-3296. DOI: 10.1063/1.1587700.
- Hofmann, H.J. and Altken, J.D. (1979). Precambrian biota from the Little Dal Group, Mackenzie Mountains, northwestern Canada. Canadian Journal of Earth Sciences, 16(1), 150-166. DOI: 10.1139/e79-014.
- Hurtgen, M.T., Arthur, M.A. and Halverson, G.P. (2005) Neoproterozoic sulfur isotopes, the evolution of microbial sulfur species, and the burial efficiency of sulfide as sedimentary pyrite. Geology, 33, 41-44; DOI: 10.1130/G20923.1.
- Jorgensen, B.B. (1979). A theoretical model of the stable sulfur isotope distribution in marine sediments. Geochimica et Cosmochimica Acta, 43(3), 363-374. DOI: 10.1016/0016-7037(79)90201-1.
- Karhu, J.A. and Holland, H.D. (1996). Carbon isotopes and the rise of atmospheric oxygen. Geology, 24(10), 867. DOI:10.1130/0091-7613(1996)024<0867:ciatro>2.3.co;2.
- Kaufman, A.J., Hayes, J.M., Knoll, A.H. and Germs, G.J. (1991). Isotopic compositions of carbonates and organic carbon from upper Proterozoic successions in Namibia: stratigraphic variation and the effects of diagenesis and metamorphism. Precambrian Research, 49(3-4), 301-327. DOI: 10.1016/0301-9268(91)90039-D.
- Keller, C.B., Husson, J.M., Mitchell, R.N., Bottke, W.F., Gernon, T.M., Boehnke, P., Bell, E.A., Swanson-Hysell, N.L. and Peters, S.E. (2018). Neoproterozoic glacial origin of the Great Unconformity. Proceedings of the National Academy of Sciences, 116(4), 1136-1145. DOI: 10.1073/pnas.1804350116.
- Keller, J.B. and Rubinow, S.I. (1981). Recurrent precipitation and Liesegang rings. The Journal of Chemical Physics, 74(9), 5000-5007. DOI: 10.1063/1.441752.
- Kinnaird, T.C., Prave, A.R., Kirkland, C., Horstwood, M., Parrish, R. and Batchelor, R.A. (2007). The late Mesoproterozoic–early Neoproterozoic tectonostratigraphic evolution of NW Scotland: the Torridonian revisited. Journal of the Geological Society, 164(3), 541-551. DOI: 10.1144/0016-76492005-096.
- Kirschvink, J.L. (1992). Late Proterozoic low-latitude global glaciation: the Snowball Earth. Cambridge University Press, Cambridge, 51-52.
- Knoll, A.H., Hayes, J.M., Kaufman, A.J., Swett, K. and Lambert, I.B. (1986). Secular variation in carbon isotope ratios from Upper Proterozoic successions of Svalbard and East Greenland. Nature, 321(6073), 832-838. DOI: 10.1038/321832a0.
- Knoll, A.H., Swett, K. and Burkhardt, E. (1989). Paleoenvironmental distribution of microfossils and stromatolites in the Upper Proterozoic Backlundtoppen Formation, Spitsbergen. Journal of Paleontology, 63(2), 129-145. DOI: 10.1017/S002233600001917X.
- Körös, E. and Orbán, M. (1978). Uncatalysed oscillatory chemical reactions. Nature, 273(5661), 371-372. DOI: 10.1038/273371b0.
- Kyser, T.K., Hiatt, E.E., Renac, C., Durocher, K., Holk, G.J. and Deckart, K. (2000). Diagenetic fluids in Paleo-and Meso-Proterozoic sedimentary basins and their implications for long protracted fluid histories. Mineralogical Association of Canada Short Course, 28, 225-262. DOI: 10.13140/2.1.1033.1847.
- Lascelles, D.F. (2007). Black smokers and density currents: A uniformitarian model for the genesis of banded iron-formations. Ore Geology Reviews, 32(1-2), 381-411. DOI: 10.1016/j.oregeorev.2006.11.005.
- Leblanc, M. and Billaud, P. (1982). Cobalt arsenide orebodies related to an upper Proterozoic ophiolite; Bou Azzer (Morocco). Economic Geology, 77(1), 162-175. DOI: 10.2113/gsecongeo.77.1.162.
- Lebron, I. and Suarez, D.L (1996). Calcite nucleation and precipitation kinetics as affected by dissolved organic matter at 25°C and pH > 7.5. Geochimica et Cosmochimica Acta, 60(15), 2765-2776. DOI: 10.1016/0016-7037(96)00137-8.
- Li, D., Luo, G., Yang, H., She, Z., Papineau, D. and Li, C. (2022). Characteristics of the carbon cycle in late Mesoproterozoic: Evidence from carbon isotope composition of paired carbonate and organic matter of the Shennongjia Group in South China. Precambrian Research, 377, 106726. DOI: 10.1016/j.precamres.2022.106726.
- Liesegang, R.E. (1910). Die Entstehung der Achate. Zentralblatt für Mineralogie, 11, 593-597.
- Liu, A.Q., Tang, D.J., Shi, X.Y., Zhou, L.M., Zhou, X.Q., Shang, M.H., Li, Y. and Song, H.Y. (2019). Growth mechanisms and environmental implications of carbonate concretions from the~ 1.4 Ga Xiamaling Formation, North China. Journal of Palaeogeography, 8(1), 20. DOI: 10.1186/s42501-019-0036-4.
- Liu, A., Tang, D., Shi, X., Zhou, X., Zhou, L., Shang, M., Li, Y. and Fang, H. (2020). Mesoproterozoic oxygenated deep seawater recorded by early diagenetic carbonate concretions from the Member IV of the Xiamaling Formation, North China. Precambrian Research, 341, 105667. DOI: 10.1016/j.precamres.2020.105667.
- Luo, J., Long, X., Bowyer, F.T., Mills, B.J.W., Li, J., Xiong, Y., Zhu, X., Zhang, K. and Poulton, S.W. (2021). Pulsed oxygenation events drove progressive oxygenation of the early Mesoproterozoic ocean. Earth and Planetary Science Letters, 559, 116754. DOI: 10.1016/j.epsl.2021.116754.
- Mason, R., Li, Y., Cao, K., Long, Y. and She, Z.B. (2017). Ediacaran macrofossils in Shunyang Valley, Sixi, Three Gorges district, Hubei Province, China. Journal of Earth Science, 28(4), 614-621. DOI: 10.1007/s12583-017-0773-1.
- McClay, K.R. and Carlile, D.G. (1978). Mid-Proterozoic sulphate evaporites at Mount Isa mine, Queensland, Australia. Nature, 274(5668), 240-241. DOI: 10.1038/274240a0.
- McCollom, T.M., Ritter, G. and Simoneit, B.R. (1999). Lipid synthesis under hydrothermal conditions by Fischer-Tropsch-type reactions. Origins of Life and Evolution of the Biosphere, 29(2), pp.153-166. DOI: 10.1023/A:1006592502746.
- Meinhold, G., Roberts, N.M.W., Arslan, A., Jensen, S., Ebbestad, J.O.R., Högström, A.E.S., Høyberget, M., Agić, H., Palacios, T. and Taylor, W.L. (2020). U–Pb dating of calcite in ancient carbonates for age estimates of syn- to post-depositional processes: a case study from the upper Ediacaran strata of Finnmark, Arctic Norway. Geological Magazine, 157(8), 1367-1372. DOI: 10.1017/S0016756820000564.
- Merdith, A.S., Collins, A.S., Williams, S.E., Pisarevsky, S., Foden, J.D., Archibald, D.B., Blades, M.L., Alessio, B.L., Armistead, S., Plavsa, D., Clark, C., and Muller, D. (2017) A full-plate global reconstruction of the Neoproterozoic. Gondwana Research 50, 84–134.
- Mitchell, R.N. and Evans, D.A.D. (2024) The balanced billion. GSA Today, February issue, 10-11.
- Mitsuchi, M. (1976). Characteristics and genesis of nodules and concretions occurring in soils of the R. Chinit area, Kompong Thom Province, Cambodia. Soil Science and Plant Nutrition, 22(4), 409-421. DOI: 10.1080/00380768.1976.10433003.
- Muyzer, G. and Stams, A.J. (2008). The ecology and biotechnology of sulphate-reducing bacteria. Nature Reviews Microbiology, 6(6), 441-454. DOI: 10.1038/nrmicro1892.
- Nabika, H., Itatani, M. and Lagzi, I. (2019). Pattern Formation in Precipitation Reactions: The Liesegang Phenomenon. Langmuir, 36(2), 481-497. DOI: 10.1021/acs.langmuir.9b03018.
- Nascimento, D.B., Schmitt, R.S., Ribeiro, A., Trouw, R.A., Passchier, C.W. and Basei, M.A. (2017). Depositional ages and provenance of the Neoproterozoic Damara Supergroup (northwest Namibia): Implications for the Angola-Congo and Kalahari cratons connection. Gondwana Research, 52, 153-171. DOI: 10.1016/j.gr.2017.09.006.
- Normington, V.J., Beyer, E.E., Whelan, J.A., Edgoose, C.J. and Woodhead, J.D. (2019). Summary of results—NTGS LA-ICP-MS Hf program: Amadeus Basin, July 2013–June 2015. Northern Territory Geological Survey Record, 5, 34.
- Och, L.M. and Shields-Zhou, G.A. (2012). The Neoproterozoic oxygenation event: Environmental perturbations and biogeochemical cycling. Earth-Science Reviews, 110(1-4), 26-57. DOI: 10.1016/j.earscirev.2011.09.004.
- Orbán, M., Kurin-Csörgei, K., Zhabotinsky, A.M. and Epstein, I.R. (2001). A new chemical system for studying pattern formation: Bromate–hypophosphite–acetone–dual catalyst. Faraday Discussions, 120, 11-19. DOI: 10.1039/B102885P.
- Ostwald, J. and Bolton, B.R. (1990). Diagenetic braunite in sedimentary rocks of the Proterozoic Manganese Group, Western Australia. Ore Geology Reviews, 5(4), 315-323. DOI: 10.1016/0169-1368(90)90036-M.
- Pandey, S.K. and Kumar, S. (2013). Organic walled microbiota from the silicified algal clasts, Bhander limestone, Satna area, Madhya Pradesh. Journal of the Geological Society of India, 82(5), 499-508. DOI: 10.1007/s12594-013-0181-9.
- Papineau, D. (2010). Global Biogeochemical Changes at Both Ends of the Proterozoic: Insights from Phosphorites. Astrobiology, 10(2), 165-181. DOI: 10.1089/ast.2009.0360.
- Papineau, D. (2020). Chemically oscillating reactions in the formation of botryoidal malachite. American Mineralogist: Journal of Earth and Planetary Materials, 105(4), 447-454. DOI: 10.2138/am-2020-7029.
- Papineau, D. (2024) Chemically oscillating reactions as a new model for the formation of mineral patterns in Agate geodes and concretions. Minerals, 14(2), 203. DOI:10.3390/min14020203.
- Papineau, D., Devine, K. and Nogueira, B.A. (2023). Self-similar patterns from abiotic decarboxylation metabolism through chemically oscillating reactions: A prebiotic model for the origin of life. Life, 13(2), 551. DOI: 10.3390/life13020551.
- Papineau, D., De Gregorio, B., Fearn, S., Kilcoyne, D., McMahon, G., Purohit, R. and Fogel, M. (2016). Nanoscale petrographic and geochemical insights on the origin of the Palaeoproterozoic stromatolitic phosphorites from Aravalli Supergroup, India. Geobiology, 14(1) 3-32. DOI: 10.1111/gbi.12164.
- Papineau, D., She, Z. and Dodd, M.S. (2017). Chemically-oscillating reactions during the diagenetic oxidation of organic matter and in the formation of granules in late Palaeoproterozoic chert from Lake Superior. Chemical Geology, 470, 33-54. DOI: 10.1016/j.chemgeo.2017.08.021.
- Papineau, D., Yin, J., Devine, K.G., Liu, D. and She, Z. (2021). Chemically Oscillating Reactions during the Diagenetic Formation of Ediacaran Siliceous and Carbonate Botryoids. Minerals, 11(10), 1060. DOI: 10.3390/min11101060.
- Postgate, J. (1959). Sulphate reduction by bacteria. Annual Reviews in Microbiology, 13, 505-520. DOI: 10.1146/annurev.mi.13.100159.002445.
- Pratt, B.R. (1998). Molar-tooth structure in Proterozoic carbonate rocks: Origin from synsedimentary earthquakes, and implications for the nature and evolution of basins and marine sediment. Geological Society of America Bulletin, 110(8), 1028-1045. DOI: 10.1130/0016-7606(1998)110<1028:MTSIPC>2.3.CO;2.
- Pufahl, P.K. and Fralick, P.W. (2004). Depositional controls on Palaeoproterozoic iron formation accumulation, Gogebic Range, Lake Superior region, USA. Sedimentology, 51(4), 791-808. DOI: 10.1111/j.1365-3091.2004.00651.x.
- Ross, G.M., Bloch, J.D. and Krouse, H.R. (1995). Neoproterozoic strata of the southern Canadian Cordillera and the isotopic evolution of seawater sulfate. Precambrian Research, 73(1-4), 71-99. DOI: 10.1016/0301-9268(94)00072-Y.
- Rowan, M.G., Hearon IV, T.E., Kernen, R.A., Giles, K.A., Gannaway-Dalton, C.E., Williams, N.J., Fiduk, J.C., Lawton, T.F., Hannah, P.T. and Fischer, M.P. (2019). A review of allochthonous salt tectonics in the Flinders and Willouran ranges, South Australia. Australian Journal of Earth Sciences, 67(6), 787-813. DOI: 10.1080/08120099.2018.1553063.
- Rowlands, N.J., Blight, P.G., Jarvis, D.M. and Von Der Borch, C.C. (1980). Sabkha and playa environments in late Proterozoic grabens, Willouran Ranges, South Australia. Journal of the Geological Society of Australia, 27(1-2), 55-68. DOI: 10.1080/00167618008729118.
- Rueter, P., Rabus, R., Wilkest, H., Aeckersberg, F., Rainey, F.A., Jannasch, H.W. and Widdel, F. (1994). Anaerobic oxidation of hydrocarbons in crude oil by new types of sulphate-reducing bacteria. Nature, 372, 455-458. DOI: 10.1038/372455a0.
- Salama, W., El Aref, M.M. and Gaupp, R. (2012). Mineral evolution and processes of ferruginous microbialite accretion - an example from the Middle Eocene stromatolitic and ooidal ironstones of the Bahariya Depression, Western Desert, Egypt. Geobiology, 11(1), 15-28. DOI: 10.1111/gbi.12011.
- Sarkar, S., Choudhuri, A., Banerjee, S., Van Loon, A.J. and Bose, P.K. (2014) Seismic and non-seismic soft-sediment deformation structures in the Proterozoic Bhander Limestone, central India. Geologos, 20(2), 89-103. DOI: 10.2478/logos-2014-0008.
- Schidlowski, M. (1989). Evolution of the sulphur cycle in the Precambrian. Evolution of the global biogeochemical sulphur cycle, 3-19.
- Schopf, J.W., Kudryavtsev, A.B., Sugitani, K. and Walter, M.R. (2010). Precambrian microbe-like pseudofossils: A promising solution to the problem. Precambrian Research, 179(1-4), 191-205. DOI: 10.1016/j.precamres.2010.03.003.
- She, Z., Strother, P., McMahon, G., Nittler, L.R., Wang, J., Zhang, J., Sang, L., Ma, C. and Papineau, D. (2013). Terminal Proterozoic cyanobacterial blooms and phosphogenesis documented by the Doushantuo granular phosphorites I: In situ micro-analysis of textures and composition. Precambrian Research, 235, 20-35. DOI: 10.1016/j.precamres.2013.05.011.
- Sheik, C.S., Cleaves, H.J., Johnson-Finn, K., Giovannelli, D., Kieft, T.L., Papineau, D., Schrenk, M.O. and Tumiati, S. (2020). Abiotic and biotic processes that drive carboxylation and decarboxylation reactions. American Mineralogist: Journal of Earth and Planetary Materials, 105(5), 609-615. DOI: 10.2138/am-2020-7166CCBYNCND.
- Shields, G.A., Mills, B.J.W., Zhu, M., Raub, T.D., Daines, S.J. and Lenton, T.M. (2019) Unique Neoproterozoic carbon isotope excursions sustained by coupled evaporite dissolution and pyrite burial. Nature Geoscience. 12, 823–827. DOI: 10.1038/s41561-019-0434-3.
- Simonson, B.M. (2003). Origin and evolution of large Precambrian iron formations. In: Chan, M.A. and Archer, A.W. (Eds.), Extreme depositional environments: Mega end members in geologic time. Geological Society of America, 231–244.
- Skotnicki, S.J. and Knauth, L.P. (2007). The Middle Proterozoic Mescal Paleokarst, Central Arizona, U.S.A.: Karst Development, Silicification, and Cave Deposits. Journal of Sedimentary Research, 77(12), 1046-1062. DOI: 10.2110/jsr.2007.094.
- Smith, A.J.B., Beukes, N.J., Gutzmer, J., Czaja, A.D., Johnson, C.M. and Nhleko, N. (2017). Oncoidal granular iron formation in the Mesoarchaean Pongola Supergroup, southern Africa: Textural and geochemical evidence for biological activity during iron deposition. Geobiology, 15(6), 731-749. DOI: 10.1111/gbi.12248.
- Smith, E.F., MacDonald, F.A., Crowley, J.L., Hodgin, E.B. and Schrag, D.P. (2016). Tectonostratigraphic evolution of the c. 780–730 Ma Beck Spring Dolomite: Basin Formation in the core of Rodinia. Geological Society, London, Special Publications, 424, 213-239. DOI: 10.1144/SP424.6.
- Southgate, P.N. (1986). Depositional environment and mechanism of preservation of microfossils, upper Proterozoic Bitter Springs Formation, Australia. Geology, 14(8), 683-686. DOI: 10.1130/0091-7613(1986)14<683:DEAMOP>2.0.CO;2.
- Stern, R.J., Avigad, D., Miller, N.R. and Beyth, M. (2006). Evidence for the Snowball Earth hypothesis in the Arabian-Nubian Shield and the East African Orogen. Journal of African Earth Sciences, 44(1), 1-20. DOI: 10.1016/j.jafrearsci.2005.10.003.
- Strauss, H. and Schieber, J. (1990). A sulfur isotope study of pyrite genesis: The mid-proterozoic Newland Formation, Belt Supergroup, Montana. Geochimica et Cosmochimica Acta, 54(1), 197-204. DOI: 10.1016/0016-7037(90)90207-2.
- Sultan, R.F. and Abdel-Rahman, M. (2013). On dynamic self-organization: examples from magmatic and other geochemical systems. Latin American Journal of Solids and Structures, 10(1), 59-73. DOI: 10.1590/S1679-78252013000100006.
- Symons, D.T.A. (2007). Paleomagnetism of the HYC Zn-Pb SEDEX deposit, Australia: evidence of an epigenetic origin. Economic Geology, 102(7), 1295-1310. DOI: 10.2113/gsecongeo.102.7.1295.
- Tang, D., Shi, X., Wang, X. and Jiang, G. (2016). Extremely low oxygen concentration in mid-Proterozoic shallow seawaters. Precambrian Research, 276, 145-157. DOI: 10.1016/j.precamres.2016.02.005.
- Timmons, J.M., Karlstrom, K.E., Heizler, M.T., Bowring, S.A., Gehrels, G.E. and Crossey, L.J. (2005). Tectonic inferences from the ca. 1255–1100 Ma Unkar Group and Nankoweap Formation, Grand Canyon: Intracratonic deformation and basin formation during protracted Grenville orogenesis. Geological Society of America Bulletin, 117(11-12), 1573-1595. DOI: 10.1130/B25538.1.
- Tucker, M.E. (1984). Calcitic, aragonitic and mixed calcitic-aragonitic ooids from the mid-Proterozoic Belt Supergroup, Montana. Sedimentology, 31(5), 627-644. DOI: 10.1111/j.1365-3091.1984.tb01227.x.
- Turner, E.C., Narbonne, G.M. and James, N.P. (1993). Neoproterozoic reef microstructures from the Little Dal Group, northwestern Canada. Geology, 21(3), 259-262. DOI: 10.1130/0091-7613(1993)021<0259:NRMFTL>2.3.CO;2.
- Valley, J.W., Eiler, J.M., Graham, C.M., Gibson, E.K., Romanek, C.S. and Stolper, E.M. (1997). Low-temperature carbonate concretions in the Martian meteorite ALH84001: Evidence from stable isotopes and mineralogy. Science, 275(5306), 1633-1638. DOI: 10.1126/science.275.5306.1633.
- Varkouhi, S. and Papineau, D. (2023). Silica botryoids from chemically oscillating reactions and as Precambrian environmental proxies. Geology, 51(7), 683–687. DOI: 10.1130/g50948.1.
- Varkouhi, S., Papineau, D., and Guo, Z. (2022) Botryoidal quartz as an abiotic signature in Palaeoarchean cherts of the Pilbara Supergroup, Western Australia. Precambrian Research. DOI: 10.1016/j.precamres.2022.106876.
- Vidal, C. and Pagola, A. (1989). Observed properties of trigger waves close to the center of the target patterns in an oscillating Belousov-Zhabotinskii reagent. The Journal of Physical Chemistry, 93(7), 2711-2716. DOI: 10.1021/j100344a004.
- Walker, R.N., Muir, M.D., Diver, W.L., Williams, N. and Wilkins, N. (1977). Evidence of major sulphate evaporite deposits in the Proterozoic McArthur Group, Northern Territory, Australia. Nature, 265, 526-529. DOI: 10.1038/265526a0.
- Walter, M.R. and Bauld, J. (1983). The association of sulphate evaporites, stromatolitic carbonates and glacial sediments: examples from the Proterozoic of Australia and the Cainozoic of Antarctica. Precambrian Research, 21(1-2), 129-148. DOI: 10.1016/0301-9268(83)90008-6.
- Walter, M.R., Goode, A.D.T. and Hall, W.D.M. (1976). Microfossils from a newly discovered Precambrian stromatolitic iron formation in Western Australia. Nature, 261, 221-223. DOI: 10.1038/261221a0.
- Wang, J., He, Z., Zhu, D., Liu, Q., Ding, Q., Li, S. and Zhang, D. (2020). Petrological and geochemical characteristics of the botryoidal dolomite of Dengying Formation in the Yangtze Craton, South China: Constraints on terminal Ediacaran “dolomite seas”. Sedimentary Geology, 406, 105722. DOI: 10.1016/j.sedgeo.2020.105722.
- Wang, X., Zhang, S., Wang, H., Canfield, D.E., Su, J., Hammarlund, E.U., and Bian, L. (2017) Remarkable preservation of microfossils and biofilms in Mesoproterozoic silicified bitumen concretions from northern China. Geofluids, DOI: 10.1155/2017/4818207.
- White, D. (1928). Algal Deposits of Unkar Proterozoic Age in the Grand Canyon, Arizona. Proceedings of the National Academy of Sciences, 14(7), 597-600. DOI: 10.1073/pnas.14.7.597.
- Wood, R.A., Grotzinger, J.P. and Dickson, J.A.D. (2002). Proterozoic modular biomineralized metazoan from the Nama Group, Namibia. Science, 296(5577), 2383-2386. DOI: 10.1126/science.1071599.
- Xiao, S., Chen, Z., Pang, K., Zhou, C. and Yuan, X. (2020). The Shibantan Lagerstätte: insights into the Proterozoic–Phanerozoic transition. Journal of the Geological Society, 178(1), 135. DOI: 10.1144/jgs2020-135.
- Xiaofeng, W., Erdtmann, B.D., Xiaohong, C. and Xiaodong, M. (1998). Integrated sequence-, bio- and chemostratigraphy of the terminal Proterozoic to Lowermost Cambrian "black rock series" from central South China. Episodes, 21(3), 178-189. DOI: 10.18814/epiiugs/1998/v21i3/007.
- Zaikin, A.N. and Zhabotinsky, A.M. (1970). Concentration Wave Propagation in Two-dimensional liquid-phase self-oscillating system. Nature, 225, 535-537. DOI: 10.1038/225535b0.
- Zhabotinsky, A.M. (1991). A history of chemical oscillations and waves. Chaos: An Interdisciplinary Journal of Nonlinear Science, 1(4), 379-386. DOI: 10.1063/1.165848.
- Zhai, X., Luo, P., Gu, Z., Jiang, H., Zhang, B., Wang, Z., Wang, T. and Wu, S. (2020). Microbial mineralization of botryoidal laminations in the Upper Ediacaran dolostones, Western Yangtze Platform, SW China. Journal of Asian Earth Sciences, 195(27), 104334. DOI: 10.1016/j.jseaes.2020.104334.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





