1. Introduction
With increasing longevity, cases of neuropathic pain have increased, largely due to sedentary lifestyles, poor nutrition leading to conditions such as obesity, diabetes, and pre-diabetes, among other environmental or genetic factors. [
1,
2] Currently, the treatment of neuropathic pain is carried out through tricyclic antidepressant medications, anticonvulsants, local anesthetic administration, topical agents, opioid analgesics, antiarrhythmics, and even neurosurgeries [
3,
4,
5]. Despite the range of pharmacological approaches, patients’ response to these treatments is still not satisfactory, in addition to having a variety of adverse side effects, such as increased risk of heart attack, subacute myopathies, hypergastrinemia, gastrointestinal tumors, among others [
3].
Non-pharmacological treatments are increasingly gaining ground due to their beneficial results, improvement in quality of life, and lack of adverse side effects. Thus, understanding the pathophysiology of pain and its processes is of huge importance for understanding the mechanisms that trigger painful processes and therefore choosing the best treatment for the patient [
3,
5,
6,
7,
8]. Given this information and based on the literature, we know that neuropathic pain can directly and indirectly affects others tissues, including the skeletal muscle, modifying its function and structure through loss of mass, function, and muscle atrophy as shown in experimental models. However, the contributions regarding nerve injury and muscle atrophy remain uncertain [
3,
9].
Due to this, the muscle adjacent to the nerve injury may be affected by impaired nerve communication, which can potentially compromise its neuromuscular junction if denervation occurs, leading to a breakdown in communication between the nerve and the muscle [
9,
10,
11,
12]. Due to this, we chose as the main focus of this study the skeletal striated muscle tissue, specifically the gastrocnemius muscle, one of the main muscles innervated by sciatic nerve.
It is already proven in some studies that neuropathic pain contributes to atrophy and decreased muscle activation, often due to lack of use of the injured limb, which generates an increase in protein degradation, a decrease in protein synthesis, or both [
9,
13,
14,
15]. The effect of non-pharmacological treatments, such as swimming and photobiomodulation, on complications induced by the neuropathic pain model as chosen in this work is still unknown.
Physical exercise recruits many muscles of the human body, consequently increasing muscle activity through increased protein synthesis due to high muscular demand [
16,
17]. Exercise treatment is applied with the aim of reducing neuropathic pain and reducing the effects caused by muscle atrophy due to nerve constriction has been demonstrated in studies and has been increasingly studied [
18,
19,
20].
Considering a more effective and less aggressive model suitable for the type of injury of our animals, we chose Swimming as treatment for this study because water induces less impact on animals, therefore making the treatment less painful. We aim to show the morphometric effects in the gastrocnemius muscle by the tissue analysis (macroscopic and microscopic). Some studies have shown that swimming training for four weeks attenuated the progression of thermal hyperalgesia as well as allodynia in rats with neuropathic pain, demonstrating higher expression of Hsp72 and lower levels of TNF-α or IL-1β in the spinal cord compared to the injured group [
18,
20]. From these studies, we can see that swimming may be an effective treatment for neuropathic pain, as it not only reduces pain behavior but is also an economical and safe therapy that has been increasingly studied for understanding its mechanisms [
2,
9,
18,
20].
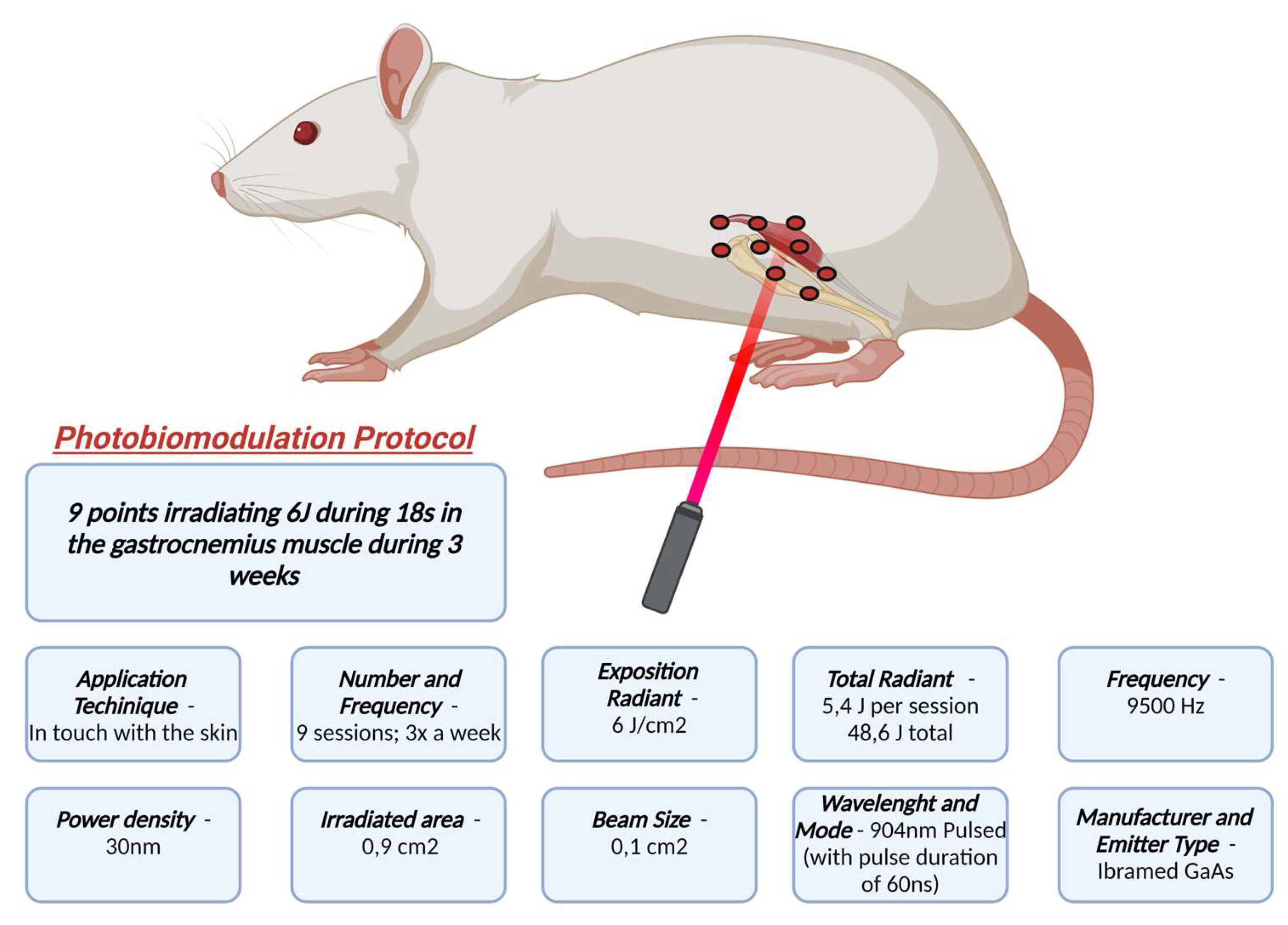
In order to complement the effects of exercise, we used Photobiomodulation Therapy (PBMT) in this study. PBMT has been increasingly studied and has been showing beneficial effects for both chronic and acute pain [
21,
22,
23]. Systematic review by Ibarra et al. (2021)[
24] showing that neurogenesis stimulated by PBMT leads to neuroprotection activation through cell oxygenation. Pigatto et al. (2020)[
25] observed in the spinal nerve injury model that the application phototherapy was able to reduce mechanical hypersensitivity and cold allodynia [
26].
Therapeutic alternatives for treating neuropathic pain are necessary, as this type of pain does not satisfactorily respond to conventional interventions such as surgical and medication-based approaches. We can suggest that the association of PBMT and SW may present potentiated effects. Both techniques have shown effectiveness in improving pain symptoms, but there are few studies detailing and exclusively studying the muscular system in these animals. Our working hypothesis is that animals with neuropathic pain treated with either therapy, or with both, may exhibit improvements in pain behaviors, muscle atrophy, contractile and motor function, and potentially benefit from enhanced factors involved in skeletal muscle maintenance and regeneration. Thus, our approach has the potential to significantly advance clinical treatments for pain resulting from conditions such as nerve compression and carpal tunnel syndrome, as well as other compressive syndromes, by utilizing non-invasive, non-pharmacological, and cost-effective techniques for patients.
2. Results
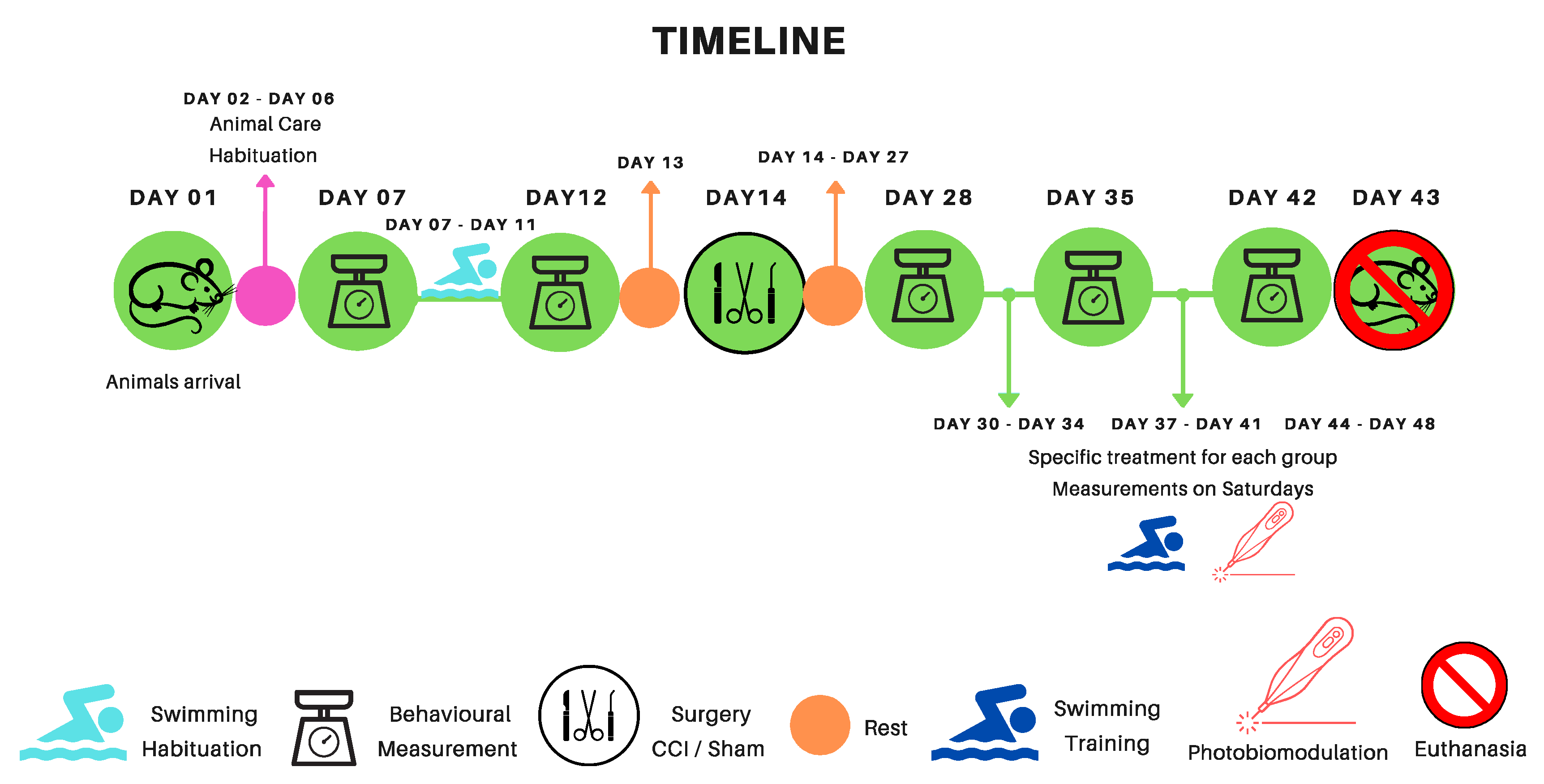
Our analysis of the behavioral test results categorizes them into three distinct groups: Exercise groups (SW), Photobiomodulation groups (PBMT), and Association groups (SW + PBMT) according to the colors below. These groups were utilized as points of comparison in all behavioral analyses and light microscopy morphometry. It’s important to note that our analyses encompassed both legs, the Experimental (Right; Leg-ipsilateral), and contralateral (Left Leg). However, as the contralateral leg did not exhibit any statistically significant differences, it is not showed in this article. In all behavioral test analyses, the following subtitle structure was employed for a better understanding:
M0 - Baseline measurements (before any procedure)
MI – Measurement after swimming habituation
M1- Post-surgical measurements (15 days after CCI or Sham - surgery)
M2, M3, M4 – After treatments measures (First week of treatment – 22 post CCI/Sham; second week of treatment – 29 post CCI/Sham; third week of treatment – 36 post CCI/Sham), respectively.
The colors below represent, in the graph, the period in which the animals underwent treatment.
■ Swimming Habituation
■ Swimming
Training (SW)
■ Photobiomodulation Treatment (PBMT)
■ Association
Treatment (SW + PBMT)
2.1. Mechanical Hyperalgesia
The results regarding the behavioral analysis of mechanical hyperalgesia utilized the Randall & Selitto tests (
Figure 1, Panels A-C) and the Electronic Von Frey Test (
Figure 1, Panels D-F). From these tests, we observed that after the injury (CCI), there was a decrease in nociceptive threshold starting from the 15th day post-surgery (M1) and persisted throughout all measurements (M2, M3, and M4) (
Figure 1). After swimming treatment (CCI + SW), we observed an improvement in the nociceptive condition from the second week of treatment, which persisted until the last session (M3; p < 0.0126 and M4; p < 0.0081) compared to the untreated CCI group (
Figure 1 A). On the other hand, no alteration was observed in the response to mechanical hyperalgesia in the group treated only with PBMT (
Figure 1 B). However, when animals were treated with both techniques (CCI + SW + PBMT), the improvement in the nociceptive condition was observed from the first session (M2; p < 0.0007; M3; p < 0.0001; M4; p < 0.0015) compared to the untreated group (CCI) (
Figure 1 C).
Regarding the Von Frey test, we observed a statistical difference only in the groups treated with photobiomodulation (CCI + PBMT) starting from the first week of treatment until the last (M2; p = 0.04; M3; p = 0.004; M4; p = 0.02) when compared to the CCI group (
Figure 1E).
2.2. Thermal Sensibility
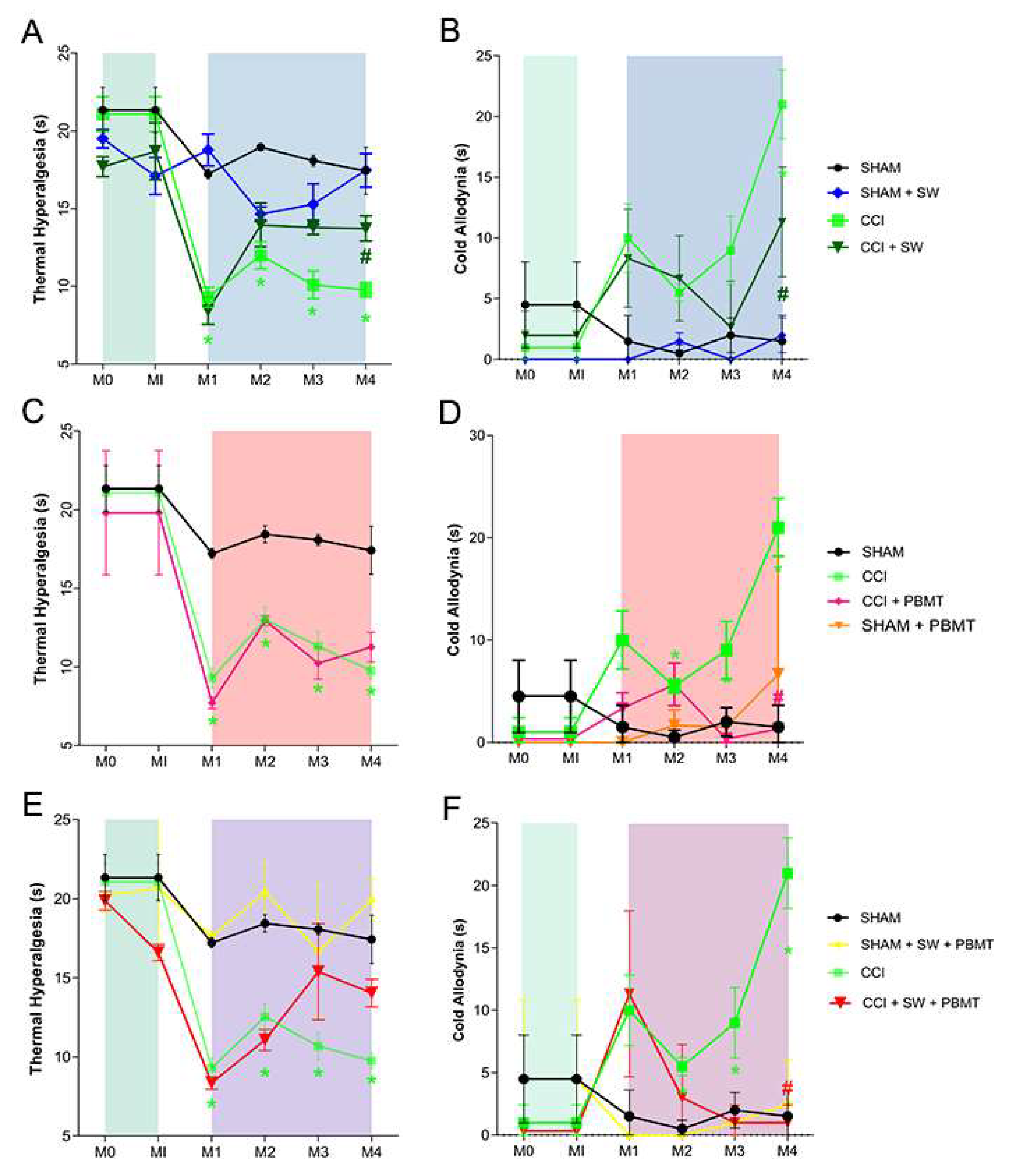
The results regarding the behavioral analysis of thermal hyperalgesia tests (
Figure 2, Panels A-C) and cold allodynia (
Figure 2, Panels D-F) demonstrated, after the injury (CCI), a decrease in nociceptive threshold starting 15th day after surgery (M1); this decrease in nociceptive threshold was maintained throughout all measurements (M2, M3, and M4) (
Figure 2) when compared with control group [
5]. Regarding the thermal hyperalgesia test (Hargreaves), it was possible to observe an improvement in the nociceptive condition in the group treated only with swimming (CCI + SW) compared to the untreated group (CCI) (
Figure 2, Panel A). It is worth mentioning that the reversal of the nociceptive condition was only observed from the third week of treatment (M4; p < 0.0311). No statistical difference was observed between the CCI and CCI treated with PBMT groups (Panel B), or even when both treatments were used together (CCI + SW + PBMT - Panel C).
Regarding the cold allodynia test, an improvement in pain condition was observed from the last week of exercise treatment (CCI + SW; Panel D) compared to untreated animals (CCI) (M4; p < 0.0020), we observed the same results when animals were treated with photobiomodulation (CCI+PBMT; Panel E (M4; p < 0.0003) and with the association of both therapies (CCI+SW+PBMT; Panel F (M4; p < 0.0001).
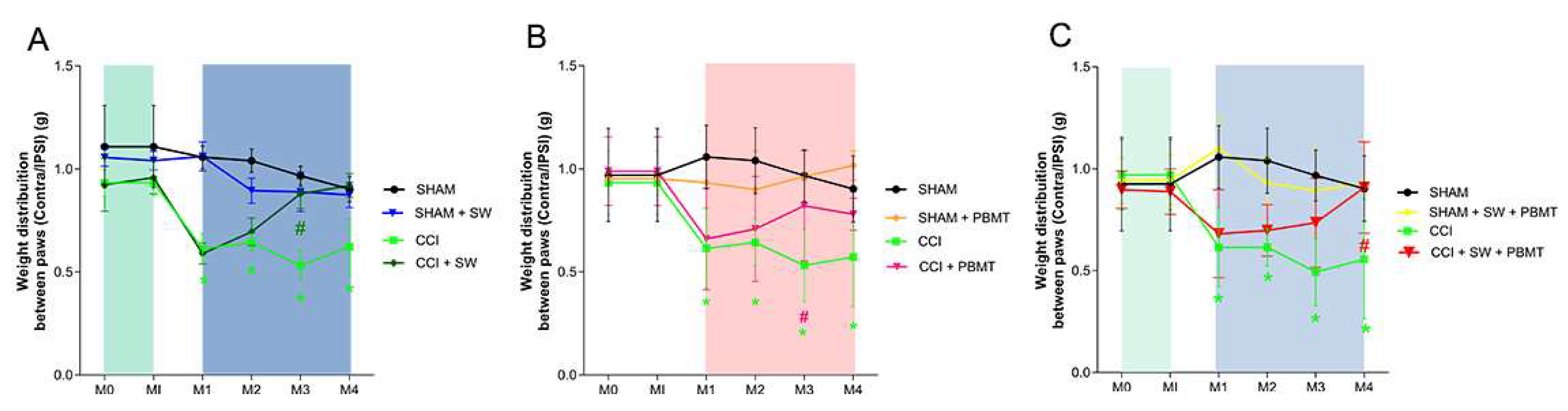
2.3. Incapacitancer Test (Static Weight Bearing)
The results regarding the analysis of the difference between the injured (ipsilateral) and non-injured (contralateral) limbs demonstrated that after the injury (CCI), there was a decrease in the difference between the limbs from the 15th day post-surgery (M1) (
Figure 3). Regarding the groups treated with swimming, photobiomodulation and association (Panel A, B and C, respectively), a reduction in the difference between the limbs was observed in the second week of treatment (M3; p < 0.0447;
Figure 3A; M3; p < 0.0240;
Figure 3B; M3; p < 0.0183;
Figure 3C) respectively (CCI + SW; CCI + PBMT; CCI + SW + PBMT).
2.4. Hematoxylin and Eosin
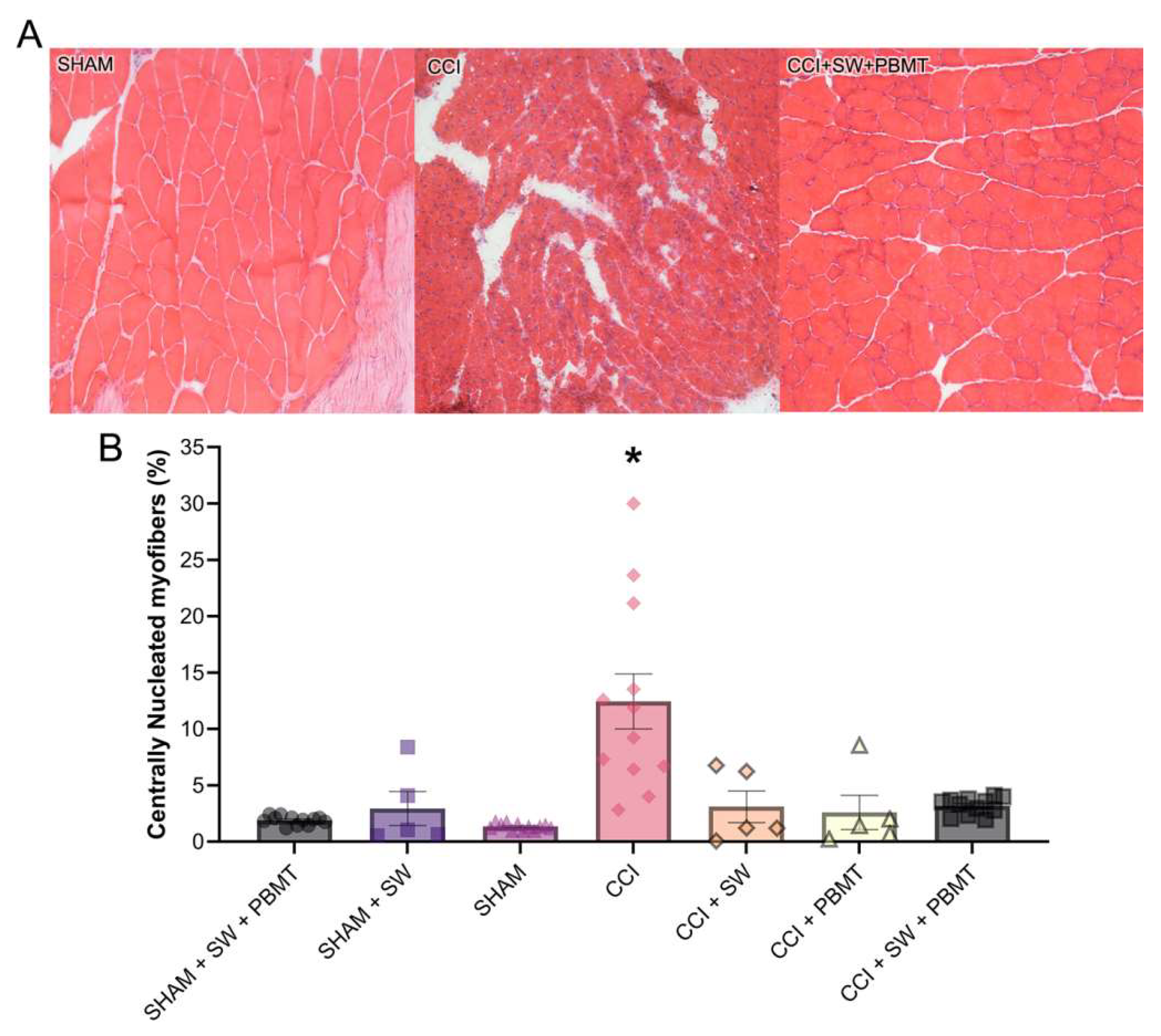
After the evaluation of the behavioral tests and specific treatments, as previously mentioned, the animals were euthanized on the 37th day post-surgery, the ipsilateral gastrocnemius muscle was collected and kept frozen. The same sample was used for the Hematoxylin and Eosin and Picro Sirius Red colorings. It was observed that in the SHAM group, the nuclei were parallel to the muscle fibers, representing the standard morphology of the skeletal striated muscle tissue of the animals (
Figure 4A). In CCI group, a massive accumulation of cellular nuclei and some fibers with centralized nuclei was observed in an attempt to recover these muscle fibers, in addition to the muscle fiber caliber (and the muscle itself) being smaller compared to the control group (
Figure 4A). Regarding the injured animals, a greater accumulation of centralized nuclei was also observed due to the neuropathy induced by CCI. However, in the exercise-treated groups (CCI + SW), it is already possible to observe that the muscle cells are returning to their usual size, also showing signs of regeneration with circular aspects, and the nuclei in the region show dissipation compared to the CCI group. When the animals were treated with Photobiomodulation (CCI + PBMT) and the combination of both techniques (CCI + SW + PBMT), they presented a similar aspect to the control group, with the nuclei parallel to the muscle fibers, representing the standard morphology of the skeletal striated muscle tissue of the animals. However, the PBMT group presents a standardized aspect of circular fibers, still with some additional nuclei in the interstitial and where the cells are not well delimited as we can see in the control and combination groups (
Figure 4A).
After taking pictures of our samples, our next step was to quantify the images. Our results demonstrate a 660% increase in inflammatory nuclei in the lesioned group (CCI) compared to the control (SHAM, considered 100%) (
Figure 4B). After the different treatments, we observed a decrease in the percentage of these centralized nuclei compared to the CCI group. This decrease was 45% for the swimming group (CCI + SW), 67% for the Photobiomodulation group (CCI + PBMT), and 76% for the combination group (CCI + SW + PBMT) (
Figure 4B).
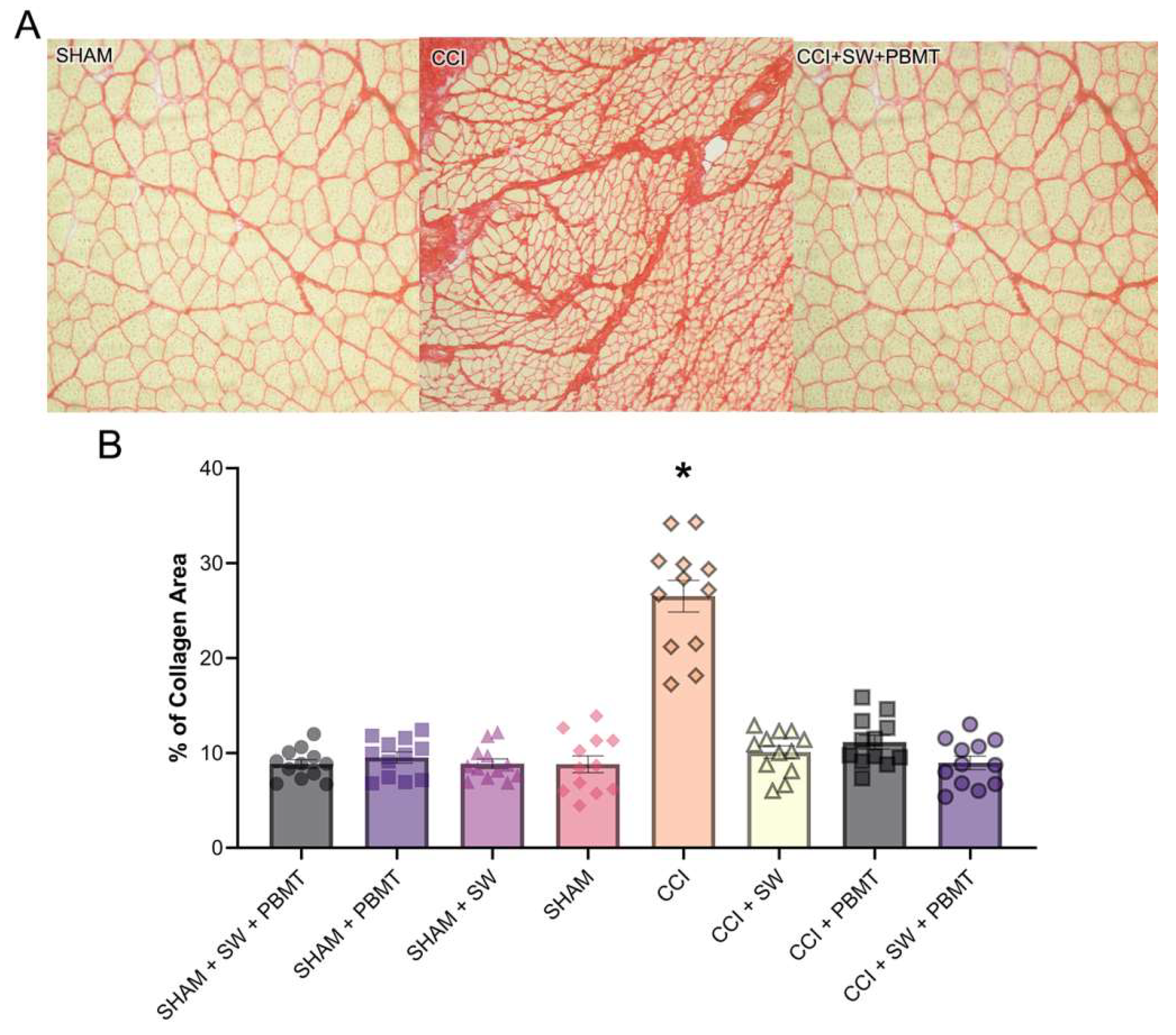
2.4.1. Interstitial Collagen - Picro Sirius Red
Standard appearance of interstitial collagen between muscle fibers and the arrangement of these fibers and congruence were observed in control group. On the other hand, after injury (CCI), an abundant accumulation of collagen and fibrotic aspects with the discharacterization of muscle fibers and a decrease in their caliber were observed (
Figure 5A). Treated groups showed a decrease in collagen expression and fibrotic aspects were observed, almost a complete reversal of the condition compared to the injured and control animals. It is also interesting to note that the treated groups (CCI + SW; CCI + PBMT; CCI + SW+ PBMT) showed a reduction in total collagen (%) and between muscle fibers compared to the SHAM and CCI groups (
Figure 5A). The same was observed in the previous experiment. Our results demonstrate a 203% increase in collagen in the injured group (CCI) compared to the control (SHAM, considered 100%). After treatments, we observed a decrease in total collagen (
Figure 5B). This decrease was 62% to the swimming treatment, 58% for the Photobiomodulation group (CCI + PBMT), and 66% for the association group (CCI + SW + PBMT) (
Figure 5B).
3. Discussion
Neuropathic pain results from damage, disease, injury, or pressure affecting the peripheral nervous system (PNS), central nervous system (CNS), and somatosensory nerves. This chronic pain condition significantly impacts individual’s quality of life and affects approximately 7-10% of the global population, with a higher prevalence in those over 50 years of age. Currently, neuropathic pain is challenging to treat and lacks a definitive cure. [
2,
3,
27]
We observed that in the CCI model, literature had some works comparing the swimming training, as well as the PBMT as treatment, although we could not find any work that focus on the association between these two treatments. Our main aim of this study was to identify if the association between Swimming and PBMT had some beneficial on pain aspects as well as in the muscle.
Given the limitations of pharmacological treatments, which are often only partially effective and may lead to adverse side effects [
7], non-pharmacological alternatives are crucial for improving patients’ quality of life. Existing literature highlights the need for effective treatments that address both the pain and associated symptoms, such as muscle atrophy and disuse. In response, our research focuses on exploring two therapeutic approaches: Photobiomodulation and Swimming. We aim to assess the effects of these therapies both individually and in combination to provide a more comprehensive solution for managing neuropathic pain.
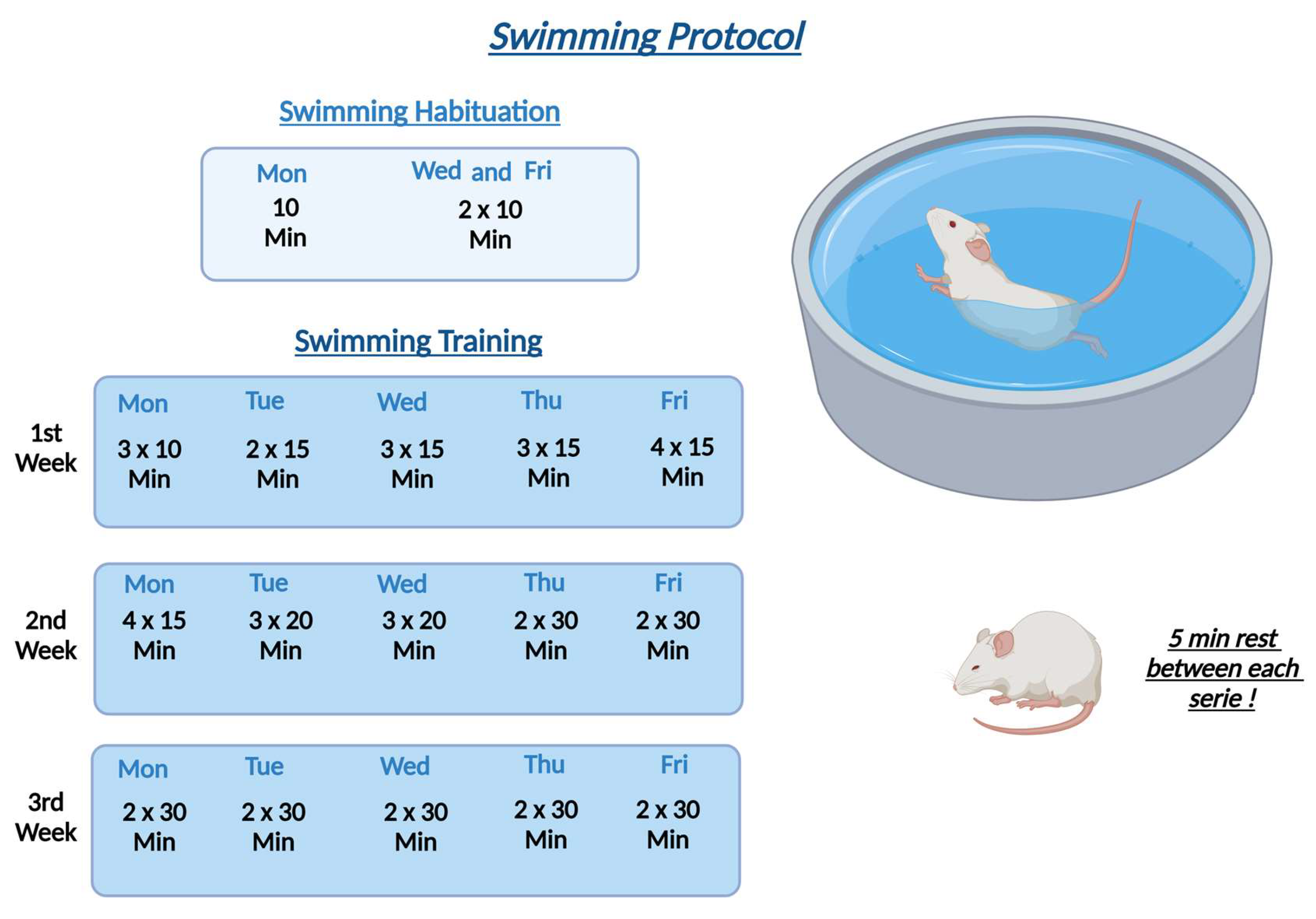
Initially, we evaluated each therapy individually and subsequently associated and compared each of them. Regarding the groups treated with swimming animals were submitted by 3 weeks of training based on the Fazard model [
18], which on the first week of swimming, animals were submitted by times of 30, 45 and 60 minutes per day on the water. From the second to third week animals spend 60 minutes of swimming per day. Our results showed an improvement in pain response in the mechanical (Randall) and thermal (hot and cold) hyperalgesia assays. This improvement in nociceptive response was observed after the second week of treatment for the mechanical hyperalgesia test (Randall & Selitto)[
28] and after the third week of treatment regarding the thermal hyperalgesia and cold allodynia.
Our results corroborate those obtained by Fazard et al. (2018) [
18], as in their study, an improvement in the nociceptive profile regarding mechanical and thermal hyperalgesia was observed from the third week of swimming treatment in injured animals (CCI model). Furthermore, other studies also demonstrate the beneficial effect of swimming in reducing nociceptive response. For example, Kuphal et al. (2007) [
19] observed that aquatic exercise reduced cold allodynia and thermal hyperalgesia.
Photobiomodulation has already shown positive results in various tissues and different conditions related to neuropathic pain [
29,
30,
31]. Similarly, Swimming has demonstrated positive outcomes in literature concerning this type of pain[
18,
19,
30]. However, little is known about the effect of these therapies on muscle tissue in the sciatic nerve constriction model. No studies were found where such therapies were associated and analyzed in this tissue. Therefore, our aim was to identify whether the combination of both therapies would have complementary effects on pain relief and a beneficial effect on muscle tissue.
When animals were subjected to Photobiomodulation therapy alone, no changes in sensitivity to mechanical stimulus were observed when using the Randall & Selitto test. However, we observed an increase in nociceptive threshold when using the Von Frey test. This improvement in pain response was observed from the first week of treatment. The same was observed when animals were exposed to thermal stimuli; we did not observe any statistical changes after the use of heat stimuli, but we observed a reversal of the pain profile when animals were exposed to cold stimuli. It’s important to note that the Randall’s test assesses withdrawal response when stimuli are applied to the anterior part of the hind paw, while the electronic von Frey test applies stimuli to the posterior part of the paw, also those test applies to different stimuli as showed by Deuis[
32] which compares some different behavioral measurements in animals and supports our results being a little different in each test.
Previous studies conducted by our research group had already demonstrated the beneficial effect of PBMT in modulating nociceptive response using the same experimental model and in other models[
21,
33,
34,
35]. However, these studies observed a reversal of the nociceptive profile using three types of behavioral assays. These discrepant results may be due to the number of sessions applied per week. Additionally, our protocol presents a different total energy, varying the number of sessions and/or power density of the device, which may lead to different results within the same research group[
29].
When we analyzed the animals subjected to the combination of both therapies, we observed improvement only when subjected to mechanical hyperalgesia and thermal stimulation. An interesting fact was that the improvement in pain profile after the combination was observed more recently, i.e., it started after the first week, demonstrating a beneficial effect when both therapies were combined. Regarding the evaluation of cold and thermal stimulation, an improvement comparable to that achieved with individual swimming treatment was observed.
These findings suggest that swimming therapy and photobiomodulation treatment may have complementary effects on pain relief, potentially offering benefits when used together. The differential effects observed between the two tests (Randall’s and von Frey electronic) could indicate varying mechanisms of action or sensitivity to treatment modalities in different pain assessment contexts[
32].
Regarding the evaluation of animal motor function, such as, the difference in weight deposited on the ipsi and contralateral hindpaws, performed in the incapacitance test, we observed an improvement from the second week of treatment in animals subjected to swimming or PBMT alone, and even during the association of both therapies. This result was somewhat surprising, as we anticipated a positive effect on nociceptive responses when both therapies were combined early, compared to individual treatments. However, our results are in line with those observed by Cobianchi et al. (2010) and Palandi (2020)[
36,
37], where they also observed an improvement in motor function after treadmill exercise.
There are no studies described in the literature with PBMT associated with Swimming in the model of neuropathic pain induced by sciatic nerve constriction, however, some results using other experimental models have shown beneficial effects in the combination of both therapies. For example, in the study by Beasi et al. (2021)[
30], where the combination of these two therapies in the process of anterior tibial muscle regeneration after cryoinjury was shown to be more efficient than when both therapies are applied individually. Furthermore, the study by Malta et al. (2020)[
38] suggested a beneficial effect of PBMT individually or in conjunction with creatine supplementation during a 12-week training program, resulting in significantly better muscle performance and lower levels of CK (a biochemical marker of muscle damage). Additionally, works by Farazi et al. (2022)[
39] also demonstrated that the combination of PBMT and a recreational environment can be effective in reducing depression and anxiety-like behaviors in mice, possibly through modulation of corticosterone levels and the hippocampal BDNF/TrkB/CREB pathway. Dutra et al. (2022)[
40] observed that, in humans, PBMT improves muscular endurance performance in single-joint exercises but is not effective for muscular strength performance.
When considered together, these data highlight the efficacy of both therapies not only in improving nociceptive behavior but also motor profiles, depending on the model and treatment employed. Additionally, it underscores the potential positive impact of these non-pharmacological therapies on patients’ quality of life. [
3,
7,
37]
The importance of histological assays, such as hematoxylin and eosin and picrosirius staining, is unquestionable in scientific and clinical research. These analyses provide a detailed view of the microscopic characteristics of tissues and organs, allowing the identification of morphological patterns, assessment of pathological changes, and understanding the effects of different treatments or experimental conditions. In the context of muscle regeneration, the application of these histological assays is crucial for examining tissue architecture and composition, as well as assessing the nature and extent of damage or repair. These techniques not only enrich scientific understanding but also play an essential role in the development of therapeutic strategies, offering significant insights for the optimization of medical interventions and regenerative therapies [
41,
42,
43].
Regarding the stained with hematoxylin and eosin, a decrease in centrally located nuclei was observed in all groups treated individually or in combination compared to CCI animals. These showed massive accumulation of cellular nuclei and some fibers with centralized nuclei as an attempt to recover such muscle fibers. In the groups treated with exercise, it is possible to observe that muscle cells are returning to their usual size, presenting signs of regeneration with circular aspects. In animals treated with PBMT and the combination of both techniques, a similar aspect to the control group is presented, where the nuclei are situated parallel to the muscle fibers, being a standard aspect of the tissue morphology of skeletal striated muscle in animals.After image quantification, we observed an increased level of inflammatory nuclei and a decrease in inflammation by 45% in the Swimming, 67% in Photobiomodulation, and 76% for combination groups. Our results corroborate with several studies suggesting the beneficial effect of photobiomodulation [
44] and swimming [
18,
37,
45,
46,
47] in reducing inflammatory response and assisting in muscle regeneration.Regarding Picro Sirius Red staining, the standard aspect of interstitial collagen between muscle fibers and the arrangement of these fibers and congruence in the control groups was observed. When chronic neuropathic injury was performed, an abundant accumulation of interstitial collagen and fibrotic aspects with the discharacterization of muscle fibers were observed, now shown as muscle atrophy, characterized by a decrease in the caliber of muscle fibers. In groups treated with isolated or combined therapies, a decrease in the expression of total collagen in percentage, reduction of interstitial collagen between muscle fibers, and fibrotic aspects were observed. Our quantifications corroborate with our visual results, where an increase of 203% in total collagen was presented in injured group. After specific treatments, we could observe a decrease in this total collagen by 62% in swimming, 58% in photobiomodulation, and 66% for combination of both treatments. Similar results were also observed in the study by Shanti et al., 2013[
48], that the combination of intramuscular collagen with an anti-inflammatory agent aided in reducing pain in a CCI model [
48,
49]. Thus, demonstrating that collagen, in addition to contributing to reducing the inflammatory response and muscle regeneration, also acts on improving the painful condition, even indirectly [
43,
49,
50,
51,
52].