Submitted:
26 August 2024
Posted:
27 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
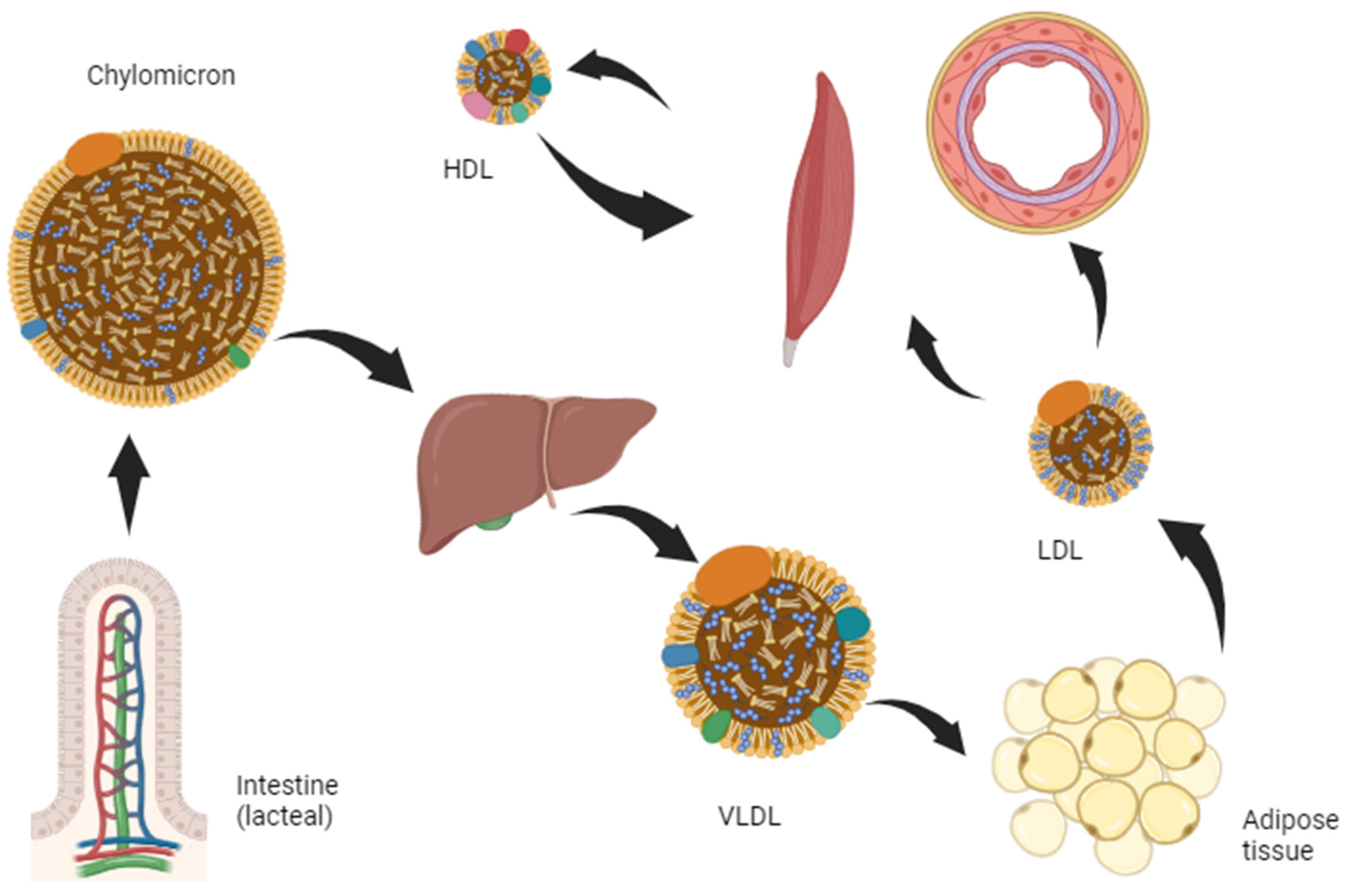
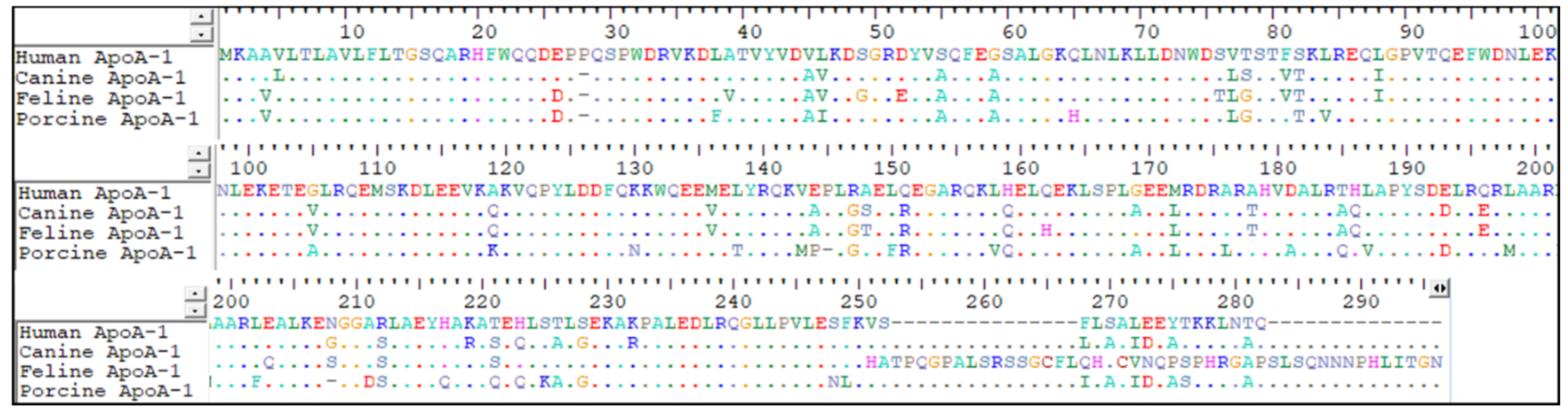
2. Materials and Methods
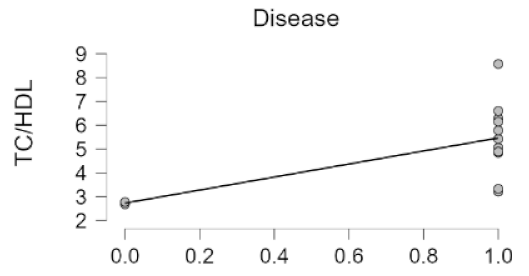
3. Results
4. Discussion
Author Contributions
Data Availability Statement
Ethical Statement
References
- Meyer, J.S. , et al., Hyperlipidemia is a risk factor for decreased cerebral perfusion and stroke. Archives of neurology. 1987, 44, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.L. , et al., Hyperlipidemia in coronary heart disease I. Lipid levels in 500 survivors of myocardial infarction. The Journal of Clinical Investigation. 1973, 52, 1533–1543. [Google Scholar] [CrossRef]
- Wierzbicki, A. , et al., Statin-fibrate combination therapy for hyperlipidaemia: a review. Current medical research and opinion 2003, 19, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Ruth McPherson, M. , Long-term efficacy and safety of fenofibrate and a statin in the treatment of combined hyperlipidemia. The American journal of cardiology. 1998, 81, 60B–65B. [Google Scholar]
- Lim, H.Y. , et al., Lymphatic vessels are essential for the removal of cholesterol from peripheral tissues by SR-BI-mediated transport of HDL. Cell metabolism. 2013, 17, 671–684. [Google Scholar] [CrossRef]
- Xiang, A.S. and B.A. Kingwell, Rethinking good cholesterol: a clinicians' guide to understanding HDL. The Lancet Diabetes & Endocrinology. 2019, 7, 575–582. [Google Scholar]
- Oda, H. , et al., Cholesterol concentrations in lipoprotein fractions separated by anion-exchange–high-performance liquid chromatography in healthy dogs and dogs with hypercholesterolemia. Research in veterinary science. 2017, 114, 163–169. [Google Scholar] [CrossRef]
- Boynosky, N.A. and L. Stokking, Atherosclerosis associated with vasculopathic lesions in a golden retriever with hypercholesterolemia. The Canadian Veterinary Journal. 2014, 55, 484. [Google Scholar]
- Guo, W. , et al., Diagnostic values and appropriate cutoff points of lipid ratios in patients with abnormal glucose tolerance status: a cross-sectional study. Lipids in health and disease. 2019, 18, 1–8. [Google Scholar] [CrossRef]
- Lemieux, I. , et al., Total cholesterol/HDL cholesterol ratio vs LDL cholesterol/HDL cholesterol ratio as indices of ischemic heart disease risk in men: the Quebec Cardiovascular Study. Archives of internal medicine. 2001, 161, 2685–2692. [Google Scholar] [CrossRef]
- Xiang, S.-K. , et al., Relationship between serum lipoprotein ratios and insulin resistance in polycystic ovary syndrome. International journal of endocrinology. 2012, 2012, 173281. [Google Scholar] [CrossRef] [PubMed]
- Sun, X. , et al., Lipid abnormalities in patients with Cushing’s disease and its relationship with impaired glucose metabolism. Frontiers in endocrinology. 2021, 11, 600323. [Google Scholar] [CrossRef]
- Zhao, X. , et al., Association between the non-HDL-cholesterol-to-HDL-cholesterol ratio and the risk of gallbladder polyp formation among men: a retrospective cohort study. Lipids in health and disease. 2020, 19, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Feely, J. , et al., Total cholesterol/HDL-cholesterol ratio in hyperthyroidism, hypothyroidism and subclinical hypothyroidism. Hormone and Metabolic Research. 1980, 12, 560–561. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.Y. , et al., Total cholesterol to high-density lipoprotein cholesterol ratio is a significant predictor of nonalcoholic fatty liver: Jinchang cohort study. Lipids in health and disease. 2019, 18, 1–7. [Google Scholar] [CrossRef]
- Yoshida, A. , et al., Usefulness of serum total cholesterol/triglyceride ratio for predicting the presence of small, dense LDL. Journal of atherosclerosis and thrombosis. 2004, 11, 215–219. [Google Scholar] [CrossRef]
- Hirayama, S. and T. Miida, Small dense LDL: an emerging risk factor for cardiovascular disease. Clinica Chimica Acta. 2012, 414, 215–224. [Google Scholar] [CrossRef]
- Superko, H. and B. Garrett, Small dense LDL: Scientific background, clinical relevance, and recent evidence still a risk even with ‘normal’LDL-C levels. Biomedicines. 2022, 10, 829. [Google Scholar] [CrossRef]
- Rizzo, M. and K. Berneis, Small, dense low-density-lipoproteins and the metabolic syndrome. Diabetes/metabolism research and reviews. 2007, 23, 14–20. [Google Scholar] [CrossRef]
- Sohda, T. , et al., Reduced expression of low-density lipoprotein receptor in hepatocellular carcinoma with paraneoplastic hypercholesterolemia. Journal of Gastroenterology and Hepatology. 2008, 23, e153–e156. [Google Scholar] [CrossRef]
- Jiang, S.-S. , et al., The clinical significance of preoperative serum cholesterol and high-density lipoprotein-cholesterol levels in hepatocellular carcinoma. Journal of Cancer. 2016, 7, 626. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, H.N. , Diabetic dyslipidemia: basic mechanisms underlying the common hypertriglyceridemia and low HDL cholesterol levels. Diabetes. 1996, 45, S27–S30. [Google Scholar] [CrossRef] [PubMed]
- Rand, J.S. and G.J. Martin, Management of feline diabetes mellitus. Veterinary Clinics of North America: Small Animal Practice. 2001, 31, 881–913. [Google Scholar] [CrossRef] [PubMed]
- Cheon, E.J. , et al., Novel association between CDKAL1 and cholesterol efflux capacity: Replication after GWAS-based discovery. Atherosclerosis. 2018, 273, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Driehuys, S. , et al., Myocardial infarction in dogs and cats: 37 cases (1985-1994). Journal of the American Veterinary Medical Association. 1998, 213, 1444–1448. [Google Scholar] [CrossRef]
- Corbalan, R., R. Verrier, and B. Lown, Psychological stress and ventricular arrhythmias during myocardial infarction in the conscious dog. The American Journal of Cardiology. 1974, 34, 692–696. [Google Scholar] [CrossRef]
- Mori, N. , et al., Predisposition for primary hyperlipidemia in Miniature Schnauzers and Shetland sheepdogs as compared to other canine breeds. Research in veterinary science. 2010, 88, 394–399. [Google Scholar] [CrossRef]
- Borgeat, K. , et al. Arterial thromboembolism in 250 cats in general practice: 2004–2012. Journal of Veterinary Internal Medicine. 2014, 28, 102–108. [Google Scholar] [CrossRef]
- Konečný, F. , Thromboembolic conditions, aetiology diagnosis and treatment in dogs and cats. Acta Veterinaria Brno. 2010, 79, 497–508. [Google Scholar] [CrossRef]
- Tran-Dinh, A. , et al., HDL and endothelial protection. British journal of pharmacology. 2013, 169, 493–511. [Google Scholar] [CrossRef]
| Dog# | Body weight (kg) | BCS(1 to 9 ) | Breed | Sex | Age | HDL (60~140) | TC (111~312) | TG (30~133) | TC/HDL | TC/TG | Visit purpose | Concurrent disease | Heart Murmur (0 to 6) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 5 | Pomeranian | Spayed female | 9y | 140 | 450 | 78 | 3.21 | 5.77 | Annual checkup | None | 0 |
| 2 | 23 | 4 | Jindo | Male castrated | 9y | 56 | 352 | 78 | 6.28 | 4.51 | Annual checkup | None | 0 |
| 3 | 2.2 | 4 | Poodle | Female | 9m | 64 | 172 | 218 | 2.68 | 0.79 | Achilles tendon repair surgery | None | 0 |
| 4 | 3.65 | 5 | Maltese | Spayed female | 3y7m | 80 | 223 | 19 | 2.78 | 11.7 | Right patella luxation surgery | None | 0 |
| 5 | 4.2 | 5.5 | Poodle | Spayed female | 7y7m | 56 | 369 | 104 | 6.6 | 3.54 | Regular checkup | Gallbladder mucocele | 0 |
| 6 | 3.7 | 4 | Poodle | Male castrated | 13y | 46 | 233 | 225 | 5.06 | 1.03 | Regular checkup | Cushing/GERD | 1 |
| 7 | 2.85 | 5 | Chiwawa | Spayed female | 11y2m | 83 | 450 | 170 | 5.42 | 2.64 | Regular checkup | Cushing | 0 |
| 8 | 5.15 | 5.5 | Maltese | Male castrated | 12y | 87 | 290 | 247 | 3.33 | 1.17 | Cognitive disorder treatment | Cognitive disorder/Cushing | 0 |
| 9 | 15.3 | 4 | Shetland Sheepdog | Male castrated | 13y5m | 65 | 315 | 500 | 4.84 | 0.63 | Cancer treatment | Liver cancer (HCC) | 2 |
| 10 | 2.95 | 4 | Maltese | Spayed female | 14y9m | 92 | 450 | 500 | 4.89 | 0.9 | Cognitive disorder treatment | Gallbladder mucocele/ Cognitive disorder | 0 |
| 11 | 8.8 | 4.5 | Jindo | Spayed female | 8y7m | 42 | 243 | 36 | 5.78 | 6.75 | Regular checkup | Hypothyroidism | 0 |
| 12 | 3.68 | 6 | Pomeranian | Male castrated | 4y9m | 42 | 360 | 402 | 8.57 | 0.89 | Regular checkup | Hypothyroidism | 0 |
| 13 | 3.5 | 6 | Pomeranian | Male castrated | 5y5m | 26 | 160 | 170 | 6.15 | 0.94 | Dental scaling | None | 0 |
| Cat# | Body weight (kg) | BCS(1 to 9 ) | Breed | Sex | Age | HDL (60~140) | TC (85~176) | TG (17~104) | TC/HDL | TC/TG | Visit purpose | Concurrent disease | Heart Murmur (0 to 6) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 8.1 | 9 | American Shorthair | Spayed female | 5y | 75 | 85 | 21 | 1.13 | 4.04 | Dysuria | Dysuria | 0 |
| 2 | 3.9 | 4 | KoreanShorthair | Male castrated | 6y 6m | 21 | 202 | 117 | 9.53 | 1.72 | Insulin treatment | Diabetes | 0 |
| 3 | 3 | 4 | KoreanShorthair | Spayed female | 10m | 93 | 138 | 36 | 1.48 | 3.83 | OHE surgery | None | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





